Abstract
Endothelial cell injury is an early event in systemic sclerosis (SSc) pathogenesis and several studies indicate oxidative stress as the trigger of SSc-associated vasculopathy. Here, we show that circulating factors present in sera of SSc patients increased reactive oxygen species (ROS) production and collagen synthesis in human pulmonary microvascular endothelial cells (HPMECs). In addition, the possibility that iloprost, a drug commonly used in SSc therapy, might modulate the above-mentioned biological phenomena has been also investigated. In this regard, as compared to sera of SSc patients, sera of iloprost-treated SSc patients failed to increased ROS levels and collagen synthesis in HPMEC, suggesting a potential antioxidant mechanism of this drug.
1. Introduction
Systemic sclerosis (SSc), also called scleroderma, is a chronic multisystemic and autoimmune disorder characterized by vasculopathy and progressive fibrosis of the skin and visceral organs, such as lungs, heart, and digestive tract [1,2]. In this regard, predisposing factors such as environmental and genetic may affect the susceptibility to scleroderma. These factors affect innate and adaptive immunity functions ultimately promoting inflammation and endothelial dysfunction, two phenomena that precede the SSc-associated fibrotic process [3,4,5,6]. Despite the enormous scientific effort in studying SSc etiology and pathogenesis, the overall etiopathogenic mechanisms picture remains obscure [7]. Along with the essential contribution of the genetic background and immune system [3,4,5,6], the last two decades’ studies have highlighted an important role of oxidative stress in SSc development. SSc patients present an altered balance between oxidant and antioxidant states, which is defined as oxidative stress [8]. Indeed, increased oxidative biomarkers and decreased anti-oxidative ones have been reported in SSc patients, clearly indicating a linkage between an altered redox homeostasis and this disease [8,9,10,11]. Indeed, as compared to healthy donor cells, several cell types coming from SSc patients, such as fibroblasts, monocytes, T lymphocytes, and erythrocytes, showed higher levels of reactive oxygen species (ROS) [12,13,14,15,16]. Moreover, fibroblast, endothelial cells, and vascular smooth muscle cells exposed to sera of SSc patients have a higher level of ROS as compared to healthy subject sera exposition [9,17]. In this regard, it now becoming evident that an overproduction of ROS can activate all cellular SSc targets (endothelial cells, fibroblasts, immune cells, and vascular smooth muscle cells) and trigger the inflammatory, autoimmune, and fibrotic processes defining SSc pathology [8,18]. Although the molecular mechanisms are unclear, vascular dysfunction appears to be an early SSc event, as most of the patients develop Raynaud’s phenomenon (RP), a small vessel disorder preceding onset disease progression, which involves both non-oxidative and oxidative pathways [19]. In this context, while non-oxidative pathways are mostly associated with vascular tone control, the oxidative ones prompt increased ROS production, triggering the excessive deposition of collagen and extracellular matrix (ECM) components leading to tissue fibrosis [8,19,20]. Indeed, during these early disease events, different ROS generating immune mediators, such as transforming growth factor beta (TGF-β), connective tissue growth factor (CTGF) endothelin (ET-1), interferons, and cytokines, are released and contribute to the disease onset and progression [8,19,20,21].
Intravenous iloprost, a stable analog of prostacyclin (PGI2), is a first-line therapeutic alternative for treating both SSc first manifestation and disease progression [22,23]. Iloprost’s beneficial effects (vasodilation, anti-platelet aggregation, cytoprotection, and immunomodulation) are largely due to the modulation of small vessel vasculopathy, one of the key factors in SSc disease [23,24]. In addition, many studies have reported the antioxidant effect of iloprost infusion in SSc patients, but the molecular mechanisms underlying it remain unknown [25,26]. We hypothesized that pro-oxidant circulating factors may trigger SSc-fibrotic process early events, such as oxidative stress and collagen I synthesis, and that the antioxidant properties of iloprost may counteract this course, thus ameliorating the SSc patients’ conditions. To verify our research question, we investigated ROS production and collagen I synthesis in human pulmonary microvascular endothelial cells (HPMECs) exposed to the sera of healthy subjects, SSc patients, and iloprost-treated SSc patients.
2. Results and Discussion
Reactive oxygen species (ROS) production by skin, visceral fibroblasts, and endothelial cells (ECs) has been suggested as an SSc background [27,28]. Indeed, SSc-associated oxidative stress causes both activation and endothelium damage, ultimately leading to vascular complications [28]. As ROS can trigger cell activation, and since ECs are one of the primary targets in SSc, endothelial dysfunction is likely to be the initiator of the cascade of events leading to SSc typical vascular remodeling [19,20]. ECs can, thus, stimulate important mediators, such as growth factors, cytokines and inflammatory agents, which play a pivotal role in the onset and progression of fibrosis [29]. Another SSc hallmark is the excessive deposition of ECM components such as collagen, caused by either increased synthesis or alterations in the degradation mechanisms [30]. In this regard, besides fibroblast and smooth muscle cells, ECs are also an important source of collagen synthesis, although less mentioned [20,31]. In comparison to sera from HD, here, we demonstrate that sera from SSc patients induce both abnormal ROS production and increased collagen synthesis in HPMECs, confirming the hypothesis that pro-oxidant circulating factors may trigger SSc early fibrotic events in an ROS-dependent fashion. Intracellular ROS levels and COL1A1 promoter activity has been kinetically determined in a 4 h time-course (Figure 1A–D), and values at the first hour have been used for comparison (Figure 1B–E). As depicted in Figure 1B, sera from SSc patients significantly increased intracellular ROS levels, compared with HD sera. In the same way, SSs sera-induced activation of collagen synthesis, which prominently occurs in the first hour, resulted significantly higher than that induced by HD sera (Figure 1E). Iloprost’s vasculoprotective properties have been also reported in a recent paper showing its ability to preserve vascular function by stabilizing endothelial adherens junctions, increasing tubulogenesis and barrier function, ultimately reducing ECs shift toward collagen-producing/αSMA-positive cells [32]. In agreement with these data, our current findings report the ability of Iloprost to counteract both ECs damage (Figure 1C–F) and collagen synthesis (Figure 1E) elicited by sera of SSc patients.
