Berberine, A Phytoalkaloid, Inhibits Inflammatory Response Induced by LPS through NF-Kappaβ Pathway: Possible Involvement of the IKKα
Abstract
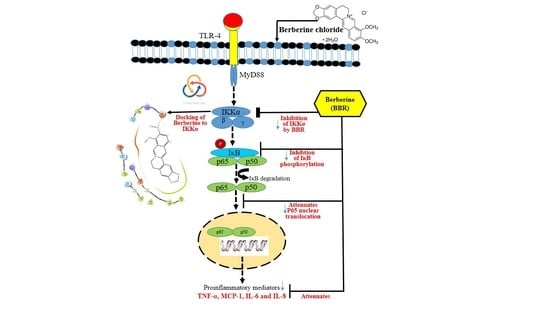
:1. Introduction
2. Results
2.1. Effect of BBR on Cell Viability of THP-1 Cells
2.2. Effect of BBR on Arachidonic Acid (AA)-Induced Proinflammatory Markers
2.3. Effect of BBR on LPS Induced Proinflammatory Markers
2.4. Effect of BBR on LPS-Induced NF-κB p-65 Translocation in THP-1 Cells
2.5. Molecular Modeling
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell culture Treatments
4.3. Measurement of Cytokines and Chemokines
4.4. Quantitative Real-Time PCR Analysis
4.5. Immunofluorescence Staining
4.6. Immunoblotting
4.7. Molecular Modeling
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Imanshahidi, M.; Hosseinzadeh, H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother. Res. 2008, 22, 999–1012. [Google Scholar] [CrossRef]
- Tang, J.; Feng, Y.; Tsao, S.; Wang, N.; Curtain, R.; Wang, Y. Berberine and Coptidis Rhizoma as novel antineoplastic agents: A review of traditional use and biomedical investigations. J. Ethnopharmacol. 2009, 126, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Rojsanga, P.; Sukhthankar, M.; Krisanapun, C.; Gritsanapan, W.; Lawson, D.B.; Baek, S.J. In vitro anti-proliferative activity of alcoholic stem extract of Coscinium fenestratum in human colorectal cancer cells. Exp. Ther. Med. 2010, 1, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Ettefagh, K.A.; Burns, J.T.; Junio, H.A.; Kaatz, G.W.; Cech, N.B. Goldenseal (Hydrastis canadensis L.) extracts synergistically enhance the antibacterial activity of berberine via efflux pump inhibition. Planta Med. 2011, 77, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.; Harsha, P.S.C.S.; Giridhar, P.; Ravishankar, G.A. Berberine and lycopene profiling during the ontogeny of Tinospora cordifolia (Willd.) Miers ex Hook. F. & Thoms fruit. Curr. Sci. 2011, 1000, 1225–1231. [Google Scholar]
- Neag, M.A.; Mocan, A.; Echeverría, J.; Pop, R.M.; Bocsan, C.I.; Crişan, G.; Buzoianu, A.D. Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders. Front. Pharmacol. 2018, 9, 557. [Google Scholar] [CrossRef] [Green Version]
- Hou, Q.; Tang, X.; Liu, H.; Tang, J.; Yang, Y.; Jing, X.; Xiao, Q.; Wang, W.; Gou, X.; Wang, Z. Berberine induces cell death in human hepatoma cells in vitro by downregulating CD147. Cancer Sci. 2011, 102, 1287–1292. [Google Scholar] [CrossRef]
- Li, M.-H.; Zhang, Y.-J.; Yu, Y.-H.; Yang, S.-H.; Iqbal, J.; Mi, Q.-Y.; Li, B.; Wang, Z.-M.; Mao, W.-X.; Xie, H.-G.; et al. Berberine improves pressure overload-induced cardiac hypertrophy and dysfunction through enhanced autophagy. Eur. J. Pharmacol. 2014, 728, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, W.S.; Kim, K.H.; Yoon, M.J.; Cho, H.J.; Shen, Y.; Ye, J.-M.; Lee, C.H.; Oh, W.K.; Kim, C.T.; et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 2006, 55, 2256–2264. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.-J.; Xu, R.-X.; Sun, J.; Tang, Y.; Li, J.