Chemometric Analysis of Fatty Acid Composition of Raw Chicken, Beef, and Pork Meat with Plant Extract Addition during Refrigerated Storage
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Plant Extract Preparation and Characterization
4.3. Meat Sample Preparation and Storage
4.4. Fatty Acid Profiles
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD Food of the United Nations Agricultural Outlook. OECD-FAO Agricultural Outlook 2020–2029; OECD-FAO Agricultural Outlook; FAO: Rom, Italy; OECD Publishing: Paris, France, 2020; ISBN 9789264317673. [Google Scholar]
- Marangoni, F.; Corsello, G.; Cricelli, C.; Ferrara, N.; Ghiselli, A.; Lucchin, L.; Poli, A. Role of poultry meat in a balanced diet aimed at maintaining health and wellbeing: An Italian consensus document. Food Nutr. Res. 2015, 59, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohrer, B.M. Review: Nutrient density and nutritional value of meat products and non-meat foods high in protein. Trends Food Sci. Technol. 2017, 65, 103–112. [Google Scholar] [CrossRef]
- McAfee, A.J.; McSorley, E.M.; Cuskelly, G.J.; Moss, B.W.; Wallace, J.M.W.; Bonham, M.P.; Fearon, A.M. Red meat consumption: An overview of the risks and benefits. Meat Sci. 2010, 84, 1–13. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef] [Green Version]
- Klensporf-Pawlik, D.; Szydlowski, M.; Kaczmarek, A.; Nowacka-Woszuk, J.; Switonski, M.; Jeleń, H. The fatty acid composition of the Longissimus dorsi muscle, subcutaneous and visceral fats differ in four commercial pig breeds. J. Anim. Feed Sci. 2012, 21, 661–676. [Google Scholar] [CrossRef]
- Gruffat, D.; Bauchart, D.; Thomas, A.; Parafita, E.; Durand, D. Fatty acid composition and oxidation in beef muscles as affected by ageing times and cooking methods. Food Chem. 2021, 343. [Google Scholar] [CrossRef]
- Medić, H.; Djurkin Kušec, I.; Pleadin, J.; Kozačinski, L.; Njari, B.; Hengl, B.; Kušec, G. The impact of frozen storage duration on physical, chemical and microbiological properties of pork. Meat Sci. 2018, 140, 119–127. [Google Scholar] [CrossRef]
- Waheed, S.; Hasnain, A.; Ahmad, A.; Tarar, O.M.; Yaqeen, Z.; Ali, T.M. Effect of botanical extracts on amino acid and fatty acid profile of broiler meat. Rev. Bras. Cienc. Avic. 2018, 20, 507–516. [Google Scholar] [CrossRef]