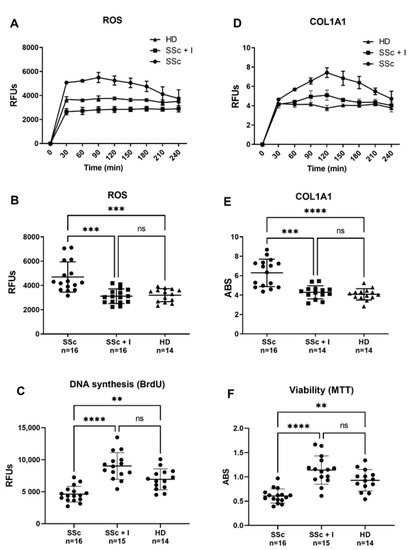
Figure 1.
(A,B) Effect of SSc and SSc iloprost-treated sera on human pulmonary microvascular endothelial cells (HPMECs) intracellular ROS levels. Before sera treatment, subconfluent HPMECs were loaded with 10 μM of H2-DCFDA, and then cultured in basal medium containing 10% (v/v) of sera from SSc patients (SSc), sera from iloprost-treated SSc patients (SSc + I), and sera from healthy donors (HD). Variations in intracellular ROS levels were kinetically determined in a 4 h time-course (A) and values at the first hour were used for comparison (B). Fluorescence data were normalized for protein content and expressed as relative fluorescence units (RFUs). (D,E) Effects of SSc and SSc iloprost-treated sera on HPMECs collagen promoter activity. Before sera treatment, subconfluent HPMECs were transduced with lentiviral particles, obtained from the pCOL1A1-LV-tGFP and EFα-LV-FP602 lentivectors, and then cultured in basal medium containing 10% (v/v) of sera from SSc patients (SSc), sera from iloprost-treated SSc patients (SSc + I), and sera from healthy donors (HD). Variations of COL1A promoter activation were kinetically followed for 4 h (C) and values at 1 h (steady state) used for comparison (D). Data are normalized for transduction efficiency by reporting the ratio of pCOL1A1-LV-tGFP to EFα-LV-FP602 relative fluorescence units (RFUs) (green/red fluorescence). (C–F) Effects of SSc and SSc iloprost-treated sera on HPMECs DNA syntheses and viability. Subconfluent HPMECs were cultured for 24 h in basal medium containing 10% (v/v) of sera from SSc patients (SSc), sera from iloprost-treated SSc patients (SSc + I), and sera from healthy donors (HD). Cells were then assessed for DNA synthesis (C) and cell viability (F), as described in materials and methods. Horizontal lines indicate the mean value and standard deviation range. Kruskall–Wallis one-way analysis of variance, followed by a post-hoc Dunn’s test for multiple comparisons, was used to detect differences among studied groups in Figure 1B,D. All statistical analyses were performed using GraphPad Prism version 9.00 for Windows (GraphPad Software, San Diego, CA, USA), and p-values < 0.05 were considered to be statistically significant. **, between p = 0.0043 and 0.0082; ***, between p = 0.0001 and 0.0009; ****, p ≤ 0.0001; ns, no significant differences have been detected.
Antioxidant-based therapies represent valid support in the treatment of SSc-associated vascular diseases. In this contest, iloprost infusion appears as the most suitable therapy for treating RP and SSc-related vasculopathy [23,33]. Several evidences suggest iloprost’s antioxidant effect at the basis of its clinical benefits [26], but the molecular mechanisms underlying this antioxidant activity are nearly unknown. Our data indicated that sera of SSc patients increase ROS levels in HPMECs. In contrast, sera of Iloprost-treated SSc patients elicits intracellular ROS levels similar to those produced by HD sera (Figure 1B). Similarly, an SSc-induced increase in COL1A1 synthesis resulted in significantly attenuated HPMECs exposed to sera of iloprost-treated SSc patients, suggesting collagen synthesis activation as a potential target of the loprost therapeutic effect (Figure 1D). A possible explanation of the iloprost treatment’s beneficial effect might be the ability to decrease the levels of circulating pro-oxidant and pro-fibrotic factors, which has been reported as increased in the SSc sera [28,34,35,36,37,38]. In this context, consonant with our data, multiple lines of evidence indicate the ability of Iloprost to modulate oxidative markers at systemic levels [25,39,40,41]. Moreover, anti-inflammatory and immunomodulatory effects of Iloprost in SSc have been reported, which highlight its ability to reduce IL1, IL12, IL23, TNF, VEGF, and endothelin-1 and increase IL-2 and RANKL [42,43,44,45]. Interestingly, in line with our hypothesis, CTGF, a well-known profibrotic cytokine, which, in concert with TGF-β, plays a pivotal role in driving collagen overproduction [46,47], has been reported elevated in SSc patients and decreased after iloprost infusion [48]. The same results have also been shown for the synthesis of collagen in fibroblasts exposed to TGF-β [48].