-J. Enhanced circulating PCSK9 concentration by berberine through SREBP-2 pathway in high fat diet-fed rats. J. Transl. Med. 2014, 12, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.-S.; Matsumoto, K.; Murakami, Y.; Hori, H.; Zhao, Q.; Obi, R. Berberine exerts neuroprotective actions against in vitro ischemia-induced neuronal cell damage in organotypic hippocampal slice cultures: Involvement of B-cell lymphoma 2 phosphorylation suppression. Biol. Pharm. Bull. 2009, 32, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šudomová, M.; Berchová-Bímová, K.; Marzocco, S.; Liskova, A.; Kubatka, P.; Hassan, S.T.S. Berberine in Human Oncogenic Herpesvirus Infections and Their Linked Cancers. Viruses 2021, 13, 1014. [Google Scholar] [CrossRef]
- Domitrović, R.; Jakovac, H.; Blagojević, G. Hepatoprotective activity of berberine is mediated by inhibition of TNF-α, COX-2, and iNOS expression in CCl(4)-intoxicated mice. Toxicology 2011, 280, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism 2008, 57, 712–717. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Du, L. Diabetes is an inflammatory disease: Evidence from traditional Chinese medicines. Diabetes. Obes. Metab. 2011, 13, 289–301. [Google Scholar] [CrossRef]
- Affuso, F.; Mercurio, V.; Fazio, V.; Fazio, S. Cardiovascular and metabolic effects of Berberine. World J. Cardiol. 2010, 2, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Vuddanda, P.R.; Chakraborty, S.; Singh, S. Berberine: A potential phytochemical with multispectrum therapeutic activities. Expert Opin. Investig. Drugs 2010, 19, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Zou, D.; Liu, W.; Yang, J.; Zhu, N.; Huo, L.; Wang, M.; Hong, J.; Wu, P.; et al. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J. Clin. Endocrinol. Metab. 2008, 93, 2559–2565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, W.-J.; Wei, J.; Zuo, Z.-Y.; Wang, Y.-M.; Song, D.-Q.; You, X.-F.; Zhao, L.-X.; Pan, H.-N.; Jiang, J.-D. Combination of simvastatin with berberine improves the lipid-lowering efficacy. Metabolism 2008, 57, 1029–1037. [Google Scholar] [CrossRef]
- Libby, P. Inflammation and Atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Ojha, D.; Mukherjee, H.; Mondal, S.; Jena, A.; Dwivedi, V.P.; Mondal, K.C.; Malhotra, B.; Samanta, A.; Chattopadhyay, D. Anti-inflammatory activity of Odina wodier Roxb, an Indian folk remedy, through inhibition of toll-like receptor 4 signaling pathway. PLoS ONE 2014, 9, e104939. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.M.; Kim, S.G. Inhibition of arachidonic acid and iron-induced mitochondrial dysfunction and apoptosis by oltipraz and novel 1,2-dithiole-3-thione congeners. Mol. Pharmacol. 2009, 75, 242–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cubero, F.J.; Nieto, N. Arachidonic acid stimulates TNFα production in Kupffer cells via a reactive oxygen species-pERK1/2-Egr1-dependent mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G228–G239. [Google Scholar] [CrossRef] [Green Version]
