Abstract
The reaction tolerance of the multicomponent process between 3-aminoazoles, 1-morpholino-2-nitroalkenes, and aldehydes was studied. The main patterns of this reaction have been established. Conditions for the oxidation of 4,7-dihydro-6-nitroazolo[1,5-a]pyrimidines were selected. Previous claims that the 4,7-dihydro-6-nitroazolo[1,5-a]pyrimidines could not be aromatised have now been refuted. Compounds with an electron-donor substituent at position seven undergo decomposition during oxidation. The phenomenon was explained based on experimental data, electro-chemical experiment, and quantum-chemical calculation. The mechanism of oxidative degradation has been proposed.
1. Introduction
Azolo[1,5-a]pyrimidines are a very diversified group of heterocycles [1,2,3]. Among them, many compounds find application due to their beneficial features, for example, photophysical properties [4,5,6,7], metal complexes [8,9,10], and as medicinal drugs for diabetes mellitus [11,12,13], viruses [14,15,16], bacteria [17,18], neurological diseases [19,20], tumor diseases [21,22,23,24,25,26], Alzheimer’s disease [27,28,29], etc.
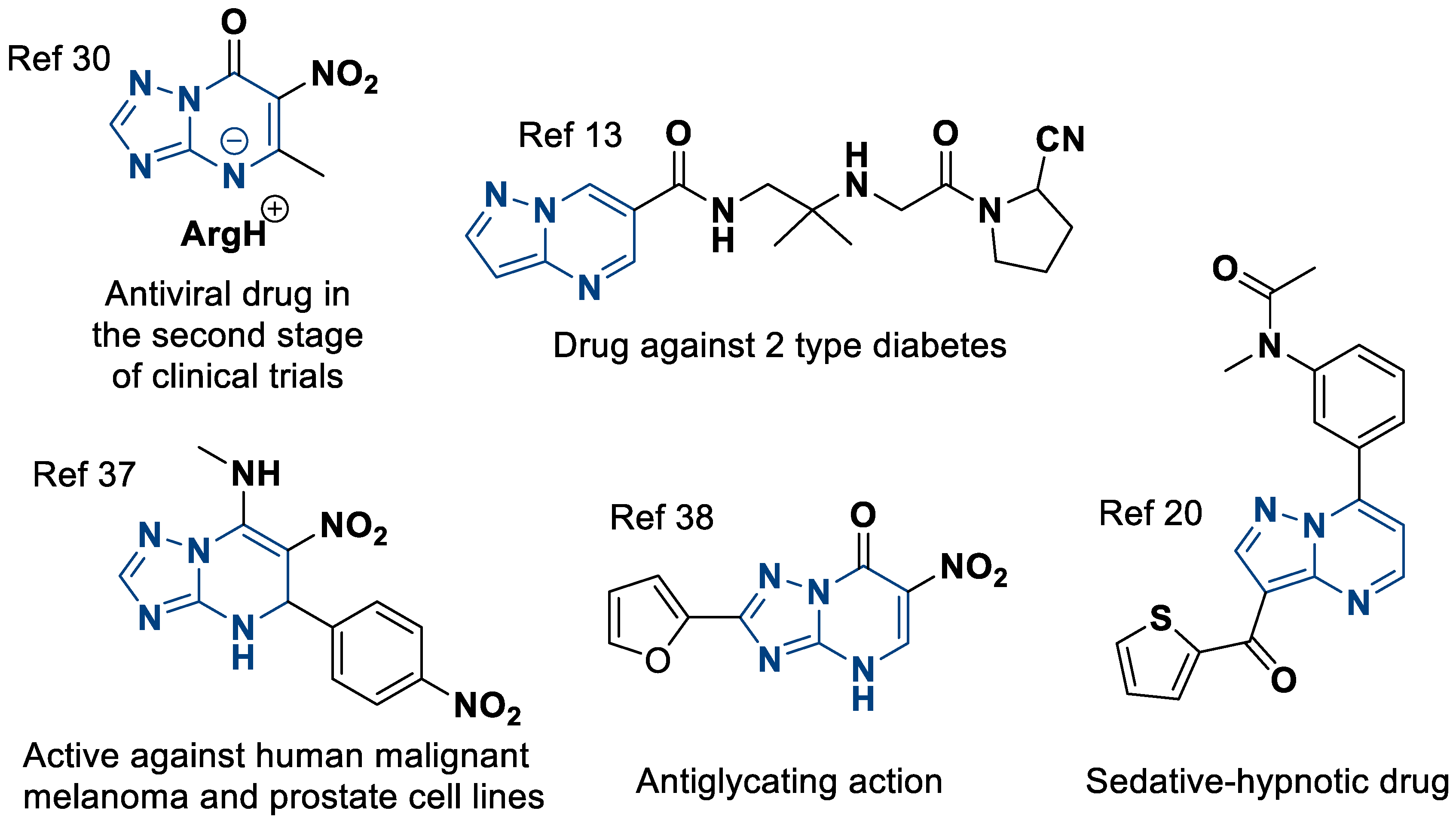
A special place is occupied by 6-nitroazolo[1,5-a]pyrimidines [30]. They are an even more specific class of heterocycles that have been explored much less thoroughly, although due to their nitro group, the variety of their properties is just as wide.
From the point of view of biological activity, these compounds exhibit antiviral [31,32], antiseptic [33,34], antioxidant [35,36], antitumor [37], and antiglycation activity [38]. From a synthetic point of view, the nitro groups are not only a quasi-form of the amino groups for creating azoloannelated purines [39,40,41], but they also, for example, open pathways for the modification of molecules by various nucleophiles [42,43]. Some useful azolo[1,5-a]pyrimidines are shown in Figure 1.
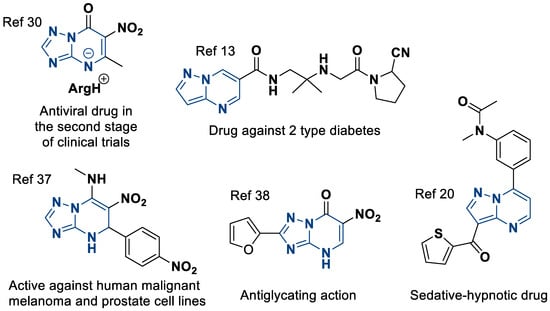
Figure 1.
Examples of biologically active azolo[1,5-a]pyrimidines.
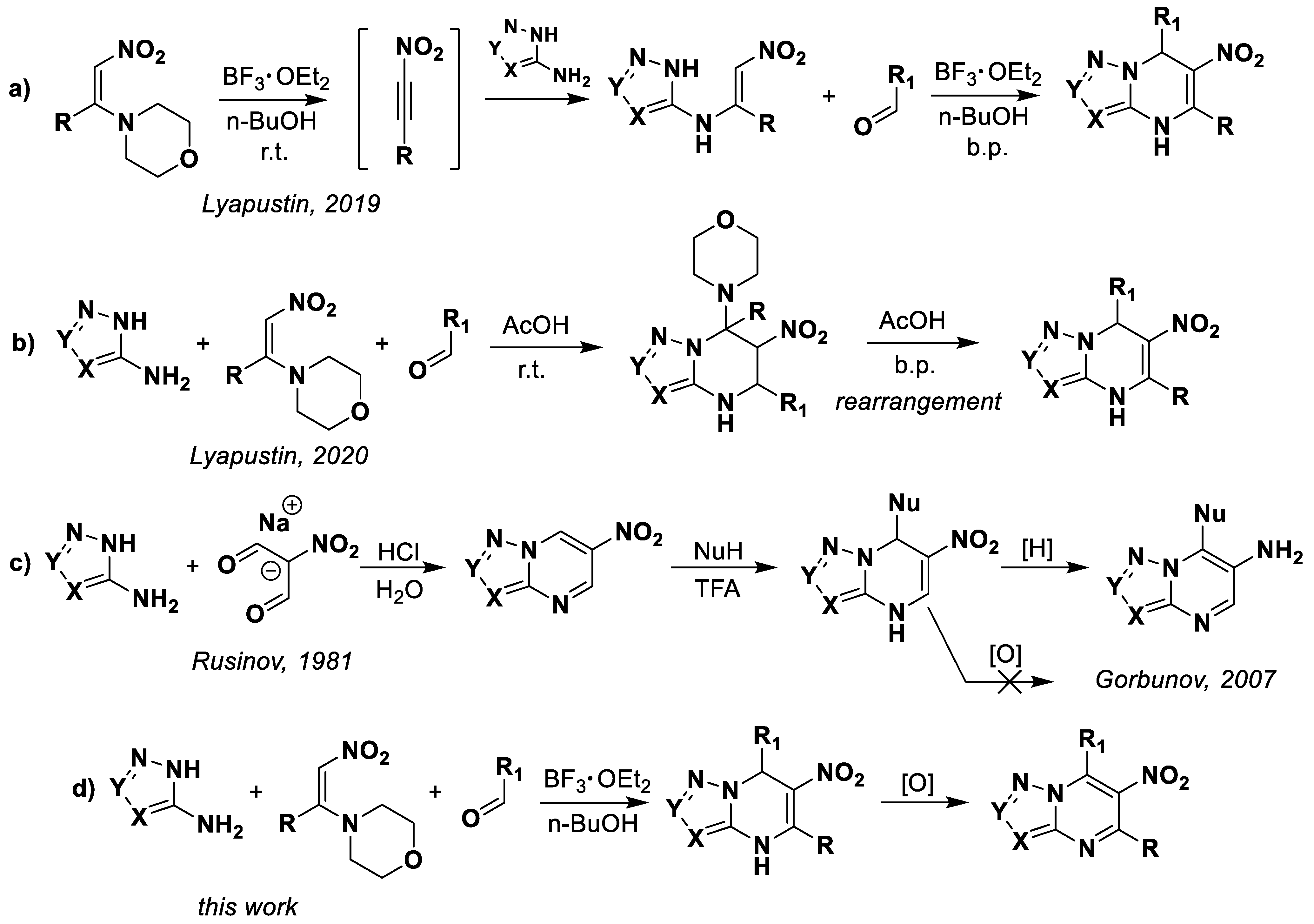
Previously we discovered the multicomponent reaction between aminoazoles, aromatic aldehydes, and 1-morpholino-2-nitroalkenes, which proceeds through different mechanisms depending on catalysis with Lewis [44] or Brønsted acids [45] (Scheme 1a,b). Formed that way, 4,7-dihydro-6-nitroazolo[1,5-a]pyrimidines have been obtained by the condensation of corresponding azoles with sodium salts of malonic dialdehyde [46] and the subsequent nucleophilic addition of π-extended (hetero)aromatic systems [43,47,48] (Scheme 1c). However, that approach has the following few disadvantages: a two-step synthesis procedure, an exclusively nucleophilic variant of structural modifications, and the limitation of possible modification positions. Moreover, the inability to aromatize 4,7-dihydro-6-nitroazolo[1,5-a]pyrimidines was established [49]. The method of “reductive autoaromatization” is used for oxidation, where the spontaneous aromatization of the heterocyclic system occurs during the reduction of the nitro group (Scheme 1c).
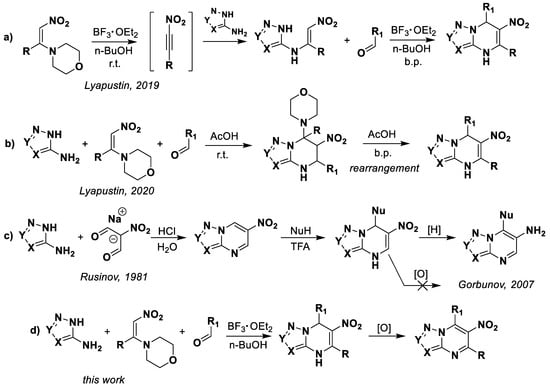
Scheme 1.
Current methods for the synthesis of 4,7-dihydroazolo[1,5-a]pyrimidines.
Thus, in this work we study the possibility of oxidation of 4,7-dihydro-6-nitroazolo[1,5-a]pyrimidines, while saving the nitro-group, and the reaction tolerance of the multicomponent reaction and oxidation process (Scheme 1d).
2. Results
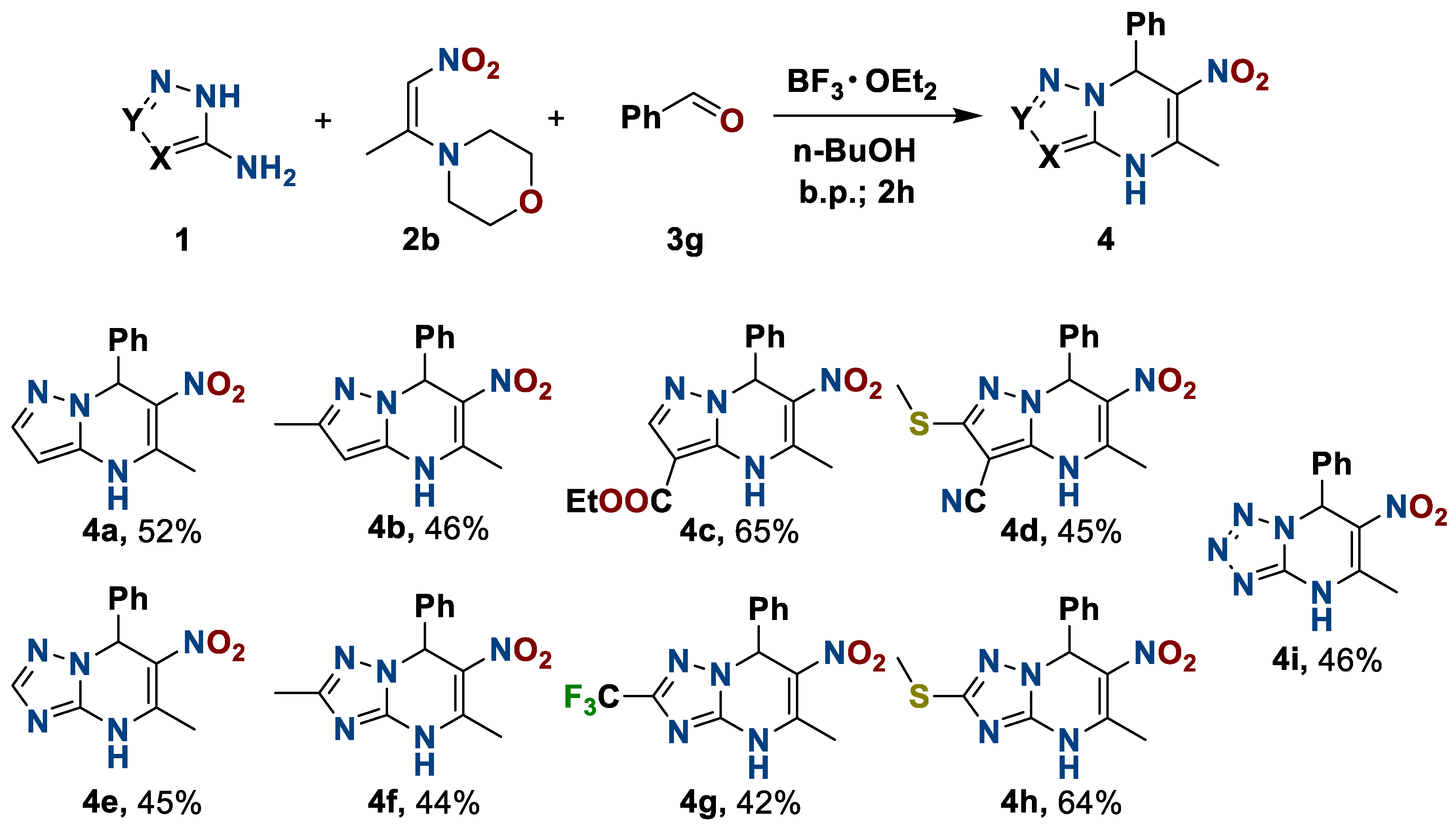
To study the substrate scope, we carried out the multicomponent process by changing one of the initial reagents, while two were unchanged. Using 1-morpholino-2-nitropropylene 2b, benzaldehyde 3g with varying 3-aminoazoles 1 and molecular fragments in their structure in the medium BF3·OEt2–n-butanol, we obtained a series of 4,7-dihydro-6-nitro-7-phenylazolo[1,5-a]pyrimidines 4 in a moderate to good yield (Scheme 2).
