The Synthesis and Photophysical Properties of Weakly Coupled Diketopyrrolopyrroles
Abstract
1. Introduction
2. Results
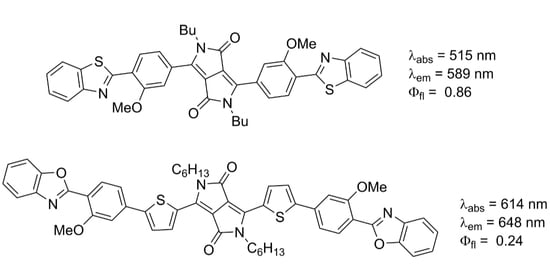
2.1. Design and Synthesis
2.2. Photophysical Properties
2.3. Theoretical Modelling
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Barzoukas, M.; Blanchard-Desce, M. Molecular engineering of push–pull dipolar and quadrupolar molecules for two-photon absorption: A multivalence-bond states approach. J. Chem. Phys. 2000, 113, 3951–3959. [Google Scholar] [CrossRef]
- Drobizhev, M.; Karotki, A.; Dzenis, Y.; Rebane, A.; Suo, Z.; Spangler, C.W. Strong Cooperative Enhancement of Two-Photon Absorption in Dendrimers. J. Phys. Chem. B 2003, 107, 7540–7543. [Google Scholar] [CrossRef]
- Meier, H. Conjugated Oligomers with Terminal Donor–Acceptor Substitution. Angew. Chem. Int. Ed. 2005, 44, 2482–2506. [Google Scholar] [CrossRef] [PubMed]
- Charlot, M.; Porrès, L.; Entwistle, C.D.; Beeby, A.; Marder, T.; Blanchard-Desce, M. Investigation of two-photon absorption behavior in symmetrical acceptor–π–acceptor derivatives with dimesitylboryl end-groups. Evidence of new engineering routes for TPA/transparency trade-off optimization. Phys. Chem. Chem. Phys. 2005, 7, 600–606. [Google Scholar] [CrossRef]
- Charlot, M.; Izard, N.; Mongin, O.; Riehl, D.; Blanchard-Desce, M. Optical limiting with soluble two-photon absorbing quad-rupoles: Structure–property relationships. Chem. Phys. Lett. 2006, 417, 297–302. [Google Scholar] [CrossRef]
- Niko, Y.; Moritomo, H.; Sugihara, H.; Suzuki, Y.; Kawamata, J.; Konishi, G.-I. A novel pyrene-based two-photon active fluo-rescent dye efficiently excited and emitting in the ‘tissue optical window (650–1100 nm)’. J. Mater. Chem. B 2015, 3, 184–190. [Google Scholar] [CrossRef]
- Tasior, M.; Bald, I.; Deperasińska, I.; Cywiński, P.J.; Gryko, D.T. An internal charge transfer-dependent solvent effect in V-shaped azacyanines. Org. Biomol. Chem. 2015, 13, 11714–11720. [Google Scholar] [CrossRef]
- Dereka, B.; Rosspeintner, A.; Li, Z.; Liska, R.; Vauthey, E. Direct visualization of excited-state symmetry breaking using ultra-fast time-resolved infrared spectroscopy. J. Am. Chem. Soc. 2016, 138, 4643–4649. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Fang, Q.; Cao, D.-X.; Wang, D.; Xu, G.-B. Triaryl Boron-Based A-π-A vs Triaryl Nitrogen-Based D-π-D Quadrupolar Compounds for Single- and Two-Photon Excited Fluorescence. Org. Lett. 2004, 6, 2933–2936. [Google Scholar] [CrossRef]
- Parent, M.; Mongin, O.; Kamada, K.; Katan, C.; Blanchard-Desce, M. New chromophores from click chemistry for two-photon absorption and tuneable photoluminescence. Chem. Commun. 2005, 2029–2031. [Google Scholar] [CrossRef]
- Kim, H.M.; Yang, W.J.; Kim, C.H.; Park, W.-H.; Jeon, S.-J.; Cho, B.R. Two-Photon Dyes Containing Heterocyclic Rings with Enhanced Photostability. Chem. A Eur. J. 2005, 11, 6386–6391. [Google Scholar] [CrossRef]
- Porrès, L.; Mongin, O.; Blanchard-Desce, M. Synthesis, fluorescence and two-photon absorption properties of multichromophoric boron-dipyrromethene fluorophores for two-photon-excited fluorescence applications. Tetrahedron Lett. 2006, 47, 1913–1917. [Google Scholar] [CrossRef][Green Version]
