The Inhibitory Effect of Sulforaphane on Bladder Cancer Cell Depends on GSH Depletion-Induced by Nrf2 Translocation
Abstract
:1. Introduction
2. Results
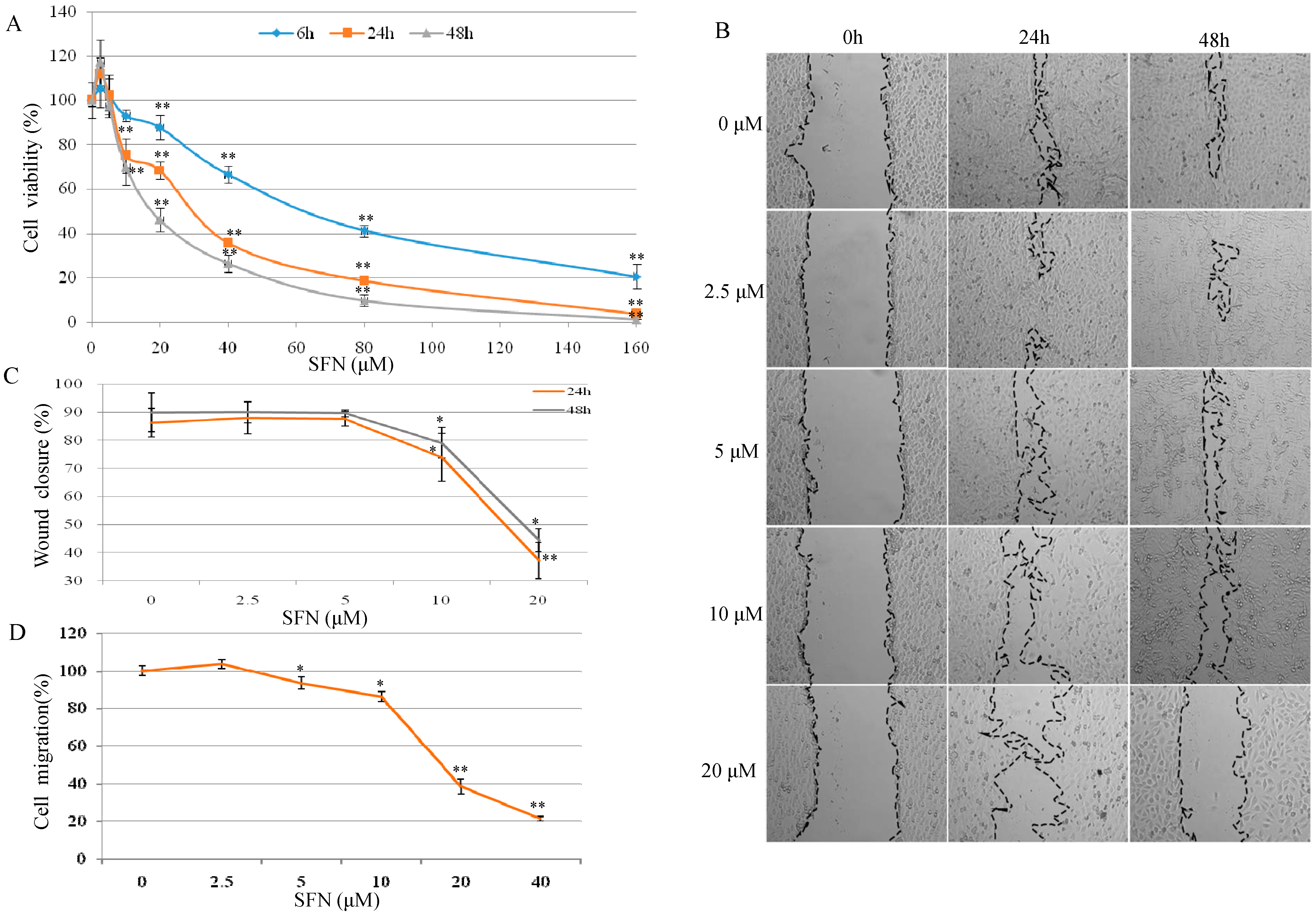
2.1. Effect of SFN on Cell Growth and Migration in T24 Cells
2.2. Effect of SFN on Nrf2 Expression and Cell Growth in T24 Cells
2.3. Effect of SFN on the Cellular Glutathione (GSH) Level in T24 Cells
2.4. Effect of γ-GCS on SFN-Induced GSH Increase
2.5. Effect of SFN on UDP-glucuronosyltransferase (UGT) and Cyclooxygenase-2 (COX-2) Expression in T24 Cells
2.6. Effect of SFN on Nrf2 Expression and Cell Growth by Targeting γ-GCS
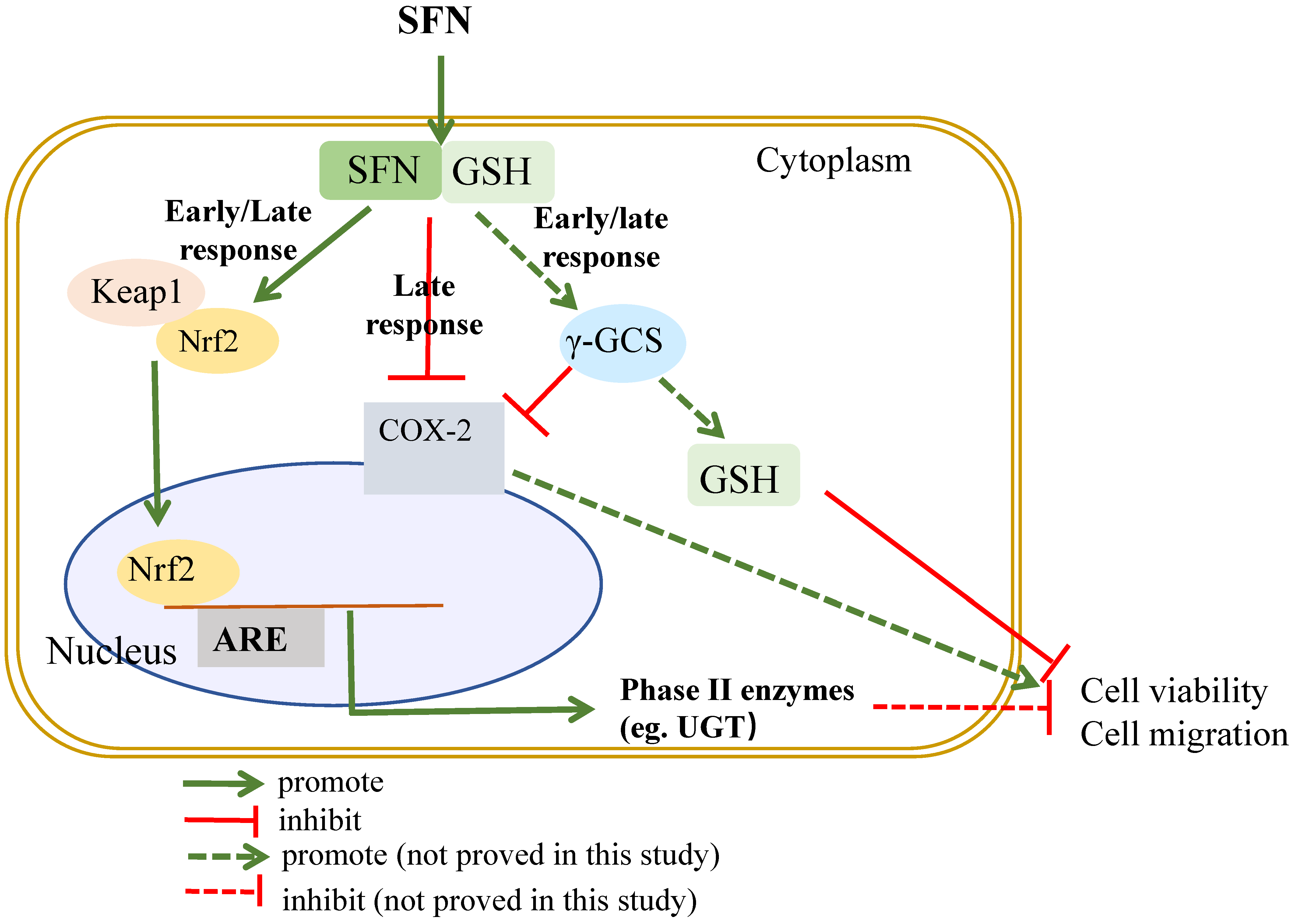
3. Discussion
4. Materials and Methods
4.1. Experiment Reagents
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Scratch Assay
4.5. Cell Migration Assay
4.6. Protein Extraction and Western Blot Analysis
4.7. HPLC Analysis of Intracellular GSH
4.8. Knockdown Gene by siRNA
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Burger, M.; Catto, J.W.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; La Vecchia, C.; Shariat, S.; et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013, 63, 234–241. [Google Scholar] [CrossRef]
- Tang, L.; Zirpoli, G.R.; Guru, K.; Moysich, K.B.; Zhang, Y.; Ambrosone, C.B.; McCann, S.E. Intake of cruciferous vegetables modifies bladder cancer survival. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1806–1811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaud, D.S.; Spiegelman, D.; Clinton, S.K.; Rimm, E.B.; Willett, W.C.; Giovannucci, E.L. Fruit and vegetable intake and incidence of bladder cancer in a male prospective cohort. J. Natl. Cancer Inst. 1999, 91, 605–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, L.; Zirpoli, G.R.; Guru, K.; Moysich, K.B.; Zhang, Y.; Ambrosone, C.B.; McCann, S.E. Consumption of raw cruciferous vegetables is inversely associated with bladder cancer risk. Cancer Epidemiol. Biomark. Prev. 2008, 17, 938–944. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Mao, Q.; Lin, Y.; Zhou, F.; Xie, L. The association of cruciferous vegetables intake and risk of bladder cancer: A meta-analysis. World J. Urol. 2013, 31, 127–133. [Google Scholar] [CrossRef]