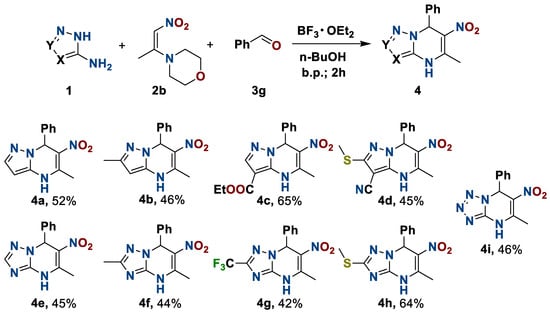
Scheme 2.
Preparation of 4,7-dihydro-6-nitro-5-methyl-7-phenylazolo[1,5-a]pyrimidines.
The next goal of the work was to examine the obtained 4,7-dihydro-5-methyl-6-nitro-7-phenylazolo[1,5-a]pyrimidines 4. Using compound 4c as an example, we studied the effect of the nature of solvents and oxidants on the yield of the model reaction product. The results are shown in Table 1.

Table 1.
Oxidation of 4c and reaction parameters.
We found that the studied compound 4c decomposes under the action of the strong inorganic oxidizer (Table 1, № 1), and is not oxidized by atmospheric oxygen under acidic conditions (Table 1, № 2), as well as dichlorodicyanobenzoquinone (Table 1, № 3,4), Dess-Martin periodate (Table 1, № 5), and diacetoxyiodobenzene (Table 1, № 7). Nevertheless, the use of pyridinechlorochromate (Table 1, № 6) and diacetoxyiodobenzene (Table 1, № 8) in acetic acid at an elevated temperature leads to the formation of heterocycle 5c with the yields 49 and 81%, respectively.
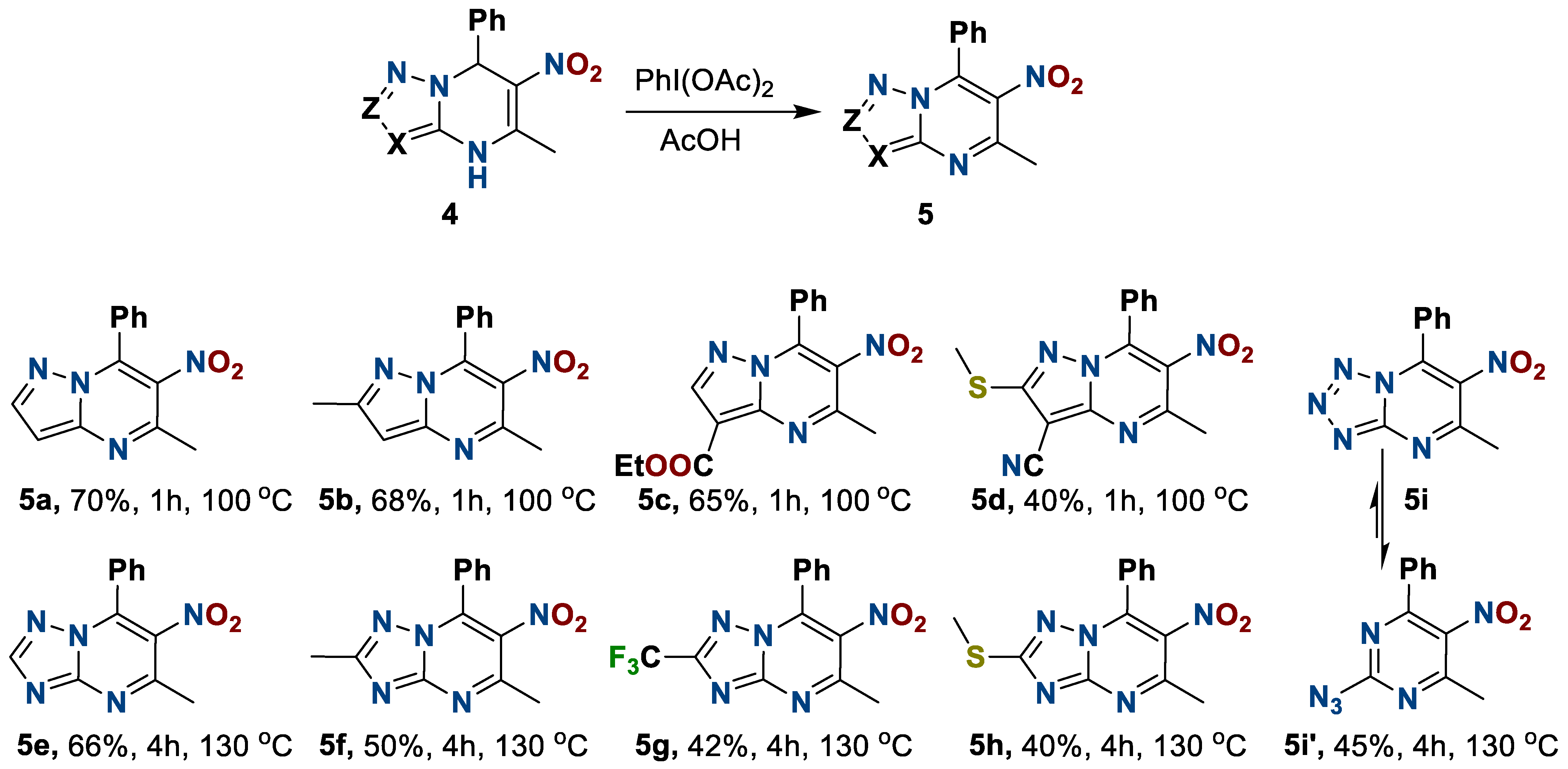
Thereby, by using diacetoxyiodobenzene in acetic acid at a corresponding temperature, we obtained a number of new 6-nitro-5-methyl-7-phenylazolo[1,5-a]pyrimidines 5 in moderate to high yields (Scheme 3).
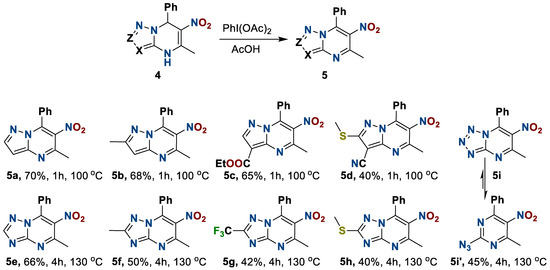
Scheme 3.
Preparation of 6-nitro-5-methyl-7-phenylazolo[1,5-a]pyrimidines.
The oxidation of triazolo- and tetrazolopyrimidines 4e–i occurs under harsher conditions than the pyrazolo- derivatives 4a–d; the process requires both a higher temperature and a longer reaction time. This is apparently due to the increasing π-deficient properties of five-membered heterocycles with the increasing numbers of N-atoms. A feature of the tetrazolopyrimidine 5i is the formation of the azido-tetrazole tautomerism product 2-azido-5-nitro-6-methyl-4-phenylpyrimidine 5i′, which was supported by IR spectroscopy data (see Supplementary Materials).
Thus, in the first part of the work, the possibility of the three-component reaction of 1-morpholino-2-nitropropylene 2a, benzaldehyde 3g, and 3-aminoazoles 1 was established with an assessment of the last reaction method. In addition, the oxidative aromatization of type 4 dihydroazolopyrimidines, which was previously considered impossible, was realized.
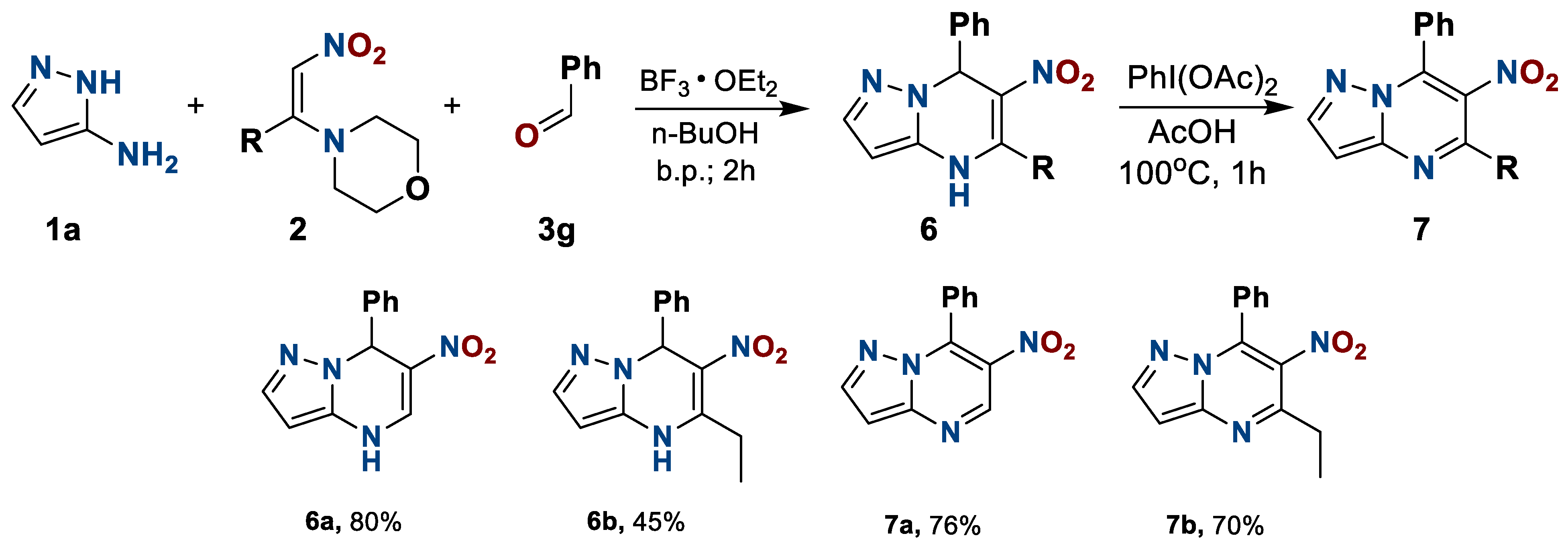
The next stage of the study was the change the 1-morplholino-2-nitroalkene component 2. The number of synthetically available nitroalkenes 2 is limited, and all of them turned out to be reactive in the preparation of dihydronitro derivatives 6, which, in turn, were successfully dehydrogenated (Scheme 4).
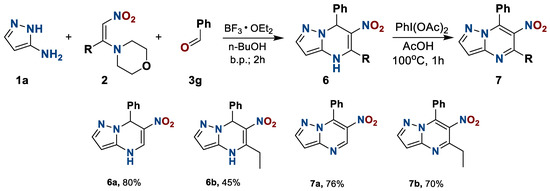
Scheme 4.
Preparation of 4,7-dihydro-6-nitro-7-phenyl-5-R-pyrazolo[1,5-a]pyrimidines 6 and 4,7-dihydro-6-nitro-7-phenyl-5-R-pyrazolo[1,5-a]pyrimidines 7.
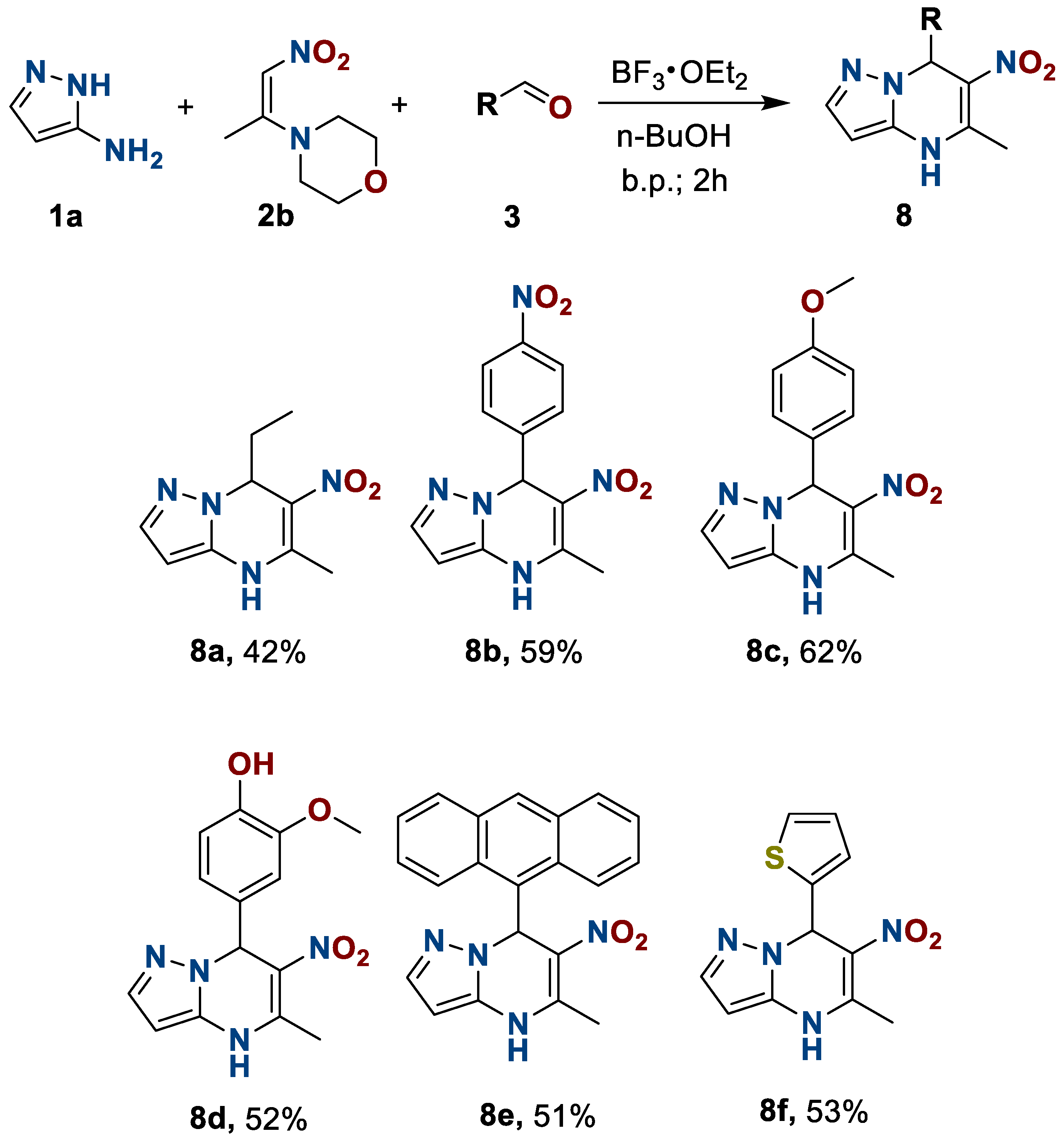
Finally, we examined the effect of the structure of the third component, aldehydes, on the formation of 4,7-dihydro-6-nitroazolo[1,5-a]pyrimidines 8 and their subsequent oxidation. The object of study was to use benzaldehyde 3g and its derivatives with electron-donating 3c,d and electron-acceptor 3b groups, polycyclic anthracenecarbaldehyde 3e, heterocyclic thophenecarbaldehyde 3f, and propanal 3a. The use of the developed reaction conditions allowed us to obtain 7-R-5-methyl-6-nitropyrazolo[1,5-a]pyrimidines 8 in moderate to good yields (Scheme 5).
