Infrared Polaritonic Biosensors Based on Two-Dimensional Materials
Abstract
1. Introduction
2. State-of-the-Art Polaritonic in 2D Materials

3. Infrared Plasmonic Biosensing in Graphene

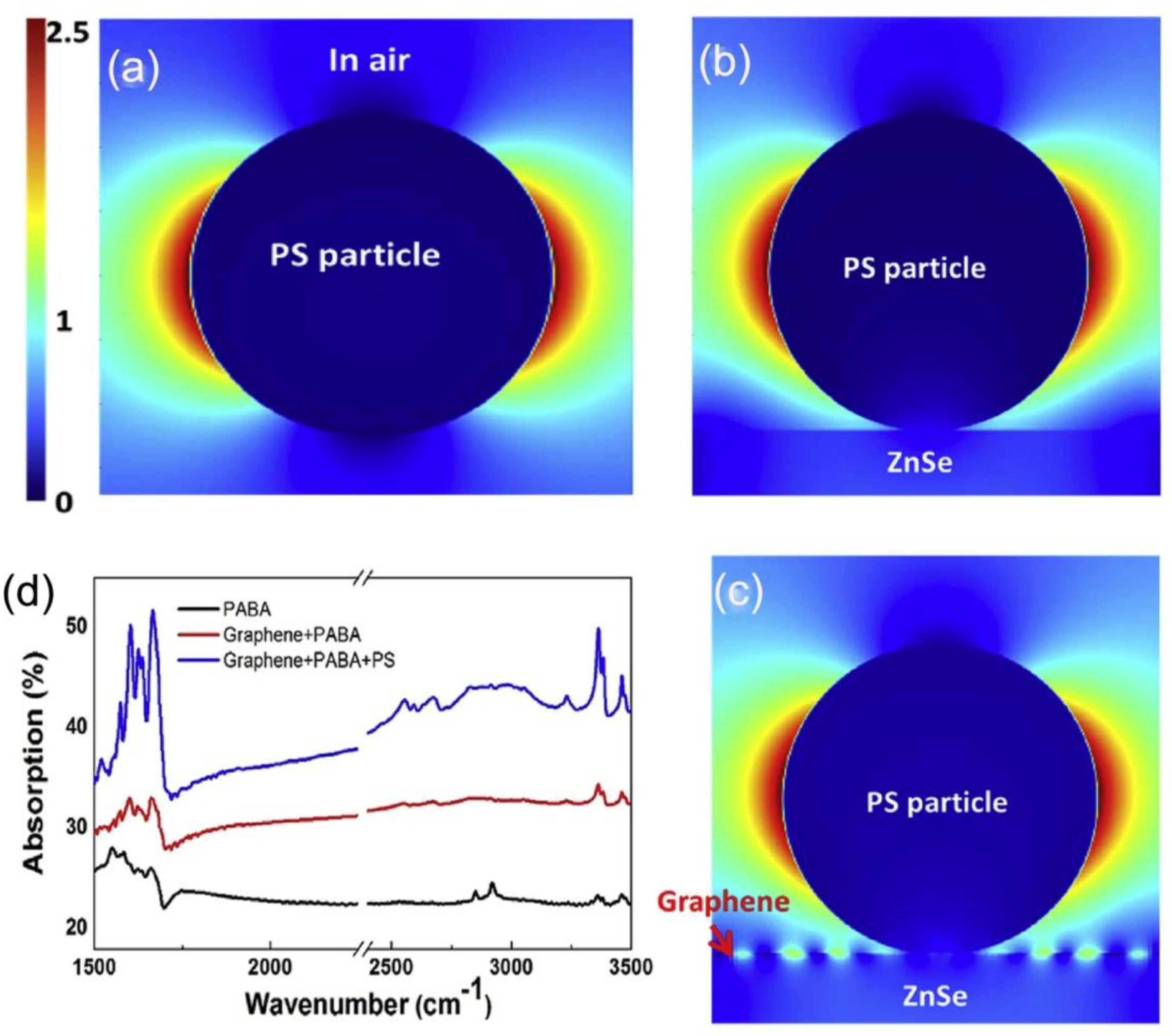
4. Infrared Plasmonic Biosensing in Hybrid Graphene-Dielectric System
5. Infrared Plasmonic Biosensing in Hybrid Graphene-Metal System
6. Novel 2D Antimonene for Plasmonic Biosensing
7. Infrared Phonon Polaritonic Biosensing in h-BN

8. Conclusions and Outlooks
Author Contributions
Funding
Conflicts of Interest
References
- Franke, K.; Vlasits, A. Unblinding with infrared nanosensors. Science 2020, 368, 1057–1058. [Google Scholar] [CrossRef]
- Deng, B.; Ma, C.; Wang, Q.; Yuan, S.; Watanabe, K.; Taniguchi, T.; Zhang, F.; Xia, F. Strong mid-infrared photoresponse in small-twist-angle bilayer graphene. Nat. Photonics 2020, 14, 549–553. [Google Scholar] [CrossRef]
- Shi, J.; Wong, T.T.W.; He, Y.; Li, L.; Zhang, R.; Yung, C.S.; Hwang, J.; Maslov, K.; Wang, L.V. High-resolution, high-contrast mid-infrared imaging of fresh biological samples with ultraviolet-localized photoacoustic microscopy. Nat. Photonics 2019, 13, 609–615. [Google Scholar] [CrossRef]
- Guo, H.; Herkommer, C.; Billat, A.; Grassani, D.; Zhang, C.; Pfeiffer, M.H.P.; Weng, W.; Brès, C.S.; Kippenberg, T.J. Mid-infrared frequency comb via coherent dispersive wave generation in silicon nitride nanophotonic waveguides. Nat. Photonics 2018, 12, 330–335. [Google Scholar] [CrossRef]
- Muraviev, A.V.; Smolski, V.O.; Loparo, Z.E.; Vodopyanov, K.L. Massively parallel sensing of trace molecules and their isotopologues with broadband subharmonic mid-infrared frequency combs. Nat. Photonics 2018, 12, 209–214. [Google Scholar] [CrossRef]
- Li, C.; Zhang, D.; Slipchenko, M.N.; Cheng, J.X. Mid-Infrared Photothermal Imaging of Active Pharmaceutical Ingredients at Submicrometer Spatial Resolution. Anal. Chem. 2017, 89, 4863–4867. [Google Scholar] [CrossRef] [PubMed]
- Holman, H.-Y.N.; Bjornstad, K.A.; Martin, M.C.; Mckinney, W.R.; Blakely, E.A.; Blankenberg, F.G. Mid-infrared reflectivity of experimental atheromas. J. Biomed. Opt. 2008, 13, 030503. [Google Scholar] [CrossRef][Green Version]
- Zhang, D.; Li, C.; Slipchenko, M.; Zhang, C.; Cheng, J.X. Depth-resolved mid-infrared photothermal imaging of living cells and organisms at sub-micron resolution. Sci. Adv. 2016, 2, e1600521. [Google Scholar] [CrossRef]
- Shivananju, B.N.; Yu, W.; Liu, Y.; Zhang, Y.; Lin, B.; Li, S.; Bao, Q. The Roadmap of Graphene-Based Optical Biochemical Sensors. Adv. Funct. Mater. 2017, 27, 1603918. [Google Scholar] [CrossRef]
- Hoh, H.Y.; Shivananju, B.N.; Li, C.M.; Bao, Q. 2D Materials for Bio-Photonic Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; ISBN 9780081026373. [Google Scholar]