Consistent with this, Gomez-Arroyo et al. also showed a reduced expression of CTGF in cardiac fibroblasts treated with iloprost [49], in addition to the increased activity of collagen synthesis mediators and ECM degradation such as metalloproteinase-9 (MMP9) [49]. Furthermore, a recent study showed an oxidative stress-induced increase in CTGF expression, which was attenuated by the use of antioxidants [50]. Finally, although not specifically in SSc, many other papers report the ability of Iloprost to modulate cytokines/chemokines production [51,52,53,54,55].
Along with the previously mentioned predisposing factors [3,4,5,6], the above-reported studies indicate oxidative stress as one of the important upstream signals responsible for driving SSc-fibrotic processes, making our hypothesis highly conceivable. Indeed, as previously reported [42,43,44,45], iloprost infusion in SSc patients can lower pro-inflammatory cytokines levels in SSc sera, which may drive the inhibition of type I collagen synthesis. It is also plausible that iloprost, concurrently with the decreasing of growth factors such as CTGF, TFG-β, and ET-1 [8,19,20,21], might also inhibit pro-oxidant factors released in the sera of SSc patients. Indeed, oxidative stress biomarkers, such as nitric oxide NO, malondialdehyde (MDA), and ROOH, resulted in elevated blood circulation in SSc patients compared to the healthy group [38]. On the contrary, the concentration of natural antioxidants, such as catalase (CAT) and superoxide dismutase SOD, instead appeared lower than in the control group [38,56]. In this regard, intravenous iloprost infusion has been shown to decrease the blood levels of pro-oxidant factors such as MDA [57] and improve the levels of natural antioxidants such as SOD and CAT [56]. Moreover, several other articles highlight the ability of Iloprost to modulate oxidative markers such as lipid peroxidation, glutathione, F8-Iso PGF2α [25,39,40,41]. In summary, several published papers have indicated the ability of Iloprost to modulate pro-fibrotic and oxidative markers at systemic levels. In this regard, our new data are consistent with the reported anti-inflammatory and immunomodulatory ability of Iloprost; therefore, we believe our proposed mechanicistic scenario highly conceivable.
3. Materials and Methods
3.1. Patients
Thirty-two serum samples were collected from two SSc patients’ groups (untreated and iloprost-treated) at the Unit of Complex Rheumatology, University of Sassari, Sassari, Italy. All SSc patients met the ACR/EULAR criteria for the classification of SSc [58]. The clinical and serological characteristics of the subjects enrolled in the study are summarized in Table 1. Iloprost-treated SSc patients underwent a monthly (5-day cycle—5–6 h/day) intravenous administration of the prostacyclin analog iloprost, at the greatest tolerated dose (0.5–2 ng/kg/min, mean dose 1.3 ng/kg/min); then, blood samples were taken after the 5-day cycle of iloprost infusion. Previous medications were maintained during the course of the study; no other drugs were started during the study period. Healthy donors were matched for gender, race, and smoking status. Study subjects were enrolled according to the protocol approved by the local ethics committee after signing the consent form. Healthy donors were recruited through posted flyers and enrolled after passing a screening questionnaire aimed at excluding the presence of any underlying vascular or autoimmune disease.

Table 1.
Patient demographics and clinical characteristics.
3.2. Cells Culture and Treatment
In this study, human pulmonary microvascular endothelial cells (HPMECs) isolated from the human lung of healthy donors were used (Innoprot, Bizkaia, Spain). Cells were cultured in HPMECs basal medium supplemented with endothelial cell growth supplement. When confluent, HPMECs were subcultured at a split ratio of 1:2 and used within three passages. Unless not specified in the text, cells were plated in 96-well black plates (Corning, Lowell, MA, USA) and processed for experiments in basal medium containing 10% (v/v) of the subjects’ sera. Sera among the different subjects were normalized based on protein content [17,59].
3.3. Intracellular ROS Determination
Intracellular ROS levels were assessed by using the ROS molecular probe 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) (Molecular Probe, Eugene, OR, USA) as previously described with minor modification [59,60]. Within the cell, esterases cleave the acetate groups on H2DCF-DA, thus trapping the reduced form of the probe (H2DCF). Intracellular ROS oxidize H2DCF, yielding the fluorescent product DCF. For ROS measurements, cultured cells were preincubated for 30 min with PBS plus containing 10 μM H2DCFDA, then washed with PBS and treated as described in the figures’ legend. Fluorescence was measured using a Tecan GENios Plus microplate reader (Tecan, Mannedorf, Switzerland) in a light-protected condition. Excitation and emission wavelengths used for fluorescence quantification were 485 nm and 535 nm, respectively. Treatment-induced variation of fluorescence was kinetically measured over a time-course of 4 h. All fluorescence measurements were corrected for background fluorescence and protein concentration expressed as a means ± SD of the relative fluorescence unit (RFU) values.
3.4. Determination of Cell Viability
Cell viability was assessed in 96-well plates (BD Falcon) using the colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT reagent) assay (Promega, Madison, WI, USA) [61,62]. The yellow MTT reagent enters the cells and passes into the mitochondria, where mitochondrial dehydrogenases of viable cells cleave the tetrazolium ring, yielding reduced purple MTT formazan crystals, which are insoluble in aqueous solutions. This reduction occurs only when mitochondrial enzymes are active; therefore, conversion can be directly related to the number of viable cells. The formazan crystals can be dissolved in acidified isopropanol. The resulting purple solution is spectrophotometrically measured at 570 nm. After 24 h of treatment, 20 µL of MTT solution (2 mg/mL) in cell medium were added to the cells and incubated at 37 °C in a cell culture incubator for 2 h. At the end of the incubation period, the solution was removed, and the purple formazan product was solubilized with acidic isopropanol (0.04 N HCl in absolute isopropanol). Then, plates were analyzed at 570 nm using a GENios plus micro-plate reader (Tecan). Results are expressed as the mean ± SD of the absorbance units (ABS).
