Saponin Stabilization via Progressive Freeze Concentration and Sterilization Treatment
Abstract
:1. Introduction
2. Results
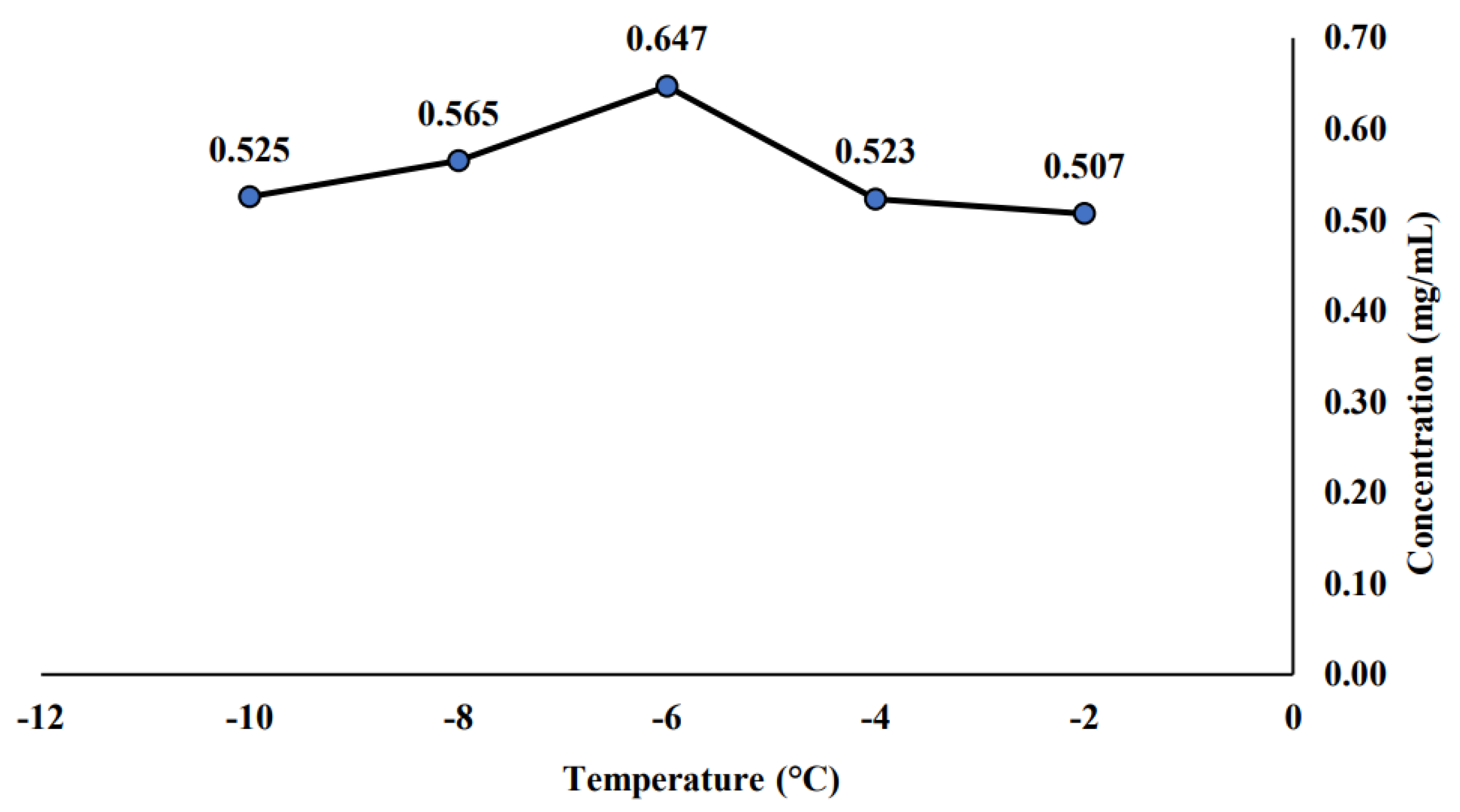
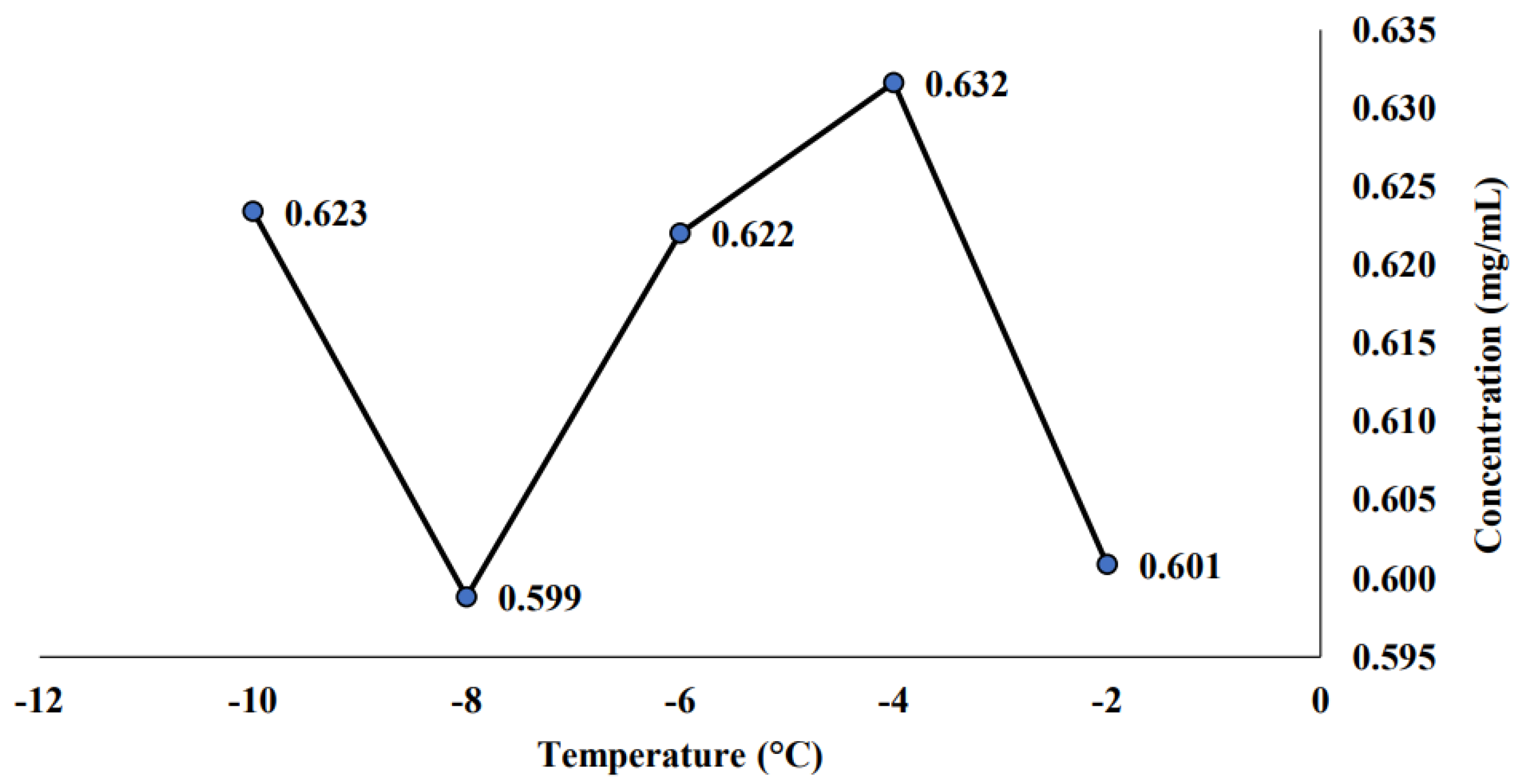
2.1. Effect of Temperature and Concentration Time of Progressive Freeze Concentration towards Saponin Concentration for the Initial PFC Sample
2.2. Stability Analysis
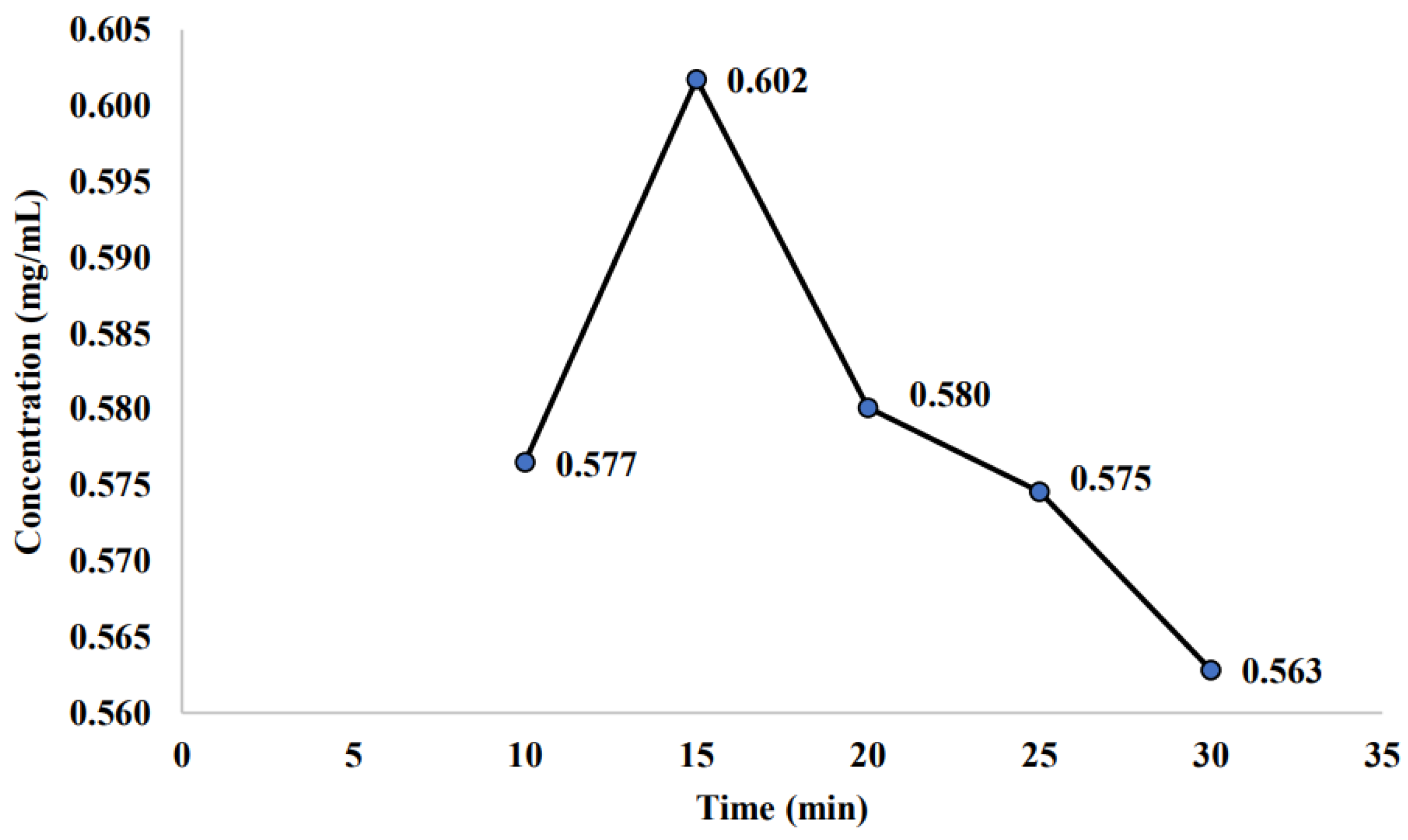
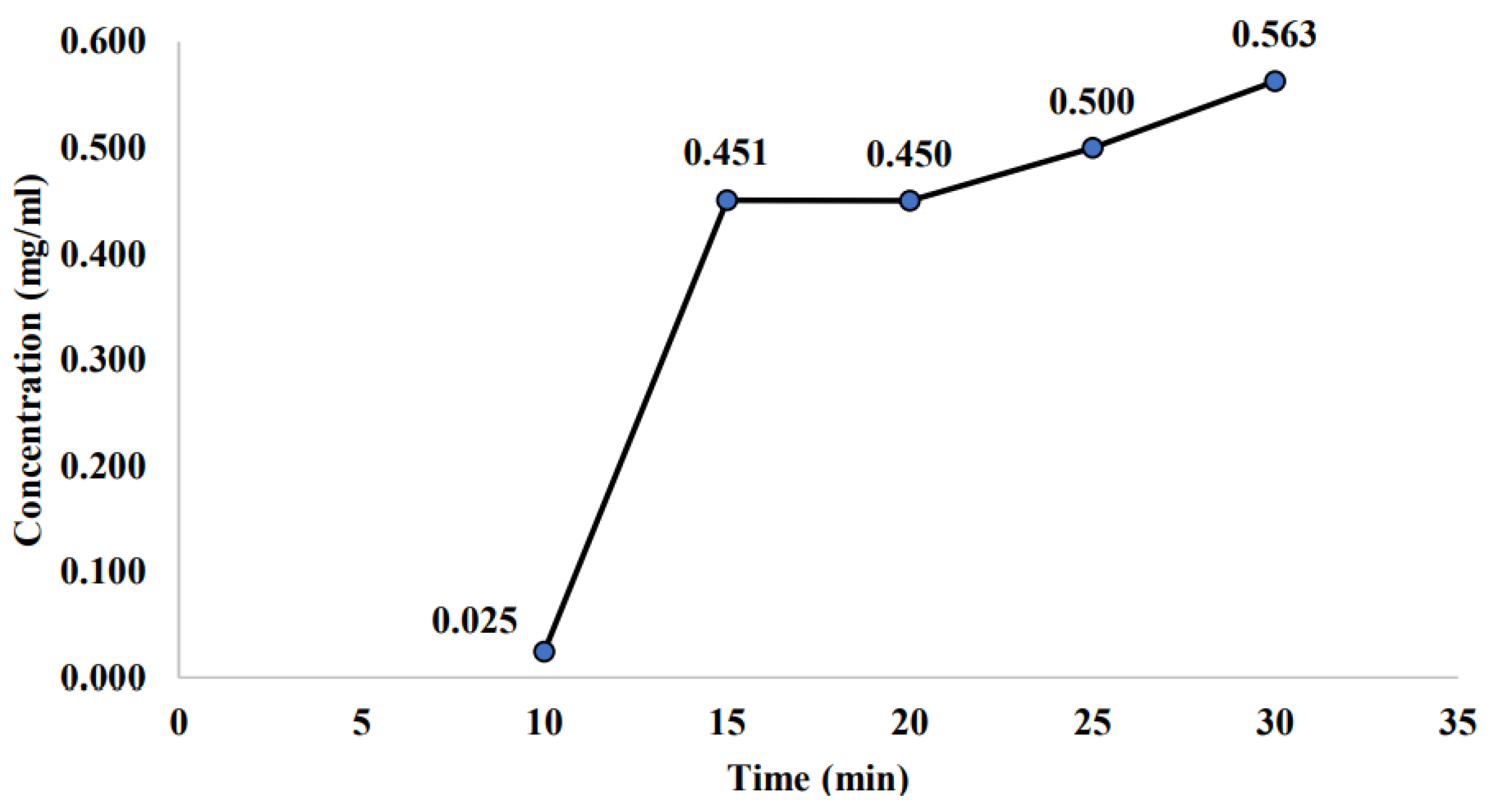
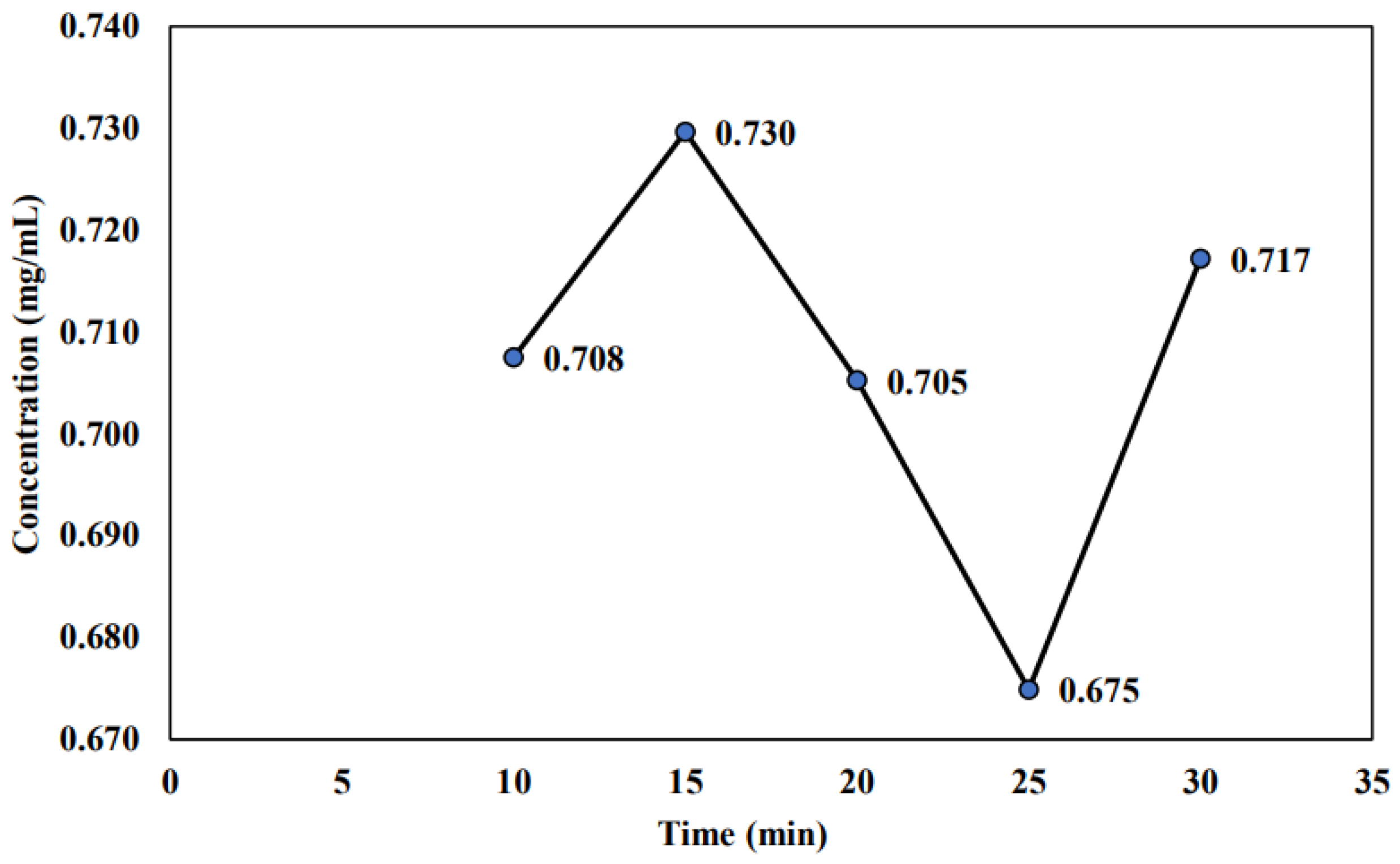
2.2.1. Effect of Room Storage on the Stability of Non-Sterilized Sample
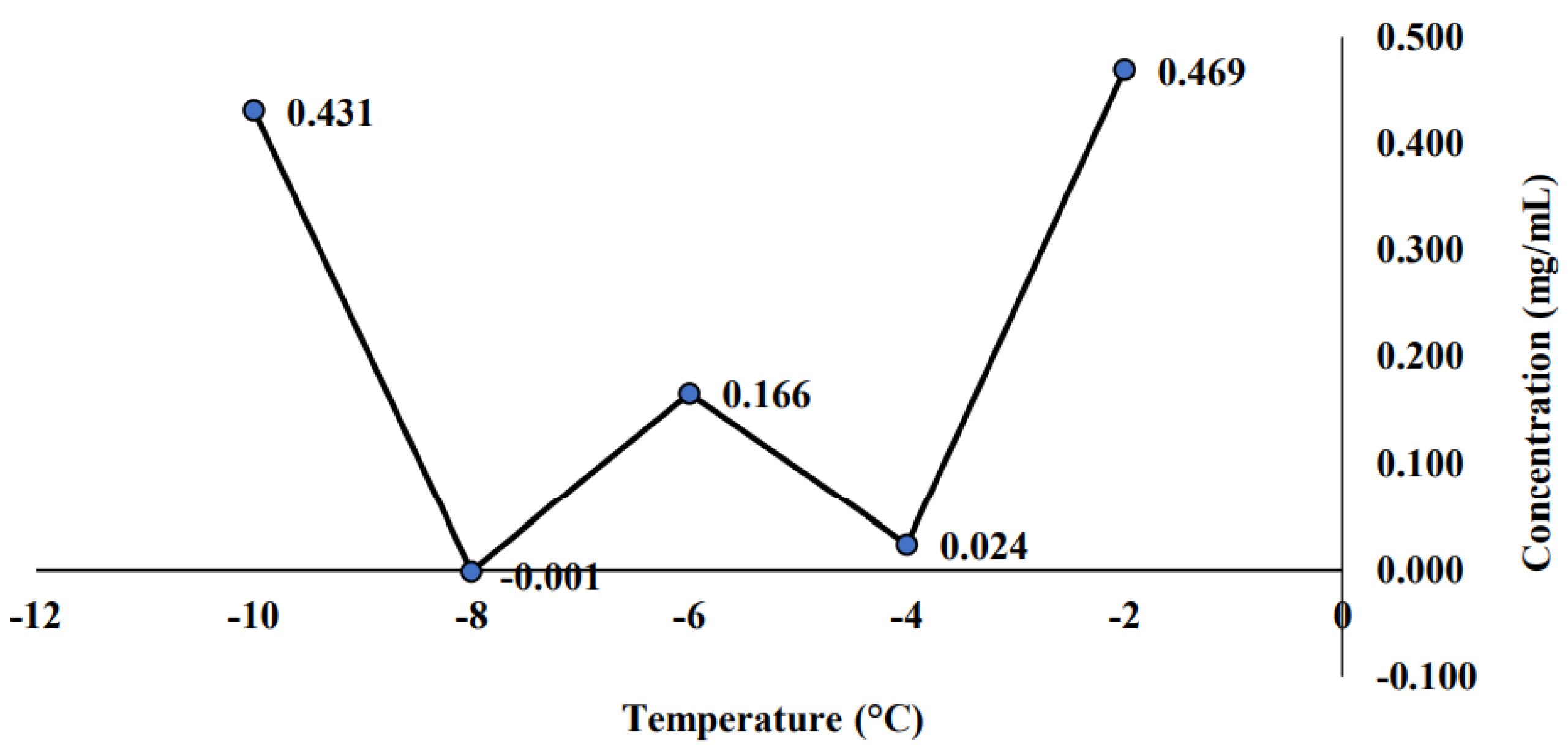
2.2.2. Effect of Cold Storage on the Stability of Sterilized Sample
3. Discussion