- Shivananju, B.N.; Hoh, H.Y.; Yu, W.; Bao, Q. Optical Biochemical Sensors based on 2D Materials; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; ISBN 9780081025772. [Google Scholar]
- Ma, W.; Shabbir, B.; Ou, Q.; Dong, Y.; Chen, H.; Li, P.; Zhang, X.; Lu, Y.; Bao, Q. Anisotropic polaritons in van der Waals materials. InfoMat 2020, 2, 777–790. [Google Scholar] [CrossRef]
- Low, T.; Chaves, A.; Caldwell, J.D.; Kumar, A.; Fang, A.K.N.X.; Avouris, P.; Heinz, T.F.; Guinea, F.; Martin-Moreno, L.; Koppens, F. Polaritons in layered two-dimensional materials. Nat. Mater. 2016, 16, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Alonso-González, P.; Li, S.; Nikitin, A.Y.; Yuan, J.; Martín-Sánchez, J.; Taboada-Gutiérrez, J.; Amenabar, I.; Li, P.; Vélez, S.; et al. In-plane anisotropic and ultra-low-loss polaritons in a natural van der Waals crystal. Nature 2018, 562, 557–562. [Google Scholar] [CrossRef]
- Lang, J.; Chang, D.; Piazza, F. Interaction-Induced Transparency for Strong-Coupling Polaritons. Phys. Rev. Lett. 2020, 125, 133604. [Google Scholar] [CrossRef]
- Hu, G.; Ou, Q.; Si, G.; Wu, Y.; Wu, J.; Dai, Z.; Krasnok, A.; Mazor, Y.; Zhang, Q.; Bao, Q.; et al. Topological polaritons and photonic magic angles in twisted α-MoO3 bilayers. Nature 2020, 582, 209–213. [Google Scholar] [CrossRef]
- Cristofolini, P.; Hatzopoulos, Z.; Savvidis, P.G.; Baumberg, J.J. Generation of Quantized Polaritons below the Condensation Threshold. Phys. Rev. Lett. 2018, 121, 67401. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.W.; Jia, N.; Schine, N.; Baum, C.; Georgakopoulos, A.; Simon, J. Interacting Floquet polaritons. Nature 2019, 571, 532–536. [Google Scholar] [CrossRef]
- Berini, P.; De Leon, I. Surface plasmon-polariton amplifiers and lasers. Nat. Photonics 2012, 6, 16–24. [Google Scholar] [CrossRef]
- Vasa, P.; Wang, W.; Pomraenke, R.; Maiuri, M.; Manzoni, C.; Cerullo, G.; Lienau, C. Optical stark Effects in J-Aggregate_Metal Hybrid Nanostructures Exhibiting a Strong. Phys. Rev. Lett. 2015, 114, 036802. [Google Scholar] [CrossRef]
- Larkin, I.A.; Keil, K.; Vagov, A.; Croitoru, M.D.; Axt, V.M. Superanomalous Skin Effect for Surface Plasmon Polaritons. Phys. Rev. Lett. 2017, 119, 176801. [Google Scholar] [CrossRef] [PubMed]
- Collini, E.; Todescato, F.; Ferrante, C.; Bozio, R.; Scholes, G.D. Photophysics and dynamics of surface plasmon polaritons-mediated energy transfer in the presence of an applied electric field. J. Am. Chem. Soc. 2012, 134, 10061–10070. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Ma, S.; Ding, K.; Zhang, S.; Pendry, J.B. Continuous topological transition from metal to dielectric. Proc. Natl. Acad. Sci. USA 2020, 117, 16739–16742. [Google Scholar] [CrossRef]
