Immunopharmacological Properties of Methacrylic Acid Polymers as Potential Polymeric Carrier Constituents of Anticancer Drugs
Abstract
1. Introduction
2. Results
2.1. Synthesis of Polymers and Their Molecular Weight Characteristics
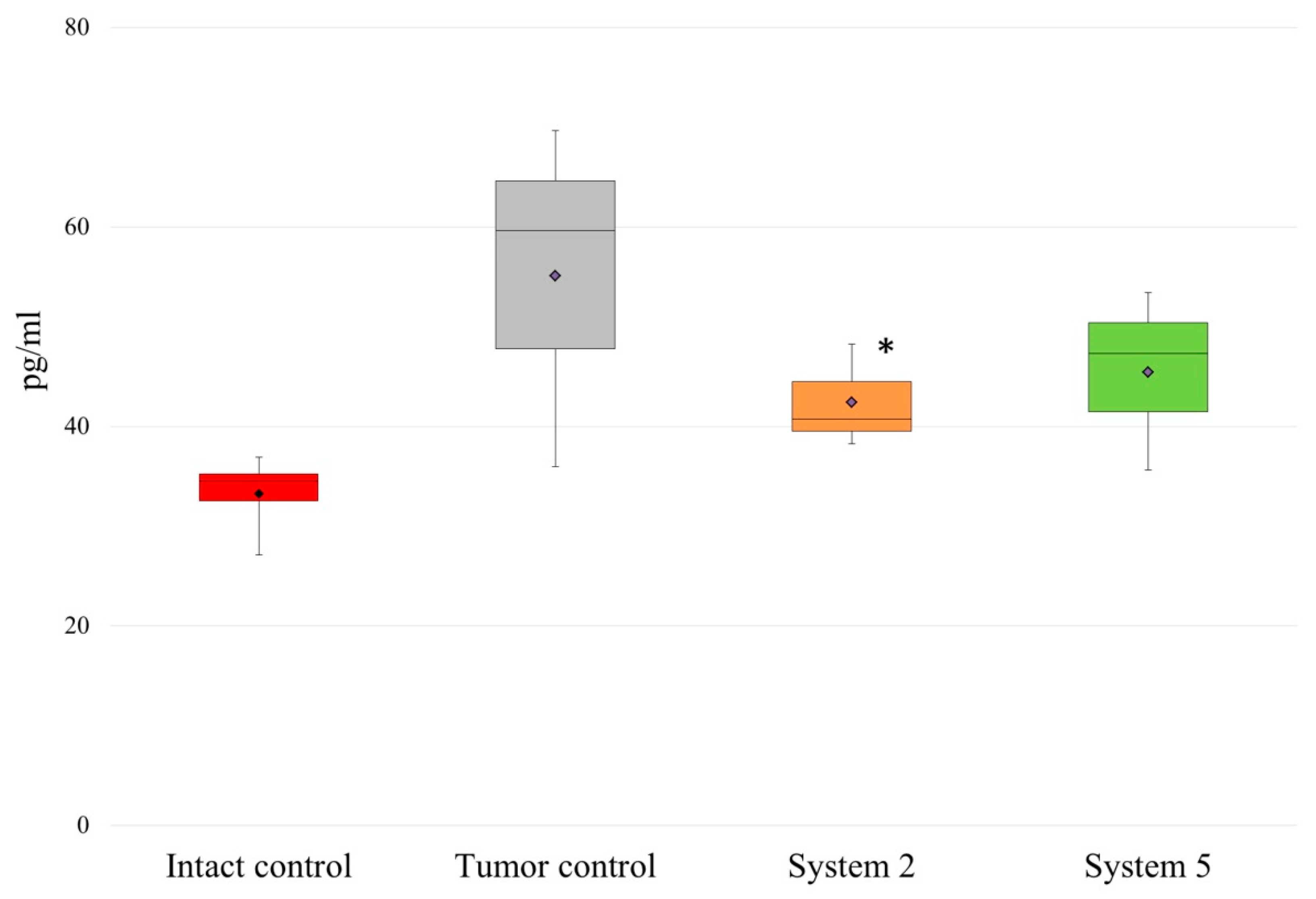
2.2. Effects of the MAA Polymers on the Cytokine Levels during Tumor Development
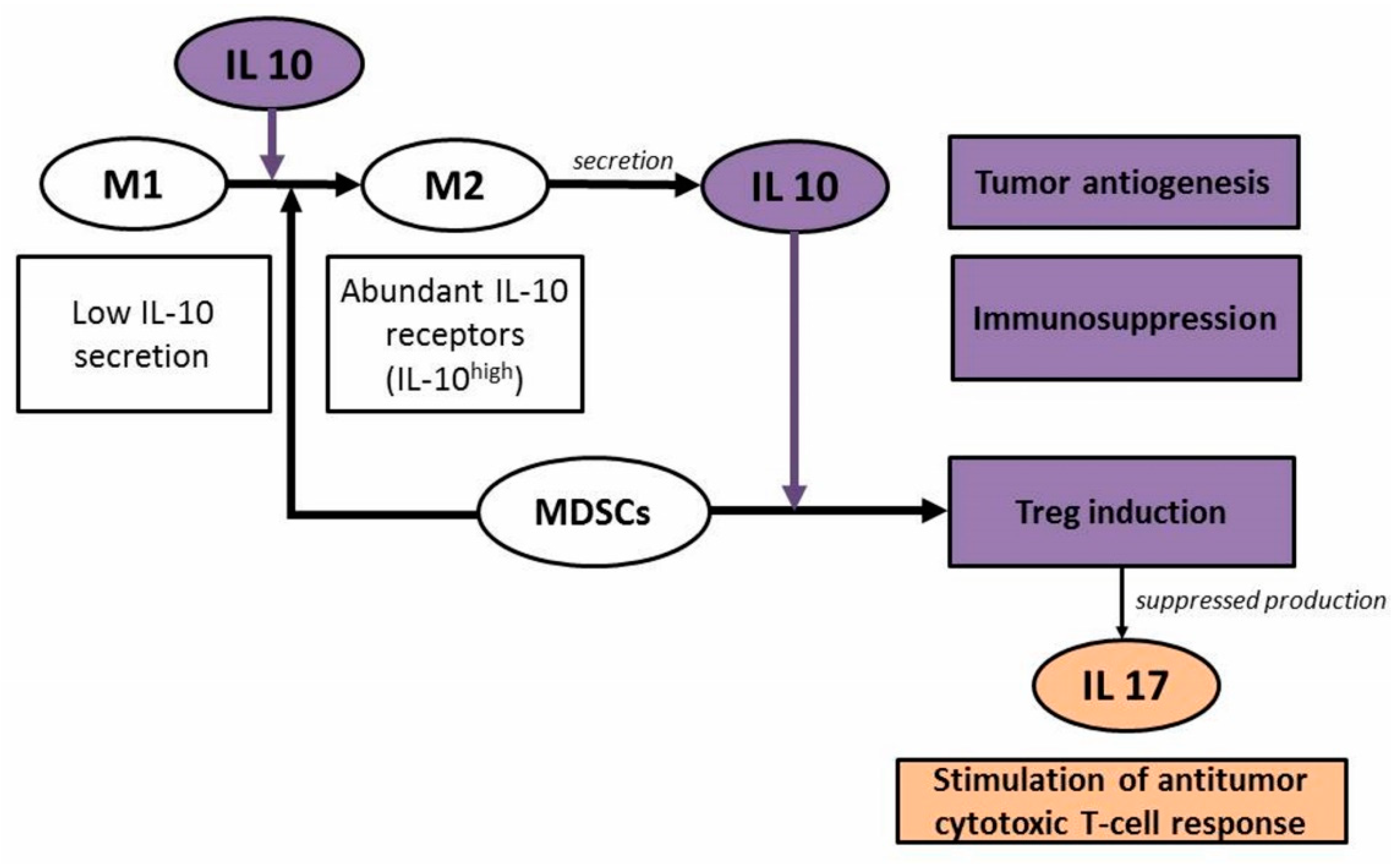
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Polymerization


4.3. Molecular Weight Characteristics of Polymers
4.4. PolyTBMA Hydrolysis
4.5. Experimental Animals
4.6. Primary Peritoneal Macrophage Cultures
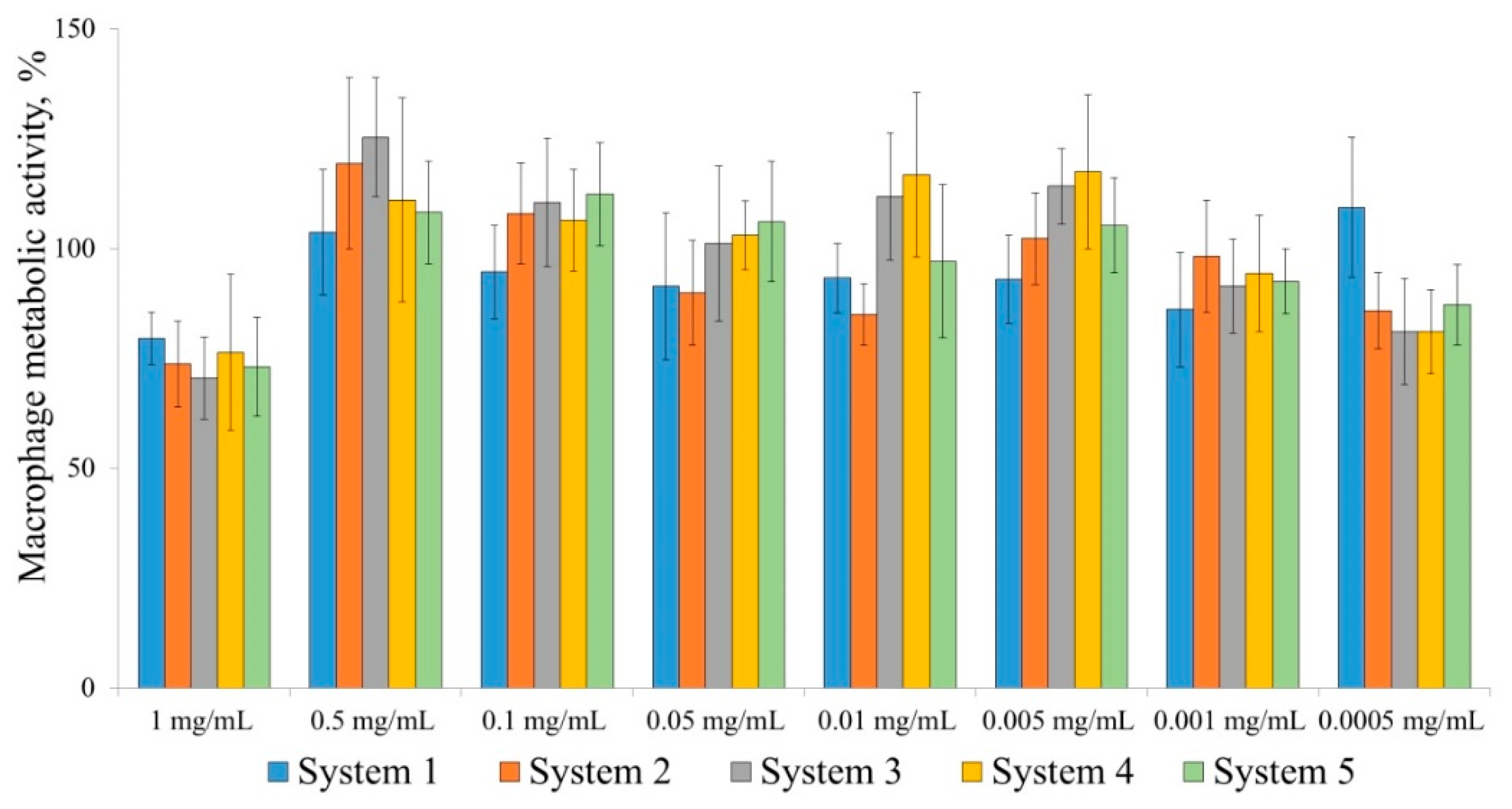
4.7. Cytotoxicity of Polymers to Immune System Cells (Peritoneal Macrophages) In Vitro; MTT Analysis
4.8. The ExperimentalModel of Cancer In Vivo
4.9. Serum Cytokine Level Measurements in Tumor-Bearing Rats by Flow Cytometry
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kitao, H.; Iimori, M.; Kataoka, Y.; Wakasa, T.; Tokunaga, E.; Saeki, H.; Oki, E.; Maehara, Y. DNA replication stress and cancer chemotherapy. Cancer Sci. 2018, 109, 264–271. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020, 5, 1–35. [Google Scholar] [CrossRef]
- Kamaruzman, N.I.; Aziz, N.A.; Poh, C.L.; Chowdhury, E.H. Oncogenic Signaling in Tumorigenesis and Applications of siRNA Nanotherapeutics in Breast Cancer. Cancers 2019, 5, 632. [Google Scholar] [CrossRef]
- Xu, Z.; Guo, M.; Yan, H.; Liu, K. Enhanced loading of doxorubicin into polymeric micelles by a combination of ionic bonding and hydrophobic effect, and the pH-sensitive and ligand-mediated delivery of loaded drug. React. Funct. Polym. 2013, 73, 564–572. [Google Scholar] [CrossRef]
- Pan, J.; Rostamizadeh, K.; Filipczak, N.; Torchilin, V.P. Polymeric Co-Delivery Systems in Cancer Treatment: An Overview on Component Drugs’ Dosage Ratio Effect. Molecules 2019, 6, 1035. [Google Scholar] [CrossRef]
- Shen, J.; Yan, B.; Li, T.; Long, Y.; Li, N.; Ye, M. Mechanical, thermal and swelling properties of poly(acrylic acid)–graphene oxide composite hydrogels. Soft Matter 2012, 8, 1831–1836. [Google Scholar] [CrossRef]