3.1. Effect of Temperature and Concentration Time of Progressive Freeze Concentration towards Saponin Concentration for the Initial PFC Sample
3.2. Effect of Room Storage on the Stability of Non-Sterilized Sample
3.3. Effect of Cold Storage on the Stability of Sterilized Sample
4. Materials and Methods
4.1. Materials and Reagents
4.2. Extraction by Maceration
4.3. Concentration by the Progressive Freeze Concentration
4.4. Sterilization by Thermal Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pareja, L.; Fernández-Alba, A.; Cesio, V.; Heinzen, H. Analytical methods for pesticide residues in rice. TrAC Trends Anal. Chem. 2011, 30, 270–291. [Google Scholar] [CrossRef]
- Prasad, B.; Prabhu, S.T.; Balikai, R. Status of insect pests and their natural enemies on rice under rainfed ecosystems. Int. J. Agric. Stat. Sci. 2011, 7, 473–482. [Google Scholar]
- Matteson, P.C. Insect Pest Management in Tropical Asian Irrigated Rice. Annu. Rev. Entomol. 2000, 45, 549–574. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, J.; Zhou, G.-H.; Zhang, H.-M.; Chen, J.-P.; Adams, M.J. The Complete Genome Sequence of Two Isolates of Southern rice black-streaked dwarf virus, a New Member of the Genus Fijivirus. J. Phytopathol. 2010, 158, 733–737. [Google Scholar] [CrossRef]
- Zhou, G.; Wen, J.; Cai, D.; Li, P.; Xu, D.; Zhang, S. Southern rice black-streaked dwarf virus: A new proposed Fijivirus species in the family Reoviridae. Chin. Sci. Bull. 2008, 53, 3677–3685. [Google Scholar] [CrossRef]