- Spatuzza, C.; Postiglione, L.; Covelli, B.; Ricciardone, M.; Benvenuti, C.; Mondola, P.; Belfiore, A. Effects of berberine and red yeast on proinflammatory cytokines IL-6 and TNF-α in peripheral blood mononuclear cells (PBMCs) of human subjects. Front. Pharmacol. 2014, 5, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uy, B.; McGlashan, S.R.; Shaikh, S.B. Measurement of reactive oxygen species in the culture media using Acridan Lumigen PS-3 assay. J. Biomol. Tech. 2011, 22, 95–107. [Google Scholar] [PubMed]
- Vladykovskaya, E.; Sithu, S.D.; Haberzettl, P.; Wickramasinghe, N.S.; Merchant, M.L.; Hill, B.G.; McCracken, J.; Agarwal, A.; Dougherty, S.; Gordon, S.A.; et al. Lipid peroxidation product 4-hydroxy-trans-2-nonenal causes endothelial activation by inducing endoplasmic reticulum stress. J. Biol. Chem. 2012, 287, 11398–11409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.-H.; Liu, Z.-Y.; Sun, L.-S.; Li, Y.-J.; Zhang, D.-S.; Pan, R.-T.; Sun, Z.-L. Effect of Danofloxacin on Reactive Oxygen Species Production, Lipid Peroxidation and Antioxidant Enzyme Activities in Kidney Tubular Epithelial Cell Line, LLC-PK1. Basic Clin. Pharmacol. Toxicol. 2013, 113, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Stangl, V.; Günther, C.; Jarrin, A.; Bramlage, P.; Moobed, M.; Staudt, A.; Baumann, G.; Stangl, K.; Felix, S.B. Homocysteine inhibits TNF-alpha-induced endothelial adhesion molecule expression and monocyte adhesion via nuclear factor-kappaB dependent pathway. Biochem. Biophys. Res. Commun. 2001, 280, 1093–1100. [Google Scholar] [CrossRef]
- Zhang, W.J.; Frei, B. Alpha-lipoic acid inhibits TNF-alpha-induced NF-kappaB activation and adhesion molecule expression in human aortic endothelial cells. FASEB J. 2001, 15, 2423–2432. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Seyedhoseini, F.S.; Asadi, J.; Yazdani, Y. Effects of berberine on the secretion of cytokines and expression of genes involved in cell cycle regulation in THP-1 monocytic cell line. Iran. J. Basic Med. Sci. 2017, 20, 530–537. [Google Scholar] [CrossRef]
- Gao, M.; Chen, L.; Yang, L.; Yu, X.; Kou, J.; Yu, B. Berberine inhibits LPS-induced TF procoagulant activity and expression through NF-κB/p65, Akt and MAPK pathway in THP-1 cells. Pharmacol. Rep. 2014, 66, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.; Peng, L.; Pan, N.; Hu, X.; Zhang, Y. The anti-atherogenic effects of berberine on foam cell formation are mediated through the upregulation of sirtuin 1. Int. J. Mol. Med. 2014, 34, 1087–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarna, L.K.; Wu, N.; Hwang, S.-Y.; Siow, Y.L.; Karmin, O. Berberine inhibits NADPH oxidase mediated superoxide anion production in macrophagesThis article is one of a selection of papers published in a Special Issue on Oxidative Stress in Health and Disease. Can. J. Physiol. Pharmacol. 2010, 88, 369–378. [Google Scholar] [CrossRef]
- Kang, J.S.; Park, S.-K.; Yang, K.-H.; Kim, H.M. Silymarin inhibits TNF-α-induced expression of adhesion molecules in human umbilical vein endothelial cells. FEBS Lett. 2003, 550, 89–93. [Google Scholar] [CrossRef] [Green Version]
