Recent Advances in the Synthesis of Ibuprofen and Naproxen
Abstract
:1. Introduction
2. Classical Synthesis of Ibuprofen
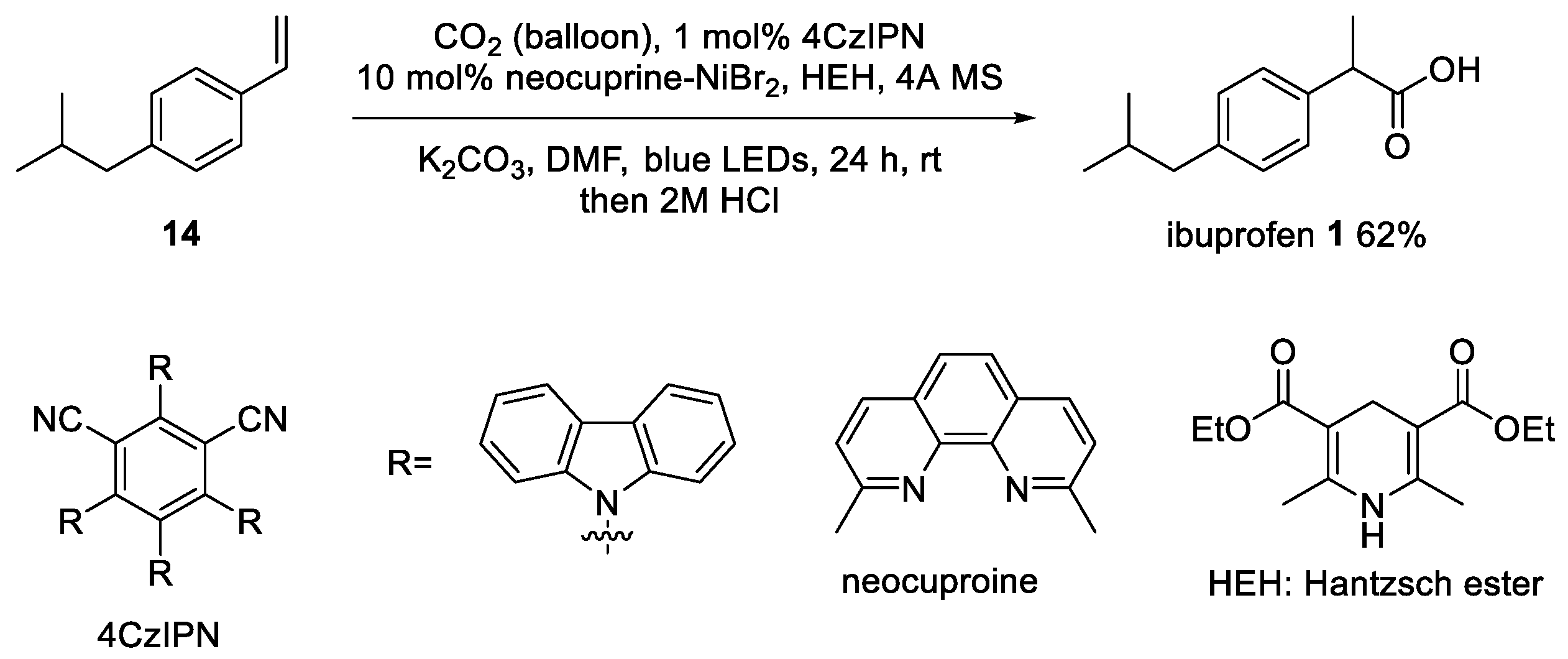
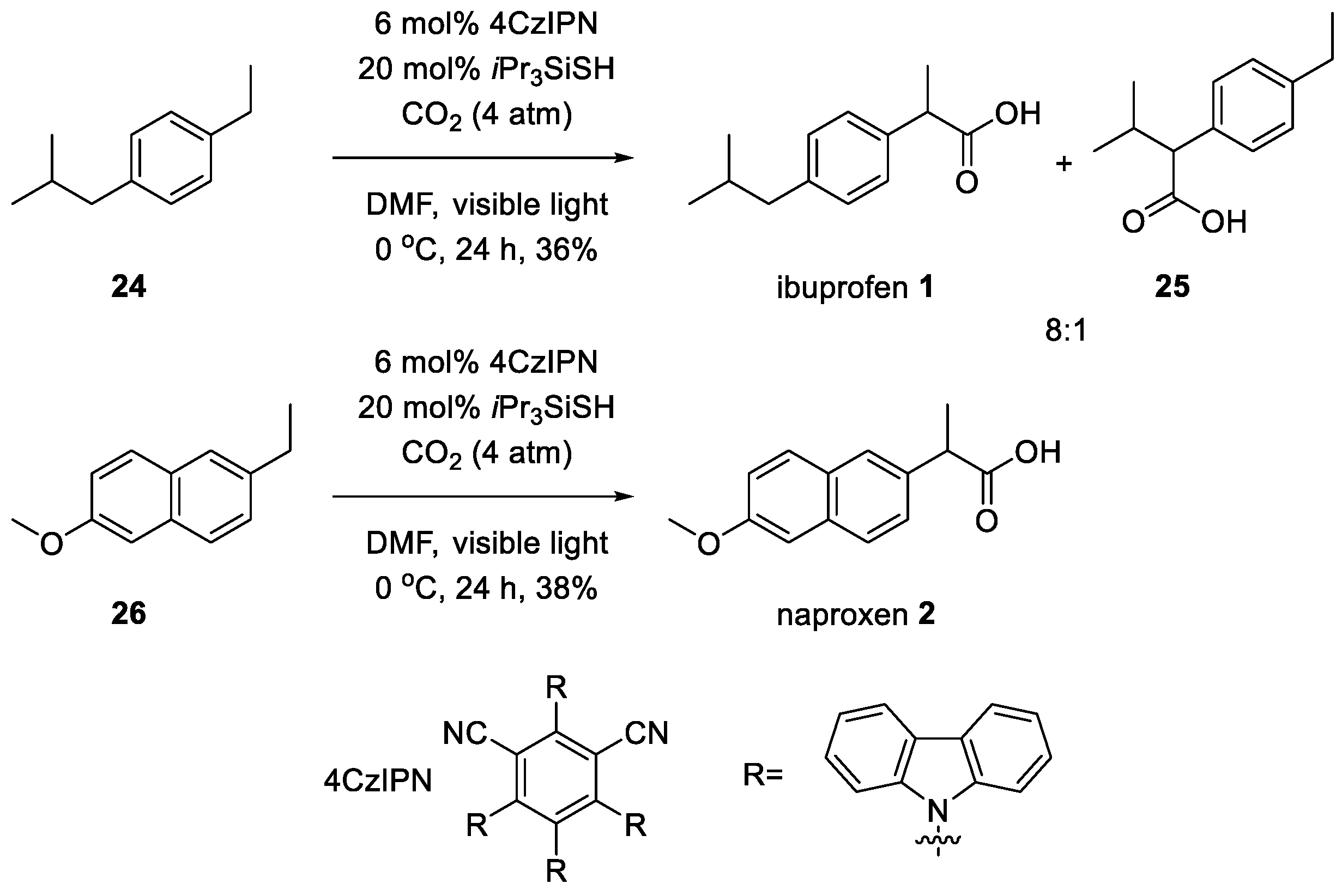
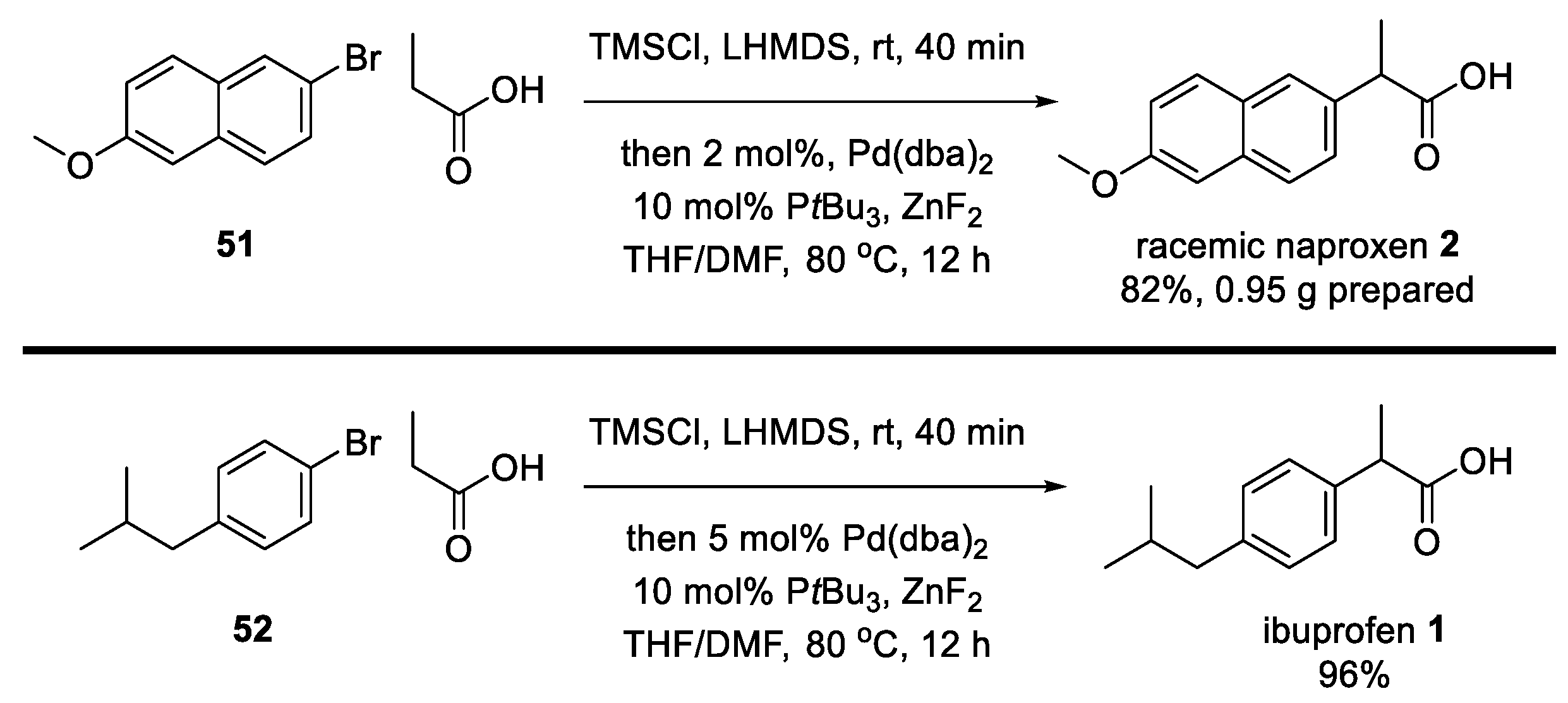
3. Recent Synthetic Advances in Ibuprofen
4. Classical Synthesis of Naproxen
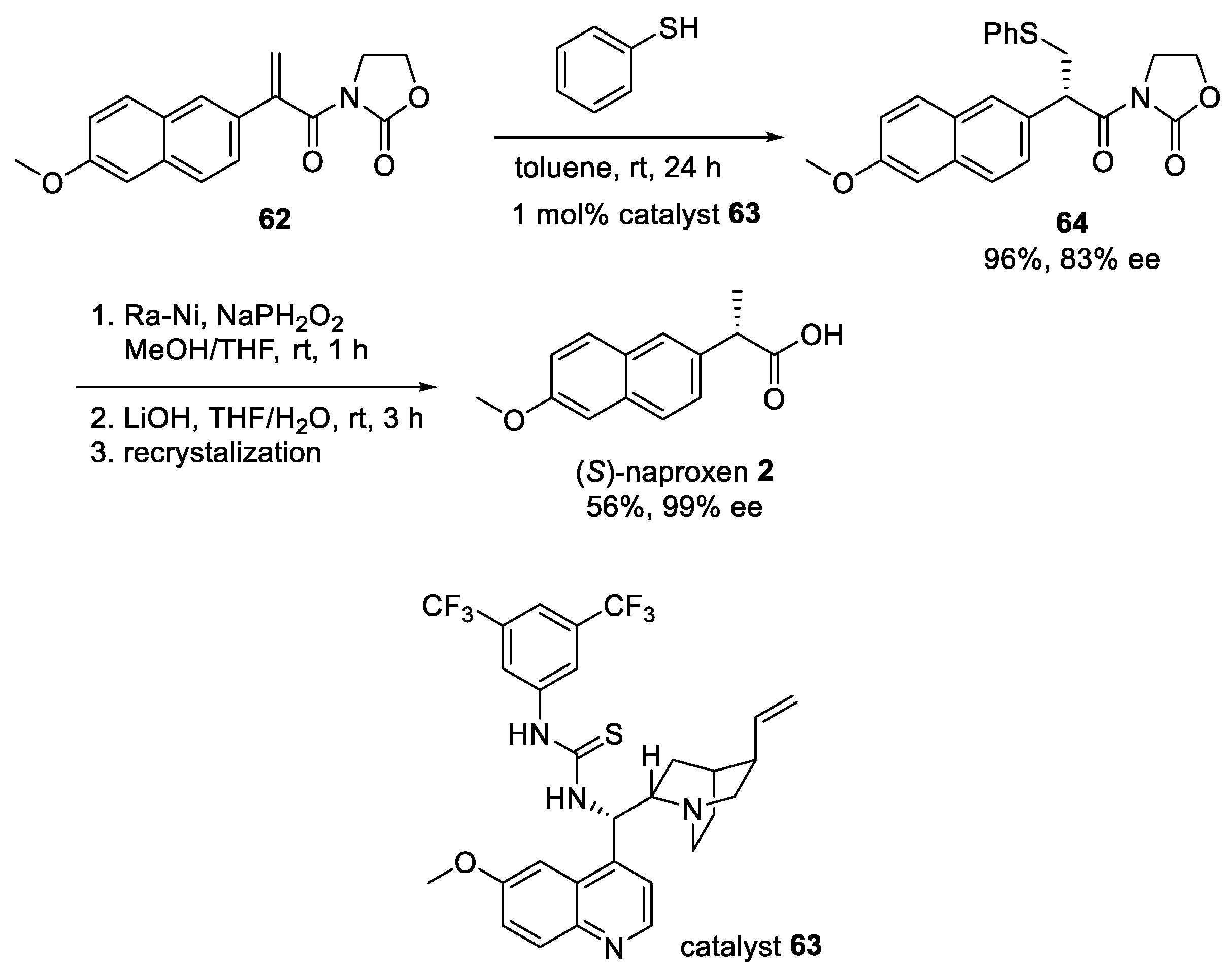
5. Recent Synthetic Advances in Naproxen
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Abdulkhaleq, L.; Assi, M.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.; Hezmee, M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marelli, G.; Sica, A.; Vannucci, L.; Allavena, P. Inflammation as target in cancer therapy. Curr. Opin. Pharmacol. 2017, 35, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.O.F.; Lee, H.J. Synthesis and pharmacology of anti-inflammatory steroidal antedrugs. Chem. Rev. 2008, 108, 5131–5145. [Google Scholar] [CrossRef] [Green Version]
- Abraham, A.; Roga, G. Topical steroid-damaged skin. Indian J. Dermatol. 2014, 59, 456. [Google Scholar] [CrossRef]
- Baggish, A.L.; Weiner, R.B.; Kanayama, G.; Hudson, J.I.; Lu, M.T.; Hoffmann, U.; Pope, H.G., Jr. Cardiovascular toxicity of illicit anabolic-androgenic steroid use. Circulation 2017, 135, 1991–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desborough, M.J.; Keeling, D.M. The aspirin story–from willow to wonder drug. Br. J. Haematol. 2017, 177, 674–683. [Google Scholar] [CrossRef] [Green Version]
- Vane, J.; Botting, R. The mechanism of action of aspirin. Thromb. Res. 2003, 110, 255–258. [Google Scholar] [CrossRef]
- Lanza, F. A review of gastric ulcer and gastroduodenal injury in normal volunteers receiving aspirin and other non-steroidal anti-inflammatory drugs. Scand. J. Gastroentero. 1989, 24 (Suppl. 163), 24–31. [Google Scholar] [CrossRef]
- Abdel-Tawab, M.; Zettl, H.; Schubert-Zsilavecz, M. Nonsteroidal anti-inflammatory drugs: A critical review on current concepts applied to reduce gastrointestinal toxicity. Curr. Med. Chem. 2009, 16, 2042–2063. [Google Scholar] [CrossRef]
- Garcia Rodriguez, L.A.; Martín-Pérez, M.; Hennekens, C.H.; Rothwell, P.M.; Lanas, A. Bleeding risk with long-term low-dose aspirin: A systematic review of observational studies. PLoS ONE 2016, 11, e0160046. [Google Scholar] [CrossRef]
- Flower, R.J. The development of COX2 inhibitors. Nat. Rev. Drug Discov. 2003, 2, 179–191. [Google Scholar] [CrossRef]
- Rao, P.P.; Kabir, S.N.; Mohamed, T. Nonsteroidal anti-inflammatory drugs (NSAIDs): Progress in small molecule drug development. Pharmaceuticals 2010, 3, 1530–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buer, J.K. Origins and impact of the term ‘NSAID’. Inflammopharmacology 2014, 22, 263–267. [Google Scholar] [CrossRef]
- Michaelidou, A.; Hadjipavlou-Litina, D. Nonsteroidal anti-inflammatory drugs (NSAIDs): A comparative QSAR study. Chem. Rev. 2005, 105, 3235–3271. [Google Scholar] [CrossRef]
