Nanocarbon-Based Flame Retardant Polymer Nanocomposites
Abstract
:1. Introduction
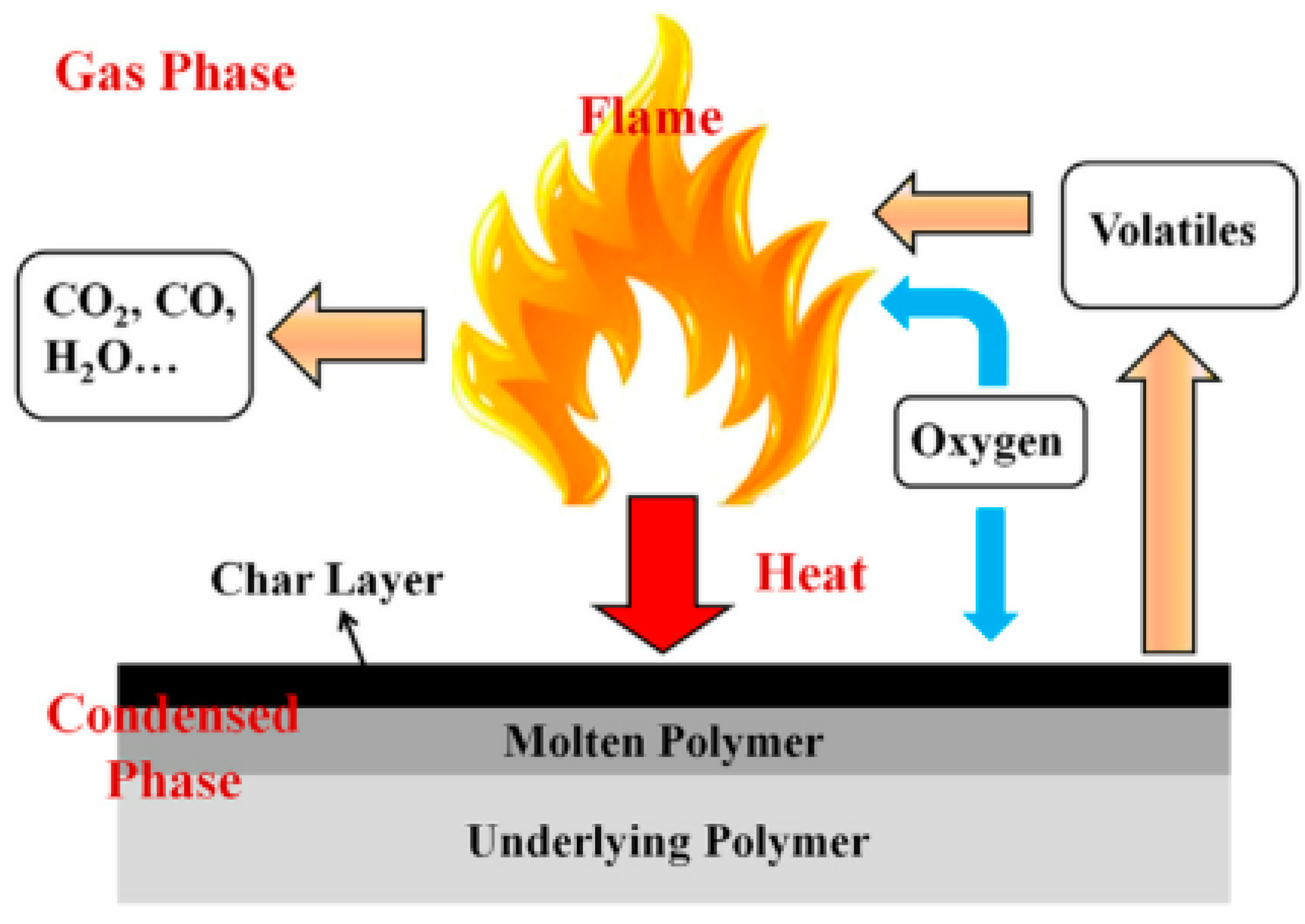
2. Graphene
2.1. Synergistic Flame Retardancy of Graphene
| Polymer | Loading of Graphene Nanomaterials | Type and Loading of Other Flame Retardant Additives | Highlights | Ref |
|---|---|---|---|---|
| EVA | 2 wt% | ATH 36 wt%, MoS2 2 wt% | PHRR decreased from 1815 kW/m2 to 377 kW/m2 | [11] |
| PP | 0.5 wt% | IFR2 4.5 wt% | PHRR decreased from 1025 kW/m2 to 140 kW/m2 | [12] |
| PI | 5 wt% | MMT 10 wt% | UL-94: V-0 rating, LOI: 55% | [13] |
| EP | 2.5 wt% | DOPO 2.5 wt% | PHRR reduced from 1194 kW/m2 to 396 kW/m2 | [14] |
| EP | 7 wt% | Al2O3 68 wt%, MH 5 wt%, | UL-94: V-0 rating, LOI: 39% | [15] |
| PLA | 0.5 wt% | phosphorus-containing flame retardant 15 wt% | UL-94: V-0 rating, LOI: 29.2% | [16] |
| PBT | 0.3 wt% | IFR 20 wt% | UL-94: V-0 rating, LOI: 25.4% | [18] |
| TPU | 0.25 wt% | MPP 14.75 wt% | PHRR decreased from 2192.6 kW/m2 to 187.2 kW/m2 | [21] |
2.2. Inorganic Hybrid Graphene
2.3. Layered Coating of Modified Grapheme
2.4. Surface Decoration of Graphene
2.5. Organic Phosphorus-Containing Flame Retardant Grafted onto Graphene
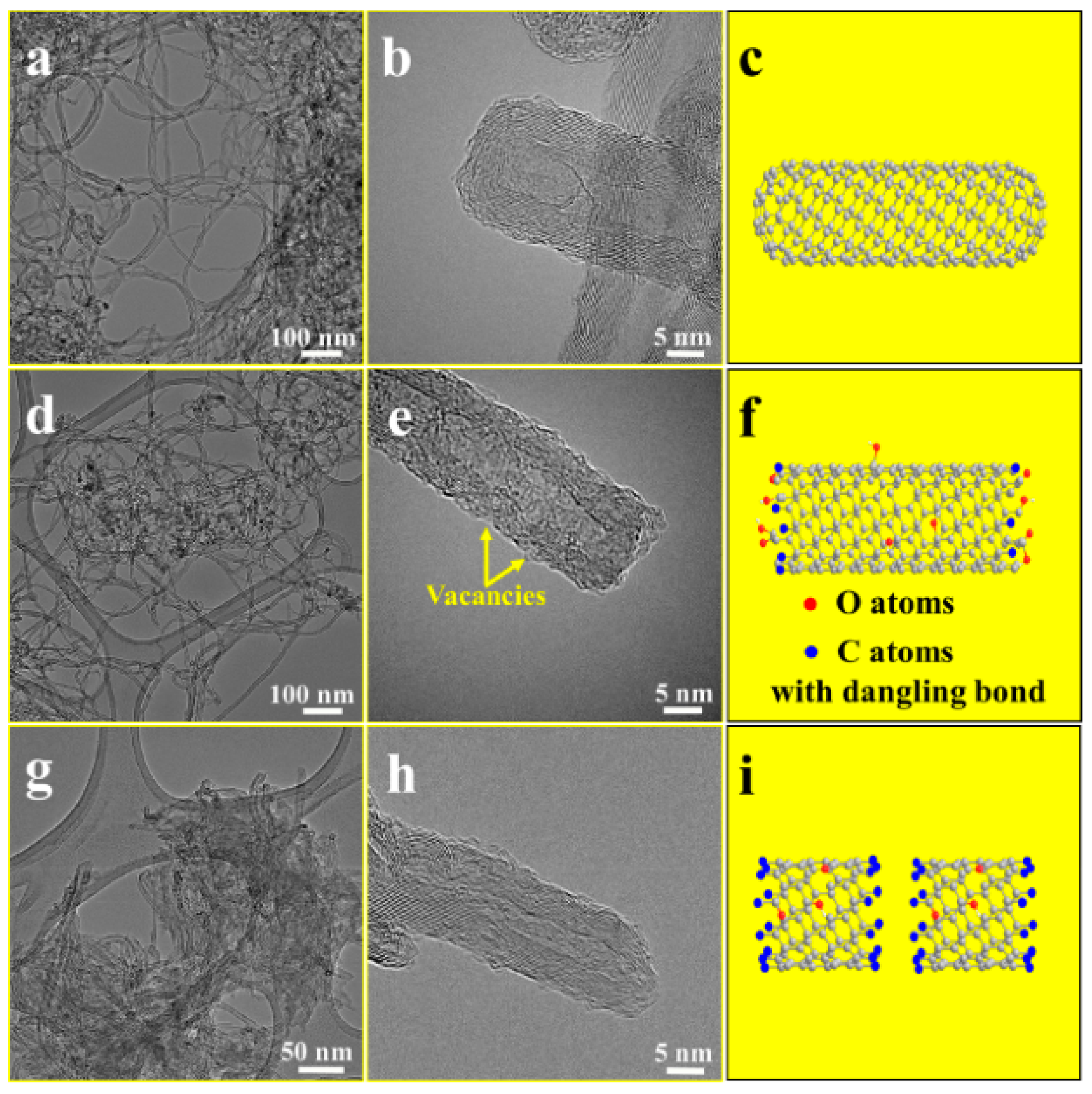
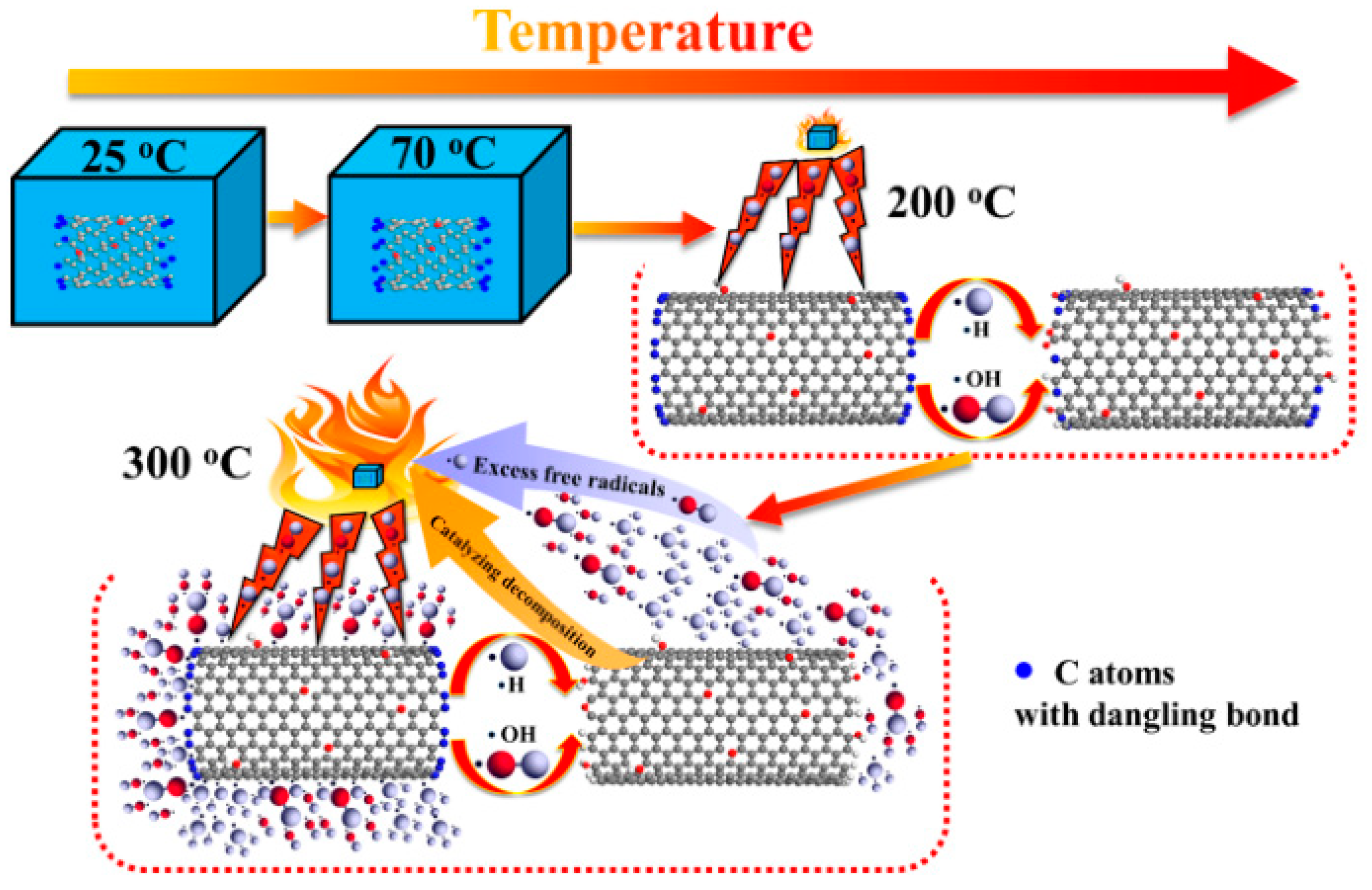
3. Carbon Nanotubes
3.1. Pristine Carbon Nanotubes
| Matrix | Flame Retardant System | Flame Retardant Performance | Ref |
|---|---|---|---|
| PA66 | intumescent fire retardant (IFR) | pHRR and THR were reduced by 76.4% and 76.5%, respectively | [59] |
| PS | intumescent fire retardant (IFR) | pHRR decreased by 30% and LOI: 34.1% | [72] |
| TPU | intumescent fire retardant (IFR) | UL-94: V-0 rating, LOI: 30.1%, pHRR and THR were reduced by 92% and 76%, respectively | [73] |
| PA6 | APP | UL-94: V-0 rating, pHRR decreased by more than 30% | [75] |
| silicone rubber (SR) | DHCP-PA (a high phosphorus content flame retardant system) | UL-94: V-0 rating, LOI: 28.4% | [76] |
3.2. Surface-Functionalized Carbon Nanotubes
4. Fullerene and Other Nanocarbons
5. Summary and Perspective
Funding
Conflicts of Interest
Abbreviations
| polymers and other compounds | POSS, Polyhedral silsesquioxane; |
| MoS2,molybdenum disulfide; | GMA, glycidyl methacrylate; |
| EVA, ethylene vinyl acetate; | BDM/DBA, 4,4-bismaleimidophenylmethane/2, 2-diallyl bi-sphenol A resins; |
| MMT, Montmorillonite; | PUA, Polyurethane acrylate; |
| GO, Graphene oxide; | PS, polystyrene; |
| LDH, layered double hydroxide; | PK, polyketone; |
| DOPO, 9,10-dihydro-9-oxa-10-phosphaphenan-threne-10-oxide; | CNTs, carbon nanotubes; |
| EP, epoxy resin; | PA66, Polyamide66; |
| PLA, Polylactide; | SR, silicone rubber; |
| α-ZrP, α-zirconium phosphate; | PPMS, melamine pentaerythritol phosphate; |
| CPO, cerium phosphate; | IFRs, intumescent flame retardants, |
| APP, Ammonium polyphosphate; | MWCNT: multi-walled carbon nanotube; |
| BPPT, Bio-based polyphosphate; | EG, Expandable graphite; |
| IG, iron–graphene; | CB, Carbon black; |
| TPU, thermoplastic polyurethane; | ND, Nanodiamond |
| MH, magnesium hydroxide; | parameters and characterization |
| PBT, polybutylene terephthalate; | Tig, ignition temperature; |
| AHP, aluminum hypophosphite; | HRR, heat release rate; |
| BP, Black phosphorene; | PHRR, peak heat release rate; |
| MZF, Mesoporous zinc ferrate; | MLR, mass loss rate; |
| MOF, metal–organic framework; | TSP, total smoke product; |
| AH, Aluminum hydroxide; | THR, total heat release; |
| RP, Red phosphorus; | EHC, effective heat of combustion; |
| LBL, layer-by-layer; | FGI, fire growth index; |
| TA, Tannic acid; | D, thermal diffusivity; |
| PDA, polydopamine; | SEM, scanning electron microscope; |
| PFR, polymeric flame retardant; | CCT, Cone calorimeter test; |
| ZH, Zinc hydroxystannate | H-TRIS, heat-transfer rate inducing system; |
| HCCP, hexachlorocyclotriphosphate; | TGA, thermogravimetric analysis; |
| ABS, acrylonitrile–butadiene–styrene; | LOI, limit oxygen index; |
| DPP, phosphaphenanthrene; | TEM, transmission electron microscope |
References