- Larsson, S.C.; Andersson, S.O.; Johansson, J.E.; Wolk, A. Fruit and vegetable consumption and risk of bladder cancer: A prospective cohort study. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2519–2522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaud, D.S.; Pietinen, P.; Taylor, P.R.; Virtanen, M.; Virtamo, J.; Albanes, D. Intakes of fruits and vegetables, carotenoids and vitamins A, E, C in relation to the risk of bladder cancer in the ATBC cohort study. Br. J. Cancer 2002, 87, 960–965. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Zeng, X.T.; Liu, T.Z.; Zhang, C.; Yang, Z.H.; Li, S.; Chen, X.Y. Fruits and vegetables intake and risk of bladder cancer: A PRISMA-compliant systematic review and dose-response meta-analysis of prospective cohort studies. Medicine 2015, 94, e759. [Google Scholar] [CrossRef]
- Lubecka-Pietruszewska, K.; Kaufman-Szymczyk, A.; Stefanska, B.; Cebula-Obrzut, B.; Smolewski, P.; Fabianowska-Majewska, K. Sulforaphane Alone and in Combination with Clofarabine Epigenetically Regulates the Expression of DNA Methylation-Silenced Tumour Suppressor Genes in Human Breast Cancer Cells. Lifestyle Genom. 2015, 8, 91–101. [Google Scholar] [CrossRef]
- Wang, Y.; Dacosta, C.; Wang, W.; Zhou, Z.; Liu, M.; Bao, Y. Synergy between sulforaphane and selenium in protection against oxidative damage in colonic CCD841 cells. Nutr. Res. 2015, 35, 610–617. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Atkinson, S.J.; Akbareian, S.E.; Zhou, Z.; Munsterberg, A.; Robinson, S.D.; Bao, Y. Sulforaphane exerts anti-angiogenesis effects against hepatocellular carcinoma through inhibition of STAT3/HIF-1alpha/VEGF signalling. Sci. Rep. 2017, 7, 12651. [Google Scholar] [CrossRef] [Green Version]
- Fahey, J.W.; Haristoy, X.; Dolan, P.M.; Kensler, T.W.; Scholtus, I.; Stephenson, K.K.; Talalay, P.; Lozniewski, A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc. Natl. Acad. Sci. USA 2002, 99, 7610–7615. [Google Scholar] [CrossRef] [Green Version]