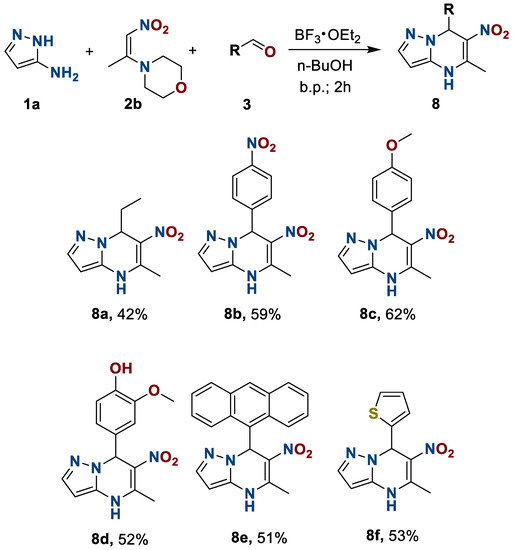
Scheme 5.
Preparation of 4,7-dihydro-6-nitro-5-methyl-7-R-azolo[1,5-a]pyrimidines 8.
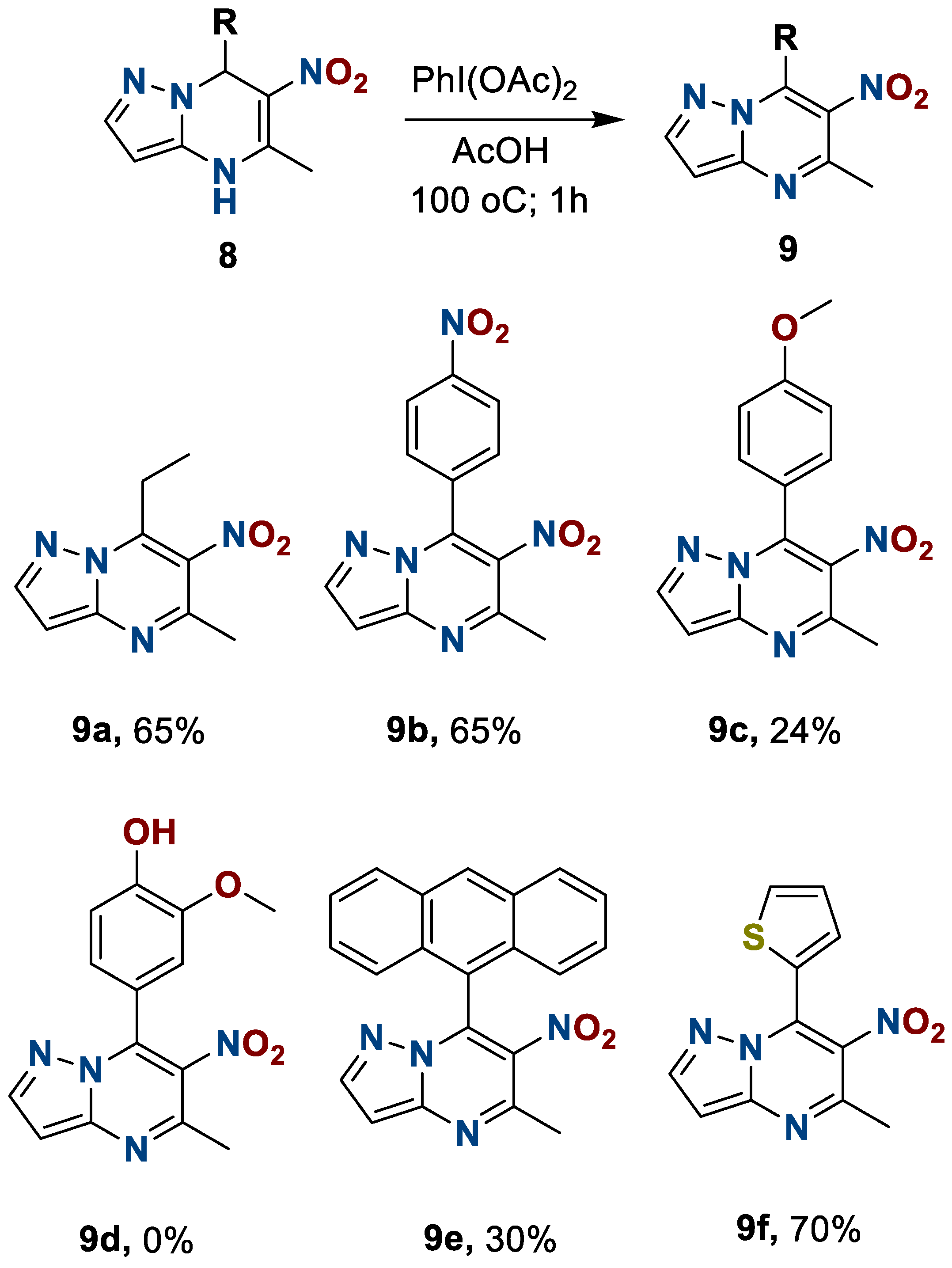
During the oxidation, we discovered that the interaction of heterocycle 8d with phenyliodozodiacetate, even at room temperature, leads to the resinification of the reaction mass. At the same time, the oxidation of heterocycles 8c and 8e was also accompanied by the formation of side products, but we have succeeded in isolating the desired compounds in yields of 24 and 30%, respectively. The results are presented in Scheme 6.
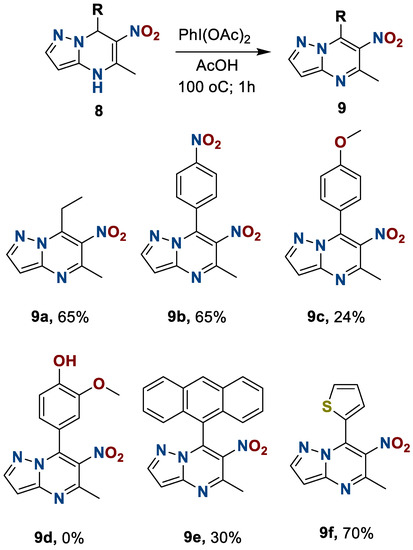
Scheme 6.
Preparation of 6-nitro-5-methyl-7-R-azolo[1,5-a]pyrimidines 9.
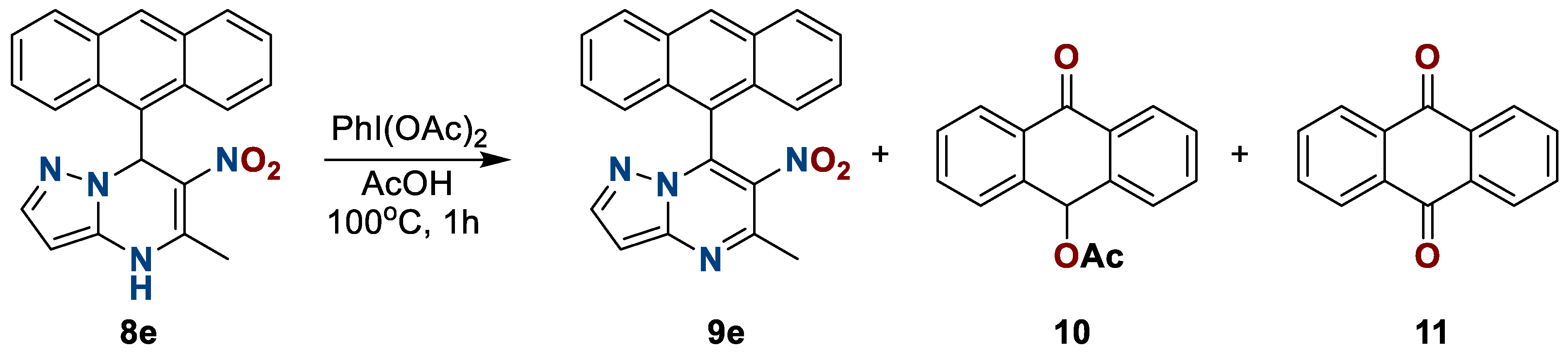
It was previously noted that it is impossible to obtain an aromatic system for similar structures, since attempts at oxidation have led either to unsuccessful results or to a mixture of unidentifiable products. However, results of this work complement the existing precedent since oxidation is complicated only in the case of compounds with an electron-donor substituent at position seven. During the oxidation of nitropyrazolopyrimdines 9e, besides the main product 9e, side products 9-acetoxy-anthracene 10 and anthraquinone 11 were also isolated using the flash chromatography method. These compounds were characterized in the composition of the mixture (Scheme 7).
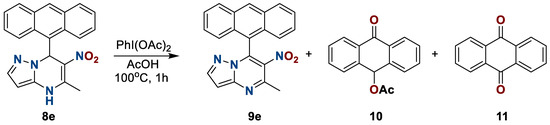
Scheme 7.
Oxidation of 6-nitro-5-methyl-7-(anthracen-9-yl)azolo[1,5-a]pyrimidine 8e.
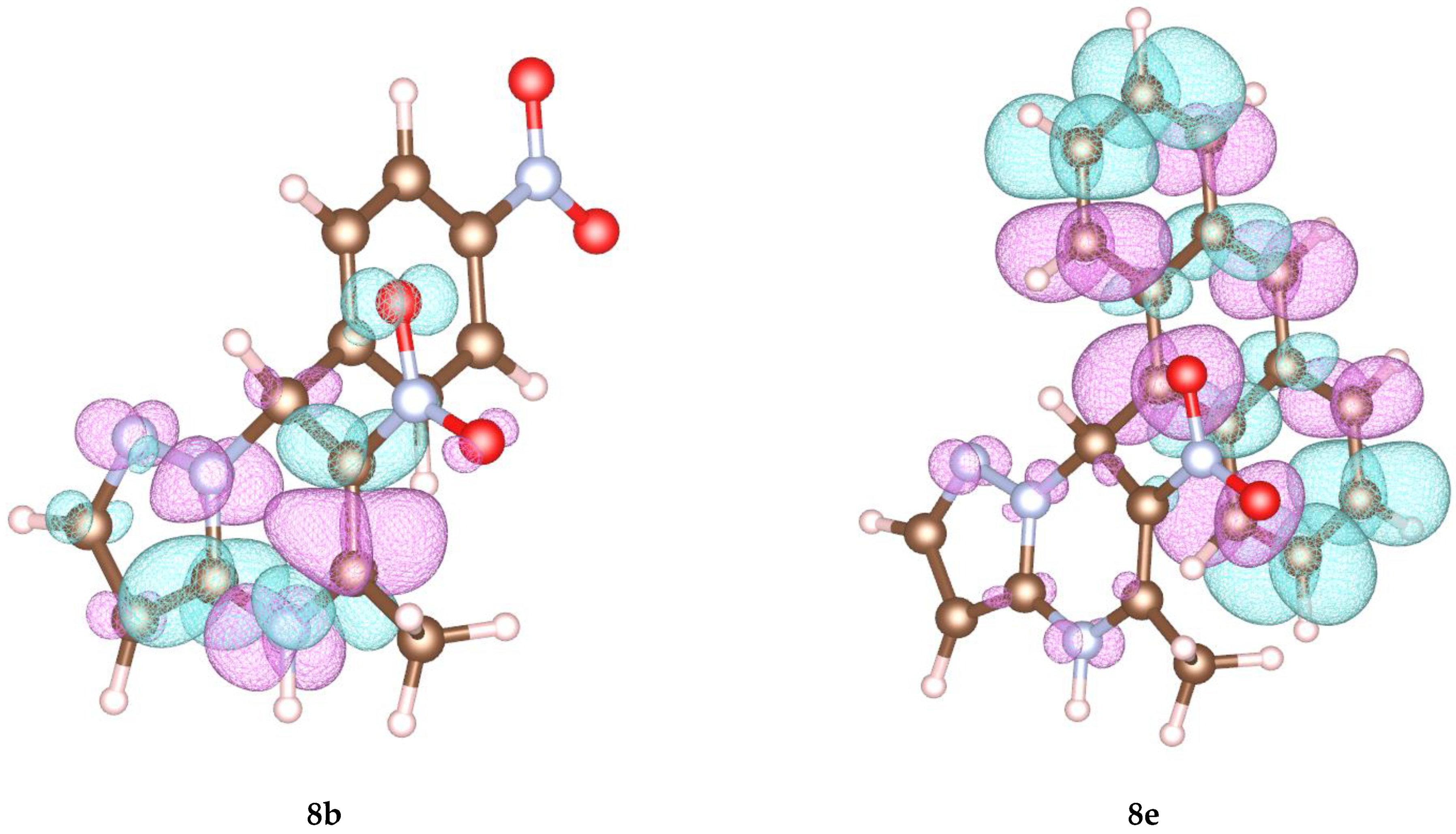
To elucidate the experimental data, we performed a quantum chemical calculation of the compounds 5a, 8a–e to obtain the charge distribution over the molecular structure. Figure 2 demonstrates the spin distribution of the highest occupied molecular orbital (HOMO) for compounds 8b and 8e. Other data are shown in the Supplementary Materials.
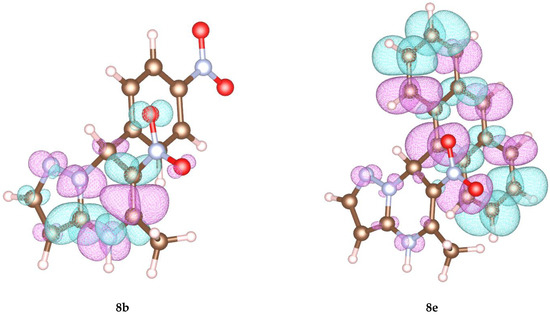
Figure 2.
Visualization of HOMO for compounds 8b and 8e obtained in PBE0 approximation.
Indeed, the calculated data demonstrate that in compounds with an electron donating substituent 8e, the substituent is involved in the electron density distribution, which can have a serious influence on the direction of oxidation. Therefore, in these structures, the covalent bond between the C-7 atom and the substituent fissions and the nonstoichiometric formation of oxidation side products occurs.