- Lin, T.-C.; Liu, Y.-Y.; Li, M.-H.; Liu, C.-Y.; Tseng, S.-Y.; Wang, Y.-T.; Tseng, Y.-H.; Chu, H.-H.; Luo, C.-W. Synthesis and Characterization of Two-Photon Chromophores Based on a Tetrasubstituted Tetraethynylethylene Scaffold. Chem. Asian J. 2014, 9, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Krzeszewski, M.; Thorsted, B.; Brewer, J.; Gryko, D.T. Tetraaryl-, Pentaaryl-, and Hexaaryl-1,4-dihydropyrrolo[3,2-b]pyrroles: Synthesis and Optical Properties. J. Org. Chem. 2014, 79, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Doval, D.A.; Molin, M.D.; Ward, S.; Fin, A.; Sakai, N.; Matile, S. Planarizable push–pull oligothiophenes: In search of the perfect twist. Chem. Sci. 2014, 5, 2819–2825. [Google Scholar] [CrossRef]
- Fin, A.; Jentzsch, A.V.; Sakai, N.; Matile, S. Oligothiophene Amphiphiles as Planarizable and Polarizable Fluorescent Membrane Probes. Angew. Chem. 2012, 124, 12908–12911. [Google Scholar] [CrossRef]
- Molin, M.D.; Matile, S. 3,4-Ethylenedioxythiophene in planarizable push–pull oligothiophenes. Org. Biomol. Chem. 2013, 11, 1952–1957. [Google Scholar] [CrossRef]
- Doval, D.A.; Matile, S. Increasingly twisted push–pull oligothiophenes and their planarization in confined space. Org. Biomol. Chem. 2013, 11, 7467. [Google Scholar] [CrossRef]
- Molin, M.D.; Verolet, Q.; Soleimanpour, S.; Matile, S. Mechanosensitive Membrane Probes. Chem. A Eur. J. 2015, 21, 6012–6021. [Google Scholar] [CrossRef]
- Molin, M.D.; Verolet, Q.; Colom, A.; Letrun, R.; Derivery, E.; Gonzalez-Gaitan, M.; Vauthey, E.; Roux, A.; Sakai, N.; Matile, S. Fluorescent Flippers for Mechanosensitive Membrane Probes. J. Am. Chem. Soc. 2015, 137, 568–571. [Google Scholar] [CrossRef]
- Grzybowski, M.; Gryko, D.T. Diketopyrrolopyrroles: Synthesis, Reactivity, and Optical Properties. Adv. Opt. Mater. 2015, 3, 280–320. [Google Scholar] [CrossRef]
- Qu, S.; Qin, C.; Islam, A.; Wu, Y.; Zhu, W.; Hua, J.; Tian, H.; Han, L. A novel D–A-π-A organic sensitizer containing a diketopyrrolopyrrole unit with a branched alkyl chain for highly efficient and stable dye-sensitized solar cells. Chem. Commun. 2012, 48, 6972–6974. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Tian, H. Diketopyrrolopyrrole (DPP)-based materials for organic photovoltaics. Chem. Commun. 2012, 48, 3039–3051. [Google Scholar] [CrossRef]
- Nielsen, C.B.; Turbiez, M.; McCulloch, I. Recent Advances in the Development of Semiconducting DPP-Containing Polymers for Transistor Applications. Adv. Mater. 2013, 25, 1859–1880. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-Y.; Shi, M.-M.; Huang, J.-C.; Jin, Z.-N.; Hu, X.-L.; Pan, J.-Y.; Li, H.-Y.; Jen, A.K.-Y.; Chen, H.-Z. C–H activation: Making diketopyrrolopyrrole derivatives easily accessible. J. Mater. Chem. A 2013, 1, 2795. [Google Scholar] [CrossRef]
- Jeong, Y.; Lee, C.; Jang, W. A Diketopyrrolopyrrole-Based Colorimetric and Fluorescent Probe for Cyanide Detection. Chem. Asian J. 2012, 7, 1562–1566. [Google Scholar] [CrossRef]