- Narciso-Gaytán, C.; Shin, D.; Sams, A.R.; Keeton, J.T.; Miller, R.K.; Smith, S.B.; Sánchez-Plata, M.X.; León, M. Lipid oxidation stability of omega-3-and conjugated linoleic acid-enriched sous vide chicken meat. Poult. Sci. 2011, 90, 473–480. [Google Scholar] [CrossRef] [Green Version]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Badiani, A.; Stipa, S.; Bitossi, F.; Gatta, P.P.; Vignola, G.; Chizzolini, R. Lipid composition, retention and oxidation in fresh and completely trimmed beef muscles as affected by common culinary practices. Meat Sci. 2002, 60, 169–186. [Google Scholar] [CrossRef]
- Huang, Y.; He, Z.; Li, H.; Li, F.; Wu, Z. Effect of antioxidant on the fatty acid composition and lipid oxidation of intramuscular lipid in pressurized pork. Meat Sci. 2012, 91, 137–141. [Google Scholar] [CrossRef]
- Chmiel, M.; Roszko, M.; Adamczak, L.; Florowski, T.; Pietrzak, D. Influence of storage and packaging method on chicken breast meat chemical composition and fat oxidation. Poult. Sci. 2019, 98, 2679–2690. [Google Scholar] [CrossRef]
- Xiong, Q.; Zhang, M.; Wang, T.; Wang, D.; Sun, C.; Bian, H.; Li, P.; Zou, Y.; Xu, W. Lipid oxidation induced by heating in chicken meat and the relationship with oxidants and antioxidant enzymes activities. Poult. Sci. 2020, 99, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orkusz, A.; Woloszyn, J.; Grajeta, H.; Haraf, G.; Okruszek, A. Changes in the fatty acid profile of intramuscular fat in goose meat packed in different atmospheres. Eur. Poult. Sci. 2015, 79, 1–10. [Google Scholar] [CrossRef]
- Cenci-Goga, B.T.; Karama, M.; Hadjichralambous, C.; Sechi, P.; Grispoldi, L. Is EU regulation on the use of antioxidants in meat preparation and in meat products still cutting edge? Eur. Food Res. Technol. 2020, 246, 661–668. [Google Scholar] [CrossRef]
- Oswell, N.J.; Thippareddi, H.; Pegg, R.B. Practical use of natural antioxidants in meat products in the U.S.: A review. Meat Sci. 2018, 145, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Haugaard, P.; Hansen, F.; Jensen, M.; Grunert, K.G. Consumer attitudes toward new technique for preserving organic meat using herbs and berries. Meat Sci. 2014, 96, 126–135. [Google Scholar] [CrossRef] [PubMed]
- UNESCO. Science for Food Safety and Sustainable Availability in Conservation Techniques. Preservatives and Antioxidants; UNESCO: Madrit, Barcelona, 2017. [Google Scholar]
- Rhee, K.S.; Anderson, L.M.; Sams, A.R. Lipid oxidation potential of beef, chicken, and pork. J. Food Sci. 1996, 61, 8–12. [Google Scholar] [CrossRef]