- Hoang, A.T.; Zhang, H.-M.; Yang, J.; Chen, J.-P.; Hebrard, E.; Zhou, G.-H.; Vinh, V.N.; Cheng, J.-A. Identification, Characterization, and Distribution of Southern rice black-streaked dwarf virus in Vietnam. Plant Dis. 2011, 95, 1063–1069. [Google Scholar] [CrossRef] [Green Version]
- Zhai, B. Macroscopic Patterns and Microscopic Mechanisms of the Outbreak of Rice Planthoppers and Epidemic SRBSDV. Biology 2011. Available online: https://www.semanticscholar.org/paper/Macroscopic-patterns-and-microscopic-mechanisms-of-Bao/0273c241a5a453bd8589667150dee65ec238b9ca (accessed on 16 June 2021).
- Matsukura, K.; Towata, T.; Sakai, J.; Onuki, M.; Okuda, M.; Matsumura, M. Dynamics of Southern rice black-streaked dwarf virus in Rice and Implication for Virus Acquisition. Phytopathology 2013, 103, 509–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Xie, X.; Gao, D.; Chen, K.; Chen, Z.; Jin, L.; Li, X.; Song, B. Dufulin Intervenes the Viroplasmic Proteins as the Mechanism of Action against Southern Rice Black-Streaked Dwarf Virus. J. Agric. Food Chem. 2019, 67, 11380–11387. [Google Scholar] [CrossRef]
- Acosta, B.O.; Pullin, R.S.V. Environmental Impact of the Golden Snail (Pomacea sp.) on Rice Farming Systems in the Philippines; Working Papers; The WorldFish Center: Penang, Malaysia, 1991; p. 5796. [Google Scholar]
- Naylor, R. Invasions in Agriculture: Assessing the Cost of the Golden Apple Snail in Asia. Undefined 1996. Available online: https://www.semanticscholar.org/paper/Invasions-in-agriculture%3A-assessing-the-cost-of-the-Naylor/7f98733afdd609c6303e1592312e35a2f3229ce9 (accessed on 22 April 2021).
- Pantua, P.C.; Mercado, S.V.; Lanting, F.O.; Nuevo, E.B. Use of ducks to control golden apple snail Ampullarius (Pomacea canaliculata) in irrigated rice. Int. Rice Res. Newsl. 1992, 17, 27. [Google Scholar]
- Ali, J.; Suryanto, E. Fish as Biological Control Agent of Golden Apple Snails-Prospects and Challenges. Presented at the Symposium on Biological Control in the Tropics, Serdang, Selangor. June 2014. Available online: http://psasir.upm.edu.my/id/eprint/31451/ (accessed on 22 April 2021).