- Kroto, H.W.; H, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Geim AK, N.K. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Xu, L.; Xiao, L.; Jia, P.; Goossens, K.; Liu, P.; Li, H.; Cheng, C.; Huang, Y.; Bielawski, C.W.; Geng, J. Lightweight and Ultrastrong Polymer Foams with Unusually Superior Flame Retardancy. ACS Appl. Mater. Interfaces 2017, 9, 26392–26399. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Yang, B.; Chen, Y.; Jia, Y. Preparation of novel phosphorus-nitrogen-silicone grafted graphene oxide and its synergistic effect on intumescent flame-retardant polypropylene composites. RSC Adv. 2018, 8, 36286–36297. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Yu, B.; Yuan, Y.; Song, L.; Hu, Y. In situ preparation of reduced graphene oxide/DOPO-based phosphonamidate hybrids towards high-performance epoxy nanocomposites. Compos. Part 2017, 123, 154–164. [Google Scholar] [CrossRef]
- Rahimi-Aghdam, T.; Shariatinia, Z.; Hakkarainen, M.; Haddadi-Asl, V. Nitrogen and phosphorous doped graphene quantum dots: Excellent flame retardants and smoke suppressants for polyacrylonitrile nanocomposites. J. Hazard Mater. 2020, 381, 121013. [Google Scholar] [CrossRef]
- Wang, X.; Kalali, E.N.; Wan, J.-T.; Wang, D.-Y. Carbon-family materials for flame retardant polymeric materials. Prog. Polym. Sci. 2017, 69, 22–46. [Google Scholar] [CrossRef]
- Ababsa, H.S.; Safidine, Z.; Mekki, A.; Grohens, Y.; Ouadah, A.; Chabane, H. Fire behavior of flame-retardant polyurethane semi-rigid foam in presence of nickel (II) oxide and graphene nanoplatelets additives. J. Polym. Res. 2021, 28, 87. [Google Scholar] [CrossRef]
- Guo, Y.; Xue, Y.; Zuo, X.; Zhang, L.; Yang, Z.; Zhou, Y.; Marmorat, C.; He, S.; Rafailovich, M. Capitalizing on the molybdenum disulfide/graphene synergy to produce mechanical enhanced flame retardant ethylene-vinyl acetate composites with low aluminum hydroxide loading. Polym. Degrad. Stab. 2017, 144, 155–166. [Google Scholar] [CrossRef]
- Yuan, B.; Fan, A.; Yang, M.; Chen, X.; Hu, Y.; Bao, C.; Jiang, S.; Niu, Y.; Zhang, Y.; He, S.; et al. The effects of graphene on the flammability and fire behavior of intumescent flame retardant polypropylene composites at different flame scenarios. Polym. Degrad. Stab. 2017, 143, 42–56. [Google Scholar] [CrossRef]
- Zuo, L.; Fan, W.; Zhang, Y.; Zhang, L.; Gao, W.; Huang, Y.; Liu, T. Graphene/montmorillonite hybrid synergistically reinforced polyimide composite aerogels with enhanced flame-retardant performance. Compos. Sci. Technol. 2017, 139, 57–63. [Google Scholar] [CrossRef]
- Liu, S.; Fang, Z.; Yan, H.; Chevali, V.S.; Wang, H. Synergistic flame retardancy effect of graphene nanosheets and traditional retardants on epoxy resin. Compos. Part 2016, 89, 26–32. [Google Scholar] [CrossRef]
- Guan, F.-L.; Gui, C.-X.; Zhang, H.-B.; Jiang, Z.-G.; Jiang, Y.; Yu, Z.-Z. Enhanced thermal conductivity and satisfactory flame retardancy of epoxy/alumina composites by combination with graphene nanoplatelets and magnesium hydroxide. Compos. Part 2016, 98, 134–140. [Google Scholar] [CrossRef]
- Wang, K.; Wang, J.; Zhao, D.; Zhai, W. Preparation of microcellular poly(lactic acid) composites foams with improved flame retardancy. J. Cell. Plast. 2016, 53, 45–63. [Google Scholar] [CrossRef]
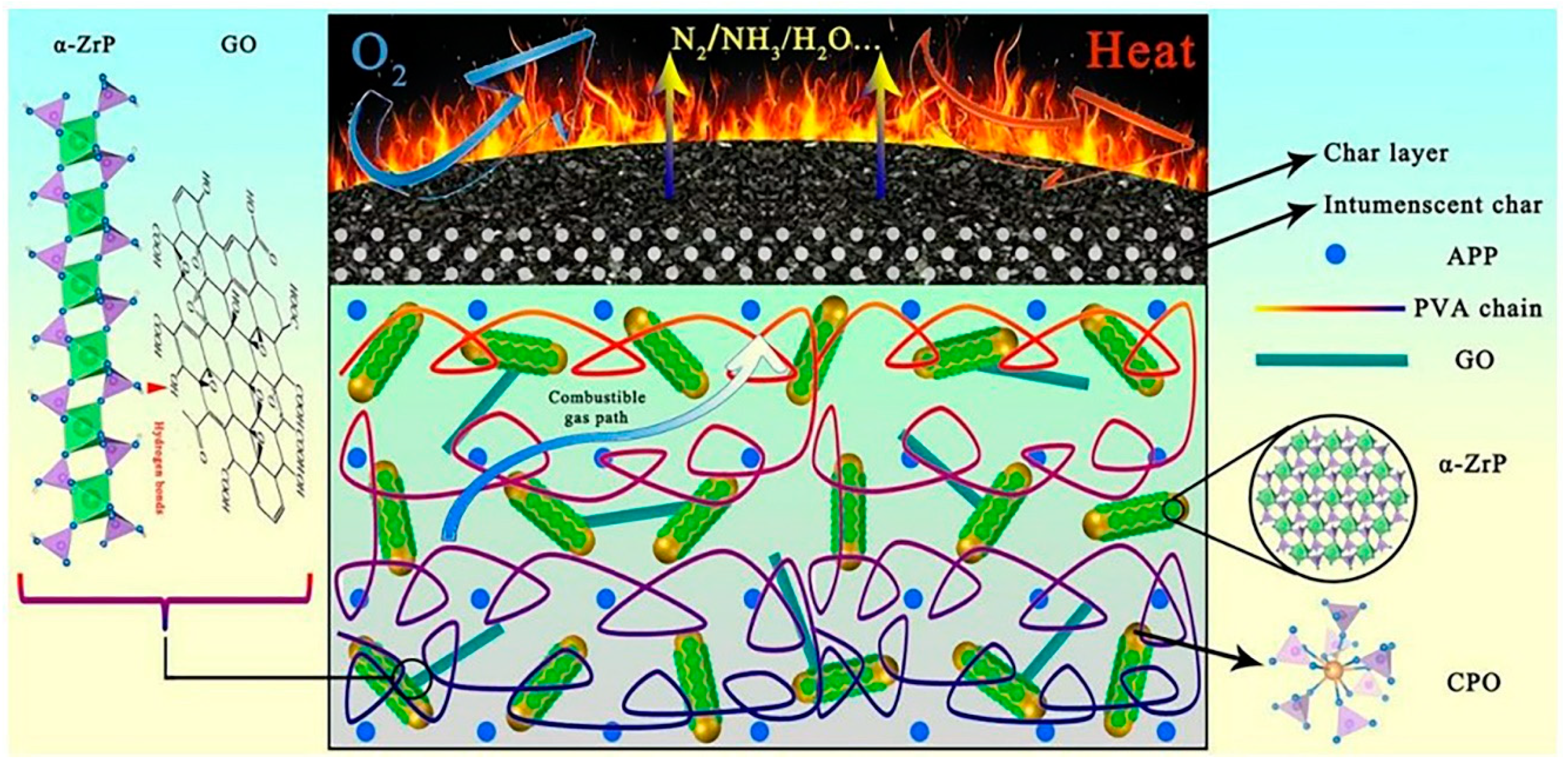
- Leng, Q.; Li, J.; Wang, Y. Structural analysis of α-zirconium phosphate/cerium phosphate/graphene oxide nanocomposites with flame-retardant properties in polyvinyl alcohol. New J. Chem. 2020, 44, 4568–4577. [Google Scholar] [CrossRef]
- Li, Z.; Li, W.; Liao, L.; Li, J.; Wu, T.; Ran, L.; Zhao, T.; Chen, B. Preparation and properties of polybutylene-terephthalate/graphene oxide in situ flame-retardant material. J. Appl. Polym. Sci. 2020, 137. [Google Scholar] [CrossRef]
- Jing, J.; Zhang, Y.; Tang, X.; Li, X.; Peng, M.; Fang, Z. Combination of a bio-based polyphosphonate and modified graphene oxide toward superior flame retardant polylactic acid. RSC Adv. 2018, 8, 4304–4313. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.-R.; Mao, M.; Li, S.-N.; Xia, Q.-Q.; Cao, C.-F.; Zhao, L.; Zhang, G.-D.; Zheng, Z.-J.; Gao, J.-F.; Tang, L.-C. Facile and green synthesis of mechanically flexible and flame-retardant clay/graphene oxide nanoribbon interconnected networks for fire safety and prevention. Chem. Eng. J. 2021, 405. [Google Scholar] [CrossRef]
- Chen, X.; Ma, C.; Jiao, C. Synergistic effects between iron-graphene and melamine salt of pentaerythritol phosphate on flame retardant thermoplastic polyurethane. Polym. Adv. Technol. 2016, 27, 1508–1516. [Google Scholar] [CrossRef]