- Guo, E.Q.; Ren, P.H.; Zhang, Y.L.; Zhang, H.C.; Yang, W.J. Diphenylamine end-capped 1,4-diketo-3,6-diphenylpyrrolo[3,4-c]pyrrole (DPP) derivatives with large two-photon absorption cross-sections and strong two-photon excitation red fluorescence. Chem. Commun. 2009, 5859–5861. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, J.; Pop, F.; Hume, P.A.; Murphy, A.S.; Lewis, W.; Davies, E.S.; Argent, S.P.; Amabilino, D.B. Solid state structure and properties of phenyl diketopyrrolopyrrole derivatives. CrystEngComm 2021, 23, 1796–1814. [Google Scholar] [CrossRef]
- Pop, F.; Humphreys, J.; Schwarz, J.; Brown, L.; Berg, A.V.D.; Amabilino, D.B. Towards more sustainable synthesis of diketopyrrolopyrroles. New J. Chem. 2019, 43, 5783–5790. [Google Scholar] [CrossRef]
- Rochat, A.C.; Cassar, L.; Iqbal, A. Preparation of pyrrolo-(3,4-c) pyrroles. Eur. Pat. Appl. 1983. EP 0094911 A3. [Google Scholar]
- Iqbal, A.; Jost, M.; Kirchmayr, R.; Pfenninger, J.; Rochat, A.; Wallquist, O. The synthesis and properties of 1,4-diketo-pyrrolo [3,4-C] pyrroles. Bull. Soc. Chim. Belg. 1988, 97, 615–644. [Google Scholar] [CrossRef]
- Hao, Z.; Iqbal, A. Some aspects of organic pigments. Chem. Soc. Rev. 1997, 26, 203–213. [Google Scholar] [CrossRef]
- Zambounis, J.S.; Hao, Z.; Iqbal, A. Latent pigments activated by heat. Nat. Cell Biol. 1997, 388, 131–132. [Google Scholar] [CrossRef]
- Wallquist, O.; Lenz, R. 20 years of DPP pigments—Future perspectives. Macromol. Symp. 2002, 187, 617–630. [Google Scholar] [CrossRef]
- Iqbal, A.; Cassar, L.; Rochat, A.C.; Pfenninger, J.; Wallquist, O. Newheterocyclic pigments. J. Coat. Technol. 1988, 60, 37–45. [Google Scholar]
- Farnum, D.G.; Mehta, G.; Moore, G.G.; Siegal, F.P. Attempted reformatskii reaction of benzonitrile, 1,4-diketo-3,6-diphenylpyrrolo[3,4-C]pyrrole. A lactam analogue of pentalene. Tetrahedron Lett. 1974, 15, 2549–2552. [Google Scholar] [CrossRef]
- Pieczykolan, M.; Sadowski, B.; Gryko, D.T. An Efficient Method for the Programmed Synthesis of Multifunctional Diketopyrrolopyrroles. Angew. Chem. 2020, 132, 7598–7605. [Google Scholar] [CrossRef]
- Alam, M.M.; Bolze, F.; Daniel, C.; Flamigni, L.; Gourlaouen, C.; Heitz, V.; Jenni, S.; Schmitt, J.; Sour, A.; Ventura, B. π-Extended diketopyrrolopyrrole–porphyrin arrays: One- and two-photon photophysical investigations and theoretical studies. Phys. Chem. Chem. Phys. 2016, 18, 21954–21965. [Google Scholar] [CrossRef]
- Ftouni, H.; Bolze, F.; De Rocquigny, H.; Nicoud, J.-F. Functionalized Two-Photon Absorbing Diketopyrrolopyrrole-Based Fluorophores for Living Cells Fluorescent Microscopy. Bioconjugate Chem. 2013, 24, 942–950. [Google Scholar] [CrossRef]
- Grzybowski, M.; Hugues, V.; Blanchard-Desce, M.; Gryko, D.T. Two-Photon-Induced Fluorescence in New π-Expanded Diketopyrrolopyrroles. Chem. Eur. J. 2014, 20, 12493–12501. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Gao, Y.; Sreejith, S.; Tian, X.; Liu, L.; Wang, Y.; Joshi, H.; Phua, S.Z.F.; Yao, S.; Lin, X.; et al. Biocompatible Two-Photon Absorbing Dipyridyldiketopyrrolopyrroles for Metal-Ion-Mediated Self-Assembly Modulation and Fluorescence Imaging. Adv. Opt. Mater. 2016, 4, 746–755. [Google Scholar] [CrossRef]