- Rouzer, C.A.; Marnett, L.J. Structural and chemical biology of the interaction of cyclooxygenase with substrates and non-steroidal anti-inflammatory drugs. Chem. Rev. 2020, 120, 7592–7641. [Google Scholar] [CrossRef]
- Chawla, G.; Ranjan, C.; Kumar, J.; Siddiqui, A.A. Chemical modifications of ketoprofen (NSAID) in search of better lead compounds: A review of literature from 2004–2016. Antiinflamm. Antiallergy Agents Med. Chem. 2016, 15, 154–177. [Google Scholar] [CrossRef]
- Rainsford, K. History and Development of Ibuprofen. In Ibuprofen; CRC Press: London, UK, 1999; pp. 1–24. [Google Scholar]
- Ibuprofen Market Research Report 2020. Available online: https://menafn.com/1099915665/Ibuprofen-Market-Research-Report-2020 (accessed on 5 July 2021).
- The Top 300 of 2021. Available online: https://clincalc.com/DrugStats/Top300Drugs.aspx (accessed on 5 July 2021).
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Acetti, D.; Brenna, E.; Fronza, G.; Fuganti, C. Monitoring the synthetic procedures of commercial drugs by 2H NMR spectroscopy: The case of ibuprofen and naproxen. Talanta 2008, 76, 651–655. [Google Scholar] [CrossRef]
- Speight, J.G. Handbook of Industrial Hydrocarbon Processes; Gulf Professional Publishing: Houston, TX, USA, 2019. [Google Scholar]
- Mason, B.P.; Price, K.E.; Steinbacher, J.L.; Bogdan, A.R.; McQuade, D.T. Greener approaches to organic synthesis using microreactor technology. Chem. Rev. 2007, 107, 2300–2318. [Google Scholar] [CrossRef]
- Bogdan, A.R.; Poe, S.L.; Kubis, D.C.; Broadwater, S.J.; McQuade, D.T. The continuous-flow synthesis of ibuprofen. Angew. Chem. 2009, 121, 8699–8702. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, H.; Kim, D.P. From p-Xylene to Ibuprofen in Flow: Three-Step Synthesis by a Unified Sequence of Chemoselective C− H Metalations. Chem. Eur. J. 2019, 25, 11641–11645. [Google Scholar] [CrossRef]
- Del Río, I.; Claver, C.; van Leeuwen, P.W. On the mechanism of the hydroxycarbonylation of styrene with palladium systems. Eur. J. Inorg. Chem. 2001, 2001, 2719–2738. [Google Scholar] [CrossRef]
- Greenhalgh, M.D.; Thomas, S.P. Iron-catalyzed, highly regioselective synthesis of α-aryl carboxylic acids from styrene derivatives and CO2. J. Am. Chem. Soc. 2012, 134, 11900–11903. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Park, G.D.; Balamurugan, M.; Seo, J.; Min, B.K.; Nam, K.T. Electrochemical β-Selective Hydrocarboxylation of Styrene Using CO2 and Water. Adv. Sci. 2020, 7, 1900137. [Google Scholar] [CrossRef] [Green Version]
- Shao, P.; Wang, S.; Chen, C.; Xi, C. Cp2TiCl2-catalyzed regioselective hydrocarboxylation of alkenes with CO2. Org. Lett. 2016, 18, 2050–2053. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Cheng, Y.; Chen, X.; Wang, H.-F.; Du, C.-X.; Li, Y. Regioselectivity inversion tuned by iron (iii) salts in palladium-catalyzed carbonylations. Chem. Commun. 2018, 54, 3967–3970. [Google Scholar] [CrossRef]