- Dagia, N.M.; Harii, N.; Meli, A.E.; Sun, X.; Lewis, C.J.; Kohn, L.D.; Goetz, D.J. Phenyl methimazole inhibits TNF-alpha-induced VCAM-1 expression in an IFN regulatory factor-1-dependent manner and reduces monocytic cell adhesion to endothelial cells. J. Immunol. 2004, 173, 2041–2049. [Google Scholar] [CrossRef] [Green Version]
- Lang, A.; Lahav, M.; Sakhnini, E.; Barshack, I.; Fidder, H.H.; Avidan, B.; Bardan, E.; Hershkoviz, R.; Bar-Meir, S.; Chowers, Y. Allicin inhibits spontaneous and TNF-alpha induced secretion of proinflammatory cytokines and chemokines from intestinal epithelial cells. Clin. Nutr. 2004, 23, 1199–1208. [Google Scholar] [CrossRef]
- Zhang, F.; Yu, W.; Hargrove, J.L.; Greenspan, P.; Dean, R.G.; Taylor, E.W.; Hartle, D.K. Inhibition of TNF-alpha induced ICAM-1, VCAM-1 and E-selectin expression by selenium. Atherosclerosis 2002, 161, 381–386. [Google Scholar] [CrossRef]
- Zhang, J.; Alcaide, P.; Liu, L.; Sun, J.; He, A.; Luscinskas, F.W.; Shi, G.-P. Regulation of endothelial cell adhesion molecule expression by mast cells, macrophages, and neutrophils. PLoS ONE 2011, 6, e14525. [Google Scholar] [CrossRef] [PubMed]
- Treede, I.; Braun, A.; Jeliaskova, P.; Giese, T.; Füllekrug, J.; Griffiths, G.; Stremmel, W.; Ehehalt, R. TNF-alpha-induced up-regulation of pro-inflammatory cytokines is reduced by phosphatidylcholine in intestinal epithelial cells. BMC Gastroenterol. 2009, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Tang, F.; Mao, G.; Bian, R. Effect of alpha-pinene on nuclear translocation of NF-kappa B in THP-1 cells. Acta Pharmacol. Sin. 2004, 25, 480–484. [Google Scholar] [PubMed]
- Zhu, C.; Xiong, Z.; Chen, X.; Peng, F.; Hu, X.; Chen, Y.; Wang, Q. Artemisinin Attenuates Lipopolysaccharide-Stimulated Proinflammatory Responses by Inhibiting NF-κB Pathway in Microglia Cells. PLoS ONE 2012, 7, e35125. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, S.S.; Bashyam, L.; Manthapuram, N.; Bitla, P.; Kollipara, P.; Tetali, S.D. Ocimum sanctum leaf extracts attenuate human monocytic (THP-1) cell activation. J. Ethnopharmacol. 2014, 154, 148–155. [Google Scholar] [CrossRef]
- Kokkiripati, P.K.; Bhakshu, L.M.; Marri, S.; Padmasree, K.; Row, A.T.; Raghavendra, A.S.; Tetali, S.D. Gum resin of Boswellia serrata inhibited human monocytic (THP-1) cell activation and platelet aggregation. J. Ethnopharmacol. 2011, 137, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Reddi, K.K.; Tetali, S.D. Dry leaf extracts of Tinospora cordifolia (Willd.) Miers attenuate oxidative stress and inflammatory condition in human monocytic (THP-1) cells. Phytomedicine 2019, 61, 152831. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.K.; Basu, S.; Lemke, H.-D.; Jankowski, J.; Kratz, K.; Lendlein, A.; Tetali, S.D. Effect of extracts of poly(ether imide) microparticles on cytotoxicity, ROS generation and proinflammatory effects on human monocytic (THP-1) cells. Clin. Hemorheol. Microcirc. 2015. [Google Scholar] [CrossRef]