- Dunkelberger, A.D.; Ellis, C.T.; Ratchford, D.C.; Giles, A.J.; Kim, M.; Kim, C.S.; Spann, B.T.; Vurgaftman, I.; Tischler, J.G.; Long, J.P.; et al. Active tuning of surface phonon polariton resonances via carrier photoinjection. Nat. Photonics 2018, 12, 50–56. [Google Scholar] [CrossRef]
- Baaske, M.D.; Vollmer, F. Optical observation of single atomic ions interacting with plasmonic nanorods in aqueous solution. Nat. Photonics 2016, 10, 733–739. [Google Scholar] [CrossRef]
- Mauranyapin, N.P.; Madsen, L.S.; Taylor, M.A.; Waleed, M.; Bowen, W.P. Evanescent single-molecule biosensing with quantum-limited precision. Nat. Photonics 2017, 11, 477–481. [Google Scholar] [CrossRef]
- Limaj, O.; Etezadi, D.; Wittenberg, N.J.; Rodrigo, D.; Yoo, D.; Oh, S.H.; Altug, H. Infrared Plasmonic Biosensor for Real-Time and Label-Free Monitoring of Lipid Membranes. Nano Lett. 2016, 16, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Guardado, A.; Barkam, S.; Peppler, M.; Biswas, A.; Dennis, W.; Das, S.; Seal, S.; Chanda, D. Enzyme-Free Plasmonic Biosensor for Direct Detection of Neurotransmitter Dopamine from Whole Blood. Nano Lett. 2019, 19, 449–454. [Google Scholar] [CrossRef]
- Assad, O.N.; Gilboa, T.; Spitzberg, J.; Juhasz, M.; Weinhold, E.; Meller, A. Light-Enhancing Plasmonic-Nanopore Biosensor for Superior Single-Molecule Detection. Adv. Mater. 2017, 29, 1605442. [Google Scholar] [CrossRef]
- Liang, F.; Guo, Y.; Hou, S.; Quan, Q. Photonic-plasmonic hybrid single-molecule nanosensor measures the effect of fluorescent labels on DNA-protein dynamics. Sci. Adv. 2017, 3, e1602991. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, T.; Chen, J.; Wang, C.; Zhang, H.; Shao, Y. Two-dimensional nanomaterial-based plasmonic sensing applications: Advances and challenges. Coord. Chem. Rev. 2020, 410, 213218. [Google Scholar] [CrossRef]
- Oh, S.H.; Altug, H. Performance metrics and enabling technologies for nanoplasmonic biosensors. Nat. Commun. 2018, 9, 5263. [Google Scholar] [CrossRef]
- Rodrigo, D.; Limaj, O.; Janner, D.; Etezadi, D.; de Abajo, F.J.G.; Pruneri, V.; Altug, H. Mid-infrared plasmonic biosensing with graphene. Science 2015, 349, 165–168. [Google Scholar] [CrossRef]
- Sreekanth, K.V.; Alapan, Y.; Elkabbash, M.; Ilker, E.; Hinczewski, M.; Gurkan, U.A.; De Luca, A.; Strangi, G. Extreme sensitivity biosensing platform based on hyperbolic metamaterials. Nat. Mater. 2016, 15, 621–627. [Google Scholar] [CrossRef]
- Dai, S.; Ma, Q.; Liu, M.K.; Andersen, T.; Fei, Z.; Goldflam, M.D.; Wagner, M.; Watanabe, K.; Taniguchi, T.; Thiemens, M.; et al. Graphene on hexagonal boron nitride as a tunable hyperbolic metamaterial. Nat. Nanotechnol. 2015, 10, 682–686. [Google Scholar] [CrossRef]