- Meng, Q.-Y.; Wang, S.; Huff, G.S.; König, B. Ligand-controlled regioselective hydrocarboxylation of styrenes with CO2 by combining visible light and nickel catalysis. J. Am. Chem. Soc. 2018, 140, 3198–3201. [Google Scholar] [CrossRef]
- Twilton, J.; Zhang, P.; Shaw, M.H.; Evans, R.W.; MacMillan, D.W. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 2017, 1, 0052. [Google Scholar] [CrossRef]
- Karkas, M.D.; Porco, J.A., Jr.; Stephenson, C.R. Photochemical approaches to complex chemotypes: Applications in natural product synthesis. Chem. Rev. 2016, 116, 9683–9747. [Google Scholar] [CrossRef]
- Hoffmann, N. Photochemical reactions as key steps in organic synthesis. Chem. Rev. 2008, 108, 1052–1103. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.-L.; Cao, G.-M.; Ye, J.-H.; Sun, G.-Q.; Zhou, W.-J.; Gui, Y.-Y.; Yan, S.-S.; Shen, G.; Yu, D.-G. Visible-light-driven external-reductant-free cross-electrophile couplings of tetraalkyl ammonium salts. J. Am. Chem. Soc. 2018, 140, 17338–17342. [Google Scholar] [CrossRef]
- Hedstrand, D.M.; Kruizinga, W.H.; Kellogg, R.M. Light induced and dye accelerated reductions of phenacyl onium salts by 1, 4-dihydropyridines. Tetrahedron Lett. 1978, 19, 1255–1258. [Google Scholar] [CrossRef]
- Wu, J.; He, L.; Noble, A.; Aggarwal, V.K. Photoinduced deaminative borylation of alkylamines. J. Am. Chem. Soc. 2018, 140, 10700–10704. [Google Scholar] [CrossRef] [Green Version]
- Klauck, F.J.; James, M.J.; Glorius, F. Deaminative Strategy for the Visible-Light-Mediated Generation of Alkyl Radicals. Angew. Chem. Int. Ed. 2017, 56, 12336–12339. [Google Scholar] [CrossRef]
- Yang, D.-T.; Zhu, M.; Schiffer, Z.J.; Williams, K.; Song, X.; Liu, X.; Manthiram, K. Direct electrochemical carboxylation of benzylic C–N bonds with carbon dioxide. ACS Catalysis 2019, 9, 4699–4705. [Google Scholar] [CrossRef]
- Moragas, T.; Gaydou, M.; Martin, R. Nickel-Catalyzed Carboxylation of Benzylic C-N Bonds with CO2. Angew. Chem. Int. Ed. 2016, 55, 5053–5057. [Google Scholar] [CrossRef]
- Yoshida, J.-i.; Kataoka, K.; Horcajada, R.; Nagaki, A. Modern strategies in electroorganic synthesis. Chem. Rrev. 2008, 108, 2265–2299. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.A. Early Industrial Roots of Green Chemistry and the history of the BHC Ibuprofen process invention and its Quality connection. Found. Chem. 2018, 20, 121–165. [Google Scholar] [CrossRef] [Green Version]
- Ishida, N.; Masuda, Y.; Uemoto, S.; Murakami, M. A light/ketone/copper system for carboxylation of allylic C-H bonds of alkenes with CO2. Chem. Eur. J. 2016, 22, 6524–6527. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.Y.; Zhou, W.J.; Ye, J.H.; Yu, D.G. Photochemical carboxylation of activated C (sp3)− H bonds with CO2. ChemSusChem 2017, 10, 1337–1340. [Google Scholar] [CrossRef] [PubMed]
- Michigami, K.; Mita, T.; Sato, Y. Cobalt-catalyzed allylic C (sp3)–H carboxylation with CO2. J. Am. Chem. Soc. 2017, 139, 6094–6097. [Google Scholar] [CrossRef]