3.5. Determination of DNA Synthesis
DNA synthesis was assessed using a chemiluminescent immunoassay method, which is based on the measurement of BrdU incorporation during DNA synthesis (Cell Proliferation ELISA BrdU, Roche Applied Science). When cells are pulsed with BrdU, it is incorporated into newly synthesized DNA strands of actively proliferating cells. The incorporation of BrdU into cellular DNA can be detected using anti-BrdU antibodies, allowing an assessment of the population of cells synthesizing DNA. Subconfluent HPMECs were treated for 24 h, as indicated in the figure legends, and BrdU was added 12 h before the end of the experiments. After that, the culture supernatant was removed, and the cells were fixed with a Fix-Denat solution for 30 min. The Fix-Denat was discarded, and cells were incubated with an anti-BrdU antibody conjugated to horseradish peroxidase for 90 min. After rinsing three times with washing buffer, a peroxidase substrate solution was added and allowed to react for 3–10 min at room temperature. The horseradish peroxidase catalyzes the oxidation of diacyl hydrazide, where the reaction product decay from its excited state yields light. Finally, light emission was read using a GENios Plus microplate reader (Tecan). Results were normalized for protein content and expressed as the mean ± SD of the relative fluorescence units (RFU) values [62,63].
3.6. Production of Lentiviral Particles
A set of lentiviral particles was created to assess the synthesis of human collagen type-I (COL1A1) in transduced HPMECs exposed to the different experimental treatments. The lentivectors used to produce the lentiviral particles were pCOL1A1-tGFP, which is a plasmid containing a green fluorescent protein (GFP) under the control of human COL1A1 promoter, and pEFα-LV-FP602, which, instead, contains a red fluorescent protein (FP602) under the control of a constitutive promoter, the elongation factor 1-alpha (EFα) promoter. While activation of pCOL1A1-tGFP is associated with collagen synthesis, pEFα-LV-FP602 activation expresses a red fluorescent protein used as a control for normalizing the cell transduction efficiency. Lentiviral particles were prepared by transient transfection of the 293T/17 packaging cells, as previously described [64,65,66]. Briefly, when cell confluence was approximately 70%, a mix of transgene expression plasmid (pCOL1A1-tGFP or EFα-FP602) and the second generation packaging plasmids (pMD2.G and pCMVR8.74) were used to transfect the cells. The transfection was carried out using a calcium-phosphate solution consisting of a 1:1 mixture of 0.25 M of CaCl2:2X BBS (0.28 M NaCl, 0.05 M N,N-bis-(2-hydroxyethyl)-2-aminoethanesulfonic acid (BES), 1.5 mM Na2HPO4). The medium was exchanged 12 to 16 h post-transfection, and virus-containing media was harvested at 24 h intervals twice, beginning 24 h after changing the medium. The collected medium was spun in a SW28 rotor for 2 h at 19,400 rpm in a L8-80M ultracentrifuge (Beckman); then, the pellet was re-suspended in 1 mL HBSS and spun again in the SW55 rotor for 2 h at 21,000 rpm in a L8-80M ultracentrifuge (Beckman). Finally, the second pellet was re-suspended in 200 μL in HBSS, and the virus suspension was vortexed for 1 to 2 h at low speed before storing at −80 °C in 20 μL aliquots. Virus titer was determined by the p24 ELISA kit (PerkinElmer) according to the manufacturer.
3.7. Generation of HPMEC/pCol1GFP-pEFα-FP602 Stable Cell Lines
Generation of stable cell line containing both pCOL1A1-tGFP and EFα-FP602 was carried out as previously described [61,66,67]. Briefly, 50–60% confluent HPMECs were transduced with the produced lentiviral particles using the optimized multiplicity of infection to reach an infection efficiency of nearly 95%. Cells were then incubated for 24 h; then, an equal amount of fresh medium with no virus was added for an additional 24 h. At around 72–96 h, the efficiency was measured by flow cytometer analysis. Stable transduced HPMECs were used for assessing the COLIA synthesis under the different experimental conditions.
3.8. Collagen Determination
As previously described, COLIA synthesis was investigated, employing the produced HPMEC/pCol1GFP-pEFα-FP602 stable cell lines [17,59]. This method allows us to perform the real-time assessment of multiple samples at the same time in a 96-well plate using a small amount of subject sera [17,59]. HPMEC/pCol1GFP-pEFα-FP602 cells were exposed to basal medium containing 10% (v/v) of sera from SSc patients (SSc), 10% sera from iloprost-treated SSc patients (SSc + I), and 10% sera from healthy donors (HD). Treatment-induced variation of GFP fluorescence was kinetically measured over a time-course of 4 h using a Tecan GENios Plus microplate reader (Tecan, Mannedorf, Switzerland). Excitation wavelengths used for fluorescence quantification were 485 nm and 535 nm, while emission wavelengths were 535 nm and 590 nm for pCOL1A1-LV-tGFP and EFα-LV-FP602, respectively. Data were normalized for transduction efficiency by reporting the ratio of pCOL1A1-LV-tGFP to EFα-LV-FP602 and expressed as a means ± SD of the relative fluorescence unit (RFU) values.
3.9. Statistical Analysis
Kruskall–Wallis one-way analysis of variance, followed by a post-hoc Dunn’s test for multiple comparisons, was used to detect differences among the studied groups. All statistical analyses were performed using GraphPad Prism version 9.00 for Windows (GraphPad Software, San Diego, CA, USA), and p-values < 0.05 were considered to be statistically significant.