- Kumar, R.K.; Heuchel, M.; Kratz, K.; Lendlein, A.; Jankowski, J.; Tetali, S.D. Effects of extracts prepared from modified porous poly(ether imide) microparticulate absorbers on cytotoxicity, macrophage differentiation and proinflammatory behavior of human monocytic (THP-1) cells. Clin. Hemorheol. Microcirc. 2018, 69, 175–185. [Google Scholar] [CrossRef]
- Kumar, R.K.; Basu, S.; Lemke, H.-D.; Jankowski, J.; Kratz, K.; Lendlein, A.; Tetali, S.D. Influence of nanoporous poly(ether imide) particle extracts on human aortic endothelial cells (HAECs). Clin. Hemorheol. Microcirc. 2016, 64, 931–940. [Google Scholar] [CrossRef]
- Shawish, H.B.; Wong, W.Y.; Wong, Y.L.; Loh, S.W.; Looi, C.Y.; Hassandarvish, P.; Phan, A.Y.L.; Wong, W.F.; Wang, H.; Paterson, I.C.; et al. Nickel(II) complex of polyhydroxybenzaldehyde N4-thiosemicarbazone exhibits anti-inflammatory activity by inhibiting NF-κB transactivation. PLoS ONE 2014, 9, e100933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christopher, J.A.; Avitabile, B.G.; Bamborough, P.; Champigny, A.C.; Cutler, G.J.; Dyos, S.L.; Grace, K.G.; Kerns, J.K.; Kitson, J.D.; Mellor, G.W.; et al. The discovery of 2-amino-3,5-diarylbenzamide inhibitors of IKK-α and IKK-β kinases. Bioorganic Med. Chem. Lett. 2007, 17, 3972–3977. [Google Scholar] [CrossRef] [PubMed]
- Polley, S.; Passos, D.O.; Huang, D.-B.; Mulero, M.C.; Mazumder, A.; Biswas, T.; Verma, I.M.; Lyumkis, D.; Ghosh, G. Structural Basis for the Activation of IKK1/α. Cell Rep. 2016, 17, 1907–1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Genes | Primer Sequences | Direction of the PCR Primer |
|---|---|---|
| TNF-α | 5′-CCCAGGGACCTCTCTCTAATC-3′ | Forward |
| 5′-ATGGGCTACAGGCTTGTCACT-3′ | Reverse | |
| MCP-1 | 5′GCCAAGGAGATCTGTGCTGAC-3′ | Forward |
| 5′-CATGGAATCCTGAACCCACTTC-3′ | Reverse | |
| IL-6 | 5′-TGGATTCAATGAGGAGACTTGC-3′ | Forward |
| 5′-CAGGAACTGGATCAGGACTT-3′ | Reverse | |
| IL-8 | 5′-GTGTAAACATGACTTCCAAGCTGG-3′ | Forward |
| 5′-GCACCTTCACACAGAGCTGC-3′ | Reverse | |
| COX-2 | 5′-CAGCACTTCACGCATCAGTT-3′ | Forward |
| 5′-CGCAGTTTACGCTGTCTAGC-3′ | Reverse |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reddi, K.K.; Li, H.; Li, W.; Tetali, S.D. Berberine, A Phytoalkaloid, Inhibits Inflammatory Response Induced by LPS through NF-Kappaβ Pathway: Possible Involvement of the IKKα. Molecules 2021, 26, 4733. https://doi.org/10.3390/molecules26164733
Reddi KK, Li H, Li W, Tetali SD. Berberine, A Phytoalkaloid, Inhibits Inflammatory Response Induced by LPS through NF-Kappaβ Pathway: Possible Involvement of the IKKα. Molecules. 2021; 26(16):4733. https://doi.org/10.3390/molecules26164733
Chicago/Turabian StyleReddi, Kiran Kumar, Hanxuan Li, Wei Li, and Sarada D. Tetali. 2021. "Berberine, A Phytoalkaloid, Inhibits Inflammatory Response Induced by LPS through NF-Kappaβ Pathway: Possible Involvement of the IKKα" Molecules 26, no. 16: 4733. https://doi.org/10.3390/molecules26164733
APA StyleReddi, K. K., Li, H., Li, W., & Tetali, S. D. (2021). Berberine, A Phytoalkaloid, Inhibits Inflammatory Response Induced by LPS through NF-Kappaβ Pathway: Possible Involvement of the IKKα. Molecules, 26(16), 4733. https://doi.org/10.3390/molecules26164733