- Meng, Q.-Y.; Schirmer, T.E.; Berger, A.L.; Donabauer, K.; König, B. Photocarboxylation of benzylic C–H bonds. J. Am. Chem. Soc. 2019, 141, 11393–11397. [Google Scholar] [CrossRef] [PubMed]
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 2012, 492, 234–238. [Google Scholar] [CrossRef]
- Gong, W.; Liu, Y.; Xue, J.; Xie, Z.; Li, Y. Unexpected Extension of Usage of PPh3/CBr4, a Versatile Reagent: Isomerization of Aromatic Allylic Alcohols. Chem. Lett. 2012, 41, 1597–1599. [Google Scholar] [CrossRef]
- Li, J.J. Appel Reaction. In Name Reactions: A Collection of Detailed Reaction Mechanisms; Springer: Berlin/Heidelberg, Germany, 2006; pp. 10–11. [Google Scholar]
- Wong, B.; Linghu, X.; Crawford, J.J.; Drobnick, J.; Lee, W.; Zhang, H. A chemoselective Reformatsky–Negishi approach to α-haloaryl esters. Tetrahedron 2014, 70, 1508–1515. [Google Scholar] [CrossRef]
- Li, C.; Chen, H.; Li, J.; Li, M.; Liao, J.; Wu, W.; Jiang, H. Palladium-Catalyzed Regioselective Aerobic Allylic C− H Oxygenation: Direct Synthesis of α, β-Unsaturated Aldehydes and Allylic Alcohols. Adv. Synth. Catal. 2018, 360, 1600–1604. [Google Scholar] [CrossRef]
- Chen, H.; Sun, S.; Liu, Y.A.; Liao, X. Nickel-catalyzed cyanation of aryl halides and hydrocyanation of alkynes via C-CN bond cleavage and cyano transfer. ACS Catal. 2019, 10, 1397–1405. [Google Scholar] [CrossRef]
- Yao, Y.-H.; Yang, H.-Y.; Chen, M.; Wu, F.; Xu, X.-X.; Guan, Z.-H. Asymmetric Markovnikov hydroaminocarbonylation of alkenes enabled by palladium-monodentate phosphoramidite catalysis. J. Am. Chem. Soc. 2021, 143, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Kempe, M.; Mosbach, K. Direct resolution of naproxen on a non-covalently molecularly imprinted chiral stationary phase. J. Chromatogr. A 1994, 664, 276–279. [Google Scholar] [CrossRef]
- Wan, K.T.; Davis, M.E. Asymmetric synthesis of naproxen by a new heterogeneous catalyst. J. Catal. 1995, 152, 25–30. [Google Scholar] [CrossRef]
- Wan, K.T.; Davis, M.E. Asymmetric synthesis of naproxen by supported aqueous-phase catalysis. J. Catal. 1994, 148, 1–8. [Google Scholar] [CrossRef]
- Brown, J.D. Asymmetric synthesis of naproxen via a pinacol-type reaction. Tetrahedron-Asymmetry 1992, 3, 1551–1552. [Google Scholar] [CrossRef]
- Muñoz-Muñiz, O.; Juaristi, E. Enantioselective protonation of prochiral enolates in the asymmetric synthesis of (S)-naproxen. Tetrahedron Lett. 2003, 44, 2023–2026. [Google Scholar] [CrossRef]
- Poulsen, T.B.; Jørgensen, K.A. Catalytic Asymmetric Friedel–Crafts Alkylation Reactions Copper Showed the Way. Chem. Rev. 2008, 108, 2903–2915. [Google Scholar] [CrossRef] [PubMed]
- Priebbenow, D.L.; Bolm, C. Recent advances in the Willgerodt–Kindler reaction. Chem. Soc. Rev. 2013, 42, 7870–7880. [Google Scholar] [CrossRef]
- Harrington, P.J.; Lodewijk, E. Twenty years of naproxen technology. Org. Process. Res. Dev. 1997, 1, 72–76. [Google Scholar] [CrossRef]