- Idumah, C.I.; Hassan, A.; Bourbigot, S. Synergistic effect of exfoliated graphene nanoplatelets and non-halogen flame retardants on flame retardancy and thermal properties of kenaf flour-PP nanocomposites. J. Therm. Anal. Calorim. 2018, 134, 1681–1703. [Google Scholar] [CrossRef]
- Wan, M.; Shen, J.; Sun, C.; Gao, M.; Yue, L.; Wang, Y. Ionic liquid modified graphene oxide for enhanced flame retardancy and mechanical properties of epoxy resin. J. Appl. Polym. Sci. 2021, 138. [Google Scholar] [CrossRef]
- Liu, Y.; Babu, H.V.; Zhao, J.; Goñi-Urtiaga, A.; Sainz, R.; Ferritto, R.; Pita, M.; Wang, D.-Y. Effect of Cu-doped graphene on the flammability and thermal properties of epoxy composites. Compos. Part 2016, 89, 108–116. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Wu, W.; Liu, X.; Qu, H.; Xu, J. Preparation and characterization of aluminum hypophosphite/reduced graphene oxide hybrid material as a flame retardant additive for PBT. Fire Mater. 2017, 41, 195–208. [Google Scholar] [CrossRef]
- Ren, X.; Mei, Y.; Lian, P.; Xie, D.; Deng, W.; Wen, Y.; Luo, Y. Fabrication and Application of Black Phosphorene/Graphene Composite Material as a Flame Retardant. Polymers 2019, 11, 193. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Xu, L.; Yang, Y.; Zhang, S.; Huang, Y.; Bielawski, C.W.; Geng, J. Core-Shell Structured Polyamide 66 Nanofibers with Enhanced Flame Retardancy. ACS Omega 2017, 2, 2665–2671. [Google Scholar] [CrossRef] [Green Version]
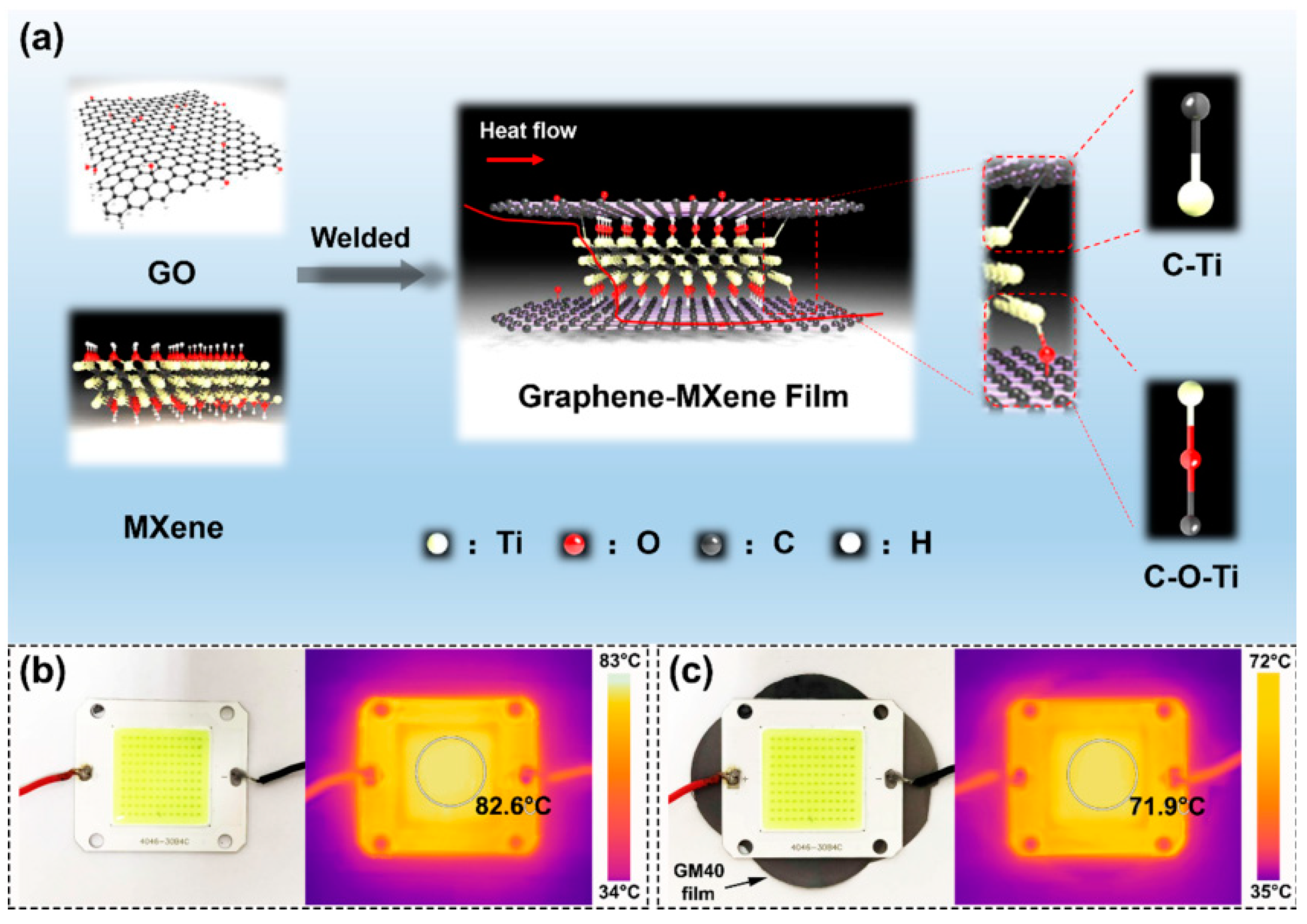
- Liu, Y.; Wu, K.; Lu, M.; Jiao, E.; Zhang, H.; Shi, J.; Lu, M. Highly thermal conductivity and flame retardant flexible graphene/MXene paper based on an optimized interface and nacre laminated structure. Compos. Part 2021, 141. [Google Scholar] [CrossRef]
- Edenharter, A.; Feicht, P.; Diar-Bakerly, B.; Beyer, G.; Breu, J. Superior flame retardant by combining high aspect ratio layered double hydroxide and graphene oxide. Polymer 2016, 91, 41–49. [Google Scholar] [CrossRef]
- Yang, C.; Li, Z.; Yu, L.; Li, X.; Zhang, Z. Mesoporous zinc ferrate microsphere-decorated graphene oxide as a flame retardant additive: Preparation, characterization, and flame retardance evaluation. Ind. Eng. Chem. Res. 2017, 56, 7720–7729. [Google Scholar] [CrossRef]
- Pan, Y.-T.; Wan, J.; Zhao, X.; Li, C.; Wang, D.-Y. Interfacial growth of MOF-derived layered double hydroxide nanosheets on graphene slab towards fabrication of multifunctional epoxy nanocomposites. Chem. Eng. J. 2017, 330, 1222–1231. [Google Scholar] [CrossRef]
- Chavali, K.S.; Pethsangave, D.A.; Patankar, K.C.; Khose, R.V.; Wadekar, P.H.; Maiti, S.; Adivarekar, R.V.; Some, S. Graphene-based intumescent flame retardant on cotton fabric. J. Mater. Sci. 2020, 55, 14197–14210. [Google Scholar] [CrossRef]
- Jeon, I.-Y.; Shin, S.-H.; Choi, H.-J.; Yu, S.-Y.; Jung, S.-M.; Baek, J.-B. Heavily aluminated graphene nanoplatelets as an efficient flame-retardant. Carbon 2017, 116, 77–83. [Google Scholar] [CrossRef]
- Li, Z.; González, A.J.; Heeralal, V.B.; Wang, D.-Y. Covalent assembly of MCM-41 nanospheres on graphene oxide for improving fire retardancy and mechanical property of epoxy resin. Compos. Part 2018, 138, 101–112. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Zhang, L.; García Molleja, J.; Wang, D.-Y. Bimetallic metal-organic frameworks and graphene oxide nano-hybrids for enhanced fire retardant epoxy composites: A novel carbonization mechanism. Carbon 2019, 153, 407–416. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.W.; Vasagar, V.; Ha, H.; Nazarenko, S.; Ellison, C.J. Polydopamine-Graphene Oxide Flame Retardant Nanocoatings Applied via an Aqueous Liquid Crystalline Scaffold. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Shi, X.; Yang, P.; Peng, X.; Huang, C.; Qian, Q.; Wang, B.; He, J.; Liu, X.; Li, Y.; Kuang, T. Bi-phase fire-resistant polyethylenimine/graphene oxide/melanin coatings using layer by layer assembly technique: Smoke suppression and thermal stability of flexible polyurethane foams. Polymer 2019, 170, 65–75. [Google Scholar] [CrossRef]
- Jing, J.; Zhang, Y.; Fang, Z.-P.; Wang, D.-Y. Core-shell flame retardant/graphene oxide hybrid: A self-assembly strategy towards reducing fire hazard and improving toughness of polylactic acid. Compos. Sci. Technol. 2018, 165, 161–167. [Google Scholar] [CrossRef]