- Grzybowski, M.; Glodkowska-Mrowka, E.; Hugues, V.; Brutkowski, W.; Blanchard-Desce, M.; Gryko, D.T. Polar Diketopyrrolopyrrole-Imidazolium Salts as Selective Probes for Staining Mitochondria in Two-Photon Fluorescence Microscopy. Chem. A Eur. J. 2015, 21, 9101–9110. [Google Scholar] [CrossRef] [PubMed]
- Purc, A.; Sobczyk, K.; Sakagami, Y.; Ando, A.; Kamada, K.; Gryko, D.T. Strategy towards large two-photon absorption cross-sections for diketopyrrolopyrroles. J. Mater. Chem. C 2015, 3, 742–749. [Google Scholar] [CrossRef]
- Heath-Apostolopoulos, I.; Vargas-Ortiz, D.; Wilbraham, L.; Jelfs, K.E.; Zwijnenburg, M.A. Using high-throughput virtual screening to explore the optoelectronic property space of organic dyes; finding diketopyrrolopyrrole dyes for dye-sensitized water splitting and solar cells. Sustain. Energy Fuels 2021, 5, 704–719. [Google Scholar] [CrossRef]
- Purc, A.; Koszarna, B.; Iachina, I.; Friese, D.H.; Tasior, M.; Sobczyk, K.; Pędziński, T.; Brewer, J.; Gryko, D.T. The impact of interplay between electronic and steric effects on the synthesis and the linear and non-linear optical properties of diketopyrrolopyrrole bearing benzofuran moieties. Org. Chem. Front. 2017, 4, 724–736. [Google Scholar] [CrossRef]
- Punzi, A.; Nicoletta, F.; Marzano, G.; Fortuna, C.G.; Dagar, J.; Brown, T.M.; Farinola, G.M. Synthetic Routes to TEG-Substituted Diketopyrrolopyrrole-Based Low Band-Gap Polymers. Eur. J. Org. Chem. 2016, 2016, 3233–3242. [Google Scholar] [CrossRef]
- Stolte, M.; Suraru, S.-L.; Diemer, P.; He, T.; Burschka, C.; Zschieschang, U.; Klauk, H.; Würthner, F. Diketopyrrolopyrrole Organic Thin-Film Transistors: Impact of Alkyl Substituents and Tolerance of Ethylhexyl Stereoisomers. Adv. Funct. Mater. 2016, 26, 7415–7422. [Google Scholar] [CrossRef]
- Tang, A.; Zhan, C.; Yao, J.; Zhou, E. Design of Diketopyrrolopyrrole (DPP)-Based Small Molecules for Organic-Solar-Cell Applications. Adv. Mater. 2017, 29, 1600013. [Google Scholar] [CrossRef]
- Zhao, C.; Guo, Y.; Zhang, Y.; Yan, N.; You, S.; Li, W. Diketopyrrolopyrrole-based conjugated materials for non-fullerene organic solar cells. J. Mater. Chem. A 2019, 7, 10174–10199. [Google Scholar] [CrossRef]
- Patil, Y.; Jadhav, T.; Dhokale, B.; Misra, R. Design and Synthesis of Low HOMO-LUMO GapN-Phenylcarbazole-Substituted Diketopyrrolopyrroles. Asian J. Org. Chem. 2016, 5, 1008–1014. [Google Scholar] [CrossRef]
- Lo, C.K.; Reynolds, J.R. Structural and morphological effects of alkyl side chains on flanking thiophenes of diketopyrrolopyrrole polymers for organic photovoltaic devices. Polymer 2016, 99, 741–747. [Google Scholar] [CrossRef]
- Tieke, B.; Rabindranath, A.R.; Zhang, K.A.I.; Zhu, Y. Conjugated polymers containing diketopyrrolopyrrole units in the main chain. Beilstein J. Org. Chem. 2010, 6, 830–845. [Google Scholar] [CrossRef]
- Wienk, M.M.; Turbiez, M.; Gilot, J.; Janssen, R.A.J. Narrow-bandgap diketo-pyrrolo-pyrrole polymer solar cells: The effect of processing on the performance. Adv. Mater. 2008, 20, 2556–2560. [Google Scholar] [CrossRef]