- Rodrigo, D.; Tittl, A.; Limaj, O.; De Abajo, F.J.G.; Pruneri, V.; Altug, H. Double-layer graphene for enhanced tunable infrared plasmonics. Light Sci. Appl. 2017, 6, e16277. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, Z.; Zhu, C.; Wang, L.; Zang, J. Out-of-Plane Designed Soft Metasurface for Tunable Surface Plasmon Polariton. Nano Lett. 2018, 18, 1435–1441. [Google Scholar] [CrossRef]
- Guo, Q.; Li, C.; Deng, B.; Yuan, S.; Guinea, F.; Xia, F. Infrared Nanophotonics Based on Graphene Plasmonics. ACS Photonics 2017, 4, 2989–2999. [Google Scholar] [CrossRef]
- Gopalan, K.K.; Paulillo, B.; Mackenzie, D.M.A.; Rodrigo, D.; Bareza, N.; Whelan, P.R.; Shivayogimath, A.; Pruneri, V. Scalable and Tunable Periodic Graphene Nanohole Arrays for Mid-Infrared Plasmonics. Nano Lett. 2018, 18, 5913–5918. [Google Scholar] [CrossRef]
- Autore, M.; Li, P.; Dolado, I.; Alfaro-Mozaz, F.J.; Esteban, R.; Atxabal, A.; Casanova, F.; Hueso, L.E.; Alonso-González, P.; Aizpurua, J.; et al. Boron nitride nanoresonators for Phonon-Enhanced molecular vibrational spectroscopy at the strong coupling limit. Light Sci. Appl. 2018, 7, 17172–17178. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Chen, R.; Li, P.; Nikitin, A.Y.; Hillenbrand, R.; Zhang, X. Extremely Confined Acoustic Phonon Polaritons in Monolayer-hBN/Metal Heterostructures for Strong Light-Matter Interactions. ACS Photonics 2020, 7, 2610–2617. [Google Scholar] [CrossRef]
- McGinnity, T.L.; Dominguez, O.; Curtis, T.E.; Nallathamby, P.D.; Hoffman, A.J.; Roeder, R.K. Hafnia (HfO2) nanoparticles as an X-ray contrast agent and mid-infrared biosensor. Nanoscale 2016, 8, 13627–13637. [Google Scholar] [CrossRef] [PubMed]
- Basov, D.N.; Fogler, M.M.; Garcia de Abajo, F.J. Polaritons in van der Waals materials. Science 2016, 354, 6309. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.D.; Vurgaftman, I.; Tischler, J.G. Mid-infrared nanophotonics: Probing hyperbolic polaritons. Nat. Photonics 2015, 9, 638–640. [Google Scholar] [CrossRef]
- Sun, Z.; Basov, D.N.; Fogler, M.M. Adiabatic Amplification of Plasmons and Demons in 2D Systems. Phys. Rev. Lett. 2016, 117, 076805. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Galfsky, T.; Sun, Z.; Xia, F.; Lin, E.C.; Lee, Y.H.; Kéna-Cohen, S.; Menon, V.M. Strong light-matter coupling in two-dimensional atomic crystals. Nat. Photonics 2014, 9, 30–34. [Google Scholar] [CrossRef]
- Splendiani, A.; Sun, L.; Zhang, Y.; Li, T.; Kim, J.; Chim, C.Y.; Galli, G.; Wang, F. Emerging photoluminescence in monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 2–5. [Google Scholar] [CrossRef]
- Jones, A.M.; Yu, H.; Ghimire, N.J.; Wu, S.; Aivazian, G.; Ross, J.S.; Zhao, B.; Yan, J.; Mandrus, D.G.; Xiao, D.; et al. Optical generation of excitonic valley coherence in monolayer WSe 2. Nat. Nanotechnol. 2013, 8, 634–638. [Google Scholar] [CrossRef]