- Kim, Y.N.; Ha, Y.-M.; Park, J.E.; Kim, Y.-O.; Jo, J.Y.; Han, H.; Lee, D.C.; Kim, J.; Jung, Y.C. Flame retardant, antimicrobial, and mechanical properties of multifunctional polyurethane nanofibers containing tannic acid-coated reduced graphene oxide. Polym. Test. 2021, 93. [Google Scholar] [CrossRef]
- Zabihi, O.; Ahmadi, M.; Li, Q.; Ferdowsi, M.R.G.; Mahmoodi, R.; Kalali, E.N.; Wang, D.-Y.; Naebe, M. A sustainable approach to scalable production of a graphene based flame retardant using waste fish deoxyribonucleic acid. J. Clean. Prod. 2020, 247. [Google Scholar] [CrossRef]
- Hu, C.; Xue, J.; Dong, L.; Jiang, Y.; Wang, X.; Qu, L.; Dai, L. Scalable Preparation of Multifunctional Fire-Retardant Ultralight Graphene Foams. ACS Nano 2016, 10, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Wu, K.; Shi, J.; Du, X.; Li, X.; Yang, L.; Lu, M. Green reduction of graphene oxide by polydopamine to a construct flexible film: Superior flame retardancy and high thermal conductivity. J. Mater. Chem. A 2017, 5, 18542–18550. [Google Scholar] [CrossRef]
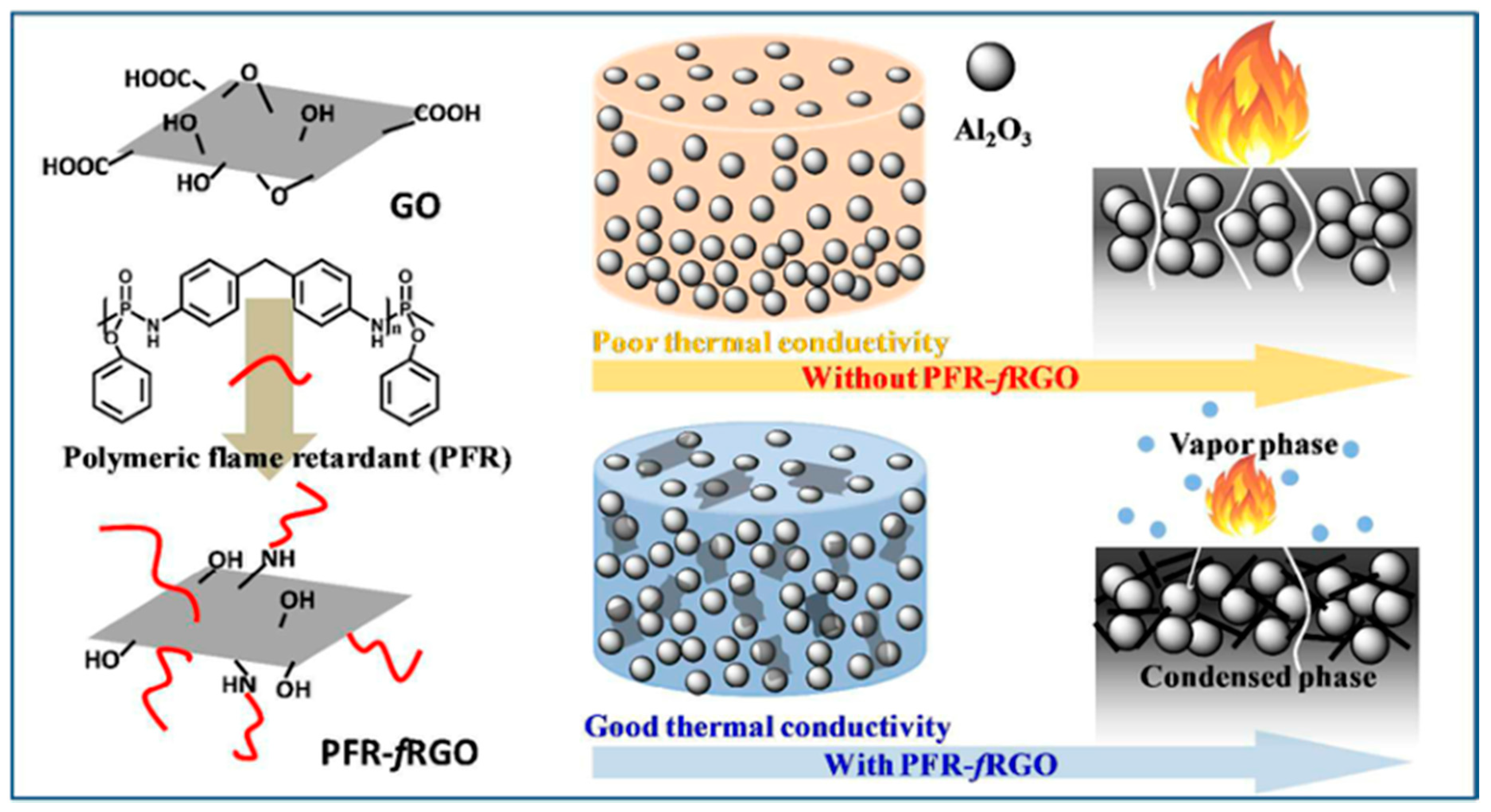
- Feng, Y.; Hu, J.; Xue, Y.; He, C.; Zhou, X.; Xie, X.; Ye, Y.; Mai, Y.-W. Simultaneous improvement in the flame resistance and thermal conductivity of epoxy/Al2O3 composites by incorporating polymeric flame retardant-functionalized graphene. J. Mater. Chem. A 2017, 5, 13544–13556. [Google Scholar] [CrossRef]
- Pethsangave, D.A.; Khose, R.V.; Wadekar, P.H.; Some, S. Novel Approach toward the Synthesis of a Phosphorus-Functionalized Polymer-Based Graphene Composite as an Efficient Flame Retardant. ACS Sustain. Chem. Eng. 2019, 7, 11745–11753. [Google Scholar] [CrossRef]
- Maddalena, L.; Carosio, F.; Gomez, J.; Saracco, G.; Fina, A. Layer-by-layer assembly of efficient flame retardant coatings based on high aspect ratio graphene oxide and chitosan capable of preventing ignition of PU foam. Polym. Degrad. Stab. 2018, 152, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Zheng, Y.; Li, M.; Fan, W.; Shi, T.; Wang, Y.; Zhang, A.; Wang, J. Enhanced flame-retardant property of epoxy composites filled with solvent-free and liquid-like graphene organic hybrid material decorated by zinc hydroxystannate boxes. Compos. Part 2016, 81, 172–181. [Google Scholar] [CrossRef]
- Liu, X.; Wu, W.; Qi, Y.; Qu, H.; Xu, J. Synthesis of a hybrid zinc hydroxystannate/reduction graphene oxide as a flame retardant and smoke suppressant of epoxy resin. J. Therm. Anal. Calorim. 2016, 126, 553–559. [Google Scholar] [CrossRef]
- Guo, W.; Wang, X.; Pan, Y.; Cai, W.; Xing, W.; Song, L.; Hu, Y. Polyaniline-coupled graphene/nickel hydroxide nanohybrids as flame retardant and smoke suppressant for epoxy composites. Polym. Adv. Technol. 2019, 30, 1959–1967. [Google Scholar] [CrossRef]
- Yuan, B.; Hu, Y.; Chen, X.; Shi, Y.; Niu, Y.; Zhang, Y.; He, S.; Dai, H. Dual modification of graphene by polymeric flame retardant and Ni(OH)2 nanosheets for improving flame retardancy of polypropylene. Compos. Part 2017, 100, 106–117. [Google Scholar] [CrossRef]
- Feng, Y.; He, C.; Wen, Y.; Ye, Y.; Zhou, X.; Xie, X.; Mai, Y.W. Superior flame retardancy and smoke suppression of epoxy-based composites with phosphorus/nitrogen co-doped graphene. J. Hazard. Mater. 2018, 346, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Z. Facile synthesis of a novel magnesium amino-tris-(methylenephosphonate)-reduced graphene oxide hybrid and its high performance in mechanical strength, thermal stability, smoke suppression and flame retardancy in phenolic foam. J. Hazard. Mater. 2018, 357, 89–99. [Google Scholar] [CrossRef]
- Xu, B.; Xu, W.; Wang, G.; Liu, L.; Xu, J. Zeolitic imidazolate frameworks-8 modified graphene as a green flame retardant for reducing the fire risk of epoxy resin. Polym. Adv. Technol. 2018, 29, 1733–1743. [Google Scholar] [CrossRef]
- Cai, W.; Feng, X.; Hu, W.; Pan, Y.; Hu, Y.; Gong, X. Functionalized Graphene from Electrochemical Exfoliation for Thermoplastic Polyurethane: Thermal Stability, Mechanical Properties, and Flame Retardancy. Ind. Eng. Chem. Res. 2016, 55, 10681–10689. [Google Scholar] [CrossRef]