- Ölmez, M.; Şahin, T.; Karadağoğlu, Ö.; Yörük, M.A.; Kara, K.; Dalğa, S. Growth performance, carcass characteristics, and fatty acid composition of breast and thigh meat of broiler chickens fed gradually increasing levels of supplemental blueberry extract. Trop. Anim. Health Prod. 2021, 53. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Muzolf-Panek, M. Predictive Modeling of Changes in TBARS in the Intramuscular Lipid Fraction of Raw Ground Beef Enriched with Plant Extracts. Antioxidants 2021, 10, 736. [Google Scholar] [CrossRef] [PubMed]
- Muzolf-Panek, M.; Kaczmarek, A.; Tomaszewska-Gras, J.; Cegielska-Radziejewska, R.; Szablewski, T.; Majcher, M.; Stuper-Szablewska, K. A Chemometric Approach to Oxidative Stability and Physicochemical Quality of Raw Ground Chicken Meat Affected by Black Seed and Other Spice Extracts. Antioxidants 2020, 9, 903. [Google Scholar] [CrossRef] [PubMed]
- Muzolf-Panek, M.; Kaczmarek, A.; Tomaszewska-Gras, J.; Cegielska-Radziejewska, R.; Majcher, M. Oxidative and microbiological stability of raw ground pork during chilled storage as affected by Plant extracts. Int. J. Food Prop. 2019, 22, 111–129. [Google Scholar] [CrossRef] [Green Version]
- Muzolf-Panek, M.; Stuper-Szablewska, K. Comprehensive study on the antioxidant capacity and phenolic profiles of black seed and other spices and herbs: Effect of solvent and time of extraction. J. Food Meas. Charact. 2021. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, Z.; Zhou, Y.; Wei, H.; Zhang, X.; Xia, M.; Deng, Z.; Zou, Y.; Jiang, S.; Peng, J. Effect of oregano essential oil supplementation to a reduced-protein, amino acid-supplemented diet on meat quality, fatty acid composition, and oxidative stability of Longissimus thoracis muscle in growing-finishing pigs. Meat Sci. 2017, 133, 103–109. [Google Scholar] [CrossRef]
- Yagoubi, Y.; Joy, M.; Ripoll, G.; Mahouachi, M.; Bertolín, J.R.; Atti, N. Rosemary distillation residues reduce lipid oxidation, increase alpha-tocopherol content and improve fatty acid profile of lamb meat. Meat Sci. 2018, 136, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Patra, A.K.; Mandal, G.P.; Debnath, B.C. Carcass characteristics, chemical and fatty acid composition and oxidative stability of meat from broiler chickens fed black cumin (Nigella sativa) seeds. J. Anim. Physiol. Anim. Nutr. 2018, 102, 769–779. [Google Scholar] [CrossRef]