- Stivala, C.E.; Zakarian, A. Highly enantioselective direct alkylation of arylacetic acids with chiral lithium amides as traceless auxiliaries. J. Am. Chem. Soc. 2011, 133, 11936–11939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz-Muñoz, G.; Miranda, I.L.; Sartori, S.K.; de Rezende, D.C.; Alves Nogueira Diaz, M. Use of chiral auxiliaries in the asymmetric synthesis of biologically active compounds: A review. Chirality 2019, 31, 776–812. [Google Scholar] [CrossRef]
- Frizzle, M.J.; Caille, S.; Marshall, T.L.; McRae, K.; Nadeau, K.; Guo, G.; Wu, S.; Martinelli, M.J.; Moniz, G.A. Dynamic biphasic counterion exchange in a configurationally stable aziridinium ion: Efficient synthesis and isolation of a Koga C2-symmetric tetraamine base. Org. Process. Res. Dev. 2007, 11, 215–222. [Google Scholar] [CrossRef]
- Harvey, J.S.; Simonovich, S.P.; Jamison, C.R.; MacMillan, D.W. Enantioselective α-arylation of carbonyls via Cu (I)-bisoxazoline catalysis. J. Am. Chem. Soc. 2011, 133, 13782–13785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, R.; Buchwald, S.L. A General Method for the Direct α-Arylation of Aldehydes with Aryl Bromides and Chlorides. Angew. Chem. 2007, 119, 7374–7377. [Google Scholar] [CrossRef]
- Allen, A.E.; MacMillan, D.W. Enantioselective α-arylation of aldehydes via the productive merger of iodonium salts and organocatalysis. J. Am. Chem. Soc. 2011, 133, 4260–4263. [Google Scholar] [CrossRef] [Green Version]
- Mazet, C. Challenges and achievements in the transition-metal-catalyzed asymmetric α-arylation of aldehydes. Synlett 2012, 23, 1999–2004. [Google Scholar] [CrossRef]
- Bigot, A.; Williamson, A.E.; Gaunt, M.J. Enantioselective α-arylation of N-acyloxazolidinones with copper (II)-bisoxazoline catalysts and diaryliodonium salts. J. Am. Chem. Soc. 2011, 133, 13778–13781. [Google Scholar] [CrossRef] [PubMed]
- Merritt, E.A.; Olofsson, B. Diaryliodonium salts: A journey from obscurity to fame. Angew. Chem. Int. Ed. 2009, 48, 9052–9070. [Google Scholar] [CrossRef] [PubMed]
- Phipps, R.J.; Grimster, N.P.; Gaunt, M.J. Cu (II)-catalyzed direct and site-selective arylation of indoles under mild conditions. J. Am. Chem. Soc. 2008, 130, 8172–8174. [Google Scholar] [CrossRef] [PubMed]
- Bielawski, M.; Aili, D.; Olofsson, B. Regiospecific one-pot synthesis of diaryliodonium tetrafluoroborates from arylboronic acids and aryl iodides. J. Org. Chem. 2008, 73, 4602–4607. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, J.S. A general method for asymmetric arylation and vinylation of silyl ketene acetals. Org. Chem. Front. 2014, 1, 365–367. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Z.; Zhou, J. An enantioselective, intermolecular α-arylation of ester enolates to form tertiary stereocenters. J. Am. Chem. Soc. 2011, 133, 15882–15885. [Google Scholar] [CrossRef]
- Lloyd-Jones, G.C. Palladium-Catalyzed α-Arylation of Esters: Ideal New Methodology for Discovery Chemistry. Angew. Chem. Int. Ed. 2002, 41, 953–956. [Google Scholar] [CrossRef]