- Attia, N.F.; Abd El-Aal, N.S.; Hassan, M.A. Facile synthesis of graphene sheets decorated nanoparticles and flammability of their polymer nanocomposites. Polym. Degrad. Stab. 2016, 126, 65–74. [Google Scholar] [CrossRef]
- Nie, L.; Liu, C.; Liu, L.; Jiang, T.; Hong, J.; Huang, J. Study of the thermal stability and flame retardant properties of graphene oxide-decorated zirconium organophosphate based on polypropylene nanocomposites. RSC Adv. 2015, 5, 92318–92327. [Google Scholar] [CrossRef]
- Rhili, K.; Chergui, S.; ElDouhaibi, A.S.; Siaj, M. Hexachlorocyclotriphosphazene Functionalized Graphene Oxide as a Highly Efficient Flame Retardant. ACS Omega 2021, 6, 6252–6260. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, W.; Wagner, M.H.; Zhang, L.; Bard, S. Synthesis of DV-GO and its effect on the fire safety and thermal stability of bismaleimide. Polym. Degrad. Stab. 2016, 128, 209–216. [Google Scholar] [CrossRef]
- Qian, X. Functionalized graphene with DOPO based organic/inorganic flame retardants: Preparation and its reinforcements on the flame retardancy of polyurea composites. Polym. Compos. 2017, 39, 4637–4645. [Google Scholar] [CrossRef]
- Shi, X.; Peng, X.; Zhu, J.; Lin, G.; Kuang, T. Synthesis of DOPO-HQ-functionalized graphene oxide as a novel and efficient flame retardant and its application on polylactic acid: Thermal property, flame retardancy, and mechanical performance. J. Colloid Interface Sci. 2018, 524, 267–278. [Google Scholar] [CrossRef]
- Sun, F.; Yu, T.; Hu, C.; Li, Y. Influence of functionalized graphene by grafted phosphorus containing flame retardant on the flammability of carbon fiber/epoxy resin (CF/ER) composite. Compos. Sci. Technol. 2016, 136, 76–84. [Google Scholar] [CrossRef]
- Dai, K.; Sun, S.; Xu, W.; Song, Y.; Deng, Z.; Qian, X. Covalently-functionalized graphene oxide via introduction of bifunctional phosphorus-containing molecules as an effective flame retardant for polystyrene. RSC Adv. 2018, 8, 24993–25000. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Liu, Y.; Liu, P.; Xu, C.; Liu, Y.; Wang, Q. The preparation and application of a graphene-based hybrid flame retardant containing a long-chain phosphaphenanthrene. Sci. Rep. 2017, 7, 8759. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Sui, Y.; Zhang, C.; Li, P.; Dai, X.; Xu, B.; Fang, D. POSS-functionalized graphene oxide hybrids with improved dispersive and smoke-suppressive properties for epoxy flame-retardant application. Eur. Polym. J. 2020, 122. [Google Scholar] [CrossRef]
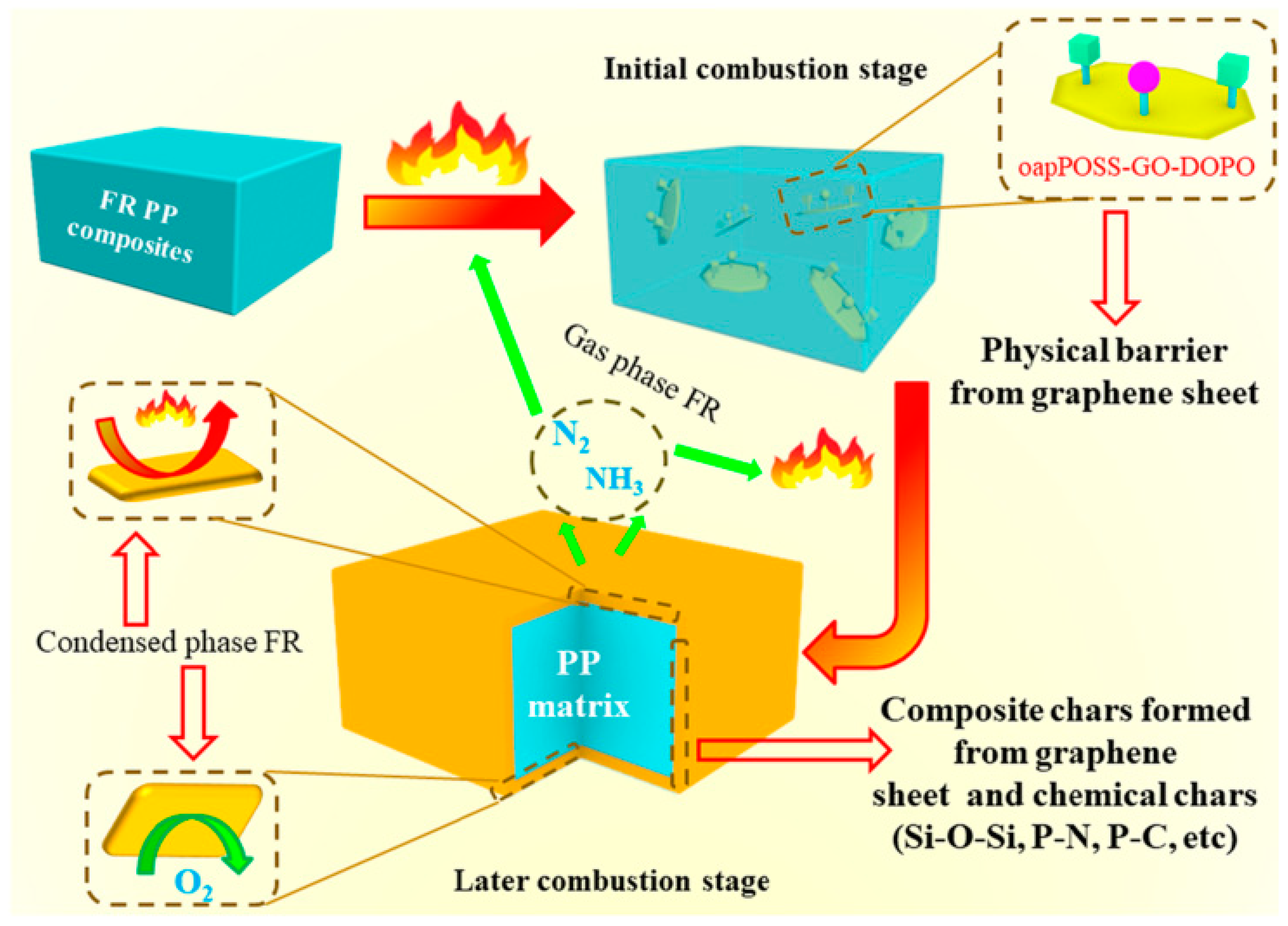
- Yuan, G.; Yang, B.; Chen, Y.; Jia, Y. Synthesis of a novel multi-structure synergistic POSS-GO-DOPO ternary graft flame retardant and its application in polypropylene. Compos. Part 2019, 117, 345–356. [Google Scholar] [CrossRef]
- Li, M.; Zhang, H.; Wu, W.; Li, M.; Xu, Y.; Chen, G.; Dai, L. A Novel POSS-Based Copolymer Functionalized Graphene: An Effective Flame Retardant for Reducing the Flammability of Epoxy Resin. Polymers 2019, 11, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, L.; Hu, C.; Song, L.; Huang, X.; Chen, N.; Qu, L. A Large-Area, Flexible, and Flame-Retardant Graphene Paper. Adv. Funct. Mater. 2016, 26, 1470–1476. [Google Scholar] [CrossRef]
- Cai, W.; Feng, X.; Wang, B.; Hu, W.; Yuan, B.; Hong, N.; Hu, Y. A novel strategy to simultaneously electrochemically prepare and functionalize graphene with a multifunctional flame retardant. Chem. Eng. J. 2017, 316, 514–524. [Google Scholar] [CrossRef]
- Feng, Y.; He, C.; Wen, Y.; Zhou, X.; Xie, X.; Ye, Y.; Mai, Y.-W. Multi-functional interface tailoring for enhancing thermal conductivity, flame retardancy and dynamic mechanical property of epoxy/Al2O3 composites. Compos. Sci. Technol. 2018, 160, 42–49. [Google Scholar] [CrossRef]
- Hu, W.; Yu, B.; Jiang, S.D.; Song, L.; Hu, Y.; Wang, B. Hyper-branched polymer grafting graphene oxide as an effective flame retardant and smoke suppressant for polystyrene. J. Hazard. Mater. 2015, 300, 58–66. [Google Scholar] [CrossRef]
- Yao, L.; Jincheng, W.; Chenyang, Z. Preparation of a novel flame retardant based on diatomite/polyethyleneimine modified MWCNT for applications in silicone rubber composites. J. Rubber Res. 2021, 24, 137–146. [Google Scholar] [CrossRef]