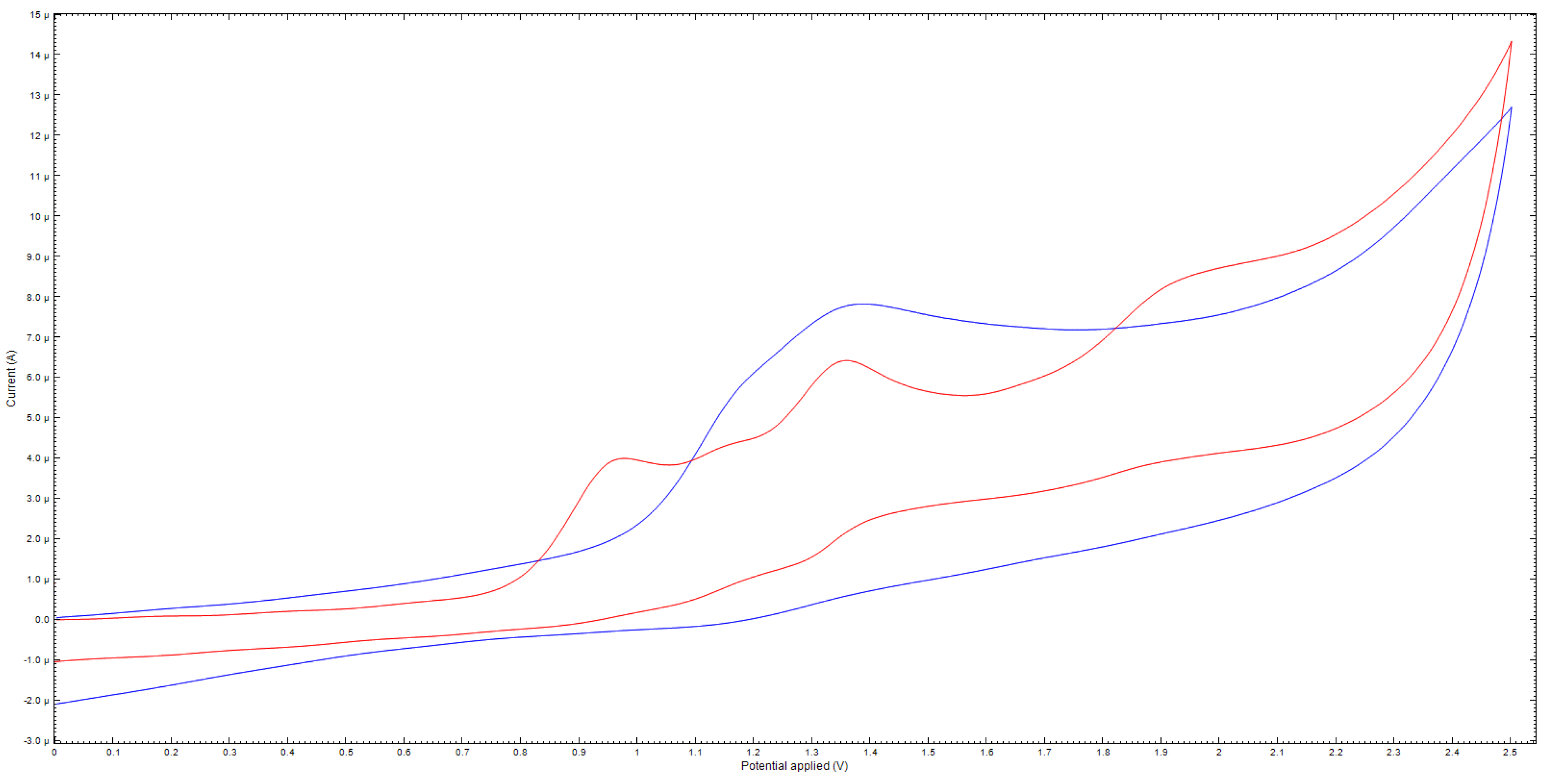
Furthermore, the results of cyclic voltammetry also indicate the different behavior of compound 8 under oxidation conditions (Figure 3). Thus, for compound 8b, one irreversible peak of two-electron oxidation is observed. In turn, compound 8e is characterized by two peaks, apparently, of one-electron oxidation. The data for other compounds are given in the Supplementary Materials.
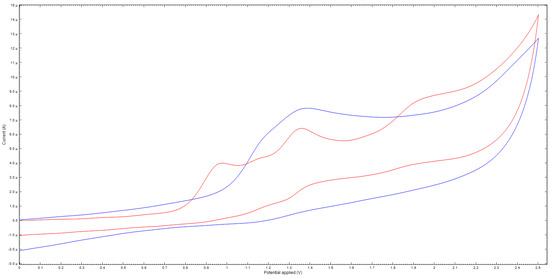
Figure 3.
Cyclic voltammogram of 8b (blue) and 8e (red), 5 × 10−3 M.
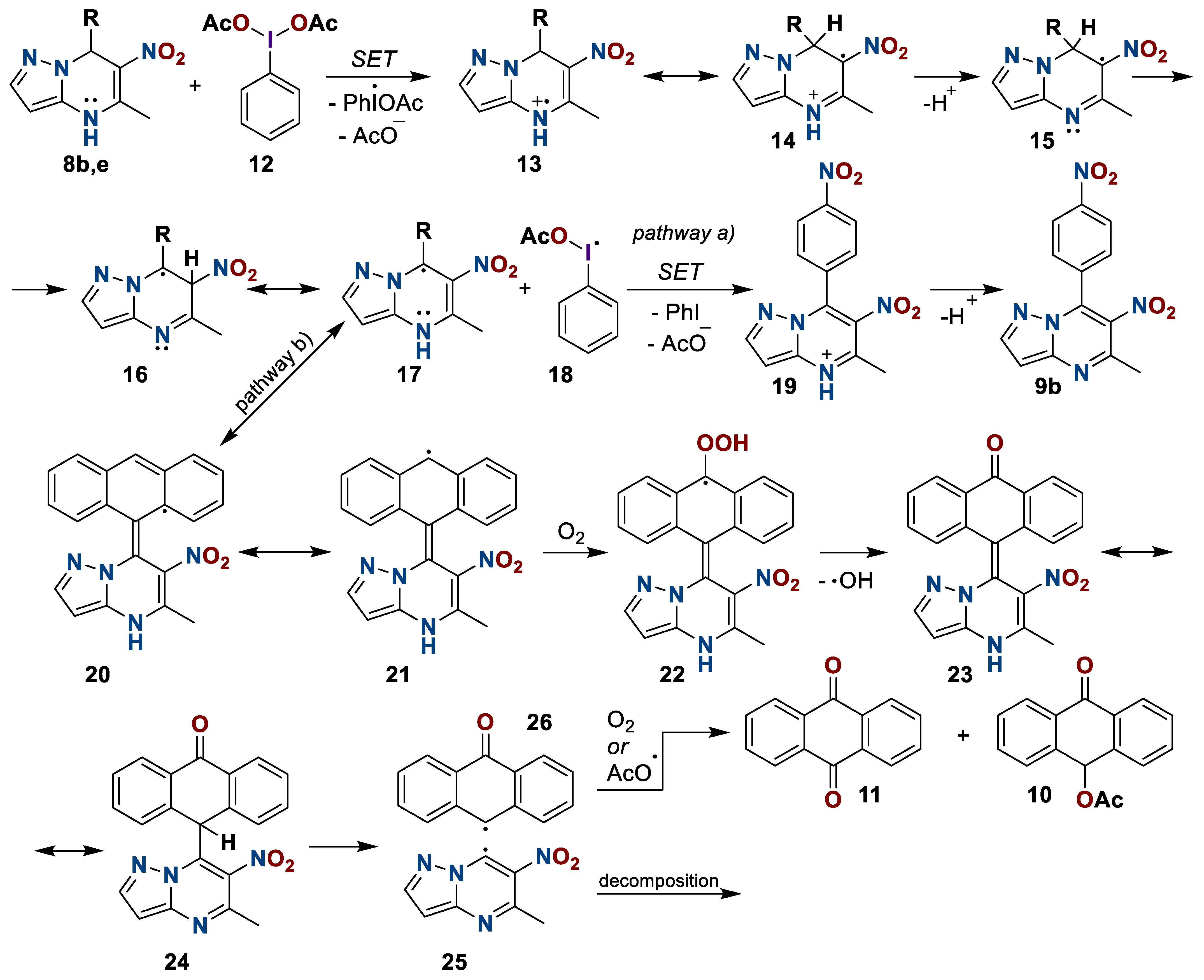
Based on the results above and the literature’s data [50,51,52], it can be assumed that initially, for both compounds 8b and 8e, single electron transfer (SET) occurs with the formation of an acetate-anion and a heterocyclic cation-radical 13 (Scheme 8). Then, the tautomeric transformation, the deprotonation of structure 14, and a shift of the hydrogen radical with the formation of radical particle 15 occur. Next, depending on the nature of the substituent, oxidation proceeds either with one more single electron transfer for compounds with an electron withdrawing substituent at position seven, or with the migration of a radical to a substituent for compounds with an electron donating substituent. Apparently, the intermediate 21 interacts with an oxygen molecule with the formation of carbonyl compound 23. Further intramolecular transformations lead to the homolytic fission of the covalent bond between the C-7 atom and the substituent in structure 24. The aromatic radical is either oxidized to anthraquinone 11 or is attacked by the acetoxy-radical from phenyliodozomonoacetate 18. The heterocyclic radical 25, apparently, decomposes nonstoichometrically.
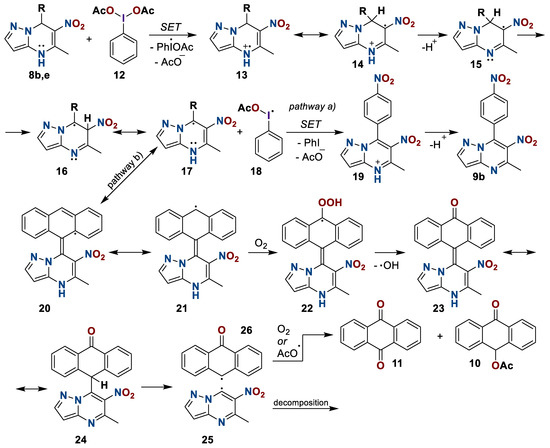
Scheme 8.
Plausible mechanism of oxidation of 6-nitro-5-methyl-7-R-azolo[1,5-a]pyrimidines 8.
3. Materials and Methods
Unless stated otherwise, all solvents and commercially available reactants/reagents were used as received. Non-commercial starting materials were prepared as described below or according to the literature’s procedures. One-dimensional 1H and 13C NMR spectra, as well as two-dimensional 1H–13C HMBC experiments were acquired on a Bruker DRX-400 instrument (400 and 101 MHz, respectively) or a Bruker Avance NEO 600 instrument (600 and 151 MHz, respectively), equipped with a Prodigy broadband gradient cryoprobe, utilizing DMSO-d6 and CDCl3 as the solvent and TMS as the internal standard. IR spectra were recorder on a Bruker Alpha FTIR spectrometer equipped with a ZnSe ATR accessory. Mass spectra were recorded on a Shimadzu GCMS-QP2010 Ultra mass spectrometer, using EI method of ionization (70 eV). Elemental analysis was performed on a PerkinElmer 2400 CHN analyzer. The reaction progress was controlled by TLC on Silufol UV-254 plates, eluent—CH3Cl. Melting points were determined on a Stuart SMP3 apparatus at the heating rate of 25 °C/min. 1-Morpholino-2-nitroethylenes 2 were prepared according to a literature procedure [53].
Density functional theory (DFT) simulation via Vienna Ab initio Simulation Package (VASP) [54] was performed for structural optimization of the molecules and obtaining of the charge distribution. The length of the supercell of every molecule was set to 22.5 Å and the cut-off energy of plane wave basis set was set to 750 eV. Structural optimization was performed using generalized gradient approximation (GGA) by Pedrew, Burke, and Ernzerhof (PBE) [55] for exchange-correlation potential. For all chemical species, projected augmented wave (PAW) pseudopotentials were used. The structures were optimized using two algorithms: conjugate gradient algorithm was implemented for initial optimization and after that RMM-DIIS (residual minimization scheme, direct inversion in the iterative subspace) algorithm was used for final optimization. The stopping criteria for optimization was |Fi| ≤ 0.01 eV/Å, where i = x, y, z, i.e., absolute value of each force component acting on nuclei should be not greater than 10 meV/Å.
For PBE-optimized structures, a hybrid PBE0 simulation was performed to obtain charge distribution, for accounting of long-range interactions Grimme DFT-D3 corrections [56] were used. Since all the performed calculation were spin polarized, it was possible to extract spin density from the charge distribution. For the interpretation of the obtained data, the program VESTA [57] was used.
Cyclic voltammetry was carried out on a Metrohm Autolab PGSTAT128N potentiostat with a standard three-electrode configuration. Typically, a three electrodes cell equipped with a glass carbon working electrode, an Ag/AgNO3 (0.01 M) reference electrode, and a glass carbon rod counter electrode was employed. The measurements were performed in acetonitrile with tetrabutylammonium hexafluorophosphate (0.1 M) as the supporting electrolyte under an argon atmosphere at a scan rate of 100 mV/s.
3.1. 4,7-dihydro-6-nitroazolo[1,5-a]pyrimidines 4,6,8; General procedure 1
To a suspension 2 mmol (1.0 equiv.) of corresponding aminoazole 1, 2 mmol (1.0 equiv.) of nitroalkene 2, and 2 mmol (1.0 equiv.) of aldehyde 3 in 5 mL of n-BuOH, was added 3 mmol (1.5 equiv., 0.37 mL) of BF3·Et2O. The reaction mixture was heated on oil bath at 120 °C for 2 h. The resulting solution was cooled to room temperature and stirred for 15 min. The obtained precipitate was filtered off, washed with 15 mL of i-PrOH. The precipitate was suspended in 50 mL of water, stirred for 5 min, filtered off again, and washed with 15 mL of water.
5-Methyl-6-nitro-7-phenyl-4,7-dihydropyrazolo[1,5-a]pyrimidine(4a). The reaction was performed according to the general procedure 1, employing 0.166 g (2 mmol, 1 equiv.) of 3-aminopyrazole 1a, 0.344 g (2 mmol, 1 equiv.) of 1-morpholino-2-nitropropylene 2b and 0.20 mL (2 mmol, 1 equiv.) of benzaldehyde 3g. Yellow solid. Yield, 0.266 g (52%); mp, 244–247 °C. IR (ATR): 1516, 1415 (NO2). 1H NMR (400 MHz, DMSO-d6): δ = 2.63 (3H, s, CH3); 5.48 (1H, s, H-7); 7.09–7.28 (5H, m, Ph); 7.41 (1H, s, H-2); 10.74 (1H, s, NH); 12.37 (1H, s, H-3). 13C {1H} NMR (101 MHz, DMSO-d6): δ = 21.9; 39.5; 106.8; 122.8; 126.0; 126.3; 128.3; 126.5; 139.0; 144.4; 146.7; 151.6. Anal. Calcd for C13H12N4O2: C, 60.93; H, 4.72; N, 21.86. Found: C, 60.80; H, 4.65; N, 21.90.
2,5-Dimethyl-6-nitro-7-phenyl-4,7-dihydropyrazolo[1,5-a]pyrimidine(4b). The reaction was performed according to the general procedure 1, employing 0.194 g (2 mmol, 1 equiv.) of 3-amino-5-methylpyrazole 1b, 0.344 g (2 mmol, 1 equiv.) of 1-morpholino-2-nitropropylene 2b, and 0.20 mL (2 mmol, 1 equiv.) of benzaldehyde 3g. Yellow solid. Yield, 0.221 g (46%); mp, 250–255 °C. IR (ATR): 1514, 1417 (NO2). 1H NMR (400 MHz, DMSO-d6): δ = 1.92 (3H, s, C-2-CH3); 2.60 (3H, s, C-5-CH3); 5.36 (1H, s, H-7); 7.06–7.31 (5H, m, Ph); 10.66 (1H, s, NH); 12.07 (1H, s, H-3). 13C {1H} NMR (101 MHz, DMSO-d6): δ = 9.4; 21.9; 39.5; 104.0; 123.7; 126.0; 127.0; 128.1; 135.5; 144.7; 145.8; 151.2. Anal. Calcd for C14H14N4O2: C, 62.21; H, 5.22; N, 20.73. Found: C, 62.28; H, 5.26; N, 20.65.