- Johansson, C.C.; Colacot, T.J. Metal-Catalyzed α-Arylation of Carbonyl and Related Molecules: Novel Trends in C-C Bond Formation by C-H Bond Functionalization. Angew. Chem. Int. Ed. 2010, 49, 676–707. [Google Scholar] [CrossRef] [PubMed]
- Bellina, F.; Rossi, R. Transition metal-catalyzed direct arylation of substrates with activated sp3-hybridized C− H bonds and some of their synthetic equivalents with aryl halides and pseudohalides. Chem. Rev. 2010, 110, 1082–1146. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hartwig, J.F. Palladium-catalyzed arylation of trimethylsilyl enolates of esters and imides. High functional group tolerance and stereoselective synthesis of α-aryl carboxylic acid derivatives. J. Am. Chem. Soc. 2004, 126, 5182–5191. [Google Scholar] [CrossRef]
- He, Z.-T.; Hartwig, J.F. Palladium-catalyzed α-arylation of carboxylic acids and secondary amides via a traceless protecting strategy. J. Am. Chem. Soc. 2019, 141, 11749–11753. [Google Scholar] [CrossRef] [Green Version]
- Netherton, M.R.; Fu, G.C. Nickel-catalyzed cross-couplings of unactivated alkyl halides and pseudohalides with organometallic compounds. Adv. Synth. Catal. 2004, 346, 1525–1532. [Google Scholar] [CrossRef]
- Rudolph, A.; Lautens, M. Secondary Alkyl Halides in Transition-Metal-Catalyzed Cross-Coupling Reactions. Angew. Chem. Int. Ed. 2009, 48, 2656–2670. [Google Scholar] [CrossRef]
- Nakamura, E.; Hatakeyama, T.; Ito, S.; Ishizuka, K.; Ilies, L.; Nakamura, M. Iron-Catalyzed Cross-Coupling Reactions. Organic Reactions 2013, 83, 1–210. [Google Scholar]
- Czaplik, W.M.; Mayer, M.; Cvengroš, J.; von Wangelin, A.J. Coming of Age: Sustainable Iron-Catalyzed Cross-Coupling Reactions. ChemSusChem 2009, 2, 396–417. [Google Scholar] [CrossRef] [PubMed]
- Sherry, B.D.; Fürstner, A. The promise and challenge of iron-catalyzed cross coupling. Acc. Chem. Res. 2008, 41, 1500–1511. [Google Scholar] [CrossRef]
- Jin, M.; Adak, L.; Nakamura, M. Iron-catalyzed enantioselective cross-coupling reactions of α-chloroesters with Aryl grignard reagents. J. Am. Chem. Soc. 2015, 137, 7128–7134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwamoto, T.; Okuzono, C.; Adak, L.; Jin, M.; Nakamura, M. Iron-catalysed enantioselective Suzuki–Miyaura coupling of racemic alkyl bromides. Chem. Commun. 2019, 55, 1128–1131. [Google Scholar] [CrossRef]
- Rana, N.K.; Singh, V.K. Enantioselective Enolate Protonation in Sulfa–Michael Addition to α-Substituted N-Acryloyloxazolidin-2-ones with Bifunctional Organocatalyst. Org. Lett. 2011, 13, 6520–6523. [Google Scholar] [CrossRef]
- Lee, Y.; Hoveyda, A.H. Efficient boron−copper additions to aryl-substituted alkenes promoted by NHC−based catalysts. Enantioselective Cu-catalyzed hydroboration reactions. J. Am. Chem. Soc. 2009, 131, 3160–3161. [Google Scholar] [CrossRef] [Green Version]