- Martins, M.S.S.; Schartel, B.; Magalhães, F.D.; Pereira, C.M.C. The effect of traditional flame retardants, nanoclays and carbon nanotubes in the fire performance of epoxy resin composites. Fire Mater. 2017, 41, 111–130. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, P.; Ma, Y.; Xu, D.; Wang, P.; Yang, R. Synergistic effect of multiwalled carbon nanotubes and an intumescent flame retardant: Toward an ideal electromagnetic interference shielding material with excellent flame retardancy. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Ji, X.; Chen, D.; Wang, Q.; Shen, J.; Guo, S. Synergistic effect of flame retardants and carbon nanotubes on flame retarding and electromagnetic shielding properties of thermoplastic polyurethane. Compos. Sci. Technol. 2018, 163, 49–55. [Google Scholar] [CrossRef]
- Zhu, F.; Feng, Q.Q.; Xu, Y.F.; Hu, J.F. Intumescent flame retardant coating for polyamide 6,6 (PA 6,6) fabrics containing carbon nanotubes: Synergistic effect of filler on thermal stability and flame retardancy. Text. Res. J. 2018, 89, 2031–2040. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, C.; Gao, X.p.; Huang, X.h.; Cao, C.l.; Yao, D.h. Synergistic flame-retarded effect between carbon nanotubes and ammonium polyphosphate in Nylon6 and Nylon6/polystyrene blends. Fire Mater. 2019, 43, 401–412. [Google Scholar] [CrossRef]
- Ye, P.; Cheng, L.; Jincheng, W.; Shiqiang, S. Preparation of a novel synergistic flame retardant and its application in silicone rubber composites. Fire Mater. 2020, 44, 1135–1148. [Google Scholar] [CrossRef]
- Chen, J.; Han, J. Comparative performance of carbon nanotubes and nanoclays as flame retardants for epoxy composites. Results Phys. 2019, 14. [Google Scholar] [CrossRef]
- Cabello-Alvarado, C.; Reyes-Rodriguez, P.; Andrade-Guel, M.; Cadenas-Pliego, G.; Perez-Alvarez, M.; Cruz-Delgado, V.J.; Melo-Lopez, L.; Quinones-Jurado, Z.V.; Avila-Orta, C.A. Melt-Mixed Thermoplastic Nanocomposite Containing Carbon Nanotubes and Titanium Dioxide for Flame Retardancy Applications. Polymers 2019, 11, 1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Kim, H.m.; Seong, D.G.; Lee, D. Synergistic improvement of flame retardant properties of expandable graphite and multi-walled carbon nanotube reinforced intumescent polyketone nanocomposites. Carbon 2019, 143, 650–659. [Google Scholar] [CrossRef]
- Shabestari, M.E.; Kalali, E.N.; González, V.J.; Wang, D.-Y.; Fernández-Blázquez, J.P.; Baselga, J.; Martin, O. Effect of nitrogen and oxygen doped carbon nanotubes on flammability of epoxy nanocomposites. Carbon 2017, 121, 193–200. [Google Scholar] [CrossRef]
- Janas, D.; Rdest, M.; Koziol, K.K.K. Flame-retardant carbon nanotube films. Appl. Surf. Sci. 2017, 411, 177–181. [Google Scholar] [CrossRef]
- Wang, Q.; Su, D.S.; Wang, D.-Y. Carbon Nanotube/Epoxy Composites for Improved Fire Safety. ACS Appl. Nano Mater. 2020, 3, 4253–4264. [Google Scholar] [CrossRef]
- Xie, H.; Ye, Q.; Si, J.; Yang, W.; Lu, H.; Zhang, Q. Synthesis of a carbon nanotubes/ZnAl-layered double hydroxide composite as a novel flame retardant for flexible polyurethane foams. Polym. Adv. Technol. 2016, 27, 651–656. [Google Scholar] [CrossRef]
- Kong, Q.; Wu, T.; Tang, Y.; Xiong, L.; Liu, H.; Zhang, J.; Guo, R.; Zhang, F. Improving Thermal and Flame Retardant Properties of Epoxy Resin with Organic NiFe-Layered Double Hydroxide-Carbon Nanotubes Hybrids. Chin. J. Chem. 2017, 35, 1875–1880. [Google Scholar] [CrossRef]
- Durkin, D.P.; Gallagher, M.J.; Frank, B.P.; Knowlton, E.D.; Trulove, P.C.; Fairbrother, D.H.; Fox, D.M. Phosphorus-functionalized multi-wall carbon nanotubes as flame-retardant additives for polystyrene and poly (methyl methacrylate). J. Therm. Anal. Calorim. 2017, 130, 735–753. [Google Scholar] [CrossRef]
- Xin, F.; Zhai, C.; Guo, C.; Chen, Y.; Qian, L.; Chen, X. A wrapped nano-flame retardant composed of carbon nanotubes and phosphorus-nitrogen containing polymer: Synthesis, properties and flame-retardant mechanism. J. Polym. Res. 2018, 25, 201. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Wang, J.; Tian, W.; Liew, K.M.; Zhang, Y.; Wang, B. Engineering carbon nanotubes wrapped ammonium polyphosphate for enhancing mechanical and flame retardant properties of poly(butylene succinate). Compos. Part 2018, 115, 215–227. [Google Scholar] [CrossRef]