- The Use of CAMB Biopesticides to Control Pests of Rice (Oryza sativa). Asian J. Plant Sci. 2003, 2, 1079–1082. [CrossRef]
- Pimentel, D. Environmental and Economic Costs of the Application of Pesticides Primarily in the United States. Environ. Dev. Sustain. 2005, 7, 229–252. [Google Scholar] [CrossRef]
- Chen, H.; Liu, X.; Deng, S.; Wang, H.; Ou, X.; Huang, L.; Li, J.; Jin, C. Pretilachlor Releasable Polyurea Microcapsules Suspension Optimization and Its Paddy Field Weeding Investigation. Front. Chem. 2020, 8, 826. [Google Scholar] [CrossRef]
- Hedaoo, R.K.; Mahulikar, P.P.; Chaudhari, A.B.; Rajput, S.D.; Gite, V.V. Fabrication of Core–Shell Novel Polyurea Microcapsules Using Isophorone Diisocyanate (IPDI) Trimer for Release System. Int. J. Polym. Mater. 2014, 63, 352–360. [Google Scholar] [CrossRef]
- Hedaoo, R.K.; Tatiya, P.D.; Mahulikar, P.P.; Gite, V.V. Fabrication of dendritic 0 G PAMAM-based novel polyurea microcapsules for encapsulation of herbicide and release rate from polymer shell in different environment. Des. Monomers Polym. 2013, 17, 111–125. [Google Scholar] [CrossRef]
- Zhang, D.-X.; Li, B.-X.; Zhang, X.-P.; Zhang, Z.-Q.; Wang, W.-C.; Liu, F. Phoxim Microcapsules Prepared with Polyurea and Urea–Formaldehyde Resins Differ in Photostability and Insecticidal Activity. J. Agric. Food Chem. 2016, 64, 2841–2846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-X.; Zhang, X.-P.; Luo, J.; Li, B.-X.; Wei, Y.; Liu, F. Causation Analysis and Improvement Strategy for Reduced Pendimethalin Herbicidal Activity in the Field after Encapsulation in Polyurea. ACS Omega 2018, 3, 706–716. [Google Scholar] [CrossRef]
- Ramli@yusof, N.H.; Yusup, S.; Bin Kueh, B.W.; Kamarulzaman, P.S.D.; Osman, N.; Rahim, M.A.; Aziz, R.; Mokhtar, S.; Ahmad, A.B. Effectiveness of biopesticides in enhancing paddy growth for yield improvement. Sustain. Chem. Pharm. 2018, 7, 1–8. [Google Scholar] [CrossRef]
- Kumar, S. Biopesticide: An Environment Friendly Pest Management Strategy. J. Fertil. Pestic. 2015, 6. [Google Scholar] [CrossRef]
- Shoaib, M.A.; Mahmoud, M.F.; Loutfy, N.; Tawfic, M.A.; Barta, M. Effect of botanical insecticide Nimbecidine® on food consumption and egg hatchability of the terrestrial snail Monacha obstructa. J. Pest Sci. 2010, 83, 27–32. [Google Scholar] [CrossRef]
- Sousa, R.M.; Rosa, J.; Silva, C.A.; Almeida, M.T.M.; Novo, M.T.; Cunha, A.; Fernandes-Ferreira, M. Larvicidal, molluscicidal and nematicidal activities of essential oils and compounds from Foeniculum vulgare. J. Pest Sci. 2015, 88, 413–426. [Google Scholar] [CrossRef]
- Baptista, D.F.; Vasconcellos, M.C.; E Lopes, F.; Silva, I.P.; Schall, V.T. Perspectives of using Euphorbia splendens as a molluscicide in schistosomiasis control programs. Southeast Asian J. Trop. Med. Public Health 1994, 25, 419–424. [Google Scholar]
- Wang, Z.; Tan, J.; Liu, J.; Wang, W. Evaluation of controlling Pomacea canaliculata with calcium oxide, ammonium bi-carbonate, Camellia oleifera powder and tea saponin. Acta Phytophylacica Sin. 2011, 38, 363–368. [Google Scholar]
- Martín, R.S.; Ndjoko, K.; Hostettmann, K. Novel molluscicide against Pomacea canaliculata based on quinoa (Chenopodium quinoa) saponins. Crop. Prot. 2008, 27, 310–319. [Google Scholar] [CrossRef]
- Wei, M.-C.; Yang, Y.-C. Kinetic studies for ultrasound-assisted supercritical carbon dioxide extraction of triterpenic acids from healthy tea ingredient Hedyotis diffusa and Hedyotis corymbosa. Sep. Purif. Technol. 2015, 142, 316–325. [Google Scholar] [CrossRef]
- Yarnell, E. Plant Chemistry in Veterinary Medicine: Medicinal Constituents and Their Mechanisms of Action. Vet. Herb. Med. 2007, 159–182. [Google Scholar] [CrossRef]
- Thompson, L.U. Potential health benefits and problems associated with antinutrients in foods. Food Res. Int. 1993, 26, 131–149. [Google Scholar] [CrossRef]
- Kim, S.-W.; Park, S.-K.; Kang, S.-L.; Kang, H.-C.; Oh, H.-J.; Bae, C.-Y.; Bae, D.-H. Hypocholesterolemic property ofYucca schidigera andQuillaja saponaria extracts in human body. Arch. Pharmacal Res. 2003, 26, 1042–1046. [Google Scholar] [CrossRef]
- Gurfinkel, D.M.; Rao, A.V. Soyasaponins: The Relationship Between Chemical Structure and Colon Anticarcinogenic Activity. Nutr. Cancer 2003, 47, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, H. Saponins in Garlic as Modifiers of the Risk of Cardiovascular Disease. J. Nutr. 2001, 131, 1000S–1005S. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, S.M. Soy saponins and the anticancer effects of soybeans and soy-based foods. Curr. Med. Chem. Agents 2004, 4, 263–272. [Google Scholar] [CrossRef]