- You, Y.; Zhang, X.X.; Berkelbach, T.C.; Hybertsen, M.S.; Reichman, D.R.; Heinz, T.F. Observation of biexcitons in monolayer WSe 2. Nat. Phys. 2015, 11, 477–481. [Google Scholar] [CrossRef]
- Dai, S.; Fei, Z.; Ma, Q.; Rodin, A.S.; Wagner, M.; McLeod, A.S.; Liu, M.K.; Gannett, W.; Regan, W.; Watanabe, K.; et al. Tunable phonon polaritons in atomically thin van der Waals crystals of boron nitride. Science 2014, 343, 1125–1129. [Google Scholar] [CrossRef]
- Caldwell, J.D.; Lindsay, L.; Giannini, V.; Vurgaftman, I.; Reinecke, T.L.; Maier, S.A.; Glembocki, O.J. Low-loss, infrared and terahertz nanophotonics using surface phonon polaritons. Nanophotonics 2015, 4, 44–68. [Google Scholar] [CrossRef]
- Gerber, J.A.; Berweger, S.; O’Callahan, B.T.; Raschke, M.B. Phase-resolved surface plasmon interferometry of graphene. Phys. Rev. Lett. 2014, 113, 055502. [Google Scholar] [CrossRef] [PubMed]
- Ozbay, E. Plasmonics: Merging photonics and electronics at nanoscale dimensions. Science 2006, 311, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Fei, Z.; Goldflam, M.D.; Wu, J.S.; Dai, S.; Wagner, M.; McLeod, A.S.; Liu, M.K.; Post, K.W.; Zhu, S.; Janssen, G.C.A.M.; et al. Edge and Surface Plasmons in Graphene Nanoribbons. Nano Lett. 2015, 15, 8271–8276. [Google Scholar] [CrossRef]
- Sanvitto, D.; Kéna-Cohen, S. The road towards polaritonic devices. Nat. Mater. 2016, 15, 1061–1073. [Google Scholar] [CrossRef]
- Tielrooij, K.J.; Orona, L.; Ferrier, A.; Badioli, M.; Navickaite, G.; Coop, S.; Nanot, S.; Kalinic, B.; Cesca, T.; Gaudreau, L.; et al. Electrical control of optical emitter relaxation pathways enabled by graphene. Nat. Phys. 2015, 11, 281–287. [Google Scholar] [CrossRef]
- Gullans, M.; Chang, D.E.; Koppens, F.H.L.; De Abajo, F.J.G.; Lukin, M.D. Single-photon nonlinear optics with graphene plasmons. Phys. Rev. Lett. 2013, 111, 247401. [Google Scholar] [CrossRef] [PubMed]
- Constant, T.J.; Hornett, S.M.; Chang, D.E.; Hendry, E. All-optical generation of surface plasmons in graphene. Nat. Phys. 2016, 12, 124–127. [Google Scholar] [CrossRef]
- Ju, L.; Geng, B.; Horng, J.; Girit, C.; Martin, M.; Hao, Z.; Bechtel, H.A.; Liang, X.; Zettl, A.; Shen, Y.R.; et al. Graphene plasmonics for tunable terahertz metamaterials. Nat. Nanotechnol. 2011, 6, 630–634. [Google Scholar] [CrossRef]
- Raikwar, S.; Srivastava, D.K.; Saini, J.P.; Prajapati, Y.K. 2D-antimonene-based surface plasmon resonance sensor for improvement of sensitivity. Appl. Phys. A Mater. Sci. Process. 2021, 127, 13–19. [Google Scholar] [CrossRef]
- Das, C.M.; Kang, L.; Chen, M.W.; Coquet, P.; Yong, K.T. Heterolayered films of monolayer WS2nanosheets on monolayer graphene embedded in poly(methyl methacrylate) for plasmonic biosensing. ACS Appl. Nano Mater. 2020, 3, 10446–10453. [Google Scholar] [CrossRef]