- Xue, C.-H.; Wu, Y.; Guo, X.-J.; Liu, B.-Y.; Wang, H.-D.; Jia, S.-T. Superhydrophobic, flame-retardant and conductive cotton fabrics via layer-by-layer assembly of carbon nanotubes for flexible sensing electronics. Cellulose 2020, 27, 3455–3468. [Google Scholar] [CrossRef]
- Gu, L.; Qiu, C.; Qiu, J.; Yao, Y.; Sakai, E.; Yang, L. Preparation and Characterization of DOPO-Functionalized MWCNT and Its High Flame-Retardant Performance in Epoxy Nanocomposites. Polymers 2020, 12, 613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, B.; Li, Y.; Guo, J.; Sun, J.; Liu, X.; Li, H.; Gu, X.; Zhang, S.; Jiang, S.; Zhang, Z. Enhancing flame retardant and antistatic properties of polyamide 6 by a grafted multiwall carbon nanotubes. J. Appl. Polym. Sci. 2020, 138, 50015. [Google Scholar] [CrossRef]
- Zhu, S.E.; Wang, L.L.; Chen, H.; Yang, W.; Yuen, A.C.; Chen, T.B.; Luo, C.; Bi, W.M.; Hu, E.Z.; Zhang, J.; et al. Comparative Studies on Thermal, Mechanical, and Flame Retardant Properties of PBT Nanocomposites via Different Oxidation State Phosphorus-Containing Agents Modified Amino-CNTs. Nanomaterials 2018, 8, 70. [Google Scholar] [CrossRef] [Green Version]
- Song, K.; Ganguly, I.; Eastin, I.; Dichiara, A.B. Lignin-Modified Carbon Nanotube/Graphene Hybrid Coating as Efficient Flame Retardant. Int. J. Mol. Sci. 2017, 18, 2368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Deng, N.; Yan, L.; Chu, Z. Functionalized multiwalled carbon nanotubes with monocomponent intumescent flame retardant for reducing the flammability and smoke emission characteristics of epoxy resins. Polym. Adv. Technol. 2018, 29, 3002–3013. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, Q.; Wang, D.-Y. Simultaneously improving the fire safety and mechanical properties of epoxy resin with Fe-CNTs via large-scale preparation. J. Mater. Chem. 2018, 6, 6376–6386. [Google Scholar] [CrossRef]
- Pan, Y.; Guo, Z.; Ran, S.; Fang, Z. Influence of fullerenes on the thermal and flame-retardant properties of polymeric materials. J. Appl. Polym. Sci. 2019, 137, 47538. [Google Scholar] [CrossRef] [Green Version]
- Song, P.a.; Liu, H.; Shen, Y.; Du, B.; Fang, Z.; Wu, Y. Fabrication of dendrimer-like fullerene (C60)-decorated oligomeric intumescent flame retardant for reducing the thermal oxidation and flammability of polypropylene nanocomposites. J. Mater. Chem. 2009, 19. [Google Scholar] [CrossRef]
- Zhou, X.; Ran, S.; Hu, H.; Fang, Z. Improving flame-retardant efficiency by incorporation of fullerene in styrene–butadiene–styrene block copolymer/aluminum hydroxide composites. J. Therm. Anal. Calorim. 2016, 125, 199–204. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, L.; Fang, Z. The flame retardant and smoke suppression effect of fullerene by trapping radicals in decabromodiphenyl oxide/Sb2O3 flame-retarded high density polyethylene. Fire Mater. 2017, 41, 916–924. [Google Scholar] [CrossRef]
- Qiu, J.; Lai, X.; Li, H.; Zeng, X.; Wu, Y. Fabrication of polymethylphenylsiloxane decorated C60 via π-π stacking interaction for reducing the flammability of silicone rubber. Mater. Lett. 2018, 229, 85–88. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, Z.; Fang, Z. Fabrication of 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-decorated fullerene to improve the anti-oxidative and flame-retardant properties of polypropylene. Compos. Part 2020, 183, 107672. [Google Scholar] [CrossRef]
- Guler, T.; Tayfun, U.; Bayramli, E.; Dogan, M. Effect of expandable graphite on flame retardant, thermal and mechanical properties of thermoplastic polyurethane composites filled with huntite&hydromagnesite mineral. Thermochim. Acta. 2017, 647, 70–80. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, Y.; Guo, X.; Chen, L.; Jia, D. The Synergistic Effect of Ionic Liquid-Modified Expandable Graphite and Intumescent Flame-Retardant on Flame-Retardant Rigid Polyurethane Foams. Materials 2020, 13, 3095. [Google Scholar] [CrossRef]