- Yilmaz, G.; Demir, B.; Timur, S.; Becer, C.R. Poly(methacrylic acid)-Coated Gold Nanoparticles: Functional Platforms for Theranostic Applications. Biomacromolecules 2016, 9, 2901–2911. [Google Scholar] [CrossRef]
- Abbad, S.; Wang, C.; Waddad, A.Y.; Lv, H.; Zhou, J. Preparation, in vitro and in vivo evaluation of polymeric nanoparticles based on hyaluronic acid-poly(butyl cyanoacrylate) and D-alpha-tocopheryl polyethylene glycol 1000 succinate for tumor-targeted delivery of morin hydrate. Int. J. Nanomed. 2015, 10, 305–320. [Google Scholar]
- Klemm, F.; Joyce, J.A. Microenviromental regulations of therapeutic response in cancer. Trends Cell Biol. 2015, 4, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jiang, Q.; Zhao, X.; Zhao, R.; Wang, Y.; Wang, Y.; Liu, J.; Shang, Y.; Zhao, S.; Wu, T.; et al. A DNA nanodevice-based vaccine for cancer immunotherapy. Nat. Mater. 2020, 3, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Simoes, M.C.F.; Sousa, J.J.S.; Pais, A.A.C.C. Skin cancer and new treatment perspectives: A review. Cancer Lett. 2015, 1, 8–42. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhu, W.; Feng, W.; Su, S.L.; Wingnang, A.L.; Shen, J.; Liqian, G.; Chuanshan, X. A Review of Resveratrol as a Potent Chemoprotective and Synergistic Agent in Cancer Chemotherapy. Front. Pharmacol. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Voulgari, E.; Bakandritsos, A.; Galtsidis, S.; Zoumpourlis, V.; Burke, B.P.; Clemente, G.S.; Cawthorne, C.; Archibald, S.J.; Tuček, J.; Zbořil, R.; et al. Synthesis, characterization and in vivo evaluation of a magnetic cisplatin delivery nanosystem based on PMAA-graft-PEG copolymers. J. Control. Release 2016, 243, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Ito, F.; Chang, A.E. Cancer immunotherapy. Current status and future directions. Surg. Oncol. Clin. N. Am. 2013, 22, 765–783. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer 2020, 2, 359–367. [Google Scholar]