- Kaczmarek, A.M.; Muzolf-Panek, M.; Rudzińska, M.; Szablewski, T.; Cegielska-Radziejewska, R. The effect of plant extracts on pork quality during storage. Ital. J. Food Sci 2017, 29, 644–656. [Google Scholar]
- Linares, M.B.; Cózar, A.; Garrido, M.D.; Vergara, H. Nutritional Attributes and Sensory Quality during Storage Time of Spiced Lamb Burgers from ManchegoSpanish Breed. Foods 2020, 9, 1466. [Google Scholar] [CrossRef]
- Holman, B.W.B.; Coombs, C.E.O.; Morris, S.; Bailes, K.; Hopkins, D.L. Effect of long term chilled (up to 5 weeks) then frozen (up to 12 months) storage at two di ff erent sub-zero holding temperatures on beef: 2. Lipid oxidation and fatty acid profiles. Meat Sci. 2018, 136, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tisues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
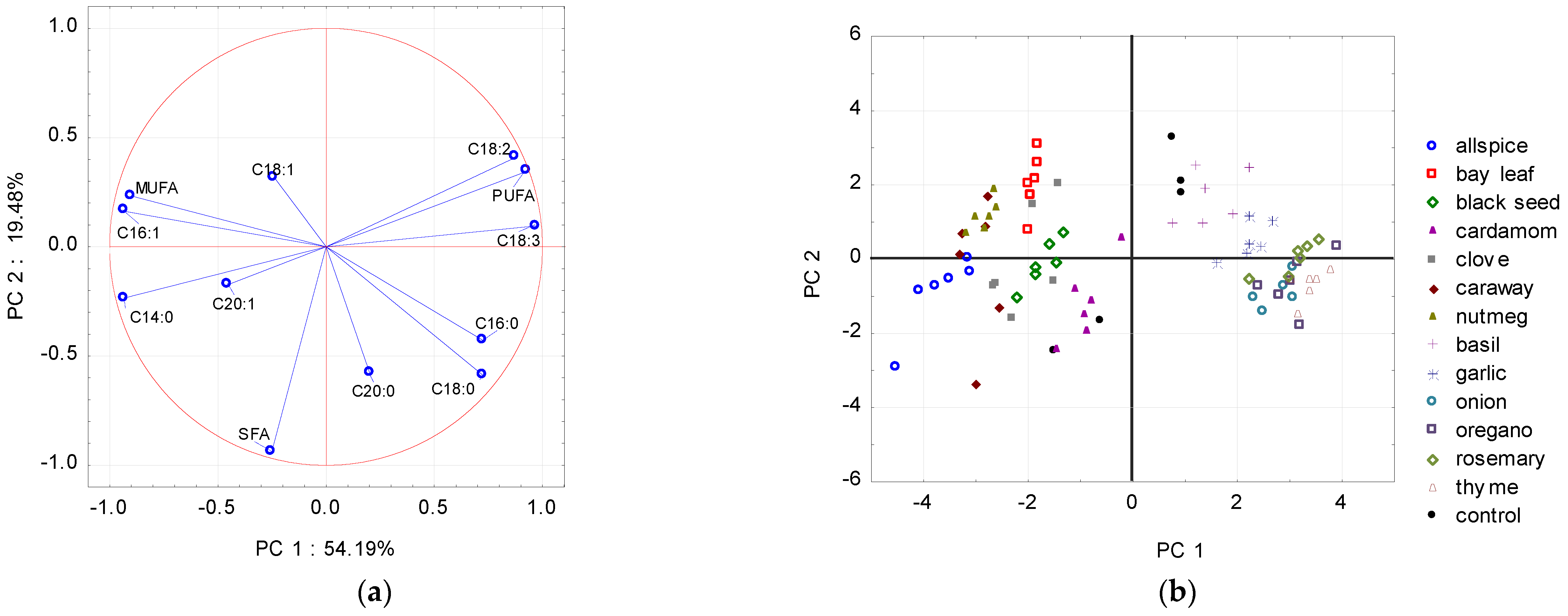
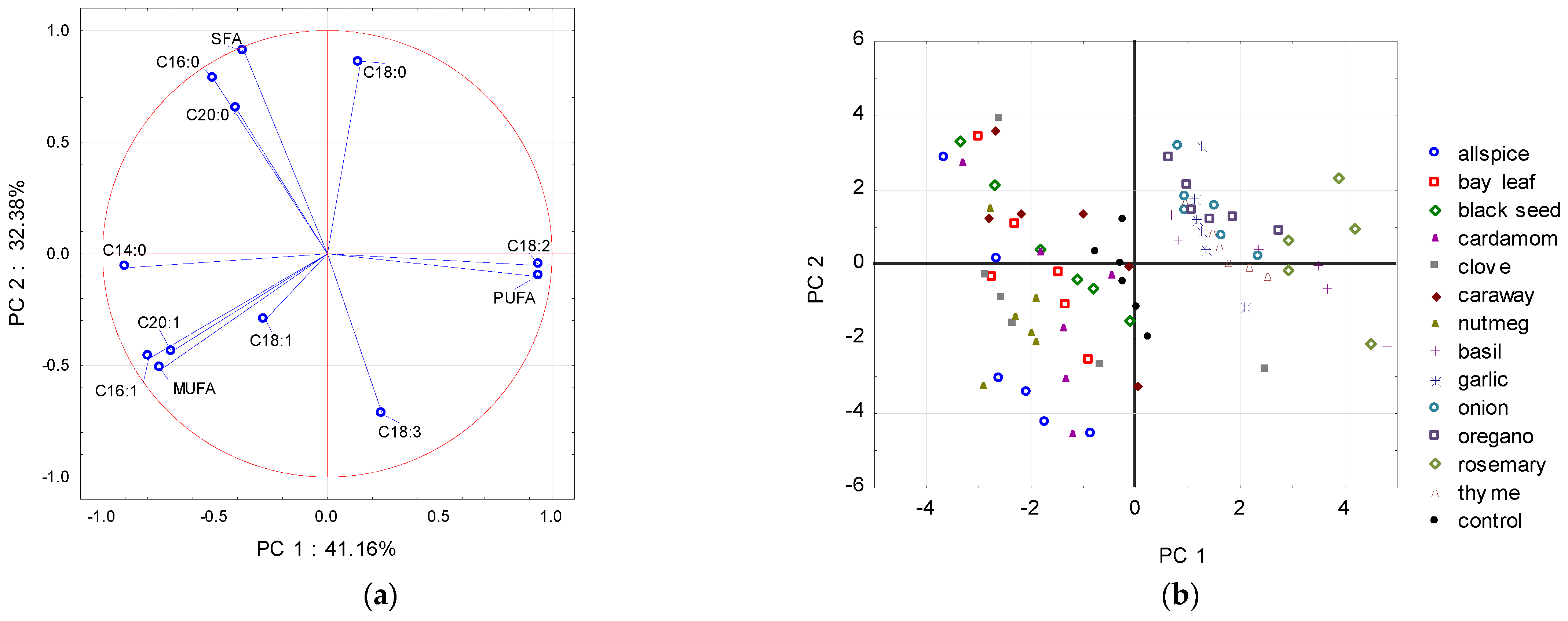

| Spice/Herb | C14:0 | C16:0 | C16:1 * | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 | C20:1 * | SFA | MUFA | PUFA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 1.72 b | 21.42 e | 5.88 cd | 4.89 de | 39.59 def | 20.49 c | 2.00 e | 0.14 b | 0.33 ab | 30.40 de | 46.15 d | 23.29 de |
| Allspice | 2.59 e | 20.86 cd | 6.68 f | 4.61 cd | 39.80 ef | 18.42 a | 1.68 a | 0.01 a | 0.49 bc | 31.58 g | 47.59 e | 20.66 a |
| Basil | 0.95 a | 21.34 e | 5.69 bc | 5.20 ef | 40.00 ef | 22.17 ef | 2.15 f | 0.05 ab | 0.31 a | 28.49 a | 46.19 d | 25.25 fg |
| Bay leaf | 2.06 c | 21.01 d | 6.44 def | 3.13 a | 40.09 f | 21.09 d | 1.82 bc | 0.01 a | 0.42 abc | 28.96 ab | 47.41 e | 23.47 e |
| Black seed | 2.22 cd | 20.60 abc | 6.32 c–f | 4.84 d | 38.83 b | 20.64 cd | 1.75 ab | 0.01 a | 0.52 c | 30.55 e | 46.24 d | 23.04 de |
| Cardamom | 2.29 d | 20.82 cd | 5.99 cde | 5.34 fg | 38.21 a | 20.41 c | 1.89 d | 0.02 a | 0.48 bc | 31.45 fg | 45.22 c | 22.98 de |
| Clove | 2.41 de | 20.30 a | 6.73 f | 4.46 c | 38.65 ab | 20.43 c | 1.83 cd | 0.04 a | 0.48 bc | 30.52 de | 46.43 d | 22.90 cd |
| Caraway | 2.31 d | 20.70 bcd | 6.59 ef | 4.12 b | 39.83 ef | 19.37 b | 1.68 a | 0.09 ab | 0.45 abc | 30.49 de | 47.46 e | 21.69 b |
| Garlic | 1.03 a | 21.80 f | 5.11 b | 5.52 gh | 39.49 cde | 22.02 ef | 2.22 fg | 0.07 ab | 0.34 ab | 29.45 bc | 45.14 bc | 25.33 fg |
| Nutmeg | 2.36 d | 20.48 ab | 6.97 f | 3.91 b | 39.77 ef | 20.17 c | 1.71 a | 0.01 a | 0.39 abc | 29.70 bcd | 47.66 e | 22.44 c |