- Shan, Y.; Zhang, L.; Bao, Y.; Li, B.; He, C.; Gao, M.; Feng, X.; Xu, W.; Zhang, X.; Wang, S. Epithelial-mesenchymal transition, a novel target of sulforaphane via COX-2/MMP2, 9/Snail, ZEB1 and miR-200c/ZEB1 pathways in human bladder cancer cells. J. Nutr. Biochem. 2013, 24, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Labsch, S.; Liu, L.; Bauer, N.; Zhang, Y.; Aleksandrowicz, E.; Gladkich, J.; Schonsiegel, F.; Herr, I. Sulforaphane and TRAIL induce a synergistic elimination of advanced prostate cancer stem-like cells. Int. J. Oncol. 2014, 44, 1470–1480. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Kang, T.W.; Jung, Y.D.; Zhang, C.; Lian, S. Sulforaphane Inhibits Nonmuscle Invasive Bladder Cancer Cells Proliferation through Suppression of HIF-1alpha-Mediated Glycolysis in Hypoxia. J. Agric. Food Chem. 2019, 67, 7844–7854. [Google Scholar] [CrossRef]
- He, C.; Huang, L.; Lei, P.; Liu, X.; Li, B.; Shan, Y. Sulforaphane Normalizes Intestinal Flora and Enhances Gut Barrier in Mice with BBN-Induced Bladder Cancer. Mol. Nutr. Food Res. 2018, 62, e1800427. [Google Scholar] [CrossRef]
- Wang, F.; Shan, Y. Sulforaphane retards the growth of UM-UC-3 xenographs, induces apoptosis, and reduces survivin in athymic mice. Nutr. Res. 2012, 32, 374–380. [Google Scholar] [CrossRef]
- Shan, Y.; Wu, K.; Wang, W.; Wang, S.; Lin, N.; Zhao, R.; Cassidy, A.; Bao, Y. Sulforaphane down-regulates COX-2 expression by activating p38 and inhibiting NF-kappaB-DNA-binding activity in human bladder T24 cells. Int. J. Oncol. 2009, 34, 1129–1134. [Google Scholar] [PubMed]
- Zhang, Y. Cancer-preventive isothiocyanates: Measurement of human exposure and mechanism of action. Mutat. Res. 2004, 555, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Veeranki, O.L.; Bhattacharya, A.; Marshall, J.R.; Zhang, Y. Organ-specific exposure and response to sulforaphane, a key chemopreventive ingredient in broccoli: Implications for cancer prevention. Br. J. Nutr. 2013, 109, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, J.; Tang, L. Cancer-preventive isothiocyanates: Dichotomous modulators of oxidative stress. Free Radic. Biol. Med. 2005, 38, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, D.; Portales-Casamar, E.; Singh, A.; Srivastava, S.; Arenillas, D.; Happel, C.; Shyr, C.; Wakabayashi, N.; Kensler, T.W.; Wasserman, W.W.; et al. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res. 2010, 38, 5718–5734. [Google Scholar] [CrossRef]
- Zhao, H.; Lin, J.; Grossman, H.B.; Hernandez, L.M.; Dinney, C.P.; Wu, X. Dietary isothiocyanates, GSTM1, GSTT1, NAT2 polymorphisms and bladder cancer risk. Int. J. Cancer 2007, 120, 2208–2213. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Wang, W.; Zhou, Z.; Sun, C. Benefits and risks of the hormetic effects of dietary isothiocyanates on cancer prevention. PLoS ONE 2014, 9, e114764. [Google Scholar]
- He, C.; Li, B.; Song, W.; Ding, Z.; Wang, S.; Shan, Y. Sulforaphane attenuates homocysteine-induced endoplasmic reticulum stress through Nrf-2-driven enzymes in immortalized human hepatocytes. J. Agric. Food Chem. 2014, 62, 7477–7485. [Google Scholar] [CrossRef]
- Liu, P.; Wang, W.; Tang, J.; Bowater, R.P.; Bao, Y. Antioxidant effects of sulforaphane in human HepG2 cells and immortalised hepatocytes. Food Chem. Toxicol. 2019, 128, 129–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y. Molecular mechanism of rapid cellular accumulation of anticarcinogenic isothiocyanates. Carcinogenesis 2001, 22, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y. The molecular basis that unifies the metabolism, cellular uptake and chemopreventive activities of dietary isothiocyanates. Carcinogenesis 2012, 33, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Kim, B.; Kim, S.H.; Srivastava, S.K. Molecular targets of isothiocyanates in cancer: Recent advances. Mol. Nutr. Food Res. 2014, 58, 1685–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, Y.; Wang, X.; Wang, W.; He, C.; Bao, Y. p38 MAPK plays a distinct role in sulforaphane-induced up-regulation of ARE-dependent enzymes and down-regulation of COX-2 in human bladder cancer cells. Oncol. Rep. 2010, 23, 1133–1138. [Google Scholar]