- Ferte, C.; Andre, F.; Soria, C. Molecular circuits of solid tumors: Prognostic and predictive tools for beside use. Nat. Rev. Clin. Oncol. 2010, 7, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Galon, G.; Angell, H.; Bedognetti, D.; Marincola, F. The continuum of cancer immunosurveillance: Prognostic, predictive and mechanistic signatures. Immunity 2013, 39, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Bolhassani, A.; Javanzad, S.; Saleh, T.; Hashemi, M.; Aghasadeghi, M.R.; Sadat, S.M. Polymeric nanoparticles. Hum. Vaccines Immunother. 2014, 2, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J. Physicochemical characterization of nanoparticles and their potential for vaccine preparation. Vaccine Res. 1992, 1, 93–98. [Google Scholar]
- Okay, S.; Özcan, Ö.Ö.; Karahan, M. Nanoparticle-based delivery platforms for mRNA vaccine development. AIMS Biophys. 2020, 4, 323–338. [Google Scholar] [CrossRef]
- Khaitov, R.M. Molecular bases for the construction of artificial immunogens. J. Biomed. Sci. 1990, 2, 122–126. [Google Scholar]
- Petrov, R.V.; Khaitov, R.M.; Ataullakhanov, R.I. Artificial antigens and vaccines based on nonnatural polyelectrolytes. Immunol. Rev. 1987, 1, 241–443. [Google Scholar]
- Gaudino, S.J.; Kumar, P. Cross-Talk Between Antigen Presenting Cells and T Cells Impacts Intestinal Homeostasis, Bacterial Infections, and Tumorigenesis. Front. Immunol. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, W.M.; Qasim, D.; Nokhodchi, A.; Al-Jabery, A.; Sallam, A. Novel salted anionic-cationic polymethacrylate polymer blends for sustained release of acidic and basic drugs. Curr. Drug Deliv. 2007, 1, 109–122. [Google Scholar] [CrossRef][Green Version]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Olingy, C.E.; Dinh, H.Q.; Hedrick, C.C. Monocyte heterogeneity and functions in cancer. J. Leukoc. Biol. 2019, 106, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.E.; Harney, A.S.; Pollard, J.W. The Multifaceted Role of Perivascular Macrophages in Tumors. Cancer Cell 2016, 1, 18–25. [Google Scholar] [CrossRef]