- Yun, G.W.; Lee, J.H.; Kim, S.H. Flame retardant and mechanical properties of expandable graphite/polyurethane foam composites containing iron phosphonate dopamine-coated cellulose. Polym. Compos. 2020, 41, 2816–2828. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Li, N.; Wen, X.; Nogales, L.A.; Li, L.; Guo, F. Study of nanocarbon black as synergist on improving flame retardancy of ethylene-vinyl acetate/brucite composites. J. Therm. Anal. Calorim. 2018, 136, 601–608. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Deng, N.; Chu, Z. Synergistic effects of mono-component intumescent flame retardant grafted with carbon black on flame retardancy and smoke suppression properties of epoxy resins. J. Therm. Anal. Calorim. 2019, 138, 915–927. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Shi, W.; Castillo-Rodríguez, M.; Su, D.S.; Wang, D.-Y. Coordinating mechanical performance and fire safety of epoxy resin via functionalized nanodiamond. Diam. Relat. Mater. 2020, 108, 107964. [Google Scholar] [CrossRef]
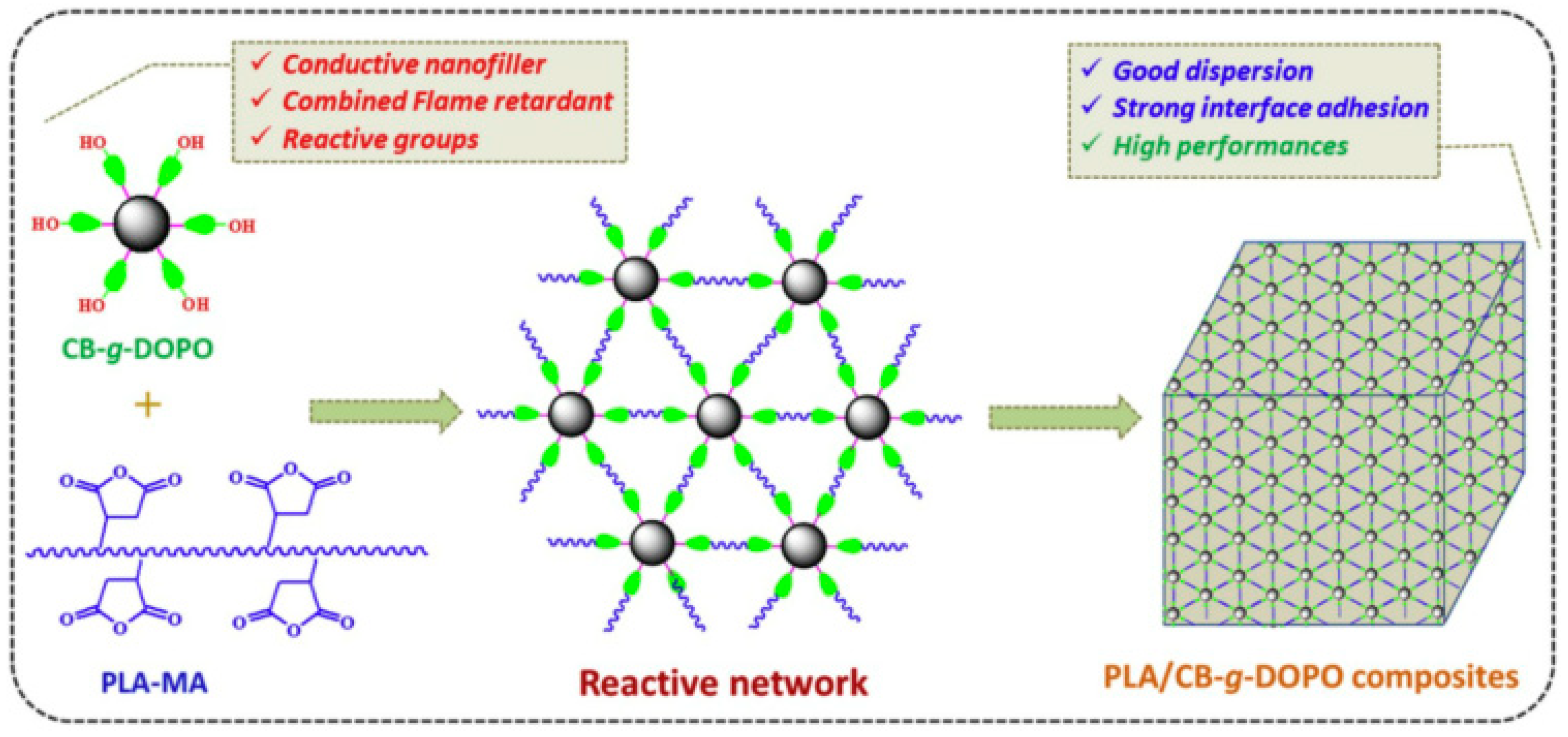
- Wen, X.; Liu, Z.; Li, Z.; Zhang, J.; Wang, D.-Y.; Szymańska, K.; Chen, X.; Mijowska, E.; Tang, T. Constructing multifunctional nanofiller with reactive interface in PLA/CB-g-DOPO composites for simultaneously improving flame retardancy, electrical conductivity and mechanical properties. Compos. Sci. Technol. 2020, 188, 107988. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, W.; Wen, X.; Chen, J.; Zhao, C.; Castillo-Rodríguez, M.; Yang, L.; Zhang, X.-Q.; Wang, R.; Wang, D.-Y. Electrospun submicron NiO fibers combined with nanosized carbon black as reinforcement for multi-functional poly(lactic acid) composites. Compos. Part 2020, 129, 105662. [Google Scholar] [CrossRef]
- Ning, H.; Ma, Z.; Zhang, Z.; Zhang, D.; Wang, Y. A novel multifunctional flame retardant MXene/nanosilica hybrid for poly(vinyl alcohol) with simultaneously improved mechanical properties. New J. Chem. 2021, 45, 4292–4302. [Google Scholar] [CrossRef]


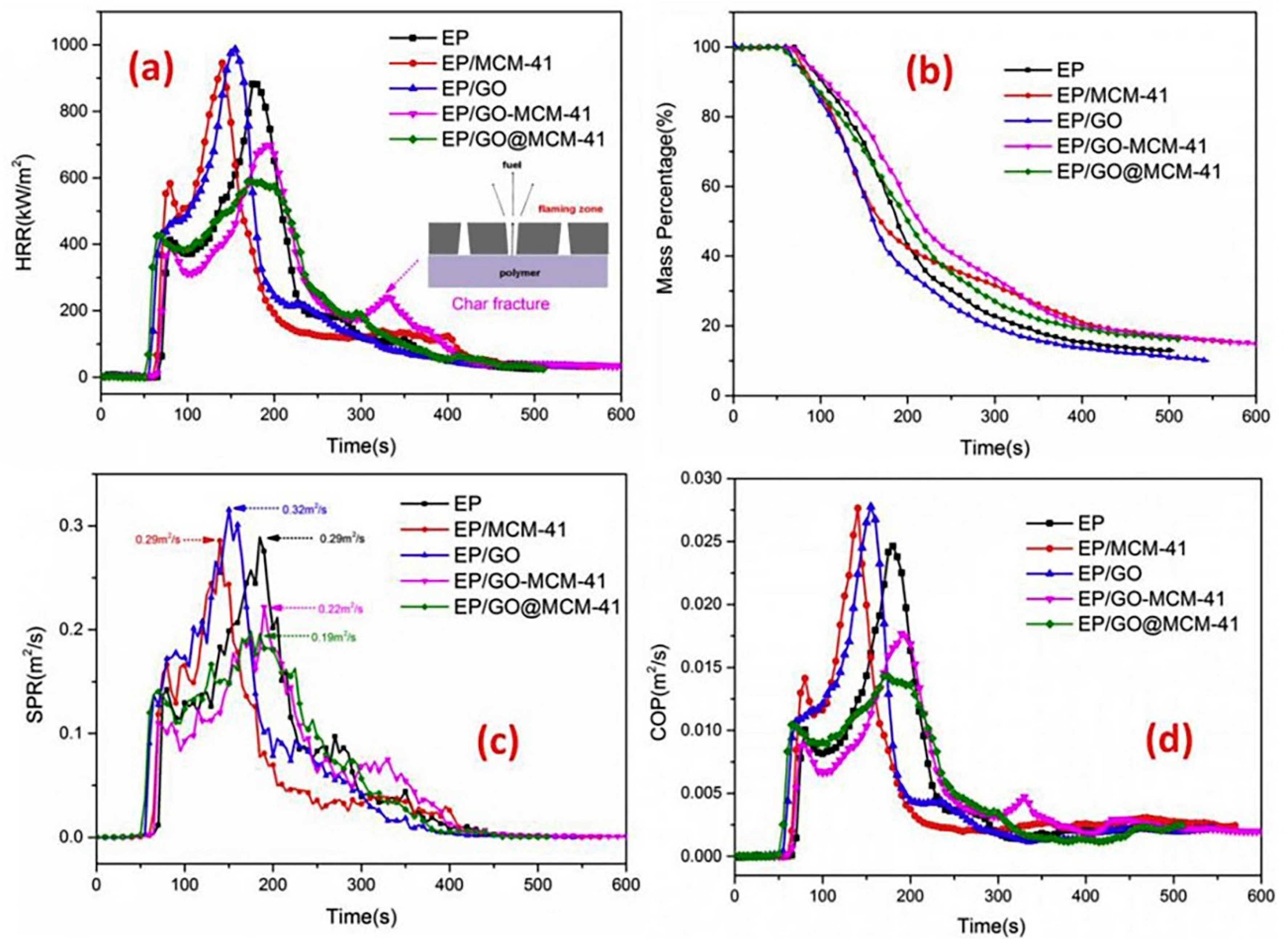
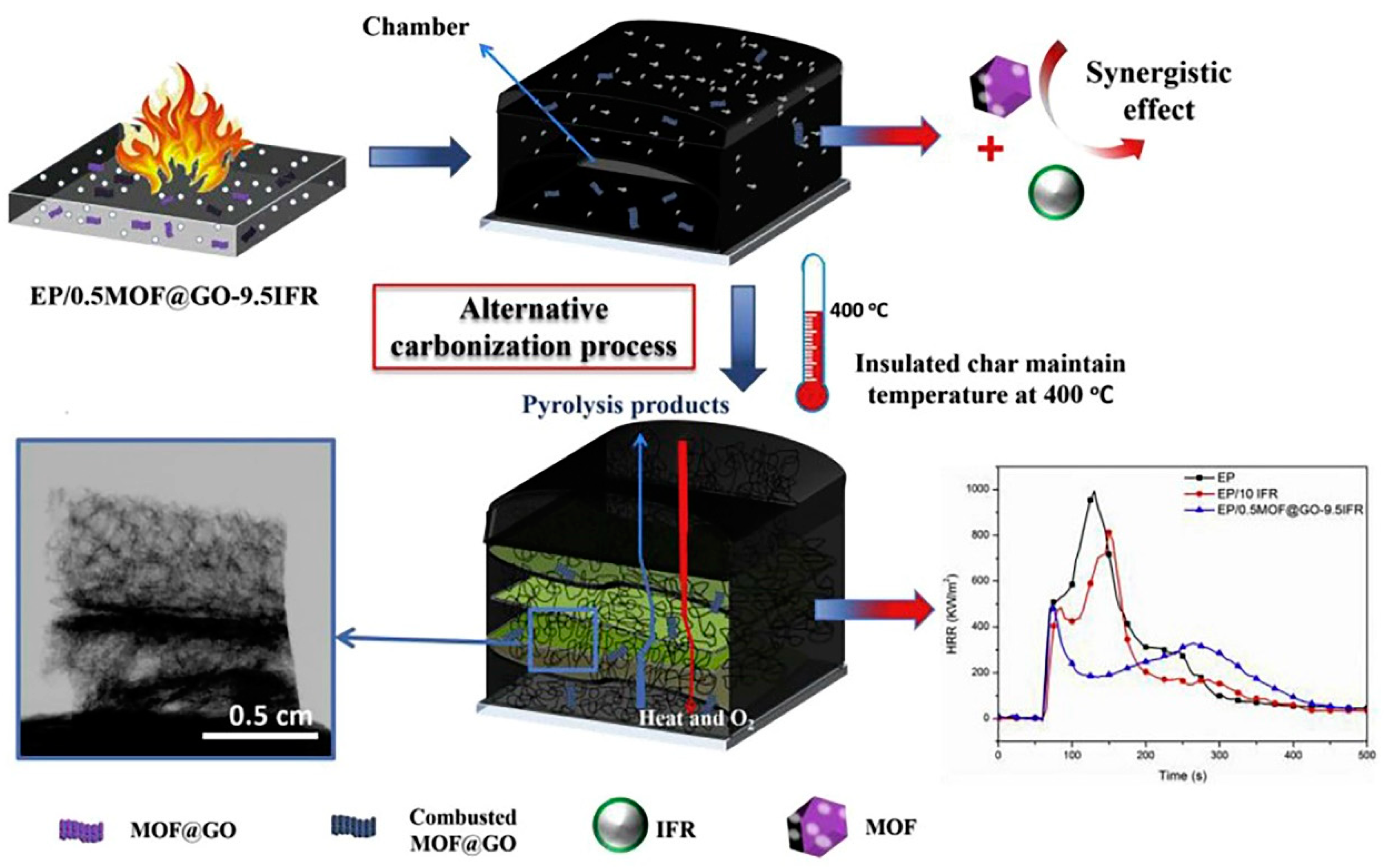
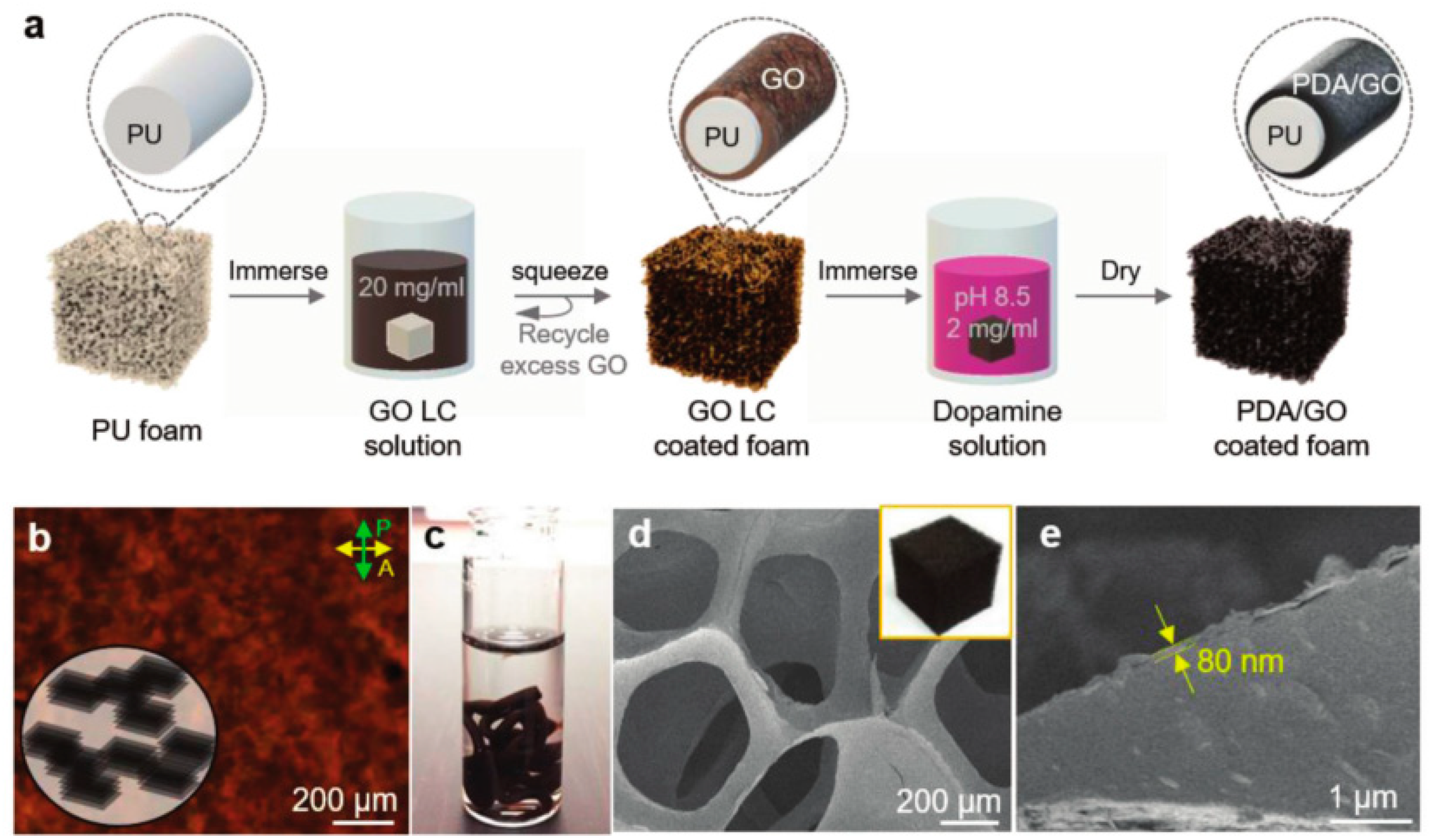
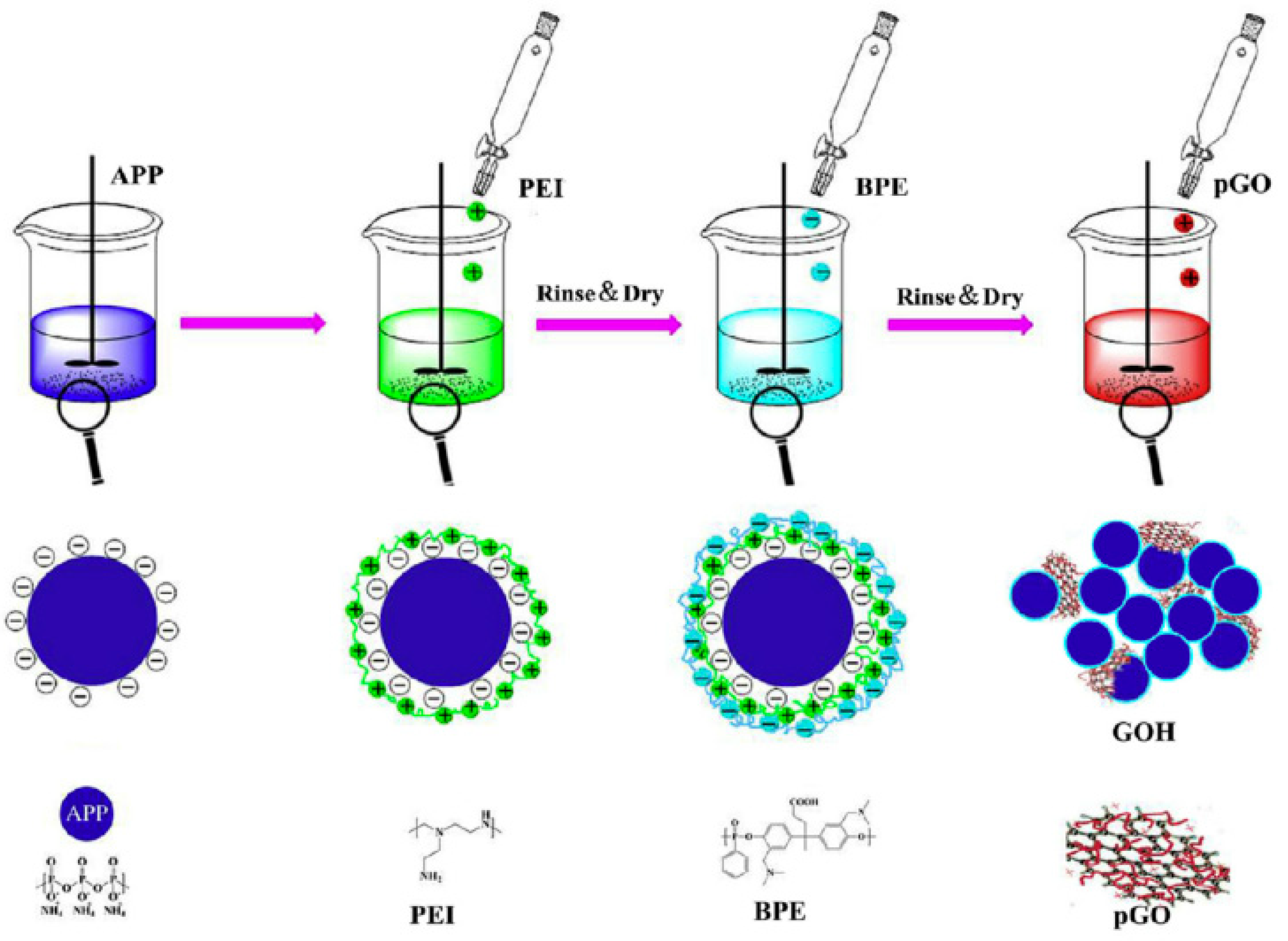

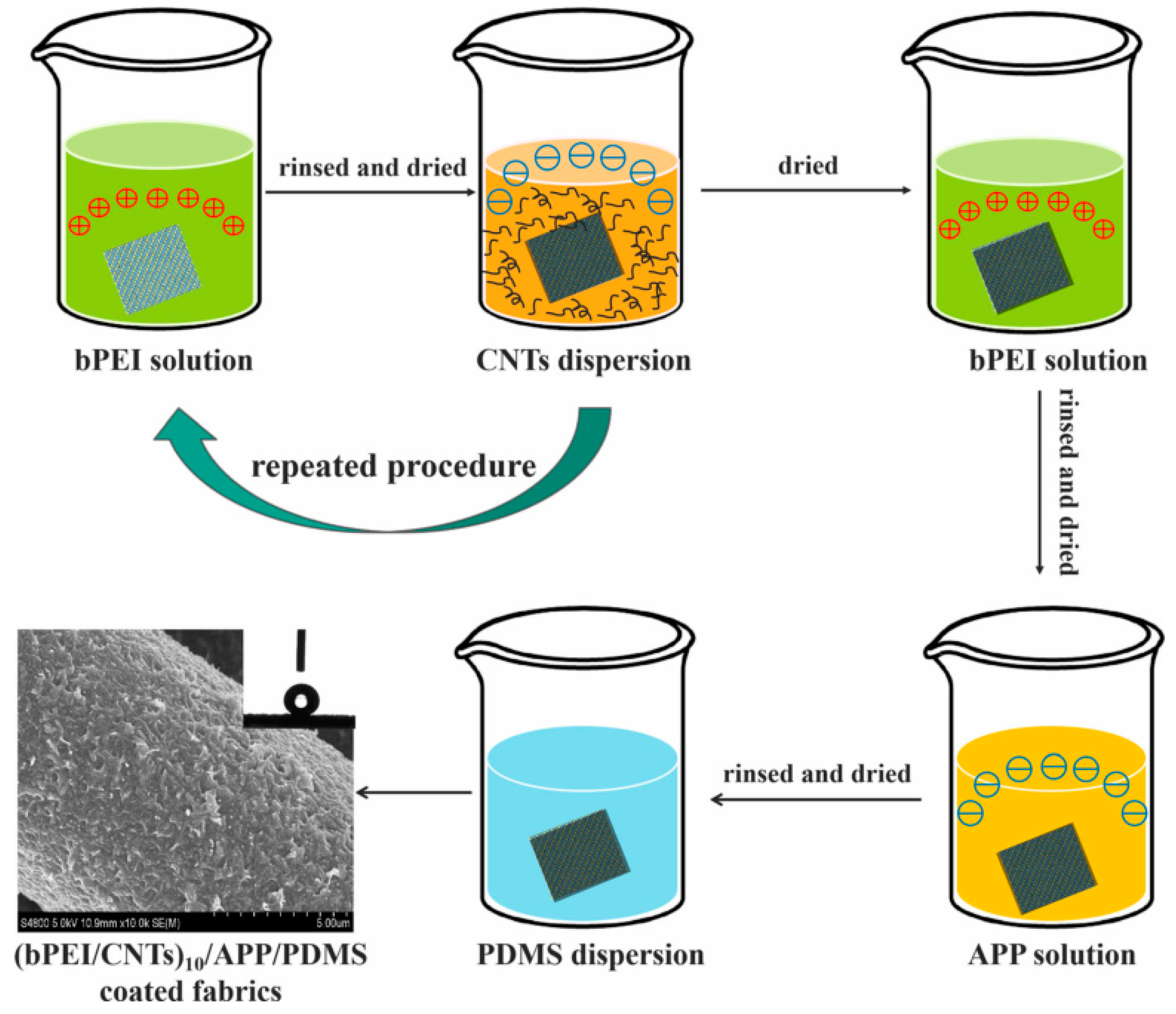
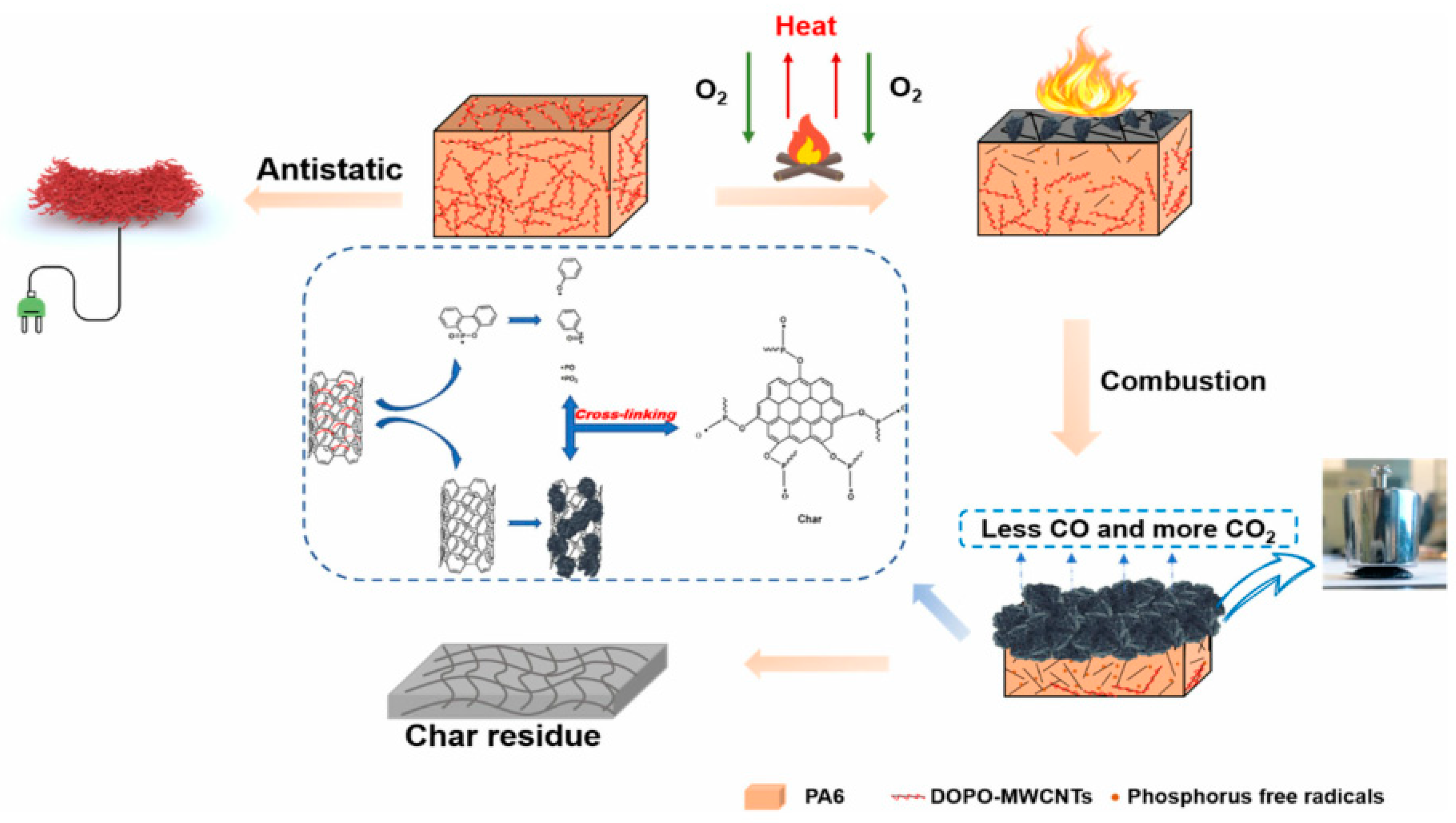
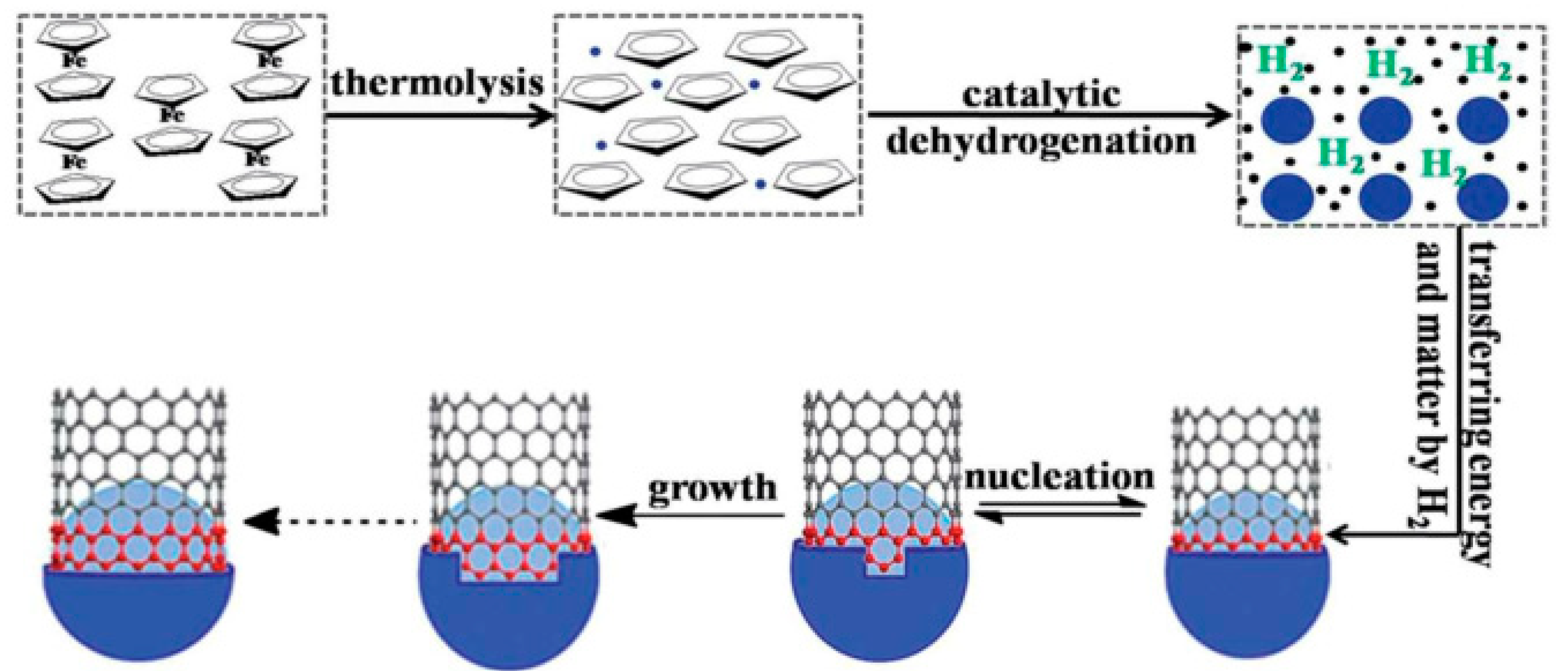



| Polymer | Loading of Modified Graphene Nanomaterials | Flame Retardant Performance | Ref |
|---|---|---|---|
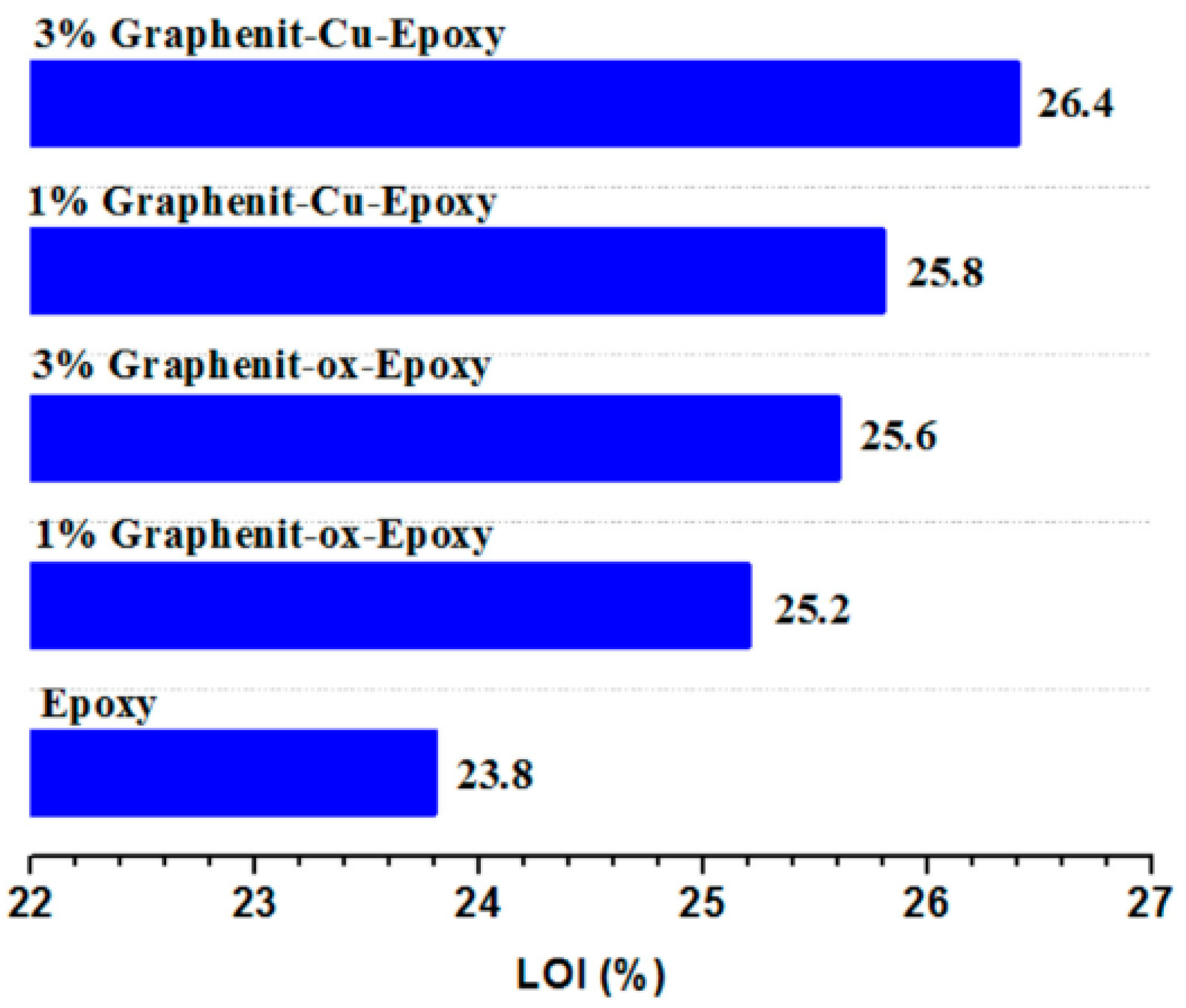
| EP | graphenite-Cu3 wt% | LOI value: 26.4%, PHRR decreased from 1193 kW/m2 (neat EP) to 786 kW/m2 | [24] |
| EP | 2 wt% rGO@LDH | 65.9% and 16.7% reduction in PHRR and SPR, respectively | [31] |
| EP | 2 wt% GO@MCM-41 | 40.0% reduction in the peak heat release rate (pHRR) | [34] |
| PU | 5 wt% PDA/GO coating | 65% reduction in the peak heat release rate (pHRR) compared with neat PU | [36] |
| EP | 5 wt% PN-rGO | 30.9% and 29.3% reduction in PHRR and THR, respectively | [50] |
| ABS | 5 wt%GRP-MDP-TIO2NP | PHRR and THR both dropped by 49%. | [54] |
| Matrix | Types of Grafting Reaction | Main Performance | Ref. |
|---|---|---|---|
| 4,4-bismaleimidophenylmethane/2, 2-diallyl bisphenol A (BDM/DBA) resins | Vinyl trie-thoxy silane and (3-isocyanatopropyl)-triethoxysilane as bridging agents | UL-94: V-0 rating, LOI: 32.8% | [57] |
| PUA | vinyltrimethoxy silane and (3-Isocyanatopropyl)-triethoxysilane as bridging agents | 49% reduction in PHRR observed in cone calorimetry | [58] |
| PLA | Reaction of 2,5-dihydroxyphenol with an acyl chloride bond | 83% reduction in TSR observed in cone calorimetry compared with neat PLA | [59] |
| CF/EP | Reaction of formaldehyde-modified DOPO with an acyl chloride bond | PHRR of composites reduced by 38.9% compared with neat CF/EP | [60] |
| PS | Paraformaldehyde, hydroxyethyl acrylate, and POCl3 as bridging agents | 39.1% reduction in PHRR observed in cone calorimetry | [61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Díaz Palencia, J.L.; Wang, N.; Jiang, Y.; Wang, D.-Y. Nanocarbon-Based Flame Retardant Polymer Nanocomposites. Molecules 2021, 26, 4670. https://doi.org/10.3390/molecules26154670
Yang Y, Díaz Palencia JL, Wang N, Jiang Y, Wang D-Y. Nanocarbon-Based Flame Retardant Polymer Nanocomposites. Molecules. 2021; 26(15):4670. https://doi.org/10.3390/molecules26154670
Chicago/Turabian StyleYang, Yuan, José Luis Díaz Palencia, Na Wang, Yan Jiang, and De-Yi Wang. 2021. "Nanocarbon-Based Flame Retardant Polymer Nanocomposites" Molecules 26, no. 15: 4670. https://doi.org/10.3390/molecules26154670
APA StyleYang, Y., Díaz Palencia, J. L., Wang, N., Jiang, Y., & Wang, D.-Y. (2021). Nanocarbon-Based Flame Retardant Polymer Nanocomposites. Molecules, 26(15), 4670. https://doi.org/10.3390/molecules26154670