- Xue, T.; Liang, W.; Li, Y.; Sun, Y.; Xiang, Y.; Zhang, Y.; Dai, Z.; Duo, Y.; Wu, L.; Qi, K.; et al. Ultrasensitive detection of miRNA with an antimonene-based surface plasmon resonance sensor. Nat. Commun. 2019, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Kravets, V.G.; Schedin, F.; Jalil, R.; Britnell, L.; Gorbachev, R.V.; Ansell, D.; Thackray, B.; Novoselov, K.S.; Geim, A.K.; Kabashin, A.V.; et al. Singular phase nano-optics in plasmonic metamaterials for label-free single-molecule detection. Nat. Mater. 2013, 12, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Giles, A.J.; Dai, S.; Glembocki, O.J.; Kretinin, A.V.; Sun, Z.; Ellis, C.T.; Tischler, J.G.; Taniguchi, T.; Watanabe, K.; Fogler, M.M.; et al. Imaging of Anomalous Internal Reflections of Hyperbolic Phonon-Polaritons in Hexagonal Boron Nitride. Nano Lett. 2016, 16, 3858–3865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Niu, Q. Chiral Phonons at High-Symmetry Points in Monolayer Hexagonal Lattices. Phys. Rev. Lett. 2015, 115, 115502. [Google Scholar] [CrossRef]
- Autore, M.; Dolado, I.; Li, P.; Esteban, R.; Alfaro-Mozaz, F.J.; Atxabal, A.; Liu, S.; Edgar, J.H.; Vélez, S.; Casanova, F.; et al. Enhanced Light—Matter Interaction in 10B Monoisotopic Boron Nitride Infrared Nanoresonators. Adv. Opt. Mater. 2020, 9, 2001958. [Google Scholar] [CrossRef]
- Fei, Z.; Iwinski, E.G.; Ni, G.X.; Zhang, L.M.; Bao, W.; Rodin, A.S.; Lee, Y.; Wagner, M.; Liu, M.K.; Dai, S.; et al. Tunneling Plasmonics in Bilayer Graphene. Nano Lett. 2015, 15, 4973–4978. [Google Scholar] [CrossRef]
- Jiang, B.Y.; Ni, G.X.; Pan, C.; Fei, Z.; Cheng, B.; Lau, C.N.; Bockrath, M.; Basov, D.N.; Fogler, M.M. Tunable Plasmonic Reflection by Bound 1D Electron States in a 2D Dirac Metal. Phys. Rev. Lett. 2016, 117, 086801. [Google Scholar] [CrossRef]
- Desouky, M.; Anisur, M.R.; Alba, M.; Singh Raman, R.K.; Swillam, M.A.; Voelcker, N.H.; Kasry, A. Near-field mapping of localized plasmon resonances in metal-free, nano-membrane graphene for mid-infrared sensing applications. ACS Appl. Nano Mater. 2018, 1, 6454–6562. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, D.; Wu, Y.C.; Yang, M.; Wang, Q.; Coileáin, C.; Xu, H.; Yang, C.; Abid, M.; Abid, M.; et al. Ultra-sensitive graphene based mid-infrared plasmonic bio-chemical sensing using dielectric beads as a medium. Carbon 2017, 122, 404–410. [Google Scholar] [CrossRef]
- Singh, S.; Singh, P.K.; Umar, A.; Lohia, P.; Albargi, H.; Castañeda, L.; Dwivedi, D.K. 2D nanomaterial-based surface plasmon resonance sensors for biosensing applications. Micromachines 2020, 11, 779. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.C.; Zhu, H.; Zhu, A.Y.; Li, C.; Cubukcu, E. Graphene-enabled silver nanoantenna sensors. Nano Lett. 2012, 12, 4090–4094. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Y.; Qu, Y.; Chen, C.H.; Chen, C.M.; Chuang, C.H.; Zhu, Y. Enhanced light-matter interaction of graphene-gold nanoparticle hybrid films for high-performance SERS detection. J. Mater. Chem. C 2014, 2, 4683–4691. [Google Scholar] [CrossRef]