- Ridout, C.L.; Price, K.R.; Dupont, M.S.; Parker, M.L.; Fenwick, G.R. Quinoa saponins—analysis and preliminary investigations into the effects of reduction by processing. J. Sci. Food Agric. 1991, 54, 165–176. [Google Scholar] [CrossRef]
- Liu, J.; Henkel, T. Traditional Chinese medicine (TCM): Are polyphenols and saponins the key ingredients triggering biological activities? Curr. Med. Chem. 2002, 9, 1483–1485. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.S.; Briones, R. Industrial uses and sustainable supply ofQuillaja saponaria (Rosaceae) saponins. Econ. Bot. 1999, 53, 302–311. [Google Scholar] [CrossRef]
- Bitencourt, R.G.; Queiroga, C.L.; Junior, Í.M.; Cabral, F. Fractionated extraction of saponins from Brazilian ginseng by sequential process using supercritical CO2, ethanol and water. J. Supercrit. Fluids 2014, 92, 272–281. [Google Scholar] [CrossRef]
- Ramli, N.; Yusup, S.; Johari, K.; Rahim, M.A. Selection of Potential Plants for Saponin Extract Using Supercritical-CO2 Extraction against Golden Apple Snails (Pomacea canaliculata) for Paddy Cultivation. Arch. Crop. Sci. 2017, 1, 30–37. [Google Scholar] [CrossRef]
- Ramli, N.H.; Yusup, S.; Quitain, A.T.; Johari, K.; Bin Kueh, B.W. Optimization of saponin extracts using microwave-assisted extraction as a sustainable biopesticide to reduce Pomacea canaliculata population in paddy cultivation. Sustain. Chem. Pharm. 2019, 11, 23–35. [Google Scholar] [CrossRef]
- Arthurs, S.; Dara, S.K. Microbial biopesticides for invertebrate pests and their markets in the United States. J. Invertebr. Pathol. 2019, 165, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, O.; Inakuma, T. Development of Progressive Freeze Concentration and Its Application: A Review. Food Bioprocess Technol. 2021, 14, 39–51. [Google Scholar] [CrossRef]
- Anuar, M.A.M.; Amran, N.A.; Ruslan, M.S.H. Optimization of Progressive Freezing for Residual Oil Recovery from a Palm Oil–Water Mixture (POME Model). ACS Omega 2021, 6, 2707–2716. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Z.; Xu, H.; Han, Y. Extraction of Saponin fromCamellia oleiferaAbel Cake by a Combination Method of Alkali Solution and Acid Isolation. J. Chem. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Yahya, N.; Zakaria, Z.Y.; Ali, N.; Jusoh, M. Effect of Coolant Temperature on Progressive Freeze Concentration of Refined, Bleached and Deodorised Palm Oil based on Process Efficiency and Heat Transfer. J. Teknol. 2015, 74. [Google Scholar] [CrossRef] [Green Version]
- Mazli, W.N.A.; Samsuri, S.; Amran, N.A.; Yáñez, E.H. Optimization of Progressive Freezing on Synthetic Produced Water by Circular Moving Cylindrical Crystallizer via Response Surface Methodology. Crystals 2021, 11, 103. [Google Scholar] [CrossRef]
- Amran, N.A.; Samsuri, S.; Jusoh, M. Effect of Freezing Time and Shaking Speed on the Performance of Progressive Freeze Concentration via Vertical Finned Crystallizer. Int. J. Automot. Mech. Eng. 2018, 15, 5356–5366. [Google Scholar] [CrossRef]
- Safiei, N.Z.; Ngadi, N.; Johari, A.; Zakaria, Z.Y.; Jusoh, M. Grape Juice Concentration by Progressive Freeze Concentrator Sequence System. J. Food Process. Preserv. 2016, 41, e12910. [Google Scholar] [CrossRef]
- Amran, N.A.; Samsuri, S.; Safiei, N.Z.; Zakaria, Z.Y.; Jusoh, M. Review: Parametric Study on the Performance of Progressive Cryoconcentration System. Chem. Eng. Commun. 2015, 203, 957–975. [Google Scholar] [CrossRef]
- Moran, S. Engineering science of water treatment unit operations. In An Applied Guide to Water and Effluent Treatment Plant Design; Elsevier: Amsterdam, The Netherlands, 2018; pp. 39–51. [Google Scholar]
- McKeen, L. Introduction to Food Irradiation and Medical Sterilization. Eff. Steriliz. Plast. Elastomers 2012, 1–40. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, W.N.A.W.; Selvarajah, D.; Samsuri, S. Saponin Stabilization via Progressive Freeze Concentration and Sterilization Treatment. Molecules 2021, 26, 4856. https://doi.org/10.3390/molecules26164856
Osman WNAW, Selvarajah D, Samsuri S. Saponin Stabilization via Progressive Freeze Concentration and Sterilization Treatment. Molecules. 2021; 26(16):4856. https://doi.org/10.3390/molecules26164856
Chicago/Turabian StyleOsman, Wan Nur Aisyah Wan, Dineshraj Selvarajah, and Shafirah Samsuri. 2021. "Saponin Stabilization via Progressive Freeze Concentration and Sterilization Treatment" Molecules 26, no. 16: 4856. https://doi.org/10.3390/molecules26164856