4. Conclusions
This study supports the hypothesis that pro-oxidant circulating factors activate collagen synthesis and drive endothelial dysfunction initiation and progression, ultimately leading to SSc pathological vascular alterations. In addition, our results demonstrate that prostacyclin (or prostacyclin analogs) employment improves ECs oxidative status and prevents collagen synthesis and ECs injury, thus providing new clues concerning the mechanisms of action of iloprost and its clinical benefits in terms of vascular damage.
Author Contributions
Conceptualization, R.G., G.L.E. and G.P.; methodology, R.G., D.T.B.T., A.M.P., A.C., A.Z. and G.P.; project administration, G.L.E., A.Z. and G.P.; Resources, G.L.E., A.M.P., A.C., A.Z. and G.P.; writing—original draft, R.G., D.T.B.T. and G.P. writing—review and editing, R.G., A.Z., G.L.E. and G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been made possible thanks to grants from the University of Sharjah (Seed 2001050151, collaborative 2101050160), fondo UNISS di Ateneo per la Ricerca 2020, and Fondo di Sviluppo e Coesione 2014–2020, Patto per lo Sviluppo della Regione Sardegna, L.R.7-2017-RASSR82005.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Azienda Sanitaria Locale #1 (ASL 1) of Sassari Ethics Committee with protocol # 779/L 2009.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the result.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef]
- Sobolewski, P.; Maślińska, M.; Wieczorek, M.; Łagun, Z.; Malewska, A.; Roszkiewicz, M.; Nitskovich, R.; Szymańska, E.; Walecka, I. Systemic sclerosis–multidisciplinary disease: Clinical features and treatment. Reumatologia 2019, 57, 221. [Google Scholar] [CrossRef] [PubMed]
- Fullard, N.; O’Reilly, S. Role of innate immune system in systemic sclerosis. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 511–517. [Google Scholar]
- Broen, J.C.; Radstake, T.R.; Rossato, M. The role of genetics and epigenetics in the pathogenesis of systemic sclerosis. Nat. Rev. Rheumatol. 2014, 10, 671–681. [Google Scholar] [CrossRef]
- Brkic, Z.; van Bon, L.; Cossu, M.; van Helden-Meeuwsen, C.G.; Vonk, M.C.; Knaapen, H.; van den Berg, W.; Dalm, V.A.; Van Daele, P.L.; Severino, A. The interferon type I signature is present in systemic sclerosis before overt fibrosis and might contribute to its pathogenesis through high BAFF gene expression and high collagen synthesis. Ann. Rheum. Dis. 2016, 75, 1567–1573. [Google Scholar] [CrossRef]
- Mora, G.F. Systemic sclerosis: Environmental factors. J. Rheumatol. 2009, 36, 2383–2396. [Google Scholar] [CrossRef]
- Asano, Y. Systemic sclerosis. J. Dermatol. 2018, 45, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Doridot, L.; Jeljeli, M.; Chêne, C.; Batteux, F. Implication of oxidative stress in the pathogenesis of systemic sclerosis via inflammation, autoimmunity and fibrosis. Redox Biol. 2019, 25, 101–122. [Google Scholar] [CrossRef]
- Servettaz, A.; Guilpain, P.; Goulvestre, C.; Chéreau, C.; Hercend, C.; Nicco, C.; Guillevin, L.; Weill, B.; Mouthon, L.; Batteux, F. Radical oxygen species production induced by advanced oxidation protein products predicts clinical evolution and response to treatment in systemic sclerosis. Ann. Rheum. Dis. 2007, 66, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Avouac, J.; Borderie, D.; Ekindjian, O.G.; Kahan, A.; Allanore, Y. High DNA oxidative damage in systemic sclerosis. J. Rheumatol. 2010, 37, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Łuczyñska, M.; Szkudlarek, U.; Dziankowska-Bartkowiak, B.; Waszczykowska, E.; Kasielski, M.; Sysa-Jȩdrzejowska, A.; Nowak, D. Elevated exhalation of hydrogen peroxide in patients with systemic sclerosis. Eur. J. Clin. Investig. 2003, 33, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Tsou, P.S.; Talia, N.N.; Pinney, A.J.; Kendzicky, A.; Piera-Velazquez, S.; Jimenez, S.A.; Seibold, J.R.; Phillips, K.; Koch, A.E. Effect of oxidative stress on protein tyrosine phosphatase 1B in scleroderma dermal fibroblasts. Arthritis Rheum. 2012, 64, 1978–1989. [Google Scholar] [CrossRef] [PubMed]
- Bourji, K.; Meyer, A.; Chatelus, E.; Pincemail, J.; Pigatto, E.; Defraigne, J.-O.; Singh, F.; Charlier, C.; Geny, B.; Gottenberg, J.-E. High reactive oxygen species in fibrotic and nonfibrotic skin of patients with diffuse cutaneous systemic sclerosis. Free Radic. Biol. Med. 2015, 87, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Sambo, P.; Jannino, L.; Candela, M.; Salvi, A.; Luchetti, M.M.; Gabrielli, A.; Donini, M.; Dusi, S. Monocytes of patients with systemic sclerosis (scleroderma) spontaneously release in vitro increased amounts of superoxide anion. J. Investig. Dermatol. 1999, 112, 78–84. [Google Scholar] [CrossRef][Green Version]