- Mantovani, A.; Bottazzi, B.; Colotta, F.; Sozzani, S.; Ruco, L. The origin and function of tumor-associated macrophages. Immunol. Today 1992, 7, 265–270. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, J.; Lan, H. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J. Hematol. Oncol. 2019, 1, 1–16. [Google Scholar] [CrossRef]
- Stein, M.; Keshav, S.; Harris, N.; Gordon, S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: A marker of alternative immunologic macrophage activation. J. Exp. Med. 1992, 1, 287–292. [Google Scholar] [CrossRef]
- Gratchev, A.; Guillot, P.; Hakiy, N.; Politz, O.; Orfanos, C.E.; Schledzewski, K.; Goerdt, S. Alternatively activated macrophages differentially express fibronectin and its splice variants and the extracellular matrix protein betaIG-H3. Scand. J. Immunol. 2001, 4, 386–392. [Google Scholar] [CrossRef]
- Porta, C.; Riboldi, E.; Ippolito, A.; Sica, A. Molecular and epigenetic basis of macrophage polarized activation. Semin. Immunol. 2015, 27, 237–248. [Google Scholar] [CrossRef]
- Glass, C.K.; Natoli, G. Molecular control of activation and priming in macrophages. Nat. Immunol. 2016, 17, 26–33. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, X.H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ngambenjawong, C.; Gustafson, H.H.; Pun, S.H. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv. Drug Deliv. Rev. 2017, 114, 206–221. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R.; Tanaka, T.; Yamamoto, Y.; Akasaki, Y.; Sasaki, H. Dual role of macrophage in tumor immunity. Immunotherapy 2018, 10, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Fabre, J.A.S.; Giustinniani, J.; Garbar, C.; Merrouche, Y.; Antonicelli, F.; Bensussan, A. The Interleukin-17 Family of Cytokines in Breast Cancer. Int. J. Mol. Sci. 2018, 12, 1–16. [Google Scholar] [CrossRef]