| Onion | 1.12 a | 22.26 h | 4.37 a | 5.99 ij | 39.10 bcd | 21.83 e | 2.24 gh | 0.06 ab | 0.40 abc | 30.62 ef | 44.14 a | 25.17 f |
| Oregano | 1.05 a | 22.01 fgh | 4.23 a | 6.11 j | 39.12 bcd | 22.14 ef | 2.24 gh | 0.08 ab | 0.40 abc | 30.34 de | 43.99 a | 25.67 fg |
| Rosemary | 1.11 a | 21.89 fg | 4.46 a | 5.71 hi | 39.19 bcd | 22.35 f | 2.35 i | 0.07 ab | 0.35 ab | 29.93 cde | 44.22 ab | 25.78 g |
| Thyme | 1.10 a | 22.17 gh | 4.12 a | 6.01 ij | 38.96 bc | 22.24 ef | 2.29 hi | 0.07 ab | 0.36 ab | 30.52 de | 43.70 a | 25.66 fg |
| Spice/Herb | C14:0 * | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 | C20:1 | SFA | MUFA | PUFA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 1.43 b | 25.14 abc | 3.16 b | 14.46 de | 45.05 cd | 8.54 abc | 0.60 efg | 0.12 abc | 0.78 b | 41.15 abc | 48.99 def | 9.14 abc |
| Allspice | 1.68 c | 25.30 bc | 4.68 d | 13.11 a | 45.28 d | 7.80 a | 0.54 b–e | 0.10 ab | 0.92 bc | 40.20 a | 50.88 g | 8.35 a |
| Basil | 1.22 a | 24.46 a | 2.21 a | 14.75 ef | 44.77 cd | 10.28 d | 0.56 cde | 0.09 a | 0.50 a | 40.52 a | 47.48 bc | 10.84 d |
| Bay leaf | 1.66 c | 25.83 cd | 3.69 bc | 14.58 de | 44.55 cd | 7.41 a | 0.64 fg | 0.13 bc | 0.97 c | 42.19 cd | 49.21 ef | 8.05 a |
| Black seed | 1.65 c | 26.13 d | 3.53 bc | 14.23 cd | 44.28 bc | 7.96 a | 0.59 def | 0.15 c | 0.92 bc | 42.16 cd | 48.73 c–f | 8.54 a |
| Cardamom | 1.66 c | 25.71 cd | 3.72 bc | 13.51 ab | 45.28 d | 8.00 a | 0.53 bcd | 0.09 a | 0.87 bc | 40.97 ab | 49.86 efg | 8.53 a |
| Clove | 1.67 c | 25.48 cd | 4.03 cd | 13.80 bc | 44.56 cd | 8.34 ab | 0.54 bcd | 0.11 abc | 0.89 bc | 41.06 abc | 49.48 efg | 8.88 ab |
| Caraway | 1.63 c | 25.83 cd | 3.95 bcd | 15.14 f | 43.63 ab | 7.65 a | 0.54 b–e | 0.09 a | 0.92 bc | 42.69 d | 48.50 cde | 8.19 a |
| Garlic | 1.31 ab | 25.88 cd | 2.18 a | 14.46 de | 44.99 cd | 9.35 bcd | 0.50 abc | 0.09 ab | 0.40 a | 41.74 bcd | 47.57 bcd | 9.85 bcd |
| Nutmeg | 1.88 d | 25.40 bcd | 4.20 cd | 13.65 b | 44.89 cd | 7.77 a | 0.59 def | 0.11 ab | 0.94 bc | 41.02 abc | 50.03 fg | 8.36 a |
| Onion | 1.32 ab | 25.86 cd | 2.07 a | 14.57 de | 45.00 cd | 9.44 bcd | 0.45 a | 0.11 ab | 0.37 a | 41.86 bcd | 47.43 bc | 9.89 bcd |
| Oregano | 1.31 ab | 25.87 cd | 2.25 a | 14.85 ef | 44.41 bcd | 9.40 bcd | 0.49 ab | 0.10 ab | 0.42 a | 42.13 bcd | 47.07 ab | 9.89 bcd |
| Rosemary | 1.25 a | 24.59 ab | 2.14 a | 14.57 de | 43.15 a | 11.60 e | 0.66 g | 0.10 ab | 0.52 a | 40.51 a | 45.80 a | 12.27 e |