- Zhao, Y.; Zeng, W.; Tao, Z.; Xiong, P.; Qu, Y.; Zhu, Y. Highly sensitive surface-enhanced Raman scattering based on multi-dimensional plasmonic coupling in Au-graphene-Ag hybrids. Chem. Commun. 2015, 51, 866–869. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Sreekanth, K.V.; Shang, J.; Yu, T.; Chen, C.K.; Yin, F.; Baillargeat, D.; Coquet, P.; Ho, H.P.; Kabashin, A.V.; et al. Graphene-Gold Metasurface Architectures for Ultrasensitive Plasmonic Biosensing. Adv. Mater. 2015, 27, 6163–6169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.M.Y.; Li, K.; Shum, P.P.; Yu, X.; Zeng, S.; Wu, Z.; Wang, Q.J.; Yong, K.T.; Wei, L. Hybrid Graphene/Gold Plasmonic Fiber-Optic Biosensor. Adv. Mater. Technol. 2017, 2, 1600185. [Google Scholar] [CrossRef]
- Alharbi, R.; Irannejad, M.; Yavuz, M. A short review on the role of the metal-graphene hybrid nanostructure in promoting the localized surface plasmon resonance sensor performance. Sensors 2019, 19, 862. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Z.; Hao, Z.; DiMarco, C.; Maturavongsadit, P.; Hao, Y.; Lu, M.; Stein, A.; Wang, Q.; Hone, J.; et al. Optical conductivity-based ultrasensitive mid-infrared biosensing on a hybrid metasurface. Light Sci. Appl. 2018, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hong, Q.; Zou, J.; He, Y.; Yuan, X.; Zhu, Z.; Qin, S. Fano-resonance in hybrid metal-graphene metamaterial and its application as mid-infrared plasmonic sensor. Micromachines 2020, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q.; Luo, J.; Wen, C.; Zhang, J.; Zhu, Z.; Qin, S.; Yuan, X. Hybrid metal-graphene plasmonic sensor for multi-spectral sensing in both near- and mid-infrared ranges. Opt. Express 2019, 27, 35914–35924. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wu, R.; Xu, N.; Li, X.; Dong, N.; Ling, G.; Liu, Y.; Zhang, P. Application and prospect of antimonene: A new two-dimensional nanomaterial in cancer theranostics. J. Inorg. Biochem. 2020, 212, 111232. [Google Scholar] [CrossRef]
- García-Mendiola, T.; Gutiérrez-Sánchez, C.; Gibaja, C.; Torres, I.; Busó-Rogero, C.; Pariente, F.; Solera, J.; Razavifar, Z.; Palacios, J.J.; Zamora, F.; et al. Functionalization of a Few-Layer Antimonene with Oligonucleotides for DNA Sensing. ACS Appl. Nano Mater. 2020, 3, 3625–3633. [Google Scholar] [CrossRef]