3-Etoxycarbonyl-5-methyl-6-nitro-7-phenyl-4,7-dihydropyrazolo[1,5-a]pyrimidine(4c). The reaction was performed according to the general procedure 1, employing 0.310 g (2 mmol, 1 equiv.) of 3-amino-4-etoxycarbonylpyrazole 1c, 0.344 g (2 mmol, 1 equiv.) of 1-morpholino-2-nitropropylene 2b, and 0.20 mL (2 mmol, 1 equiv.) of benzaldehyde 3g. Bright yellow solid. Yield, 0.426 g (65%); mp, 215–218 °C. IR (ATR): 1685 (C=O); 1576, 1300 (NO2). 1H NMR (400 MHz, DMSO-d6): δ = 1.32 (3H, t, CH2-CH3, J = 7.1 Hz); 2.79 (3H, s, C-5-CH3); 4.27 (2H, dd, CH2-CH3, J = 7.0, 2.5 Hz); 6.56 (1H, s, H-7); 7.23–7.38 (5H, m, Ph); 7.66 (1H, s, H-2); 10.29 (1H, s, NH). 13C {1H} NMR (101 MHz, DMSO-d6): δ = 14.3; 19.8; 59.5; 59.8; 97.5; 122.6; 127.2; 128.4; 128.6; 137.4; 139.6; 140.8; 148.1; 161.7. Anal. Calcd for C16H16N4O4: C, 58.53; H, 4.91; N, 17.06. Found: C, 58.41; H, 4.81; N, 17.19.
3-Cyano-5-methyl-2-methylthio-6-nitro-7-phenyl-4,7-dihydropyrazolo[1,5-a]pyrimidine (4d). The reaction was performed according to the general procedure 1, employing 0.308 g (2 mmol, 1 equiv.) of 3-amino-4-cyano-5-methylthiopyrazole 1d, 0.344 g (2 mmol, 1 equiv.) of 1-morpholino-2-nitropropylene 2b, and 0.20 mL (2 mmol, 1 equiv.) of benzaldehyde 3g. Sand color solid. Yield, 0.294 g (45%); mp, 248–256 °C with decomp. IR (ATR): 2229 (CN); 1575, 1306 (NO2). 1H NMR (400 MHz, DMSO-d6): δ = 2.42 (3H, s, S-CH3); 2.67 (3H, s, C-5-CH3); 6.53 (1H, s, H-7); 7.27–7.47 (5H, m, Ph); 11.90 (1H, s, NH). 13C {1H} NMR (101 MHz, DMSO-d6): δ = 13.8; 19.6; 59.8; 75.3; 112.1; 122.6; 127.4 (2C); 128.6; 139.0; 140.4; 147.4; 150.9. Anal. Calcd for C15H13N5O2S: C, 55.04; H, 4.00; N, 21.39. Found: C, 55.00; H, 4.04; N, 21.50.
5-Methyl-6-nitro-7-phenyl-4,7-dihydro-1,2,4-triazolo[1,5-a]pyrimidine(4e). The reaction was performed according to the general procedure 1, employing 0.168 g (2 mmol, 1 equiv.) of 3-amino-1,2,4-triazole 1e, 0.344 g (2 mmol, 1 equiv.) of 1-morpholino-2-nitropropylene 2b, and 0.20 mL (2 mmol, 1 equiv.) of benzaldehyde 3g. Light yellow solid. Yield, 0.231 g (45%); mp, 270 °C with decomp. IR (ATR): 1582, 1312 (NO2). 1H NMR (400 MHz, DMSO-d6): δ = 2.66 (3H, s, CH3); 6.63 (1H, s, H-7); 7.26–7.40 (5H, m, Ph); 7.78 (1H, s, H-2); 11.87 (1H, s, NH). 13C {1H} NMR (101 MHz, DMSO-d6): δ = 20.2; 59.7; 121.9; 127.3; 128.5; 128.5; 139.3; 145.5; 149.3; 150.9. Anal. Calcd for C12H11N5O2: C, 56.03; H, 4.31; N, 27.22. Found: C, 56.10; H, 4.26; N, 27.27.
2,5-Dimethyl-6-nitro-7-phenyl-4,7-dihydro-1,2,4-triazolo[1,5-a]pyrimidine(4f). The reaction was performed according to the general procedure 1, employing 0.196 g (2 mmol, 1 equiv.) of 3-amino-5-methyl-1,2,4-triazole 1f, 0.344 g (2 mmol, 1 equiv.) of 1-morpholino-2-nitropropylene 2b, and 0.20 mL (2 mmol, 1 equiv.) of benzaldehyde 3g. Yellow solid. Yield, 0.238 g (44%); mp, 270 °C with decomp. IR (ATR): 1586, 1314 (NO2). 1H NMR (400 MHz, DMSO-d6): δ = 2.11 (3H, s, C-2-CH3), 2.64 (3H, s, C-5-CH3); 6.52 (1H, s, H-7); 7.26–7.38 (5H, m, Ph); 11.74 (1H, s, NH). 13C {1H} NMR (101 MHz, DMSO-d6): δ = 13.9; 20.2; 59.5; 121.9; 127.3; 128.4; 128.5; 139.5; 145.5; 149.1; 159.3. Anal. Calcd for C13H13N5O2: C, 57.56; H, 4.83; N, 25.82. Found: C, 57.49; H, 4.90; N, 25.75.
5-Methyl-6-nitro-7-phenyl-2-trifluoromethyl-4,7-dihydro-1,2,4-triazolo[1,5-a]pyrimidine(4g).
To a suspension of 0.304 g (2 mmol, 1 equiv.) of 3-amino-5-trifluoromethyl-1,2,4-triazole 1g, 0.344 g (2 mmol, 1 equiv.) of 1-morpholino-2-nitropropylene 2b and 0.20 mL (2 mmol, 1 equiv.) of benzaldehyde 3g in 5 mL of n-BuOH was added 3 mmol (1.5 equiv., 0.37 mL) of BF3·Et2O. The reaction mixture was heated on oil bath at 120 °C for 2 h. The resulting solution was cooled to room temperature, and 5 mL of n-heptane was added. The obtained suspension was stirred for 10 min, filtered off, and washed with 20 mL of water. The product was recrystallized from i-PrOH-H2O 1/1. Yellow solid. Yield, 0.273 g (42%); mp, 231–234 °C. IR (ATR): 1573, 1321 (NO2). 1H NMR (400 MHz, DMSO-d6): δ = 2.66 (3H, s, C-5-CH3); 6.74 (1H, s, H-7); 7.31–7.45 (5H, m, Ph); 12.13 (1H, s, NH). 13C {1H} NMR (101 MHz, DMSO-d6): δ = 20.1; 60.4; 118.9 (q, J = 269.7 Hz); 122.5; 127.6; 128.7; 128.9; 138.4; 147.0; 148.8; 151.1 (q, J = 39.1 Hz). Anal. Calcd for C13H10F3N5O2: C, 48.01; H, 3.10; N, 21.53. Found: C, 48.15; H, 3.24; N, 21.40.
5-Methyl-2-methylthio-6-nitro-7-phenyl-4,7-dihydro-1,2,4-triazolo[1,5-a]pyrimidine(4h). The reaction was performed according to the general procedure 1, employing 0.260 g (2 mmol, 1 equiv.) of 3-amino-5-methylthio-1,2,4-triazole 1h, 0.344 g (2 mmol, 1 equiv.) of 1-morpholino-2-nitropropylene 2b, and 0.20 mL (2 mmol, 1 equiv.) of benzaldehyde 3g. Pale yellow solid. Yield, 0.388 g (64%); mp, 273–276 °C. IR (ATR): 1557, 1320 (NO2). 1H NMR (400 MHz, DMSO-d6): δ = 2.42 (3H, s, S-CH3); 2.64 (3H, s, C-5-CH3); 6.56 (1H, s, H-7); 7.27–7.38 (5H, m, Ph); 11.90 (1H, s, NH). 13C {1H} NMR (101 MHz, DMSO-d6): δ = 13.5; 20.2; 59.8; 122.2; 127.4; 128.5; 128.6; 139.1; 146.2; 148.8; 160.0. Anal. Calcd for C13H13N5O2S: C, 51.49; H, 4.41; N, 23.02. Found: C, 51.47; H, 4.32; N, 23.09.
5-Methyl-6-nitro-7-phenyl-4,7-dihydrotetrazolo[1,5-a]pyrimidine(4i). The reaction was performed according to the general procedure 1, employing 0.206 g (2 mmol, 1 equiv.) of 3-aminotetrazole monohydrate 1i, 0.344 g (2 mmol, 1 equiv.) of 1-morpholino-2-nitropropylene 2b, and 0.20 mL (2 mmol, 1 equiv.) of benzaldehyde 3g. To a suspension of 0.206 g (2 mmol, 1 equiv.) of 3-aminotetrazole monohydrate 1i, 0.344 g (2 mmol, 1 equiv.) of 1-morpholino-2-nitropropylene 2b and 0.20 mL (2 mmol, 1 equiv.) of benzaldehyde 3g in 5 mL of n-BuOH was added 3 mmol (1.5 equiv., 0.37 mL) of BF3·Et2O. The reaction mixture was heated on oil bath at 120 °C for 2 h. The resulting solution was cooled to room temperature, and 5 mL of n-heptane was added. The obtained suspension was stirred for 10 min, decanted, and 5 mL of i-PrOH was added. The obtained suspension was filtered off and washed with 20 mL of water. The product was recrystallized from i-PrOH. White yellow solid. Yield, 0.237 g (46%); mp, 236–239 °C IR (ATR): 1576, 1295 (NO2). 1H NMR (400 MHz, DMSO-d6): δ = 2.68 (3H, s, CH3); 7.02 (1H, s, H-7); 7.30–7.50 (5H, m, Ph); 12.30 (1H, s, NH). 13C {1H} NMR (101 MHz, DMSO-d6): δ = 20.2; 58.9; 122.2; 127.5; 128.9; 129.1; 138.2; 147.4; 149.4. Anal. Calcd for C11H10N6O2: C, 51.16; H, 3.90; N, 32.54. Found: C, 51.10; H, 3.95; N, 32.51.
3.2. 6-Nitroazolo[1,5-a]pyrimidines 5,7,9; General procedure 2
To a suspension of 100 mg of corresponding heterocycle 4,6,8 in 5 mL of acetic acid, 1.25 equiv. of PhI(OAc)2 was added. The reaction mixture was heated on oil bath at 100 °C for pyrazolopyrimidines and 130 °C for tetra- and triazolopyrimidines for 1.5 h and 4 h, respectively. To the resulting solution, another 0.5 equiv. of PhI(OAc)2 was added and stirred for 30 min. After this, 5 mL of EtOH was added and stirred again for 15 min. After heating, the resulting solution was concentrated under reduced pressure. To the residue, 3 mL of n-heptane was added and evaporated again. The residue was purified using flash-chromatography on silica gel 60 with CHCl3 as the eluent to give the desirable product.
5-Methyl-6-nitro-7-phenylpyrazolo[1,5-a]pyrimidine(5a). The reaction was performed according to the general procedure 2, employing 0.39 mmol of heterocycle 4a and 0.157 + 0.063 g (0.48 + 0.19 mmol) of PhI(OAc)2. Sand color solid. Yield, 69 mg (70%); mp, 232–235 °C. IR (ATR): 1527, 1364 (NO2). 1H NMR (400 MHz, CDCl3): δ = 2.80 (3H, s, CH3); 7.48–7.62 (5H, m, Ph); 8.03 (1H, s, H-2); 12.05 (1H, s, H-3). 13C {1H} NMR (101 MHz, CDCl3): δ = 21.8; 113,3; 128,4; 129.7; 130.7; 132.3; 135.7; 138.2; 142.8; 150.6; 151.5. Anal. Calcd for C13H10N4O2: C, 61.41; H, 3.96; N, 22.04. Found: C, 61.44; H, 3.93; N, 22.00.
2,5-Dimethyl-6-nitro-7-phenylpyrazolo[1,5-a]pyrimidine(5b). The reaction was performed according to the general procedure 2, employing 0.39 mmol of heterocycle 4a and 0.150 + 0.060 g (0.47 + 0.19 mmol) of PhI(OAc)2. Sand color solid. Yield, 69 mg (55%); mp, 199–212 °C. IR (ATR): 1525, 1367 (NO2). 1H NMR (400 MHz, CDCl3): δ = 2.04 (3H, s, C-2-CH3); 2.77 (3H, s, C-5-CH3); 7.32–7.58 (5H, m, Ph); 11.61 (1H, br.s., NH). 13C {1H} NMR (101 MHz, CDCl3): δ = 14.4; 21.5; 111.5; 128.5; 128.7; 129.9; 131.3; 139.2; 143.0; 144.7; 150.5; 151.1. Anal. Calcd for C14H12N4O2: C, 62.68; H, 4.51; N, 20.88. Found: C, 62.75; H, 4.43; N, 20.82.
3-Etoxycarbonyl-5-methyl-6-nitro-7-phenylpyrazolo[1,5-a]pyrimidine(5c). The reaction was performed according to the general procedure 2, employing 0.30 mmol of heterocycle 4c and 0.123 + 0.049 g (0.38 + 0.15 mmol) of PhI(OAc)2. Pale yellow solid. Yield, 64 mg (65%); mp, 147–150 °C. IR (ATR): 1714 (C=O); 1534, 1364 (NO2). 1H NMR (400 MHz, CDCl3): δ = 1.42 (3H, t, CH2-CH3, J = 7.1 Hz); 2.79 (3H, s, C-5-CH3); 4.44 (2H, q, CH2-CH3, J = 7.2, 2.5 Hz); 7.55–7.71 (5H, m, Ph); 8.59 (1H, s, H-2). 13C {1H} NMR (101 MHz, CDCl3): δ = 14.8; 22.4; 61.1; 104.5; 125.5; 129.3; 129.5; 132.6; 137.6; 142.5; 147.2; 150.1; 154.9; 162.2. Anal. Calcd for C16H14N4O4: C, 58.89; H, 4.32; N, 17.17. Found: C, 58.94; H, 4.35; N, 17.10.
3-Cyano-5-methyl-2-methylthio-6-nitro-7-phenylpyrazolo[1,5-a]pyrimidine(5d). The reaction was performed according to the general procedure 2, employing 0.31 mmol of heterocycle 4d and 0.123 + 0.049 g (0.38 + 0.15 mmol) of PhI(OAc)2. Yellow solid. Yield, 39 mg (40%); mp, 168–172 °C. IR (ATR): 2226 (CN); 1551, 1349 (NO2). 1H NMR (400 MHz, CDCl3): δ = 2.55 (3H, s, S-CH3); 2.72 (3H, s, C-5-CH3); 7.54–7.69 (5H, m, Ph). 13C {1H} NMR (101 MHz, CDCl3): δ = 13.8; 22.1; 83.3; 111.7; 125.1; 129.4 (2C); 132.8; 137.4; 141.9; 150.8; 155.5; 162.4. Anal. Calcd for C15H11N5O2S: C, 55.38; H, 3.41; N, 21.53. Found: C, 55.34; H, 4.44; N, 21.49.
5-Methyl-6-nitro-7-phenyl-1,2,4-triazolo[1,5-a]pyrimidine(5e). The reaction was performed according to the general procedure 2, employing 0.39 mmol of heterocycle 4e and 0.157 + 0.063 g (0.49 + 0.19 mmol) of PhI(OAc)2. Beige solid. Yield, 65 mg (66%); mp, 228–232 °C. IR (ATR): 1537, 1363 (NO2). 1H NMR (400 MHz, CDCl3): δ = 2.80 (3H, s, CH3); 7.58–7.72 (5H, m, Ph); 8.54 (1H, s, H-2). 13C {1H} NMR (101 MHz, CDCl3): δ = 22.3; 125.3; 129.2; 129.7; 133.0; 137.8; 143.0; 154.4; 157.8; 158.7. Anal. Calcdfor C12H9N5O2: C, 56.47; H, 3.55; N, 27.44. Found: C, 56.53; H, 3.49; N, 27.49.