- Kanibolotsky, A.L.; Vilela, F.; Forgie, J.C.; Elmasly, S.E.T.; Skabara, P.J.; Zhang, K.A.I.; Tieke, B.; McGurk, J.; Belton, C.R.; Stavrinou, P.N.; et al. Well-Defined and Monodisperse Linear and Star-Shaped Quaterfluorene-DPP Molecules: The Significance of Conjugation and Dimensionality. Adv. Mater. 2011, 23, 2093–2097. [Google Scholar] [CrossRef]
- Li, Y.; Sonar, P.; Murphy, L.; Hong, W. High mobility diketopyrrolopyrrole (DPP)-based organic semiconductor materials for organic thin film transistors and photovoltaics. Energy Environ. Sci. 2013, 6, 1684–1710. [Google Scholar] [CrossRef]
- Hendriks, K.H.; Heintges, G.H.L.; Gevaerts, V.S.; Wienk, M.M.; Janssen, R.A.J. High-Molecular-Weight Regular Alternating Diketopyrrolopyrrole-based Terpolymers for Efficient Organic Solar Cells. Angew. Chem. Int. Ed. 2013, 52, 8341–8344. [Google Scholar] [CrossRef]
- Coffin, R.C.; Peet, J.; Rogers, J.; Bazan, G.C.; Peet, R. Streamlined microwave-assisted preparation of narrow-bandgap conjugated polymers for high-performance bulk heterojunction solar cells. Nat. Chem. 2009, 1, 657–661. [Google Scholar] [CrossRef]
- Li, Y.; Sonar, P.; Singh, S.P.; Ooi, Z.E.; Lek, E.S.H.; Loh, M.Q.Y. Poly (2, 5-bis (2-octyldodecyl)-3, 6-di (furan-2-yl)-2, 5-dihydro-pyrrolo [3, 4-c] pyrrole-1, 4-dione-co-thieno [3, 2-b] thiophene): A high performance polymer semiconductor for both organic thin film transistors and organic photovoltaics. Phys. Chem. Chem. Phys. 2012, 14, 7162–7169. [Google Scholar] [CrossRef]
- Sonar, P.; Singh, S.P.; Williams, E.L.; Li, Y.; Soh, M.S.; Dodabalapur, A. Furan containing diketopyrrolopyrrolecopolymers: Synthesis, characterization, organic field effect transistor performance and photovoltaic properties. J. Mater. Chem. 2012, 22, 4425–4435. [Google Scholar] [CrossRef]
- Murphy, A.S.; Killalea, C.E.; Humphreys, J.; Hume, P.A.; Cliffe, M.J.; Murray, G.J.; Davies, E.S.; Lewis, W.; Amabilino, D.B. Ground and Excited States of Bis-4-Methoxybenzyl-Substituted Diketopyrrolopyrroles: Spectroscopic and Electrochemical Studies. ChemPlusChem 2019, 84, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Ponsot, F.; Desbois, N.; Bucher, L.; Berthelot, M.; Mondal, P.; Gros, C.P.; Romieu, A. Near-infrared emissive bacteriochlorin-diketopyrrolopyrrole triads: Synthesis and photophysical properties. Dye. Pigment. 2019, 160, 747–756. [Google Scholar] [CrossRef]
- Langhals, H.; Potrawa, T.; Noth, H.; Linti, G. Der Einfluß von Packungseffekten auf die Feststoffluoreszenz von Diketopyrrolopyrrolen. Angew. Chem. 1989, 101, 497–499. [Google Scholar] [CrossRef]
- Langhals, H.; Demmig, S.; Potrawa, T. The Relation between Packing Effects and Solid State Fluorescence of Dyes. J. Prakt. Chem. 1991, 333, 733–748. [Google Scholar] [CrossRef]
- Lorenz, I.-P.; Limmert, M.; Mayer, P.; Piotrowski, H.; Langhals, H.; Poppe, M.; Polborn, K. DPP Dyes as Ligands in Transition-Metal Complexes. Chem. A Eur. J. 2002, 8, 4047–4055. [Google Scholar] [CrossRef]