- Bourn, J.; Rathore, K.; Donnell, R.; White, W.; Uddin, M.J.; Marnett, L.; Cekanova, M. Detection of carcinogen-induced bladder cancer by fluorocoxib A. BMC Cancer 2019, 19, 1152. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; Sanchez, A.; Pilar Martinez, M.; Benedito, S.; Lopez-Oliva, M.E.; Garcia-Sacristan, A.; Hernandez, M.; Prieto, D. COX-2 is involved in vascular oxidative stress and endothelial dysfunction of renal interlobar arteries from obese Zucker rats. Free Radic. Biol. Med. 2015, 84, 77–90. [Google Scholar] [CrossRef]
- Liu, P.; Behray, M.; Wang, Q.; Wang, W.; Zhou, Z.; Chao, Y.; Bao, Y. Anti-cancer activities of allyl isothiocyanate and its conjugated silicon quantum dots. Sci. Rep. 2018, 8, 1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabrese, E.J.; Kozumbo, W.J. The phytoprotective agent sulforaphane prevents inflammatory degenerative diseases and age-related pathologies via Nrf2-mediated hormesis. Pharmacol. Res. 2021, 163, 105283. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, C.; Buongiorno, L.P.; Wang, W.; Tang, J.C.Y.; Miceli, N.; Taviano, M.F.; Shan, Y.; Bao, Y. The Inhibitory Effect of Sulforaphane on Bladder Cancer Cell Depends on GSH Depletion-Induced by Nrf2 Translocation. Molecules 2021, 26, 4919. https://doi.org/10.3390/molecules26164919
He C, Buongiorno LP, Wang W, Tang JCY, Miceli N, Taviano MF, Shan Y, Bao Y. The Inhibitory Effect of Sulforaphane on Bladder Cancer Cell Depends on GSH Depletion-Induced by Nrf2 Translocation. Molecules. 2021; 26(16):4919. https://doi.org/10.3390/molecules26164919
Chicago/Turabian StyleHe, Canxia, Luigina P. Buongiorno, Wei Wang, Jonathan C. Y. Tang, Natalizia Miceli, Maria Fernanda Taviano, Yujuan Shan, and Yongping Bao. 2021. "The Inhibitory Effect of Sulforaphane on Bladder Cancer Cell Depends on GSH Depletion-Induced by Nrf2 Translocation" Molecules 26, no. 16: 4919. https://doi.org/10.3390/molecules26164919