- Amico, D.; Spadoni, T.; Rovinelli, M.; Serafini, M.; D’Amico, G.; Campelli, N.; Baroni, S.S.; Gabrielli, A. Intracellular free radical production by peripheral blood T lymphocytes from patients with systemic sclerosis: Role of NADPH oxidase and ERK1/2. Arthritis Res. Ther. 2015, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Devrim, E.; Erten, Ş.; Ergüder, İ.B.; Namuslu, M.; Turgay, M.; Durak, İ. Malondialdehyde and nitric oxide levels in erythrocytes from patients with systemic sclerosis. Med. Princ. Pract. 2008, 17, 349–350. [Google Scholar] [CrossRef]
- Boin, F.; Erre, G.L.; Posadino, A.M.; Cossu, A.; Giordo, R.; Spinetti, G.; Passiu, G.; Emanueli, C.; Pintus, G. Oxidative stress-dependent activation of collagen synthesis is induced in human pulmonary smooth muscle cells by sera from patients with scleroderma-associated pulmonary hypertension. Orphanet J. Rare Dis. 2014, 9, 1–5. [Google Scholar] [CrossRef]
- Svegliati, S.; Spadoni, T.; Moroncini, G.; Gabrielli, A. NADPH oxidase, oxidative stress and fibrosis in systemic sclerosis. Free Radic. Biol. Med. 2018, 125, 90–97. [Google Scholar] [CrossRef]
- Abdulle, A.E.; Diercks, G.F.; Feelisch, M.; Mulder, D.J.; Goor, H.V. The role of oxidative stress in the development of systemic sclerosis related vasculopathy. Front. Physiol. 2018, 9, 1177. [Google Scholar] [CrossRef]
- Thuan, D.T.B.; Zayed, H.; Eid, A.H.; Abou-Saleh, H.; Nasrallah, G.K.; Mangoni, A.A.; Pintus, G. A potential link between oxidative stress and endothelial-to-mesenchymal transition in systemic sclerosis. Front. Immunol. 2018, 9, 1985. [Google Scholar] [CrossRef]
- Vona, R.; Giovannetti, A.; Gambardella, L.; Malorni, W.; Pietraforte, D.; Straface, E. Oxidative stress in the pathogenesis of systemic scleroderma: An overview. J. Cell. Mol. Med. 2018, 22, 3308–3314. [Google Scholar] [CrossRef]
- Scorza, R.; Caronni, M.; Mascagni, B.; Berruti, V.; Bazzi, S.; Micallef, E.; Arpaia, G.; Sardina, M.; Origgi, L.; Vanoli, M. Effects of long-term cyclic iloprost therapy in systemic sclerosis with Raynaud’s phenomenon. A randomized, controlled study. Clin. Exp. Rheumatol. 2001, 19, 503–508. [Google Scholar] [PubMed]
- Foti, R.; Visalli, E.; Amato, G.; Benenati, A.; Converso, G.; Farina, A.; Bellofiore, S.; Mulè, M.; Di Gangi, M. Long-term clinical stabilization of scleroderma patients treated with a chronic and intensive IV iloprost regimen. Rheumatol. Int. 2017, 37, 245–249. [Google Scholar] [CrossRef]
- Wigley, F.M.; Seibold, J.R.; Wise, R.A.; McCLOSKEY, D.A.; Dole, W. Intravenous iloprost treatment of Raynaud’s phenomenon and ischemic ulcers secondary to systemic sclerosis. J Rheumatol. 1992, 19, 1407–1414. [Google Scholar]
- Erre, G.L.; De Muro, P.; Dellaca, P.; Fenu, P.; Cherchi, G.M.; Faedda, R.; Passiu, G. Iloprost therapy acutely decreases oxidative stress in patients affected by systemic sclerosis. Clin. Exp. Rheumatol. 2008, 26, 1095. [Google Scholar] [PubMed]
- Erre, G.; Passiu, G. Antioxidant effect of Iloprost: Current knowledge and therapeutic implications for systemic sclerosis. Reumatismo 2009, 61, 90–97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sambo, P.; Baroni, S.S.; Luchetti, M.; Paroncini, P.; Dusi, S.; Orlandini, G.; Gabrielli, A. Oxidative stress in scleroderma: Maintenance of scleroderma fibroblast phenotype by the constitutive up-regulation of reactive oxygen species generation through the NADPH oxidase complex pathway. Arthritis Rheum. 2001, 44, 2653–2664. [Google Scholar] [CrossRef]
- Grygiel-Górniak, B.; Puszczewicz, M. Oxidative damage and antioxidative therapy in systemic sclerosis. Mediat. Inflamm. 2014. [Google Scholar] [CrossRef]
- Abraham, D.; Distler, O. How does endothelial cell injury start? The role of endothelin in systemic sclerosis. Arthritis Res. Ther. 2007, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Scheja, A.; Wildt, M.; Wollheim, F.; Åkesson, A.; Saxne, T. Circulating collagen metabolites in systemic sclerosis. Differ. Between Ltd. Diffus. Relatsh. Pulm. Involvement. Rheumatol. 2000, 39, 1110–1113. [Google Scholar]
- Jimenez, S.A. Role of endothelial to mesenchymal transition in the pathogenesis of the vascular alterations in systemic sclerosis. Int. Sch. Res. Not. 2013, 2013, 835948. [Google Scholar] [CrossRef]
- Tsou, P.S.; Palisoc, P.J.; Flavahan, N.A.; Khanna, D. Dissecting the Cellular Mechanism of Prostacyclin Analog Iloprost in Reversing Vascular Dysfunction in Scleroderma. Arthritis Rheumatol. 2021, 73, 520–529. [Google Scholar] [CrossRef]