- Kirkus, M.; Knippenberg, S.; Beljonne, D.; Cornil, J.; Janssen, R.A.J.; Meskers, S.C.J. Synthesis and Optical Properties of Pyrrolo[3,2-b]pyrrole-2,5(1H,4H)-dione (iDPP)-Based Molecules. J. Phys. Chem. A 2013, 117, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Walker, B.; Tamayo, A.; Zhang, Y.; Nguyen, T.-Q. Effects of Heteroatom Substitutions on the Crystal Structure, Film Formation, and Optoelectronic Properties of Diketopyrrolopyrrole-Based Materials. Adv. Funct. Mater. 2012, 23, 47–56. [Google Scholar] [CrossRef]
- Fischer, G.M.; Ehlers, A.P.; Zumbusch, A.; Daltrozzo, E. Near-Infrared Dyes and Fluorophores Based on Diketopyrrolopyrroles. Angew. Chem. Int. Ed. 2007, 46, 3750–3753. [Google Scholar] [CrossRef]
- Fischer, G.M.; Isomäki-Krondahl, M.; Göttker-Schnetmann, I.; Daltrozzo, E.; Zumbusch, A. Pyrrolopyrrole Cyanine Dyes: A New Class of Near-Infrared Dyes and Fluorophores. Chem. A Eur. J. 2009, 15, 4857–4864. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Iino, T.; Araki, Y.; Kobayashi, N. Pyrrolopyrrole aza-BODIPY analogues: A facile synthesis and intense fluorescence. Chem. Commun. 2013, 49, 1621. [Google Scholar] [CrossRef]
- Marks, T.; Daltrozzo, E.; Zumbusch, A. Azacyanines of the Pyrrolopyrrole Series. Chem. A Eur. J. 2014, 20, 6494–6504. [Google Scholar] [CrossRef]
- Yue, W.; Suraru, S.-L.; Bialas, D.; Müller, M.; Würthner, F. Synthesis and Properties of a New Class of Fully Conjugated Azahexacene Analogues. Angew. Chem. 2014, 126, 6273–6276. [Google Scholar] [CrossRef]
- Yu, J.; Li, N.; Chen, D.-F.; Luo, S.-W. Catalytic enantioselective C(sp3)-H functionalization: Intramolecular ben-zylic[1,5]-hydride shift. Tetrahedron Lett. 2014, 55, 2859–2864. [Google Scholar] [CrossRef]
- Luňák, S., Jr.; Vyňuchal, J.; Vala, M.; Havel, L.; Hrdina, R. The synthesis, absorption and fluorescence of polar diketo-pyrrolo-pyrroles. Dyes Pigm. 2009, 82, 102–108. [Google Scholar] [CrossRef]
- Vala, M.; Krajčovič, J.; Luňák, S., Jr.; Ouzzane, I.; Bouillon, J.-P.; Weiter, M. HOMO and LUMO energy levels of N, N′-dinitrophenyl-substituted polar diketopyrrolopyrroles (DPPs). Dyes Pigm. 2014, 106, 136–142. [Google Scholar] [CrossRef]
- Vala, M.; Weiter, M.; Vyňuchal, J.; Toman, P.; Lunák, S. Comparative Studies of Diphenyl-Diketo-Pyrrolopyrrole Derivatives for Electroluminescence Applications. J. Fluoresc. 2008, 18, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Luňák, S., Jr.; Vala, M.; Vyňuchal, J.; Ouzzane, I.; Horáková, P.; Možíšková, P.; Eliáš, Z.; Weiter, M. Absorption and fluorescence of soluble polar diketo-pyrrolo-pyrroles. Dye. Pigm. 2011, 91, 269–278. [Google Scholar] [CrossRef]
- Kanbara, T.; Yamagata, T.; Kuwabara, J. Synthesis and Photophysical Properties of Diketopyrrolopyrrole-Based Near-Infrared Dyes. Heterocycles 2014, 89, 1173. [Google Scholar] [CrossRef]
- Zhang, L.; Zou, L.-Y.; Guo, J.-F.; Ren, A.-M. Theoretical investigation on the one-and two-photon responsive behavior of fluo-ride ion probes based on diketopyrrolopyrrole and its π-expanded derivatives. New J. Chem. 2016, 40, 4899–4910. [Google Scholar] [CrossRef]