- Lin, X.; Yang, Y.; Rivera, N.; López, J.J.; Shen, Y.; Kaminer, I.; Chen, H.; Zhang, B.; Joannopoulos, J.D.; Soljačic, M. All-angle negative refraction of highly squeezed plasmon and phonon polaritons in graphene-boron nitride heterostructures. Proc. Natl. Acad. Sci. USA 2017, 114, 6717–6721. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lewin, M.; Kretinin, A.V.; Caldwell, J.D.; Novoselov, K.S.; Taniguchi, T.; Watanabe, K.; Gaussmann, F.; Taubner, T. Hyperbolic phonon-polaritons in boron nitride for near-field optical imaging and focusing. Nat. Commun. 2015, 6, 7507. [Google Scholar] [CrossRef]
- Alfaro-Mozaz, F.J.; Rodrigo, S.G.; Alonso-González, P.; Vélez, S.; Dolado, I.; Casanova, F.; Hueso, L.E.; Martín-Moreno, L.; Hillenbrand, R.; Nikitin, A.Y. Deeply subwavelength phonon-polaritonic crystal made of a van der Waals material. Nat. Commun. 2019, 10, 42. [Google Scholar] [CrossRef]
- Pons-Valencia, P.; Alfaro-Mozaz, F.J.; Wiecha, M.M.; Biolek, V.; Dolado, I.; Vélez, S.; Li, P.; Alonso-González, P.; Casanova, F.; Hueso, L.E.; et al. Launching of hyperbolic phonon-polaritons in h-BN slabs by resonant metal plasmonic antennas. Nat. Commun. 2019, 10, 3242. [Google Scholar] [CrossRef]
- Li, P.; Dolado, I.; Alfaro-Mozaz, F.J.; Casanova, F.; Hueso, L.E.; Liu, S.; Edgar, J.H.; Nikitin, A.Y.; Vélez, S.; Hillenbrand, R. Infrared hyperbolic metasurface based on nanostructured van der Waals materials. Sensors 2019, 896, 892–896. [Google Scholar] [CrossRef]
- Bylinkin, A.; Schnell, M.; Autore, M.; Calavalle, F.; Li, P.; Taboada-Gutièrrez, J.; Liu, S.; Edgar, J.H.; Casanova, F.; Hueso, L.E.; et al. Real-space observation of vibrational strong coupling between propagating phonon polaritons and organic molecules. Nat. Photonics 2020, 15, 162–168. [Google Scholar] [CrossRef]
- Caldwell, J.D.; Aharonovich, I.; Cassabois, G.; Edgar, J.H.; Gil, B.; Basov, D.N. Photonics with hexagonal boron nitride. Nat. Rev. Mater. 2019, 4, 552–567. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, G.; Bao, X.; Lin, S.; Pang, H.; Bannur Nanjunda, S.; Bao, Q. Infrared Polaritonic Biosensors Based on Two-Dimensional Materials. Molecules 2021, 26, 4651. https://doi.org/10.3390/molecules26154651
Du G, Bao X, Lin S, Pang H, Bannur Nanjunda S, Bao Q. Infrared Polaritonic Biosensors Based on Two-Dimensional Materials. Molecules. 2021; 26(15):4651. https://doi.org/10.3390/molecules26154651
Chicago/Turabian StyleDu, Guangyu, Xiaozhi Bao, Shenghuang Lin, Huan Pang, Shivananju Bannur Nanjunda, and Qiaoliang Bao. 2021. "Infrared Polaritonic Biosensors Based on Two-Dimensional Materials" Molecules 26, no. 15: 4651. https://doi.org/10.3390/molecules26154651
APA StyleDu, G., Bao, X., Lin, S., Pang, H., Bannur Nanjunda, S., & Bao, Q. (2021). Infrared Polaritonic Biosensors Based on Two-Dimensional Materials. Molecules, 26(15), 4651. https://doi.org/10.3390/molecules26154651