2,5-Dimethyl-6-nitro-7-phenyl-1,2,4-triazolo[1,5-a]pyrimidine(5f). The reaction was performed according to the general procedure 2, employing 0.37 mmol of heterocycle 4f and 0.149 + 0.059 g (0.46 + 0.18 mmol) of PhI(OAc)2. Pale orange solid. Yield, 49 mg (50%); mp, 180–183 °C. IR (ATR): 1542, 1368 (NO2). 1H NMR (400 MHz, CDCl3): δ = 2.59 (3H, s, C-2-CH3); 2.80 (3H, s, C-5-CH3); 7.55–7.70 (5H, m, Ph); 8.54 (1H, s, H-2). 13C {1H} NMR (101 MHz, CDCl3): δ = 15.7; 22.2; 125.6; 129.2; 129.6; 132.8; 137.4; 142.2; 154.9; 157.0; 169.6. Anal. Calcd for C13H11N5O2: C, 57.99; H, 4.12; N, 26.01. Found: C, 58.04; H, 4.20; N, 25.93.
5-Methyl-6-nitro-7-phenyl-2-trifluoromethyl-1,2,4-triazolo[1,5-a]pyrimidine(5g). The reaction was performed according to the general procedure 2, employing 0.31 mmol of heterocycle 4g and 0.124 + 0.50 g (0.38 + 0.15 mmol) of PhI(OAc)2. White yellow solid. Yield, 41 mg (42%); mp, 172–175 °C. IR (ATR): 1548, 1367 (NO2). 1H NMR (400 MHz, CDCl3): δ = 2.83 (3H, s, C-5-CH3); 7.59–7.74 (5H, m, Ph). 13C {1H} NMR (101 MHz, CDCl3): δ = 22.5; 119.0 (q, J = 271.7 Hz); 124.3; 129.4; 129.8; 133.5; 138.7; 143.6; 154.6, 159.8, 159.9 (q, J = 40.5 Hz). Anal. Calcd for C13H8F3N5O2: C, 48.31; H, 2.49; N, 21.67. Found: C, 48.36; H, 2.52; N, 21.61.
5-Methyl-2-methylthio-6-nitro-7-phenyl-1,2,4-triazolo[1,5-a]pyrimidine(5f). The reaction was performed according to the general procedure 2, employing 0.33 mmol of heterocycle 4h and 0.133 + 0.053 g (0.41 + 0.17 mmol) of PhI(OAc)2. Beige solid. Yield, 39 mg (40%); mp, 193–198 °C. IR (ATR): 1542, 1369 (NO2). 1H NMR (400 MHz, CDCl3): δ = 2.81 (3H, s, C-5-CH3); 3.12 (3H, s, S-CH3); 7.56–7.73 (5H, m, Ph). 13C {1H} NMR (101 MHz, CDCl3): δ = 22.5; 40.3; 124.4; 129.4; 129.8; 133.4; 138.3; 143.6; 154.7; 159.5; 173.9. Anal. Calcd for C13H11N5O2S: C, 51.82; H, 3.68; N, 23.24. Found: C, 51.91; H, 3.59; N, 23.30.
2-Azido-6-methyl-5-nitro-4-phenylpyrimidine (5i′). The reaction was performed according to the general procedure 2, employing 0.39 mmol of heterocycle 4i and 0.156 + 0.062 g (0.48 + 0.19 mmol) of PhI(OAc)2. White yellow solid. Yield, 44 mg (45%); mp, 118–120 °C. IR (ATR): 2143 (N3); 1519, 1329 (NO2). 1H NMR (400 MHz, CDCl3): δ = 2.59 (3H, s, CH3); 7.43–7.70 (5H, m, Ph). 13C {1H} NMR (101 MHz, CDCl3): δ = 21.0; 128.4; 159.5; 132.1; 133.4; 142.2; 160.5; 161.6; 163.4. Anal. Calcd for C11H8N6O2: C, 51.56; H, 3.15; N, 32.80. Found: C, 51.50; H, 3.13; N, 32.82.
6-Nitro-7-phenyl-4,7-dihydropyrazolo[1,5-a]pyrimidine(6a). The reaction was performed according to the general procedure 1, employing 0.166 g (2 mmol, 1 equiv.) of 3-aminopyrazole 1a, 0.316 g (2 mmol, 1 equiv.) of 1-morpholino-2-nitroethylene 2a, and 0.20 mL (2 mmol, 1 equiv.) of benzaldehyde 3g. Yellow solid. Yield, 0.387 g (80%); mp, 289–295 °C. IR (ATR): 1528, 1417 (NO2). 1H NMR (400 MHz, DMSO-d6): δ = 5.43 (1H, s, H-7); 7.10–7.30 (5H, m, Ph); 7.41 (1H, s, H-2); 8.36 (1H, d, H-5, J = 6.1 Hz); 10.88 (1H, d, NH, J = 6.4 Hz); 12.45 (1H, s, H-3). 13C {1H} NMR (101 MHz, DMSO-d6): δ = 38.2; 106.8; 124.3; 126.2; 126.5; 128.4; 127.3; 139.0; 144.2; 146.0. Anal. Calcd for C12H10N4O2: C, 59.50; H, 4.16; N, 23.13. Found: C, 59.61; H, 4.20; N, 23.01.
5-Ethyl-6-nitro-7-phenyl-4,7-dihydropyrazolo[1,5-a]pyrimidine(6b). The reaction was performed according to the general procedure 1, employing 0.166 g (2 mmol, 1 equiv.) of 3-aminopyrazole 1a, 0.372 g (2 mmol, 1 equiv.) of 1-morpholino-2-nitrobuthylene 2c, and 0.20 mL (2 mmol, 1 equiv.) of benzaldehyde 3g. Green yellow solid. Yield, 0.243 (45%); mp, 231–234 °C. IR (ATR): 1511, 1426 (NO2). 1H NMR (400 MHz, DMSO-d6): δ = 1.29 (3H, t, CH3, J = 7.2 Hz); 2.90–3.05 (2H, m, CH2); 5.48 (1H, s, H-7); 7.09–7.32 (5H, m, Ph); 7.41 (1H, s, H-2); 10.73 (1H, s, NH); 12.37 (1H, s, H-3). 13C {1H} NMR (101 MHz, DMSO-d6): δ = 13.0; 27.3; 39.5; 106.8; 122.1; 122.1; 126.0; 126.2; 126.4; 128.3; 144.3; 146.8; 156.4. Anal. Calcd for C14H14N4O2: C, 62.21; H, 5.22; N, 20.73. Found: C, 62.28; H, 5.18; N, 20.65.
6-Nitro-7-phenylpyrazolo[1,5-a]pyrimidine(7a). The reaction was performed according to the general procedure 2, employing 0.41 mmol of heterocycle 6a and 0.166 + 0.067 g (0.52 + 0.21 mmol) of PhI(OAc)2. Light orange solid. Yield, 75 mg (76%); mp, 205–213 °C. IR (ATR): 1523, 1358 (NO2). 1H NMR (400 MHz, CDCl3): δ = 7.46–7.60 (5H, m, Ph); 8.10 (1H, s, H-2); 9.25 (1H, d, H-5); 12.24 (1H, s, H-3). 13C {1H} NMR (101 MHz, CDCl3): δ = 115.6; 128.4; 129.5; 130.4; 132.8; 136.7; 140.1; 141.5; 146.4; 152.1. Anal. Calcd for C12H18N4O2: C, 60.00; H, 3.36; N, 23.32. Found: C, 59.89; H, 3.28; N, 23.40.
5-Ethyl-6-nitro-7-phenylpyrazolo[1,5-a]pyrimidine (7b). The reaction was performed according to the general procedure 2, employing 0.37 mmol of heterocycle 6b and 0.149 + 0.060 g (0.46 + 0.19 mmol) of PhI(OAc)2. Pale yellow solid. Yield, 69 mg (70%); mp, 198–205 °C. IR (ATR): 1522, 1341 (NO2). 1H NMR (400 MHz, CDCl3): δ = 1.53 (3H, t, CH3, J = 7.5 Hz); 3.08 (2H, q, CH2, J = 7.5 Hz); 7.50–7.60 (5H, m, Ph); 8.06 (1H, s, H-2); 12.75 (1H, s, H-3). 13C {1H} NMR (101 MHz, CDCl3): δ = 13.5; 28.0; 113.3; 128.4; 129.7; 130.6; 132.4; 135.6; 138.1; 142.6; 150.8; 156.0. Anal. Calcd for C14H12N4O2: C, 62.68; H, 4.51; N, 20.88. Found: C, 62.61; H, 4.54; N, 20.81.
7-Ethyl-6-nitro-5-methyl-4,7-dihydropyrazolo[1,5-a]pyrimidine(8a). The reaction was performed according to the general procedure 1, employing 0.166 g (2 mmol, 1 equiv.) of 3-aminopyrazole 1a, 0.344 g (2 mmol, 1 equiv.) of 1-morpholino-2-nitropropylene 2b, and 0.14 mL (2 mmol, 1 equiv.) of propionaldehyde 3a. Reaction mixture was heated in a glass autoclave. Orange solid. Yield, 0.175 g (42%); mp, 235 °C with decomp. IR (ATR): 1515, 1278 (NO2). 1H NMR (400 MHz, DMSO-d6): δ = 0.72 (3H, t, CH2-CH3, J = 7.4 Hz); 1.45–1.65 (2H, m, CH2-CH3); 2.52 (3H, s, C-5-CH3); 4.23–4.34 (1H, m, H-7); 7.54 (1H, s, H-2); 10.54 (1H, s, NH); 12.36 (1H, s, H-3). 13C {1H} NMR (101 MHz, DMSO-d6): δ = 9.8; 21.9; 28.4; 34.4; 105.0; 122.6; 125.9; 145.7; 152.0. Anal. Calcd for C9H12N4O2: C, 51.92; H, 5.81; N, 26.91. Found: C, 52.08; H, 5.92; N, 26.75.
6-Nitro-5-methyl-7-(4ʹ-nitrophenyl)-4,7-dihydropyrazolo[1,5-a]pyrimidine(8b). To a suspension of 0.166 g (2 mmol, 1 equiv.) of 3-aminopyrazole 1a, 0.344 g (2 mmol, 1 equiv.) of 1-morpholino-2-nitropropylene 2b in 5 mL of n-BuOH, 3 mmol (1.5 equiv., 0.37 mL) of BF3·Et2O was added. The reaction mixture was heated on oil bath at 120 °C for 15 min. To the obtained solution, 0.302 g (2 mmol, 1 equiv.) of 4-nitrobenzaldehyde 3b was added. The reaction mixture was heated on oil bath at 120 °C for 2 h. The resulting solution was cooled to room temperature and stirred for 15 min. The obtained precipitate was filtered off and washed with 15 mL of i-PrOH. The precipitate was suspended in 50 mL of water, stirred for 5 min, filtered off again, and washed with 15 mL of water. To the residue, 20 mL of 2 M Na2CO3 and 50 mL of water were added and stirred for 20 min. The solution was extracted twice with 20 mL of EtOAc. To the water phase, 15 mL of hexane was added, and the mixture was neutralized by diluted HCl to pH 7. The resulting mixture was stirred overnight, filtered off, and washed with water. Yellow solid. Yield, 0.355 g (59%); mp, 197 °C with decomp.
IR (ATR): 1535, 1352 (NO2); 1508, 1268 (NO2). 1H NMR (600 MHz, DMSO-d6): δ = 2.66 (3H, s, C-5-CH3); 5.64 (1H, s, H-5); 7.45 (1H, s, H-2); 7.50 (2H, d, H-2ʹ, J = 8.3 Hz); 8.12 (2H, d, H-3ʹ, J = 8.3 Hz); 10.95 (1H, s, NH); 12.49 (1H, s, H-3). 13C {1H} NMR (151 MHz, DMSO-d6): δ = 22.0; 39.8; 105.6; 121.8; 123.8; 127.0; 127.6; 144.2; 145.9; 152.5; 154.1. Anal. Calcd for C13H11N5O4: C, 51.83; H, 3.68; N, 23.25 Found: C, 51.89; H, 3.73; N, 23.19.
6-Nitro-5-methyl-7-(4ʹ-metoxyphenyl)-4,7-dihydropyrazolo[1,5-a]pyrimidine (8c). The reaction was performed according to the general procedure 1, employing 0.166 g (2 mmol, 1 equiv.) of 3-aminopyrazole 1a, 0.344 g (2 mmol, 1 equiv.) of 1-morpholino-2-nitropropylene 2b, and 0.24 mL (2 mmol, 1 equiv.) of 4-metoxybenzaldehyde 3c. Yellow solid. Yield, 0.355 (62%); mp, 264–266 °C. IR (ATR): 1509, 1260 (NO2). 1H NMR (600 MHz, DMSO-d6): δ = 2.61 (3H, s, C-5-CH3); 3.68 (3H, s, O-CH3); 5.43 (1H, s, H-5); 6.80 (2H, d, H-3ʹ, J = 8.7 Hz); 7.10 (2H, d, H-2ʹ, J = 8.7 Hz); 7.39 (1H, s, H-2); 10.72 (1H, s, NH); 12.37 (1H, s, H-3). 13C {1H} NMR (151 MHz, DMSO-d6): δ = 22.0; 38.7; 55.0; 107.1; 113.7; 123.3; 126.4; 127.5; 139.0; 144.5; 151.2; 157.6. Anal. Calcd for C14H14N4O3: C, 58.74; H, 4.93; N, 19.57. Found: C, 58.78; H, 4.91; N, 19.61.
6-Nitro-5-methyl-7-(4ʹ-hydroxy-3ʹ-metoxyphenyl)-4,7-dihydropyrazolo[1,5-a]pyrimidine(8d). The reaction was performed according to the general procedure 1, employing 0.166 g (2 mmol, 1 equiv.) of 3-aminopyrazole 1a, 0.344 g (2 mmol, 1 equiv.) of 1-morpholino-2-nitropropylene 2b, and 0.24 g (2 mmol, 1 equiv.) of 4-hydroxy-3-metoxybenzaldehyde 3d. Yellow solid. Yield, 0.314 (52%); mp, 224-226 °C. IR (ATR): 3170 (OH); 1515, 1264 (NO2). 1H NMR (400 MHz, DMSO-d6): δ = 2.61 (3H, s, C-5-CH3); 3.73 (3H, s, O-CH3); 5.39 (1H, s, H-5); 6.50 (1H, dd, H-5ʹ, J1 = 8.1 Hz, J1 = 2.1 Hz); 6.62 (1H, d, H-6ʹ, J = 8.1 Hz); 6.79 (1H, s, H-2ʹ); 7.43 (1H, s, H-2); 8.68 (1H, s, OH); 10.65 (1H, s, NH); 12.33 (1H, s, H-3). 13C {1H} NMR (151 MHz, DMSO-d6): δ = 21.8; 38.9; 55.6; 107.2; 111.0; 115.3; 118.1; 123.0; 126.3; 137.9; 144.3; 144.8; 147.4; 151.1. Anal. Calcd for C14H14N4O4: C, 55.63; H, 4.67; N, 18.53. Found: C, 55.69; H, 4.71; N, 18.50.