- Bürckstümmer, H.; Weissenstein, A.; Bialas, D.; Würthner, F. Synthesis and Characterization of Optical and Redox Properties of Bithiophene-Functionalized Diketopyrrolopyrrole Chromophores. J. Org. Chem. 2011, 76, 2426–2432. [Google Scholar] [CrossRef]
- Zhang, K.; Wucher, P.; Marszalek, T.; Babics, M.; Ringk, A.; Blom, P.W.M.; Beaujuge, P.M.; Pisula, W. Long-Range Molecular Self-Assembly from π-Extended Pyrene-Functionalized Diketopyrrolopyrroles. Chem. Mater. 2018, 30, 5032–5040. [Google Scholar] [CrossRef]
- Patil, Y.; Misra, R. Rational molecular design towards NIR absorption: Efficient diketopyrrolopyrrole derivatives for organic solar cells and photothermal therapy. J. Mater. Chem. C 2019, 7, 13020–13031. [Google Scholar] [CrossRef]
- Dhar, J.; Venkatramaiah, N.; Patil, S. Photophysical, electrochemical and solid state properties of diketopyrrolopyrrole based molecular materials: Importance of the donor group. J. Mater. Chem. C 2014, 2, 3457–3466. [Google Scholar] [CrossRef]
- Canivet, J.; Yamaguchi, J.; Ban, I.; Itami, K. Nickel-Catalyzed Biaryl Coupling of Heteroarenes and Aryl Halides/Triflates. Org. Lett. 2009, 11, 1733–1736. [Google Scholar] [CrossRef]
- Ackermann, L.; Vicente, R.; Kapdi, A.R. Transition-Metal-Catalyzed Direct Arylation of (Hetero)Arenes by C-H Bond Cleavage. Angew. Chem. Int. Ed. 2009, 49, 9792–9826. [Google Scholar] [CrossRef] [PubMed]
- Cardoza, S.; Shrivash, M.K.; Das, P.; Tandon, V. Strategic Advances in Sequential C-Arylations of Heteroarenes. J. Org. Chem. 2021, 86, 1330–1356. [Google Scholar] [CrossRef]
- Zhang, J.; Kang, D.-Y.; Barlow, S.; Marder, S.R. Transition metal-catalyzed C–H activation as a route to structurally diverse di(arylthiophenyl)-diketopyrrolopyrroles. J. Mater. Chem. 2012, 22, 21392–21394. [Google Scholar] [CrossRef]
- Wakioka, M.; Takahashi, R.; Ichihara, N.; Ozawa, F. Mixed-Ligand Approach to Palladium-Catalyzed Direct Arylation Polymerization: Highly Selective Synthesis of π-Conjugated Polymers with Diketopyrrolopyrrole Units. Macromolecules 2017, 50, 927–934. [Google Scholar] [CrossRef]
- Ni, Z.; Wang, H.; Dong, H.; Dang, Y.; Zhao, Q.; Zhang, X.; Hu, W. Mesopolymer synthesis by ligand-modulated direct arylation polycondensation towards n-type and ambipolar conjugated systems. Nat. Chem. 2019, 11, 271–277. [Google Scholar] [CrossRef]
- Hagui, W.; Doucet, H.; Soulé, J.-F. Application of Palladium-Catalyzed C(sp2)–H Bond Arylation to the Synthesis of Polycyclic (Hetero)Aromatics. Chem 2019, 5, 2006–2078. [Google Scholar] [CrossRef]
- Roger, J.; Požgan, F.; Doucet, H. Ligand-Free Palladium-Catalyzed Direct Arylation of Thiazoles at Low Catalyst Loadings. J. Org. Chem. 2009, 74, 1179–1186. [Google Scholar] [CrossRef]
- Purc, A.; Espinoza, E.M.; Nazir, R.; Romero, J.J.; Skonieczny, K.; Jeżewski, A.; Larsen, J.M.; Gryko, D.T.; Vullev, V.I. Gating That Suppresses Charge Recombination–The Role of Mono-N-Arylated Diketopyrrolopyrrole. J. Am. Chem. Soc. 2016, 138, 12826–12832. [Google Scholar] [CrossRef] [PubMed]
- Krzeszewski, M.; Espinoza, E.M.; Červinka, C.; Derr, J.B.; Clark, J.A.; Borchardt, D.; Beran, G.J.O.; Gryko, D.T.; Vullev, V.I. Dipole Effects on Charge Transfer are Enormous. Angew. Chem. Int. Ed. 2018, 57, 12365–12369. [Google Scholar] [CrossRef]