| Thyme | 1.30 ab | 25.34 bcd | 2.26 a | 14.40 de | 44.96 cd | 9.73 cd | 0.56 b–e | 0.11 ab | 0.45 a | 41.14 abc | 47.66 bcd | 10.28 cd |
| Spice/Herb | C14:0 | C16:0 * | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 | C20:1 * | SFA * | MUFA * | PUFA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 2.11 e | 26.55 e | 2.22 a | 17.87 d | 45.87 c | 3.05 bf | 0.43 c | 0.07 a | 0.18 a | 47.38 c | 48.27 cd | 3.73 b |
| Allspice | 1.89 bc | 25.67 cd | 2.36 ab | 17.17 c | 48.00 ef | 3.17 bcf | 0.56 de | 0.08 a | 0.16 a | 45.69 b | 50.52 fg | 4.25 c |
| Basil | 2.26 f | 26.82 e | 2.99 c | 20.04 gh | 44.10 b | 2.35 a | 0.20 a | 0.08 a | 0.18 a | 49.96 de | 47.27 abc | 2.80 a |
| Bay leaf | 1.95 d | 25.64 cd | 2.35 ab | 17.21 c | 46.92 cde | 3.84 d | 0.62 fg | 0.08 a | 0.18 a | 45.58 b | 49.45 def | 4.69 de |
| Black seed | 1.72 a | 25.19 ab | 2.34 ab | 16.60 b | 47.99 ef | 4.56 e | 0.51 d | 0.08 a | 0.17 a | 44.39 a | 50.49 fg | 5.51 f |
| Cardamom | 1.91 cd | 26.00 d | 2.23 a | 18.16 d | 46.71 cd | 3.20 bc | 0.58 ef | 0.08 a | 0.15 a | 46.95 c | 49.09 de | 4.37 cd |
| Clove | 1.85 b | 25.05 a | 2.19 a | 16.65 b | 47.15 def | 3.96 d | 0.65 g | 0.08 a | 0.22 a | 44.45 a | 49.56 ef | 4.92 e |
| Caraway | 1.95 cd | 25.55 bc | 2.22 a | 17.36 c | 47.56 def | 3.43 c | 0.60 efg | 0.08 a | 0.16 a | 45.73 b | 49.94 efg | 4.33 cd |
| Garlic | 2.56 i | 27.59 f | 3.19 d | 19.22 f | 43.38 ab | 2.38 a | 0.28 b | 0.08 a | 0.18 a | 50.26 e | 46.75 ab | 2.88 a |
| Nutmeg | 2.21 f | 25.94 cd | 2.49 b | 16.10 a | 48.13 f | 3.16 bc | 0.71 i | 0.08 a | 0.18 a | 45.07 ab | 50.80 g | 4.05 bc |
| Onion | 2.46 h | 27.31 f | 3.07 cd | 19.68 g | 43.46 ab | 2.43 a | 0.25 ab | 0.09 a | 0.18 a | 50.32 e | 46.71 ab | 2.89 a |
| Oregano | 2.36 g | 27.37 f | 3.17 cd | 18.81 e | 44.05 b | 2.59 af | 0.27 b | 0.08 a | 0.18 a | 49.40 d | 47.39 bc | 3.12 a |
| Rosemary | 2.48 h | 27.63 f | 3.17 cd | 19.21 f | 43.45 ab | 2.42 af | 0.27 b | 0.08 a | 0.18 a | 50.20 e | 46.79 ab | 2.93 a |
| Thyme | 2.42 h | 27.38 f | 3.04 cd | 20.35 h | 42.87 a | 2.33 af | 0.25 ab | 0.09 a | 0.18 a | 51.02 f | 46.08 a | 2.79 a |
| Allspice | Basil | Bay Leaf | Black Seed | Caraway | Cardamom | Clove | Garlic | Nutmeg | Onion | Oregano | Rosemary | Thyme | Control | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chicken | ||||||||||||||