- Meng, F.; Haeffner, F.; Hoveyda, A.H. Diastereo-and enantioselective reactions of bis (pinacolato) diboron, 1, 3-enynes, and aldehydes catalyzed by an easily accessible bisphosphine–Cu complex. J. Am. Chem. Soc. 2014, 136, 11304–11307. [Google Scholar] [CrossRef] [Green Version]
- Corberán, R.; Mszar, N.W.; Hoveyda, A.H. NHC-Cu-Catalyzed Enantioselective Hydroboration of Acyclic and Exocyclic 1,1-Disubstituted Aryl Alkenes. Angew. Chem. 2011, 123, 7217–7220. [Google Scholar] [CrossRef]
- Jang, H.; Jung, B.; Hoveyda, A.H. Catalytic enantioselective protoboration of disubstituted allenes. Access to alkenylboron compounds in high enantiomeric purity. Org. Lett. 2014, 16, 4658–4661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zuo, Z.; Wan, X.; Huang, Z. Cobalt-catalyzed enantioselective hydroboration of 1,1-disubstituted aryl alkenes. J. Am. Chem. Soc. 2014, 136, 15501–15504. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Eno, M.S.; Annis, A.H.; Morken, J.P. Enantioselective Hydroformylation of 1-Alkenes with Commercial Ph-BPE Ligand. Org. Lett. 2015, 17, 3264–3267. [Google Scholar] [CrossRef] [Green Version]
- Abrams, M.L.; Foarta, F.; Landis, C.R. Asymmetric hydroformylation of Z-enamides and enol esters with rhodium-bisdiazaphos catalysts. J. Am. Chem. Soc. 2014, 136, 14583–14588. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, W.; Yan, L.; Ding, Y. A mini review on strategies for heterogenization of rhodium-based hydroformylation catalysts. Front. Chem. Sci. Eng. 2018, 12, 113–123. [Google Scholar] [CrossRef]
- Abrams, M.L.; Buser, J.Y.; Calvin, J.R.; Johnson, M.D.; Jones, B.R.; Lambertus, G.; Landis, C.R.; Martinelli, J.R.; May, S.A.; McFarland, A.D. Continuous liquid vapor reactions part 2: Asymmetric hydroformylation with rhodium-bisdiazaphos catalysts in a vertical pipes-in-series reactor. Org. Process. Res. Dev. 2016, 20, 901–910. [Google Scholar] [CrossRef]
- Rajurkar, K.B.; Tonde, S.S.; Didgikar, M.R.; Joshi, S.S.; Chaudhari, R.V. Environmentally Benign Catalytic Hydroformylation− Oxidation Route for Naproxen Synthesis. Ind. Eng. Chem. Res. 2007, 46, 8480–8489. [Google Scholar] [CrossRef]
- Halpern, J. Mechanism and stereoselectivity of asymmetric hydrogenation. Science 1982, 217, 401–407. [Google Scholar] [CrossRef]
- Crépy, K.V.; Imamoto, T. Recent developments in catalytic asymmetric hydrogenation employing P-chirogenic diphosphine ligands. Adv. Synth. Catal. 2003, 345, 79–101. [Google Scholar] [CrossRef]
- Du, X.; Xiao, Y.; Huang, J.-M.; Zhang, Y.; Duan, Y.-N.; Wang, H.; Shi, C.; Chen, G.-Q.; Zhang, X. Cobalt-catalyzed highly enantioselective hydrogenation of α,β-unsaturated carboxylic acids. Nat. Commun. 2020, 11, 3239. [Google Scholar] [CrossRef]
- Li, J.; Shen, J.; Xia, C.; Wang, Y.; Liu, D.; Zhang, W. Asymmetric hydrogenation of α-substituted acrylic acids catalyzed by a ruthenocenyl phosphino-oxazoline–ruthenium complex. Org. Lett. 2016, 18, 2122–2125. [Google Scholar] [CrossRef] [PubMed]
- WaáChung, L. Ferrocenyl chiral bisphosphorus ligands for highly enantioselective asymmetric hydrogenation via noncovalent ion pair interaction. Chem. Sci. 2016, 7, 6669–6673. [Google Scholar]