- Ryu, H.G.; Mayther, M.F.; Tamayo, J.; Azarias, C.; Espinoza, E.M.; Banasiewicz, M.; Łukasiewicz, Ł.G.; Poronik, Y.M.; Jeżewski, A.; Clark, J.; et al. Bidirectional Solvatofluorochromism of a Pyrrolo[3,2-b]pyrrole–Diketopyrrolopyrrole Hybrid. J. Phys. Chem. C 2017, 122, 13424–13434. [Google Scholar] [CrossRef]
- McCusker, C.E.; Hablot, D.; Ziessel, R.; Castellano, F.N. Triplet State Formation in Homo- and Heterometallic Diketo-pyrrolopyrrole Chromophores. Inorg. Chem. 2014, 53, 12564–12571. [Google Scholar] [CrossRef] [PubMed]
- McCusker, C.E.; Hablot, D.; Ziessel, R.; Castellano, F.N. Metal Coordination Induced π-Extension and Triplet State Produc-tion in Diketopyrrolopyrrole Chromophores. Inorg. Chem. 2012, 51, 7957–7959. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, A.B.; Tantiwiwat, M.; Walker, B.; Nguyen, T.-Q. Design, Synthesis, and Self-assembly of Oligothiophene Derivatives with a Diketopyrrolopyrrole Core. J. Phys. Chem. C 2008, 112, 15543–15552. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, non-covalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class func-tionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]









| DPP | Solvent | λabs (nm) | λem(nm) | Φfl | (ns) | kf × 107/s−1 | knr × 107/s−1 |
|---|---|---|---|---|---|---|---|
| 5 | TOL | 528 | 593 | 0.89 | 4.16 | 21.1 | 2.95 |
| DCM | 515 | 589 | 0.86 | 4.35 | 19.7 | 3.27 | |
| DMF | 512 | 591 | 0.72 | 4.39 | 16.0 | 6.09 | |
| 13 | TOL | 622 | 655 | 0.31 | 2.87 | 10.8 | 24.1 |
| DCM | 614 | 648 | 0.24 | 2.94 | 8.23 | 25.8 | |
| DMF | 619 | 645 | 0.25 | 2.71 | 9.83 | 27.6 | |
| 15 | TOL | 611 | 635 | 0.34 | 3.35 | 10.1 | 19.7 |
| DCM | 606 | 633 | 0.32 | 3.23 | 9.92 | 21.0 | |
| DMF | 610 | 633 | 0.19 | 3.10 | 6.26 | 26.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieczykolan, M.; Derr, J.B.; Chrayteh, A.; Koszarna, B.; Clark, J.A.; Vakuliuk, O.; Jacquemin, D.; Vullev, V.I.; Gryko, D.T. The Synthesis and Photophysical Properties of Weakly Coupled Diketopyrrolopyrroles. Molecules 2021, 26, 4744. https://doi.org/10.3390/molecules26164744
Pieczykolan M, Derr JB, Chrayteh A, Koszarna B, Clark JA, Vakuliuk O, Jacquemin D, Vullev VI, Gryko DT. The Synthesis and Photophysical Properties of Weakly Coupled Diketopyrrolopyrroles. Molecules. 2021; 26(16):4744. https://doi.org/10.3390/molecules26164744
Chicago/Turabian StylePieczykolan, Michał, James B. Derr, Amara Chrayteh, Beata Koszarna, John A. Clark, Olena Vakuliuk, Denis Jacquemin, Valentine I. Vullev, and Daniel T. Gryko. 2021. "The Synthesis and Photophysical Properties of Weakly Coupled Diketopyrrolopyrroles" Molecules 26, no. 16: 4744. https://doi.org/10.3390/molecules26164744
APA StylePieczykolan, M., Derr, J. B., Chrayteh, A., Koszarna, B., Clark, J. A., Vakuliuk, O., Jacquemin, D., Vullev, V. I., & Gryko, D. T. (2021). The Synthesis and Photophysical Properties of Weakly Coupled Diketopyrrolopyrroles. Molecules, 26(16), 4744. https://doi.org/10.3390/molecules26164744