- Ingegnoli, F.; Schioppo, T.; Allanore, Y.; Caporali, R.; Colaci, M.; Distler, O.; Furst, D.E.; Hunzelmann, N.; Iannone, F.; Khanna, D. Practical suggestions on intravenous iloprost in Raynaud’s phenomenon and digital ulcer secondary to systemic sclerosis: Systematic literature review and expert consensus. In Seminars in Arthritis and Rheumatism; Elsevier: Amsterdam, The Netherlands, 2019; pp. 686–693. [Google Scholar]
- Adami, E.; Viswanathan, S.; Widjaja, A.A.; Ng, B.; Chothani, S.; Zhihao, N.; Tan, J.; Lio, P.M.; George, B.L.; Altunoglu, U. IL11 is elevated in systemic sclerosis and IL11-dependent ERK signalling underlies TGFβ-mediated activation of dermal fibroblasts. Rheumatology 2021. [Google Scholar] [CrossRef]
- Shima, Y. The benefits and prospects of interleukin-6 inhibitor on systemic sclerosis. Mod. Rheumatol. 2019, 29, 294–301. [Google Scholar] [CrossRef]
- Xu, D.; Mu, R.; Wei, X. The roles of IL-1 family cytokines in the pathogenesis of systemic sclerosis. Front. Immunol. 2019, 10, 2025. [Google Scholar] [CrossRef] [PubMed]
- Benfaremo, D.; Baroni, S.S.; Manfredi, L.; Moroncini, G.; Gabrielli, A. Putative functional pathogenic autoantibodies in systemic sclerosis. Eur. J. Rheumatol. 2020, 7 (Suppl. S3), S181. [Google Scholar] [CrossRef]
- Luo, J.-Y.; Liu, X.; Jiang, M.; Zhao, H.-P.; Zhao, J.-J. Oxidative stress markers in blood in systemic sclerosis: A meta-analysis. Mod. Rheumatol. 2017, 27, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Lessiani, G.; Vazzana, N.; Cuccurullo, C.; Di Michele, D.; Laurora, G.; Sgrò, G.; Di Ruscio, P.; Simeone, E.; Di Iorio, P.; Lattanzio, S. Inflammation, oxidative stress and platelet activation in aspirin-treated critical limb ischaemia: Beneficial effects of iloprost. Thromb. Haemost. 2011, 105, 321–328. [Google Scholar]
- Aytac, E.; Teksöz, S.; Saygili, S.; Tortum, O.B.; Yavuz, N. Iloprost reduces colitis induced oxidative stress: An experimental study in rats. Turk. J. Gastroenterol. 2013, 24, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Aytac, E.; Seymen, H.O.; Uzun, H.; Dikmen, G.; Altug, T. Effects of iloprost on visual evoked potentials and brain tissue oxidative stress after bilateral common carotid artery occlusion. Prostaglandinsleukotrienes Essent. Fat. Acids 2006, 74, 373–378. [Google Scholar] [CrossRef]
- D’Amelio, P.; Cristofaro, M.A.; D’Amico, L.; Veneziano, L.; Roato, I.; Sassi, F.; Bisignano, G.; Saracco, M.; Pellerito, R.; Patanè, S. Iloprost modulates the immune response in systemic sclerosis. BMC Immunol. 2010, 11, 1–7. [Google Scholar] [CrossRef]
- Della Bella, S.; Molteni, M.; Mascagni, B.; Zulian, C.; Compasso, S.; Scorza, R. Cytokine production in scleroderma patients: Effects of therapy with either iloprost or nifedipine. Clin. Exp. Rheumatol. 1997, 15, 135–141. [Google Scholar]
- Auriemma, M.; Vianale, G.; Reale, M.; Costantini, E.; Di Nicola, M.; Romani, G.; Merla, A.; Muraro, R.; Amerio, P. Iloprost treatment summer-suspension: Effects on skin thermal properties and cytokine profile in systemic sclerosis patients. G Ital. Derm. Venereol 2013, 148, 209–216. [Google Scholar]
- Mittag, M.; Beckheinrich, P.; Haustein, U. Systemic sclerosis-related Raynaud’s phenomenon: Effects of iloprost infusion therapy on serum cytokine, growth factor and soluble adhesion molecule levels. Acta Derm. Venereol. 2001, 81, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Cheong, M.-L.; Lai, T.-H.; Wu, W.-B. Connective tissue growth factor mediates transforming growth factor β-induced collagen expression in human endometrial stromal cells. PLoS ONE 2019, 14, e0210765. [Google Scholar] [CrossRef]
- Quan, T.; Shao, Y.; He, T.; Voorhees, J.J.; Fisher, G.J. Reduced expression of connective tissue growth factor (CTGF/CCN2) mediates collagen loss in chronologically aged human skin. J. Investig. Dermatol. 2010, 130, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Stratton, R.; Shiwen, X.; Martini, G.; Holmes, A.; Leask, A.; Haberberger, T.; Martin, G.R.; Black, C.M.; Abraham, D. Iloprost suppresses connective tissue growth factor production in fibroblasts and in the skin of scleroderma patients. J. Clin. Investig. 2001, 108, 241–250. [Google Scholar] [CrossRef]
- Gomez-Arroyo, J.; Sakagami, M.; Syed, A.A.; Farkas, L.; Van Tassell, B.; Kraskauskas, D.; Mizuno, S.; Abbate, A.; Bogaard, H.J.; Byron, P.R. Iloprost reverses established fibrosis in experimental right ventricular failure. Eur. Respir. J. 2015, 45, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-C.; Wu, S.-B.; Chang, P.-C.; Wei, Y.-H. Alteration of connective tissue growth factor (CTGF) expression in orbital fibroblasts from patients with Graves’ ophthalmopathy. PLoS ONE 2015, 10, e0143514. [Google Scholar] [CrossRef]