| SFA | 0.182 * | 0.216 * | 0.189 * | 0.161 * | 0.235 * | 0.184 * | 0.303 * | 0.148 * | 0.120 * | 0.100 * | 0.153 * | 0.111 * | 0.078 * | 0.747 |
| MUFA | 0.038 * | 0.167 N | −0.012 * | 0.056 * | −0.096 | 0.038 | 0.120 *N | 0.115 * | 0.053 * | 0.076 * | 0.053 * | 0.117 * | 0.026 * | −0.065 |
| PUFA | −0.193 * | −0.093 * | −0.080 * | −0.123 * | −0.126 * | −0.115 * | −0.171 * | −0.100 * | −0.055 * | −0.085 * | −0.128 * | −0.130 * | −0.094 * | −0.458 |
| Pork | ||||||||||||||
| SFA | 0.502 * | 0.296 * | 0.364 * | 0.367 * | 0.431 * | 0.439 * | 0.399 * | 0.188 | 0.239 | 0.195 | 0.146 | 0.147 | 0.178 | 0.145 |
| MUFA | −0.309 | 0.225 * | −0.075 N | 0.149 * | 0.151 *N | −0.268 * | 0.146 N | −0.179 | −0.272 * | 0.081 * | 0.158 * | −0.343 * | 0.120 * | −0.128 |
| PUFA | −0.147 | −0.240 * | −0.048 | −0.097 | −0.053 | −0.071 N | −0.285 | −0.064 | −0.058 | −0.089 | −0.117 | −0.211 | −0.063 | −0.099 |
| Beef | ||||||||||||||
| SFA | 0.178 | 0.165 | 0.179 | 0.213 | 0.200 | 0.259 * | 0.161 | 0.109 * | 0.167 | 0.203 | 0.222 | 0.134 | 0.212 | 0.171 |
| MUFA | −0.110 * | −0.015 * | −0.190 | −0.145 | −0.168 | −0.047 N | 0.082 * | −0.042 * | 0.013 * | −0.091 | −0.144 | −0.125 | −0.110 | −0.172 |
| PUFA | −0.047 | −0.049 | 0.027 * | −0.094 * | −0.037 | −0.080 | −0.072 | −0.037 | −0.027 | −0.044 | −0.068 | −0.023 | −0.037 | −0.045 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muzolf-Panek, M.; Kaczmarek, A. Chemometric Analysis of Fatty Acid Composition of Raw Chicken, Beef, and Pork Meat with Plant Extract Addition during Refrigerated Storage. Molecules 2021, 26, 4952. https://doi.org/10.3390/molecules26164952
Muzolf-Panek M, Kaczmarek A. Chemometric Analysis of Fatty Acid Composition of Raw Chicken, Beef, and Pork Meat with Plant Extract Addition during Refrigerated Storage. Molecules. 2021; 26(16):4952. https://doi.org/10.3390/molecules26164952
Chicago/Turabian StyleMuzolf-Panek, Małgorzata, and Anna Kaczmarek. 2021. "Chemometric Analysis of Fatty Acid Composition of Raw Chicken, Beef, and Pork Meat with Plant Extract Addition during Refrigerated Storage" Molecules 26, no. 16: 4952. https://doi.org/10.3390/molecules26164952
APA StyleMuzolf-Panek, M., & Kaczmarek, A. (2021). Chemometric Analysis of Fatty Acid Composition of Raw Chicken, Beef, and Pork Meat with Plant Extract Addition during Refrigerated Storage. Molecules, 26(16), 4952. https://doi.org/10.3390/molecules26164952