- Chen, Q.; Daniel, V.; Maher, D.W.; Hersey, P. Production of IL-10 by melanoma cells: Examination of its role in immunosuppression mediated by melanoma. Int. J. Cancer 1994, 4, 755–760. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, Z.; Dong, C.; Luan, Y.; Zhang, A.; Moore, C.; Fu, K.; Peng, J.; Wang, Y.; Ren, Z.; et al. Targeting Tumors with IL-10 Prevents Dendritic Cell-Mediated CD8+ T Cell Apoptosis. Cancer Cell 2019, 6, 901–915. [Google Scholar] [CrossRef] [PubMed]
- McKay, K.; Moore, P.C.; Smoller, B.R.; Hiatt, K.M. Association between natural killer cells and regression in melanocytic lesions. Hum. Pathol. 2011, 42, 1960–1964. [Google Scholar] [CrossRef]
- Mannino, M.H.; Zhu, Z.; Xiao, H.; Bai, Q.; Wakefield, M.R.; Fang, Y. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. 2015, 2, 103–107. [Google Scholar] [CrossRef]
- Petersson, M.; Charo, J.; Salazar-Onfray, F.; Noffz, G.; Mohaupt, M.; Qin, Z.; Klein, G.; Blankenstein, T.; Kiessling, R. Constitutive IL-10 production accounts for the high NK sensitivity, low MHC class I expression, and poor transporter associated with antigen processing (TAP)-1/2 function in the prototype NK target YAC-1. J. Immunol 1998, 161, 2099–2105. [Google Scholar]
- Yang, S.; Jian, M.Y. Role of interleukin (IL)-17 and T-helper (Th)17 cells in cancer. Biochem. Biophys. Res. Commun. 2017, 493, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, T.; Wilke, C.M.; Kryczek, I.; Chen, G.Y.; Kao, J.; Núñez, G.; Zou, W. Interleukin-10 Ablation Promotes Tumor Development, Growth, and Metastasis. Cancer Res. 2012, 2, 420–429. [Google Scholar] [CrossRef]
- Baseler, W.A.; Davies, L.C.; Quigley, L.; Ridnour, L.A.; Weiss, J.M.; Hussain, S.P.; Wink, D.A.; McVicar, D.W. Autocrine IL-10 functions as a rheostat for M1 macrophage glycolytic commitment by tuning nitric oxide production. Redox Biol. 2016, 10, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Nagata, E.; Masuda, H.; Nakayama, T.; Netsu, S.; Yuzawa, H.; Fujii, N.; Kohara, S.; Sorimachi, T.; Osada, T.; Imazeki, R.; et al. Insufficient production of IL-10 from M2 macrophages impairs in vitro endothelial progenitor cell differentiation in patients with Moyamoya disease. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Yu, H.; Zhang, Y.; Zhao, D.; Lv, P.; Zhong, Y.; Xu, Y. IL-10 secreted by M2 macrophage promoted tumorigenesis through interaction with JAK2 in glioma. Oncotarget 2016, 44, 71673–71685. [Google Scholar] [CrossRef]