- Etayo, P.; Vidal-Ferran, A. Rhodium-catalysed asymmetric hydrogenation as a valuable synthetic tool for the preparation of chiral drugs. Chem. Soc. Rev. 2013, 42, 728–754. [Google Scholar] [CrossRef]
- Friedfeld, M.R.; Zhong, H.; Ruck, R.T.; Shevlin, M.; Chirik, P.J. Cobalt-catalyzed asymmetric hydrogenation of enamides enabled by single-electron reduction. Science 2018, 360, 888–893. [Google Scholar] [CrossRef] [Green Version]
- Zhong, H.; Shevlin, M.; Chirik, P.J. Cobalt-catalyzed asymmetric hydrogenation of α,β-unsaturated carboxylic acids by homolytic H2 cleavage. J. Am. Chem. Soc. 2020, 142, 5272–5281. [Google Scholar] [CrossRef]
- Mandrelli, F.; Blond, A.; James, T.; Kim, H.; List, B. Deracemizing α-Branched Carboxylic Acids by Catalytic Asymmetric Protonation of Bis-Silyl Ketene Acetals with Water or Methanol. Angew. Chem. Int. Ed. 2019, 58, 11479–11482. [Google Scholar] [CrossRef]
- Blanchet, J.; Baudoux, J.; Amere, M.; Lasne, M.C.; Rouden, J. Asymmetric malonic and acetoacetic acid syntheses–a century of enantioselective decarboxylative protonations. Eur. J. Org. Chem. 2008, 2008, 5493–5506. [Google Scholar] [CrossRef]
- Mohr, J.T.; Hong, A.Y.; Stoltz, B.M. Enantioselective protonation. Nat. Chem. 2009, 1, 359–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oudeyer, S.; Brière, J.F.; Levacher, V. Progress in catalytic asymmetric protonation. Eur. J. Org. Chem. 2014, 2014, 6103–6119. [Google Scholar] [CrossRef]
- Li, J.; An, S.; Yuan, C.; Li, P. Enantioselective Protonation of Silyl Enol Ethers Catalyzed by a Chiral Pentacarboxycyclopentadiene-Based Brønsted Acid. Synlett 2019, 30, 1317–1320. [Google Scholar] [CrossRef] [Green Version]
- Michel, N.W.; Jeanneret, A.D.; Kim, H.; Rousseaux, S.A. Nickel-catalyzed cyanation of benzylic and allylic pivalate esters. J. Org. Chem. 2018, 83, 11860–11872. [Google Scholar] [CrossRef] [PubMed]
- Kleemiss, F.; Justies, A.; Duvinage, D.; Watermann, P.; Ehrke, E.; Sugimoto, K.; Fugel, M.; Malaspina, L.A.; Dittmer, A.; Kleemiss, T.; et al. Sila-Ibuprofen. J. Med. Chem. 2020, 63, 12614–12622. [Google Scholar] [CrossRef] [PubMed]





























Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, M.-W.; Paek, S.-M. Recent Advances in the Synthesis of Ibuprofen and Naproxen. Molecules 2021, 26, 4792. https://doi.org/10.3390/molecules26164792
Ha M-W, Paek S-M. Recent Advances in the Synthesis of Ibuprofen and Naproxen. Molecules. 2021; 26(16):4792. https://doi.org/10.3390/molecules26164792
Chicago/Turabian StyleHa, Min-Woo, and Seung-Mann Paek. 2021. "Recent Advances in the Synthesis of Ibuprofen and Naproxen" Molecules 26, no. 16: 4792. https://doi.org/10.3390/molecules26164792
APA StyleHa, M. -W., & Paek, S. -M. (2021). Recent Advances in the Synthesis of Ibuprofen and Naproxen. Molecules, 26(16), 4792. https://doi.org/10.3390/molecules26164792