6-Nitro-5-methyl-7-(anthracene-9ʹ-yl)-4,7-dihydropyrazolo[1,5-a]pyrimidine (8e). The reaction was performed according to the general procedure 1, employing 0.166 g (2 mmol, 1 equiv.) of 3-aminopyrazole 1a, 0.344 g (2 mmol, 1 equiv.) of 1-morpholino-2-nitropropylene 2b, and 0.412 g (2 mmol, 1 equiv.) of 9-anthraldehyde 3e. The product was recrystallized from n-BuOH. The substance was dried over P2O5 at 140 °C. Pale green solid. Yield, 0.363 (51%); mp, 222 °C with decomp. IR (ATR): 1522, 1290 (NO2). 1H NMR (400 MHz, DMSO-d6): δ = 2.66 (3H, s, C-5-CH3); 7.02 (1H, s, H-5); 7.13 (1H, s, H-10ʹ); 7.29–7.40 (2H, m, H-3ʹ, H-6); 7.50–7.67 (2H, m, H-2ʹ, H-7ʹ); 8.03 (1H, d, H-1ʹ, J = 8.3 Hz); 8.05–8.15 (2H, m, H-4ʹ, H-5ʹ); 8.48 (1H, s, H-2); 8.79 (1H, d, H-8ʹ, J = 9.0 Hz); 11.01 (1H, s, NH); 12.32 (1H, s, H-3). 13C {1H} NMR (151 MHz, DMSO-d6): δ = 21.7; 34.1; 106.4; 123.7; 124.1; 124.4; 124.9; 125.0 (2C); 126.3; 126.4; 126.6; 128.2; 128.9; 129.5; 131.0; 131.7; 136.9; 144.7; 150.5. Anal. Calcd for C21H16N4O2: C, 70.77; H, 4.53; N, 15.72. Found: C, 70.74; H, 4.51; N, 15.76.
6-Nitro-5-methyl-7-(thiophen-2ʹ-yl)-4,7-dihydropyrazolo[1,5-a]pyrimidine (8f). The reaction was performed according to the general procedure 1, employing 0.166 g (2 mmol, 1 equiv.) of 3-aminopyrazole 1a, 0.344 g (2 mmol, 1 equiv.) of 1-morpholino-2-nitropropylene 2b, and 0.184 mL (2 mmol, 1 equiv.) of thiophen-2-carbaldehyde 3d. Pale green solid. Yield, 0.278 (53%); mp, 208 °C with decomp. IR (ATR): 1511, 1256 (NO2). 1H NMR (400 MHz, DMSO-d6): δ = 2.58 (3H, s, C-5-CH3); 5.84 (1H, s, H-7); 6.80–6.82 (1H, m, H-3ʹ); 6.83–6.87 (1H, m, H-4ʹ); 7.23 (1H, d, H-5ʹ, J = 5.0 Hz); 7.56 (1H, s, H-2); 10.85 (1H, s, NH); 12.49 (1H, s, H-3). 13C {1H} NMR (101 MHz, DMSO-d6): δ = 21.8; 34.3; 106.2; 122.9; 123.1; 123.6; 126.5; 126.7; 144.5; 150.6; 151.1. Anal. Calcd for C11H10N4O2S: C, 50.37; H, 3.84; N, 21.36. Found: C, 50.20; H, 3.99; N, 21.49.
7-Ethyl-6-nitro-5-methylpyrazolo[1,5-a]pyrimidine(9a). The reaction was performed according to the general procedure 2, employing 0.48 mmol of heterocycle 8a and 0.194 + 0.077 g (0.60 + 0.24 mmol) of PhI(OAc)2. White yellow solid. Yield, 49 mg (50%); mp, 175–179 °C. IR (ATR): 1521, 1360 (NO2). 1H NMR (400 MHz, CDCl3): δ = 1.42 (3H, t, CH2-CH3, J = 7.6 Hz); 2.76 (3H, s, C-5-CH3); 1.42 (2H, q, CH2-CH3, J = 7.6 Hz); 8.23 (1H, s, H-2); 13.02 (1H, s, H-3). 13C {1H} NMR (101 MHz, CDCl3): δ = 15.1; 21.9; 23.6; 113.4; 134.5; 141.9; 143.6; 150.5; 151.4. Anal. Calcd for C9H10N4O2: C, 52.42; H, 4.89; N, 27.17. Found: C, 52.39; H, 4.89; N, 27.20.
6-Nitro-5-methyl-7-(4ʹ-nitrophenyl)-pyrazolo[1,5-a]pyrimidine(9b). The reaction was performed according to the general procedure 2, employing 0.33 mmol of heterocycle 8b and 0.134 + 0.054 g (0.42 + 0.17 mmol) of PhI(OAc)2. Pale yellow solid. Yield, 49 mg (50%); mp, 243 °C with decomp. IR (ATR): 1542, 1344 (NO2); 1522, 1369 (NO2). 1H NMR (400 MHz, DMSO-d6): δ = 2.68 (3H, s, CH3); 7.82 (2H, d, H-2ʹ, J = 8.6 Hz); 8.09 (1H, s, H-2); 8.41 (2H, d, H-3ʹ, J = 8.6 Hz); 14.26 (1H, s, H-3). 13C {1H} NMR (101 MHz, DMSO-d6): δ = 21.5; 111.6; 124.3; 129.6; 134.2; 135.0; 138.5; 140.8; 148.3; 150.1; 150.2. Anal. Calcd for C13H9N5O4: C, 52.18; H, 3.03; N, 23.40. Found: C, 52.25; H, 2.99; N, 23.38.
6-Nitro-5-methyl-7-(anthracene-9ʹ-yl)-pyrazolo[1,5-a]pyrimidine (9e), 9-acetoxy-10-oxoanthracene (10), anthraquinone (11). The reaction was performed according to the general procedure 2, employing 0.28 mmol of heterocycle 8e and 0.113 + 0.045 g (0.35 + 0.14 mmol) of PhI(OAc)2. 6-Nitro-5-methyl-7-(anthracene-9ʹ-yl)-pyrazolo[1,5-a]pyrimidine (9e). The substance was dried over P2O5 at 140 oC. Sand color solid. Yield, 29 mg (30%); mp, 250 °C with decomp. IR (ATR): 1529, 1373 (NO2). 1H NMR (400 MHz, DMSO-d6): δ = 2.78 (3H, s, C-5-CH3); 7.36 (2H, d, H-4ʹ, H-5ʹ, J = 8.8 Hz); 7.40–7.47 (2H, m, H-2ʹ, H-7ʹ); 7.50 (1H, s, H-10ʹ); 7.52–7.58 (2H, m, H-3ʹ, H-6ʹ); 8.21 (2H, d, H-1ʹ, H-8ʹ, J = 8.6 Hz); 8.84 (1H, s, H-2); 14.30 (1H, s, H-3). 13C {1H} NMR (151 MHz, DMSO-d6): δ = 21.7; 113.8; 124.9 (2C); 125.1; 125.7 (2C); 127.0 (2C); 128.6; 128.8; 128.9 (2C); 129.1; 130.6; 134.3; 134.4; 134.9; 143.4; 150.1; 150.5.Anal. Calcdfor C21H14N4O2: C, 71.18; H, 3.98; N, 15.81. Found: C, 71.26; H, 3.93; N, 15.85.
9-acetoxy-10-oxoanthracene (10). Yellow crystals. Yield, 25 mg (35%). 1H NMR (400 MHz, DMSO-d6): δ = 2.19 (3H, s, CH3); 7.15 (1H, s, CH); 7.56–7.64 (4H, m, H-2ʹ, H-3ʹ, H-6ʹ, H-7ʹ); 7.70–7.76 (2H, m, H-1ʹ, H-8ʹ); 8.15–8.21 (2H, m, H-4ʹ, H-5ʹ). Mass spectrum, m/z (Irel, %): 208 (100) [M]+; 180 (80), 152 (79), 126 (14), 90 (10), 76 (62), 63 (16), 50 (20), 39 (3).
Anthraquinone (11). Yellow crystals. Yield, 15 mg (25%). 1H NMR (400 MHz, DMSO-d6): δ = 7.88-7.94 (4H, m, H-2ʹ, H-3ʹ, H-6ʹ, H-7ʹ); 8.21–8.26 (4H, m, H-1ʹ, H-4ʹ, H-5ʹ, H-8ʹ). Mass spectrum, m/z (Irel, %): 252 (13) [M]+; 210 (100); 193 (43); 181 (14); 165 (33); 152 (16); 139 (6); 82 (18); 69 (9); 43 (15).
6-Nitro-5-methyl-7-(thiophen-2ʹ-yl)pyrazolo[1,5-a]pyrimidine (9f). The reaction was performed according to the general procedure 2, employing 0.38 mmol of heterocycle 8f and 0.154 + 0.061 g (0.48 + 0.19 mmol) of PhI(OAc)2. Light orange solid. Yield, 59 mg (60%); mp, 219 °C with decomp. IR (ATR): 1520, 1366 (NO2). 1H NMR (400 MHz, DMSO-d6): δ = 2.59 (3H, s, C-5-CH3); 5.84 (1H, s, H-5); 7.29–7.34 (1H, m, H-3ʹ); 7.48–7.53 (1H, m, H-4ʹ); 7.98–8.02 (1H, d, H-5ʹ); 8.33 (1H, s, H-2); 14.19 (1H, s, H-3). 13C {1H} NMR (151 MHz, DMSO-d6): δ = 20.8; 110.8; 128.5; 128.9; 130.0; 131.2; 131.3; 134.3; 140.4; 149.5; 150.2. Anal. Calcd for C11H8N4O2S: C, 50.76; H, 3.10; N, 21.53. Found: C, 50.73; H, 3.05; N, 21.60.
4. Conclusions
Thus, we can conclude that 6-nitroazolo[1,5-a]pyrimidines, even containing an electron donating substituent at position seven, are capable of oxidation with the formation of an aromatic structure. On the other hand, during oxidation, it is necessary to take into consideration the features of these structures since the reaction is often complicated by a side process. However, there is no reason to suppose that the same compounds cannot be obtained by any alternative synthetic approach that excludes the destructive nature of key intermediates. Nevertheless, the obtained experimental and theoretical data correlate well with each other, which indicates the possibility of using this set of methods to study the oxidation reactions of this class of organic compounds.
Supplementary Materials
Figures with HOMO distribution, Cyclic voltammograms, and NMR Spectra of compounds 4–11.
Author Contributions
Synthesis, D.N.L.; quantum-chemical calculation, I.A.B.; electro-chemical experiment, A.V.S.; methodology, E.N.U., O.N.C. and D.N.L.; writing, V.L.R. and D.N.L. All authors have read and agreed to the published version of the manuscript.
Funding
The synthetic part was supported by the Ministry of Science and Higher Education of the Russian Federation, State Contract no FEUZ-2020-0058 (H687.42B.223/20). The electrochemical research and the quantum chemical calculations was funded by Russian Foundation for Basic Research (RFBR), project number 20-03-00814.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within article.
Acknowledgments
DFT computations were performed on the Uran supercomputer at the IMM UB RAS. The team of authors would like to thank the Laboratory for Comprehensive Research and Expert Evaluation of Organic Materials under the direction of O. S. Eltsov.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 4–11 are available from the authors.
References
- Pinheiro, S.; Pinheiro, E.M.C.; Muri, E.M.F.; Pessôa, J.C.; Cadorini, A.M.; Greco, S.J. Biological Activities of [1,2,4]Triazolo[1,5-a]Pyrimidines and Analogues. Med. Chem. Res. 2020, 29, 1751–1776. [Google Scholar] [CrossRef]
- Oukoloff, K.; Lucero, B.; Francisco, K.R.; Brunden, K.R.; Ballatore, C. 1,2,4-Triazolo[1,5-a]Pyrimidines in Drug Design. Eur. J. Med. Chem. 2019, 165, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G. Recent Advances in 1,2,4-Triazolo[1,5-a]Pyrimidine Chemistry. In Advances in Heterocyclic Chemistry; Academic Press Inc.: Cambridge, MA, USA, 2019; Volume 128, pp. 1–101. [Google Scholar] [CrossRef]
- Tigreros, A.; Aranzazu, S.-L.; Bravo, N.-F.; Zapata-Rivera, J.; Portilla, J. Pyrazolo[1,5-a]Pyrimidines-Based Fluorophores: A Comprehensive Theoretical-Experimental Study. RSC Adv. 2020, 10, 39542–39552. [Google Scholar] [CrossRef]
- Tigreros, A.; Castillo, J.C.; Portilla, J. Cyanide Chemosensors Based on 3-Dicyanovinylpyrazolo[1,5-a]Pyrimidines: Effects of Peripheral 4-Anisyl Group Substitution on the Photophysical Properties. Talanta 2020, 215, 120905. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, R.; Lan, J.; Zhang, H.; Yan, L.; Pu, X.; Huang, Z.; Wu, D.; You, J. Oxidative C-H/C-H Cross-Coupling of [1,2,4]Triazolo[1,5-a]Pyrimidines with Indoles and Pyrroles: Discovering Excited-State Intramolecular Proton Transfer (ESIPT) Fluorophores. Org. Lett. 2019, 21, 4058–4062. [Google Scholar] [CrossRef]
- Tigreros, A.; Rosero, H.A.; Castillo, J.C.; Portilla, J. Integrated Pyrazolo[1,5-a]Pyrimidine–Hemicyanine System as a Colorimetric and Fluorometric Chemosensor for Cyanide Recognition in Water. Talanta 2019, 196, 395–401. [Google Scholar] [CrossRef]
- Łakomska, I.; Śmiłowicz, D.; Jakubowski, M.; Sitkowski, J.; Wojtczak, A. Materials Platinum(II) Complexes with Bulky Disubstitute Triazolopyrimidines as Promising Materials for Anticancer Agents. Materials 2020, 13, 5312. [Google Scholar] [CrossRef]
- Esteban-Parra, G.M.; Sebastián, E.S.; Cepeda, J.; Sánchez-González, C.; Rivas-García, L.; Llopis, J.; Aranda, P.; Sánchez-Moreno, M.; Quirós, M.; Rodríguez-Diéguez, A. Anti-Diabetic and Anti-Parasitic Properties of a Family of Luminescent Zinc Coordination Compounds Based on the 7-Amino-5-Methyl-1,2,4-Triazolo[1,5-a]Pyrimidine Ligand. J. Inorg. Biochem. 2020, 212, 111235. [Google Scholar] [CrossRef]
- Fandzloch, M.; Augustyniak, A.W.; Dobrzańska, L.; Jędrzejewski, T.; Sitkowski, J.; Wypij, M.; Golińska, P. First Dinuclear Rhodium(II) Complexes with Triazolopyrimidines and the Prospect of Their Potential Biological Use. J. Inorg. Biochem. 2020, 210, 111072. [Google Scholar] [CrossRef]
- Shen, J.; Deng, X.; Sun, R.; Tavallaie, M.S.; Wang, J.; Cai, Q.; Lam, C.; Lei, S.; Fu, L.; Jiang, F. Structural Optimization of Pyrazolo[1,5-a]Pyrimidine Derivatives as Potent and Highly Selective DPP-4 Inhibitors. Eur. J. Med. Chem. 2020, 208, 112850. [Google Scholar] [CrossRef]