- Saito, K.; Pignon, P.; Ayyoub, M.; Valmori, D. Modulation of Cytokine Secretion Allows CD4 T- Cells Secreting IL-10 and IL-17 to Simultaneously Participatein Maintaining Toleranceand Immunity. PLoS ONE 2015, 12, e0145788. [Google Scholar] [CrossRef]
- Ottenbrite, R.M.; Regelson, W.; Kaplan, A.; Carchman, R.; Morahan, P.; Munson, A. Polymer Drugs; Donaruma, L.G., Vogel, O., Eds.; John Wiley & Sons: New York, NY, USA, 1978; p. 263. [Google Scholar]
- Zhukova, O.V.; Kovaleva, T.F.; Arkhipova, E.V.; Ryabov, S.A.; Mukhina, I.V. Tumor associated macrophages: Role in the pathological process of tumorigenesis and prospective therapeutic use (Review). Biomed. Rep. 2020, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, S.; Panelli, M.C.; Wang, E.; Nagorsen, D.; Marincola, F.M. The dual role of IL-10. Trends Immunol. 2003, 24, 36–43. [Google Scholar] [CrossRef]
- Xin, Q.; Hankui, C.; Xiaofeng, W.; Ling, H.; Qi, H.; Yang, J. Interleukin-17 acts as double-edged sword in anti-tumor immunity and tumorigenesis. Cytokine 2017, 89, 34–44. [Google Scholar]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar]
- Chong, Y.K.; Moad, G.; Rizzardo, E.; Thang, S.H. Reversible Addition Fragmentation Chain Transfer Polymerization of Methyl Methacrylate in the Presence of Lewis Acids: An Approach to Stereocontrolled Living Radical Polymerization. Macromolecules 2007, 13, 4446–4455. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.C.L. Purification of Laboratory Chemicals, 7th ed.; Elsevier/Butterworth-Heinemann: London, UK, 2013; p. 1024. [Google Scholar]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 2, 271–277. [Google Scholar] [CrossRef]
- Konoplev, V.P.; Lagova, N.D. Characteristics of the rat breast cancer to be transferred. Bull. Exp. Biol. Med. 1960, 7, 79–81. [Google Scholar]

| RAFT, mol/L | CPDT | CDSPA | ||||