- Karatepe, O.; Cakir, A.; Unal, O.; Battal, M.; Adas, G.; Kamali, G.; Kemik, A.; Aydin, T.; Kamali, S.; Karahan, S.R. Iloprost reduces colonic injury in ischemic colitis in rats. Acta Cir. Bras. 2011, 26, 220–226. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Zhou, W.; Xiang, R.; Jiang, L.; Huang, K.; Xiao, Y.; Guo, Z.; Gao, J. A prostacyclin analogue, iloprost, protects from bleomycin-induced pulmonary fibrosis in mice. Respir. Res. 2010, 11, 1–12. [Google Scholar] [CrossRef]
- Di Renzo, M.; Pieragalli, D.; Meini, S.; De Franco, V.; Pompella, G.; Auteri, A.; Pasqui, A. Iloprost treatment reduces TNF-alpha production and TNF-RII expression in critical limb ischemia patients without affecting IL6. Prostaglandinsleukotrienes Essent. Fat. Acids 2005, 73, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.-J.; Fu, M.; Lo, F.R. Prostaglandin I2Analogue, Iloprost, down regulates Mitogen-activated protein kinases of macrophages. J. Surg. Res. 1998, 76, 159–164. [Google Scholar] [CrossRef]
- Jung, S.; Donhauser, T.; Toyka, K.V.; Hartung, H.-P. Propentofylline and Iloprost Suppress the Production of TNF-αby Macrophages but Fail to Ameliorate Experimental Autoimmune Encephalomyelitis in Lewis Rats. J. Autoimmun. 1997, 10, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Balbir-Gurman, A.; Braun-Moscovici, Y.; Livshitz, V.; Schapira, D.; Markovits, D.; Rozin, A.; Boikaner, T.; Nahir, A.M. Antioxidant status after iloprost treatment in patients with Raynaud’s phenomenon secondary to systemic sclerosis. Clin. Rheumatol. 2007, 26, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Karaçor, T.; Dogan, Z.; Elibol, E.; Bulbul, M.; Nacar, M. Effects of iloprost on experimental ischemia and reperfusion injury in rat ovary. Biotech. Histochem. 2020, 95, 373–380. [Google Scholar] [CrossRef]
- Valentini, G.; Marcoccia, A.; Cuomo, G.; Iudici, M.; Vettori, S. The concept of early systemic sclerosis following 2013 ACR\EULAR criteria for the classification of systemic sclerosis. Curr. Rheumatol. Rev. 2014, 10, 38–44. [Google Scholar] [CrossRef]
- Fois, A.G.; Posadino, A.M.; Giordo, R.; Cossu, A.; Agouni, A.; Rizk, N.M.; Pirina, P.; Carru, C.; Zinellu, A.; Pintus, G. Antioxidant activity mediates pirfenidone antifibrotic effects in human pulmonary vascular smooth muscle cells exposed to sera of idiopathic pulmonary fibrosis patients. Oxidative Med. Cell. Longev. 2018. [Google Scholar] [CrossRef]
- Posadino, A.M.; Phu, H.T.; Cossu, A.; Giordo, R.; Fois, M.; Thuan, D.T.B.; Piga, A.; Sotgia, S.; Zinellu, A.; Carru, C. Oxidative stress-induced Akt downregulation mediates green tea toxicity towards prostate cancer cells. Toxicol. Vitr. 2017, 42, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Cossu, A.; Posadino, A.M.; Giordo, R.; Emanueli, C.; Sanguinetti, A.M.; Piscopo, A.; Poiana, M.; Capobianco, G.; Piga, A.; Pintus, G. Apricot melanoidins prevent oxidative endothelial cell death by counteracting mitochondrial oxidation and membrane depolarization. PLoS ONE 2012, 7, e48817. [Google Scholar] [CrossRef]
- Posadino, A.M.; Giordo, R.; Cossu, A.; Nasrallah, G.K.; Shaito, A.; Abou-Saleh, H.; Eid, A.H.; Pintus, G. Flavin oxidase-induced ROS generation modulates PKC biphasic effect of resveratrol on endothelial cell survival. Biomolecules 2019, 9, 209. [Google Scholar] [CrossRef]
- Vono, R.; Fuoco, C.; Testa, S.; Pirrò, S.; Maselli, D.; McCollough, D.F.; Sangalli, E.; Pintus, G.; Giordo, R.; Finzi, G. Activation of the pro-oxidant PKCβII-p66Shc signaling pathway contributes to pericyte dysfunction in skeletal muscles of patients with diabetes with critical limb ischemia. Diabetes 2016, 65, 3691–3704. [Google Scholar] [CrossRef] [PubMed]
- Begemann, S.; Galimi, F.; Karlseder, J. Moderate expression of TRF2 in the hematopoietic system increases development of large cell blastic T-cell lymphomas. Aging 2009, 1, 122. [Google Scholar] [CrossRef]
- Dissen, G.A.; Lomniczi, A.; Neff, T.L.; Hobbs, T.R.; Kohama, S.G.; Kroenke, C.D.; Galimi, F.; Ojeda, S.R. In vivo manipulation of gene expression in non-human primates using lentiviral vectors as delivery vehicles. Methods 2009, 49, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Giordo, R.; Nasrallah, G.K.; Posadino, A.M.; Galimi, F.; Capobianco, G.; Eid, A.H.; Pintus, G. Resveratrol-elicited pkc inhibition counteracts nox-mediated endothelial to mesenchymal transition in human retinal endothelial cells exposed to high glucose. Antioxidants 2021, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Posadino, A.M.; Cossu, A.; Giordo, R.; Zinellu, A.; Sotgia, S.; Vardeu, A.; Hoa, P.T.; Carru, C.; Pintus, G. Resveratrol alters human endothelial cells redox state and causes mitochondrial-dependent cell death. Food Chem. Toxicol. 2015, 78, 10–16. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).