- Peytam, F.; Adib, M.; Shourgeshty, R.; Firoozpour, L.; Rahmanian-Jazi, M.; Jahani, M.; Moghimi, S.; Divsalar, K.; Faramarzi, M.A.; Mojtabavi, S.; et al. An Efficient and Targeted Synthetic Approach towards New Highly Substituted 6-Amino-Pyrazolo[1,5-a]Pyrimidines with α-Glucosidase Inhibitory Activity. Sci. Rep. 2020, 10, 2595. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Oka, M.; Murase, T.; Yoshida, M.; Sakairi, M.; Yamashita, S.; Yasuda, Y.; Yoshikawa, A.; Hayashi, Y.; Makino, M.; et al. Discovery and Pharmacological Characterization of N-[2-({2-[(2S)-2- Cyanopyrrolidin-1-Yl]-2-Oxoethyl}amino)-2-Methylpropyl]-2-Methylpyrazolo[1,5-a]Pyrimidine-6-Carboxamide Hydrochloride (Anagliptin Hydrochloride Salt) as a Potent and Selective DPP-IV Inhibitor. Bioorg. Med. Chem. 2011, 19, 7221–7227. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.H.; Wu, W.Y.; Guo, S.X.; He, S.J.; Tang, X.D.; Wu, X.Y.; Nandakumar, K.S.; Zou, M.; Li, L.; Chen, X.G.; et al. [1,2,4]Triazolo[1,5-a]Pyrimidine Derivative (Mol-5) Is a New NS5-RdRp Inhibitor of DENV2 Proliferation and DENV2-Induced Inflammation. Acta Pharm. Sin. 2020, 41, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Sasaki, T.; Tamura, Y.; Ogata, Y.; Kawaguchi, T.; Kurosaka, J.; Sugaya, Y.; Iwakiri, K.; Busujima, T.; Takahashi, R.; Ueda-Yonemoto, N.; et al. Design and Synthesis of 2-(1-Alkylaminoalkyl)Pyrazolo[1,5-a]Pyrimidines as New Respiratory Syncytial Virus Fusion Protein Inhibitors. Chem. Pharm, Bull. 2020, 68, 345–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massari, S.; Nannetti, G.; Desantis, J.; Muratore, G.; Sabatini, S.; Manfroni, G.; Mercorelli, B.; Cecchetti, V.; Palù, G.; Cruciani, G.; et al. A Broad Anti-Influenza Hybrid Small Molecule That Potently Disrupts the Interaction of Polymerase Acidic Protein−Basic Protein 1 (PA-PB1) Subunits. J. Med. Chem. 2015, 58, 3830–3842. [Google Scholar] [CrossRef]
- Wang, H.; Lee, M.; Peng, Z.; Blázquez, B.; Lastochkin, E.; Kumarasiri, M.; Bouley, R.; Chang, M.; Mobashery, S. Synthesis and Evaluation of 1,2,4-Triazolo[1,5-a]Pyrimidines as Antibacterial Agents against Enterococcus Faecium. J. Med. Chem. 2015, 58, 4194–4203. [Google Scholar] [CrossRef] [Green Version]
- Zuniga, E.S.; Korkegian, A.; Mullen, S.; Hembre, E.J.; Ornstein, P.L.; Cortez, G.; Biswas, K.; Kumar, N.; Cramer, J.; Masquelin, T.; et al. The Synthesis and Evaluation of Triazolopyrimidines as Anti-Tubercular Agents. Bioorg. Med. Chem. 2017, 25, 3922–3946. [Google Scholar] [CrossRef]
- Ding, J.; Cao, F.D.; Geng, Y.R.; Tian, Y.; Li, P.; Li, X.F.; Huang, L.J. Synthesis and in Vitro Anti-Epileptic Activities of Novel[1,2,4]-Triazolo[1,5-a]Pyrimidin-7(4H)-One Derivatives. J. Asian Nat. Prod. Res. 2019, 21, 1190–1204. [Google Scholar] [CrossRef]
- Sullivan, S.K.; Petroski, R.E.; Verge, G.; Gross, R.S.; Foster, A.C.; Grigoriadis, D.E. Characterization of the Interaction of Indiplon, a Novel Pyrazolopyrimidine Sedative-Hypnotic, with the GABAA Receptor. J. Pharmacol. Exp. Ther. 2004, 311, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Huo, J.L.; Wang, S.; Yuan, X.H.; Yu, B.; Zhao, W.; Liu, H.M. Discovery of [1,2,4]Triazolo[1,5-a]Pyrimidines Derivatives as Potential Anticancer Agents. Eur. J. Med. Chem. 2021, 211, 113108. [Google Scholar] [CrossRef]
- Krämer, A.; Kurz, C.G.; Berger, B.T.; Celik, I.E.; Tjaden, A.; Greco, F.A.; Knapp, S.; Hanke, T. Optimization of Pyrazolo[1,5-a]Pyrimidines Lead to the Identification of a Highly Selective Casein Kinase 2 Inhibitor. Eur. J. Med. Chem. 2020, 208, 112770. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, S.-Q.; Teng, Q.-X.; Yang, L.; Lei, Z.-N.; Yuan, X.-H.; Huo, J.-F.; Chen, X.-B.; Wang, M.; Yu, B.; et al. Structure-Based Design, Synthesis, and Biological Evaluation of New Triazolo[1,5-a]Pyrimidine Derivatives as Highly Potent and Orally Active ABCB1 Modulators. J. Med. Chem. 2020, 63, 15979–15996. [Google Scholar] [CrossRef]
- Li, G.; Wang, Y.; Li, L.; Ren, Y.; Deng, X.; Liu, J.; Wang, W.; Luo, M.; Liu, S.; Chen, J. Design, Synthesis, and Bioevaluation of Pyrazolo[1,5-a]Pyrimidine Derivatives as Tubulin Polymerization Inhibitors Targeting the Colchicine Binding Site with Potent Anticancer Activities. Eur. J. Med. Chem. 2020, 202. [Google Scholar] [CrossRef] [PubMed]
- Mathison, C.; Chianelli, D.; Rucker, P.; Nelson, J.; Roland, J.; Huang, Z.; Yang, Y.; Jiang, J.; Feng Xie, Y.; Epple, R.; et al. Efficacy and Tolerability of Pyrazolo[1,5-a]Pyrimidine RET Kinase Inhibitors for the Treatment of Lung Adenocarcinoma. ACS Med. Chem. Lett. 2020, 11, 558–565. [Google Scholar] [CrossRef]
- Badolato, M.; Manetti, F.; Garofalo, A.; Aiello, F. Triazolopyrimidinium Salts: Discovery of a New Class of Agents for Cancer Therapy. Future Med. Chem. 2020, 12, 387–402. [Google Scholar] [CrossRef]
- Oukoloff, K.; Nzou, G.; Varricchio, C.; Lucero, B.; Alle, T.; Kovalevich, J.; Monti, L.; Cornec, A.-S.; Yao, Y.; James, M.; et al. Evaluation of the Structure–Activity Relationship of Microtubule-Targeting 1,2,4-Triazolo[1,5-a]Pyrimidines Identifies New Candidates for Neurodegenerative Tauopathies. J. Med. Chem. 2021, 64, 1073–1102. [Google Scholar] [CrossRef]
- Uryu, S.; Tokuhiro, S.; Murasugi, T.; Oda, T. A Novel Compound, RS-1178, Specifically Inhibits Neuronal Cell Death Mediated by β-Amyloid-Induced Macrophage Activation in Vitro. Brain Res. 2002, 946, 298–306. [Google Scholar] [CrossRef]
- Lou, K.; Yao, Y.; Hoye, A.T.; James, M.J.; Cornec, A.-S.; Hyde, E.; Gay, B.; Lee, V.; Trojanowski, J.Q.; Smith, A.B.; et al. Brain-Penetrant, Orally Bioavailable Microtubule-Stabilizing Small Molecules Are Potential Candidate Therapeutics for Alzheimer’s Disease and Related Tauopathies. J. Med. Chem. 2014, 57, 6116–6127. [Google Scholar] [CrossRef] [PubMed]
- Rusinov, V.L.; Charushin, V.N.; Chupakhin, O.N. Biologically Active Azolo-1,2,4-Triazines and Azolopyrimidines. Russ. Chem.Bull. 2018, 67, 573–599. [Google Scholar] [CrossRef]
- Chupakhin, O.N. 5-Methyl-6-nitro-7-oxo-4,7-dihydro-1,2,4-triazolo[1,5-alpha]pyrimidine L-argininium monohydrate. RF Patent 2529487, 20 October 2014. [Google Scholar]
- Deyeva, E.G.; Shevchik, Y.I.; Shaldghan, A.A.; Zagorodnikova, K.A.; Tumashov, A.A.; Baklykov, A.V.; Kotovskaya, S.K.; Chupahin, O.N.; Charushin, V.N.; Rusinov, V.L.; et al. New Antiviral Drug Triazid. Results of First Phase of Clinical Trial. Drug Dev. Registration 2018, 3, 172–180. (In Russ) [Google Scholar]
- Savateev, K.V.; Ulomsky, E.N.; Fedotov, V.V.; Rusinov, V.L.; Sivak, K.V.; Lyubishin, M.M.; Kuzmich, N.N.; Aleksandrov, A.G. 6-Nitrotriazolo[1,5-a]Pyrimidines as Promising Structures for Pharmacotherapy of Septic Conditions. Russ. J. Bioorg. Chem. 2017, 43, 421–428. [Google Scholar] [CrossRef]
- Savateev, K.V.; Ulomsky, E.N.; Rusinov, V.L.; Isenov, M.L.; Chupakhin, O.N. Structural Analogs of Adenosine Receptor Inhibitors in the Series of 1,2,4-Triazolo[1,5-a]Pyrimidines. Russ. Chem. Bull. 2015, 64, 1378–1384. [Google Scholar] [CrossRef]
- Ulomskiy, E.N.; Ivanova, A.V.; Gorbunov, E.B.; Esaulkova, I.L.; Slita, A.V.; Sinegubova, E.O.; Voinkov, E.K.; Drokin, R.A.; Butorin, I.I.; Gazizullina, E.R.; et al. Synthesis and Biological Evaluation of 6-Nitro-1,2,4-Triazoloazines Containing Polyphenol Fragments Possessing Antioxidant and Antiviral Activity. Bioorganic Med. Chem. Lett. 2020, 30, 127216. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Gerasimova, E.; Gazizullina, E.; Borisova, M.; Drokin, R.; Gorbunov, E.; Ulomskiy, E.; Rusinov, V. The Antioxidant Screening of Potential Materials for Drugs Based on 6-Nitro-1,2,4-Triazoloazines Containing Natural Polyphenol Fragments. Anal. Bioanal. Chem. 2020, 412, 5147–5155. [Google Scholar] [CrossRef]
- Safari, F.; Bayat, M.; Nasri, S.; Karami, S. Synthesis and Evaluation of Anti-Tumor Activity of Novel Triazolo[1,5-a] Pyrimidine on Cancer Cells by Induction of Cellular Apoptosis and Inhibition of Epithelial-to-Mesenchymal Transition Process. Bioorganic Med. Chem. Lett. 2020, 30, 127111. [Google Scholar] [CrossRef] [PubMed]
- Spasov, A.A.; Babkov, D.A.; Sysoeva, V.A.; Litvinov, R.A.; Shamshina, D.D.; Ulomsky, E.N.; Savateev, K.V.; Fedotov, V.V.; Slepukhin, P.A.; Chupakhin, O.N.; et al. 6-Nitroazolo[1,5-a]Pyrimidin-7(4H)-Ones as Antidiabetic Agents. Arch. Pharm. 2017, 350, 1700226. [Google Scholar] [CrossRef]
- Gazizov, D.A.; Gorbunov, E.B.; Rusinov, G.L.; Ulomsky, E.N.; Charushin, V.N. A New Family of Fused Azolo[1,5-A]Pteridines and Azolo[5,1-b]Purines. ACS Omega 2020, 5, 18226–18233. [Google Scholar] [CrossRef]
- Savateev, K.V.; Ulomsky, E.N.; Borisov, S.S.; Voinkov, E.K.; Fedotov, V.V.; Rusinov, V.L. 8-Alkyl[1,2,4]Triazolo[5,1-b]Purines. Chem. Heterocycl. Comp. 2014, 50, 880–887. [Google Scholar] [CrossRef]
- Fedotov, V.V.; Ulomsky, E.N.; Savateev, K.V.; Mukhin, E.M.; Gazizov, D.A.; Gorbunov, E.B.; Rusinov, V.L. A PASE Approach to the Synthesis of Benzimidazopurines as Polycondensed Purine Derivatives. Synth 2020, 52, 3622–3631. [Google Scholar] [CrossRef]
- Gorbunov, E.B.; Rusinov, G.L.; Ulomskii, E.N.; Isenov, M.L.; Charushin, V.N. Synthesis of 2H-Azolo[1,5-a][1,2,3]Triazolo[4,5-e]Pyrimidines. Chem. Heterocycl. Comp. 2015, 51, 491–495. [Google Scholar] [CrossRef]
- Gorbunov, E.B.; Rusinov, G.L.; Chupakhin, O.N.; Charushin, V.N. Design of Fused Systems Based on Σh-Adducts of 6-Nitro-1,2,4- Triazolo[1,5-a]Pyrimidine with π-Excessive Heteroaromatic Compounds. Russ. Chem. Bull. 2009, 58, 1309–1314. [Google Scholar] [CrossRef]
- Lyapustin, D.N.; Ulomsky, E.N.; Zanakhov, T.O.; Rusinov, V.L. Three-Component Coupling of Aromatic Aldehydes, 1-Morpholino-2-Nitroalkenes, and 3-Aminoazoles via Boron Trifluoride Etherate Catalysis: Reaction Pathway and Features of the Formation of Intermediates. J. Org. Chem. 2019, 84, 15267–15275. [Google Scholar] [CrossRef]
- Lyapustin, D.N.; Ulomsky, E.N.; Rusinov, V.L. 6-Nitro-4,7-Dihydroazolo [1,5-a]Pyrimidines: An Alternative Mechanism of Formation and Studies of Alkylation. Chem. Heterocycl. Compd. 2020, 56, 1465–1472. [Google Scholar] [CrossRef]
- Rusinov, V.L.; Postovskii, I.Y.; Petrov, A.Y.; Sidorov, E.O.; Azev, Y.A. Synthesis and study of the covalent solvation of 6-nitroazalopyrimidines. Chem. Heterocycl. Comp. 1981, 17, 1139–1141. [Google Scholar] [CrossRef]
- Pilicheva, T.L.; Rusinov, V.L.; Chupakhin, O.N.; Klyuev, N.A.; Aleksandrov, G.G.; Eslpov, S.E.; Kirov, S.M. Nitroazines. 6. Direct introduction of indole residues into 6-nitroazolo[1,5-a]pyrimidines. Chem. Heterocycl. Comp. 1986, 22, 1250–1255. [Google Scholar] [CrossRef]
- Rusinov, V.L.; Pilicheva, T.L.; Myasnikov, A.V.; Klyuev, N.A.; Chupakhin, O.N. Direct Introduction of Azoloazine Residues into Resorcinol. Chem. Heterocycl. Comp. 1986, 22, 928. [Google Scholar] [CrossRef]
- Rusinov, G.L.; Gorbunov, E.B.; Charushin, V.N.; Chupakhin, O.N. An Unusual Aromatisation of Dihydropyrimidines Facilitated by Reduction of the Nitro Group. Tetrahedron Lett. 2007, 48, 5873–5876. [Google Scholar] [CrossRef]
- Chupakhin, O.N.; Shchepochkin, A.V.; Charushin, V.N.; Maiorova, A.V.; Kulikova, T.V.; Shunyaev, K.Y.; Enyashin, A.N.; Slepukhin, P.A.; Suvorova, A.I. Electrochemical Oxidative Aromatizationof 9-Substituted 9,10-Dihydroacridines: Cleavage of C–H vs C–X Bond. Chem. Heterocycl. Compd. 2019, 55, 956–963. [Google Scholar] [CrossRef]
- Gallardo, I.; Guirado, G. Thermodynamic Study of ΣH Complexes in Nucleophilic Aromatic Substitution Reactions: Relative Stabilities of Electrochemically Generated Radicals. Eur. J. Org. Chem. 2008, 14, 2463–2472. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, A.; Hussain, K. Iodobenzene Diacetate (IBD) Catalyzed an Quick Oxidative Aromatization of Hantzsch-1,4-Dihydropyridines to Pyridines under Ultrasonic Irradiation. Ultrason. Sonochem. 2012, 19, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Rusinov, V.L.; Drokin, R.A.; Tiufiakov, D.V.; Voinkov, E.K.; Ulomsky, E.N. Synthesis and Properties of the Salts of 1-Nitropropan-2-One and 1-Nitrobutan-2-One. Mendeleev Commun. 2020, 30, 177–179. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).