|---|---|---|---|---|---|---|
| Mn∙103 | Mw∙103 | Mw/Mn | Mn∙103 | Mw∙103 | Mw/Mn | |
| 0.01 | 87.8 | 149.8 | 1.71 | 99.2 | 160.4 | 1.57 |
| 0.04 | 31.3 | 45.9 | 1.47 | 31.5 | 40.0 | 1.27 |
| 0.08 | 19.0 | 27.7 | 1.45 | 19.5 | 23.6 | 1.19 |
| 0.10 | 16.0 | 22.7 | 1.47 | 14.6 | 16.6 | 1.13 |
| RAFT, mol/L | CPDT | ||
|---|---|---|---|
| Mn∙103 | Mw∙103 | Mw/Mn | |
| 0.01 | 46.9 | 53.5 | 1.14 |
| 0.04 | 21.5 | 28.7 | 1.33 |
| 0.08 | 13.8 | 15.6 | 1.13 |
| 0.10 | 11.5 | 13.2 | 1.15 |
| Polymer | IC50, mg/mL |
|---|---|
| System 1 | 0.707 |
| System 2 | 0.612 |
| System 3 | 0.652 |
| System 4 | 0.657 |
| System 5 | 0.567 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhukova, O.V.; Arkhipova, E.V.; Kovaleva, T.F.; Ryabov, S.A.; Ivanova, I.P.; Golovacheva, A.A.; Zykova, D.A.; Zaitsev, S.D. Immunopharmacological Properties of Methacrylic Acid Polymers as Potential Polymeric Carrier Constituents of Anticancer Drugs. Molecules 2021, 26, 4855. https://doi.org/10.3390/molecules26164855
Zhukova OV, Arkhipova EV, Kovaleva TF, Ryabov SA, Ivanova IP, Golovacheva AA, Zykova DA, Zaitsev SD. Immunopharmacological Properties of Methacrylic Acid Polymers as Potential Polymeric Carrier Constituents of Anticancer Drugs. Molecules. 2021; 26(16):4855. https://doi.org/10.3390/molecules26164855
Chicago/Turabian StyleZhukova, Olga V., Evgenia V. Arkhipova, Tatyana F. Kovaleva, Sergey A. Ryabov, Irina. P. Ivanova, Anna A. Golovacheva, Daria A. Zykova, and Sergey D. Zaitsev. 2021. "Immunopharmacological Properties of Methacrylic Acid Polymers as Potential Polymeric Carrier Constituents of Anticancer Drugs" Molecules 26, no. 16: 4855. https://doi.org/10.3390/molecules26164855
APA StyleZhukova, O. V., Arkhipova, E. V., Kovaleva, T. F., Ryabov, S. A., Ivanova, I. P., Golovacheva, A. A., Zykova, D. A., & Zaitsev, S. D. (2021). Immunopharmacological Properties of Methacrylic Acid Polymers as Potential Polymeric Carrier Constituents of Anticancer Drugs. Molecules, 26(16), 4855. https://doi.org/10.3390/molecules26164855






