Preparation of Curcumin-Eudragit® E PO Solid Dispersions with Gradient Temperature through Hot-Melt Extrusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermal Analysis
2.3. Miscibility Study of Drug and Carriers
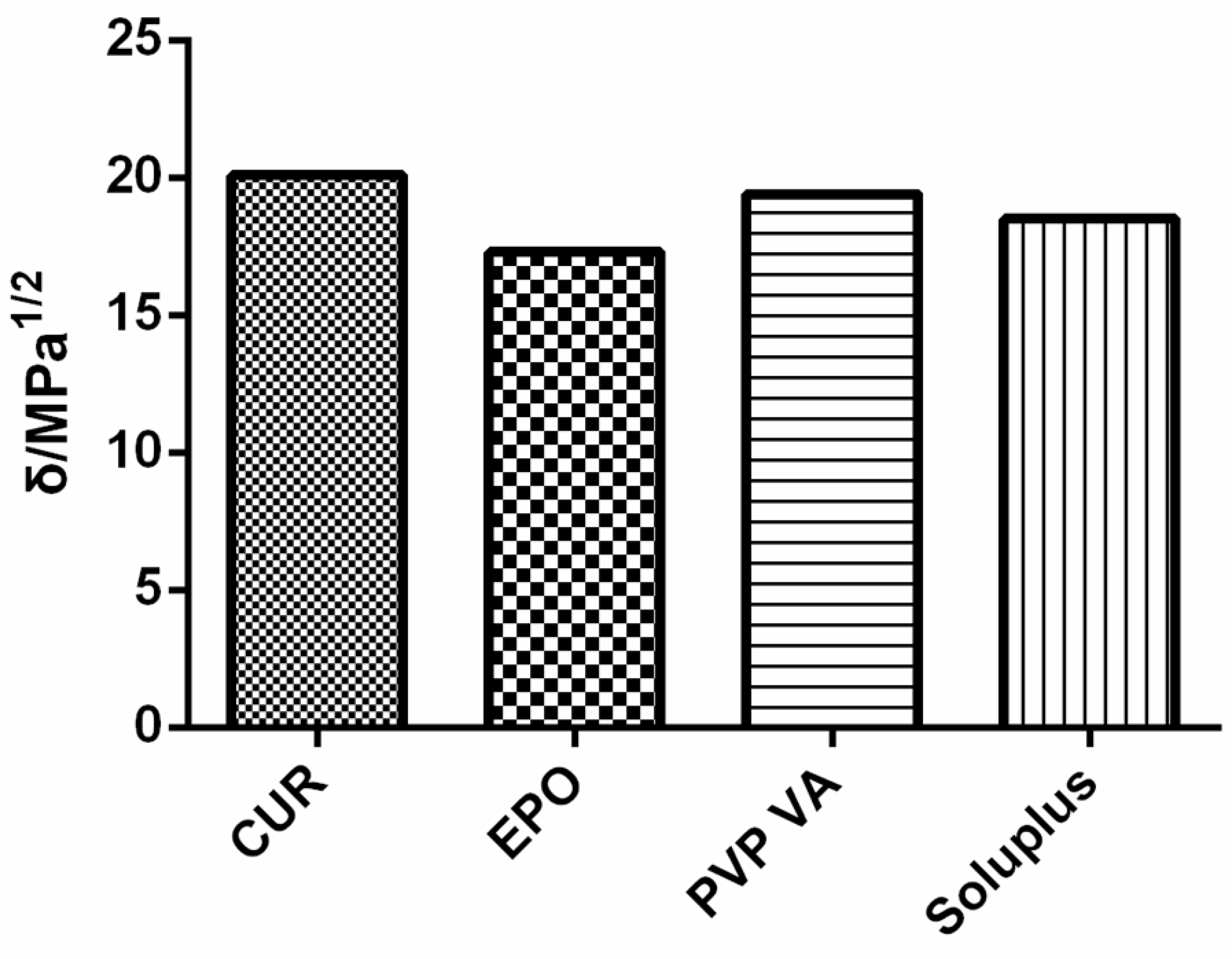
Solubility Parameters
2.4. The Preparation of the Cur-EPO System Using HME
2.4.1. Preparation of Physical Mixture (PM)
2.4.2. Preparation of Cur-EPO SD
2.5. Determination of Drug Content
2.6. X-ray Powder Diffraction (XRD)
2.7. Fourier Transform Infrared Spectroscopy (FTIR)
2.8. In Vitro Dissolution Study
2.9. Equilibrium Solubility Study
2.10. Accelerate Stability Study
2.11. In Vivo Pharmacokinetic Studies
3. Results and Discussion
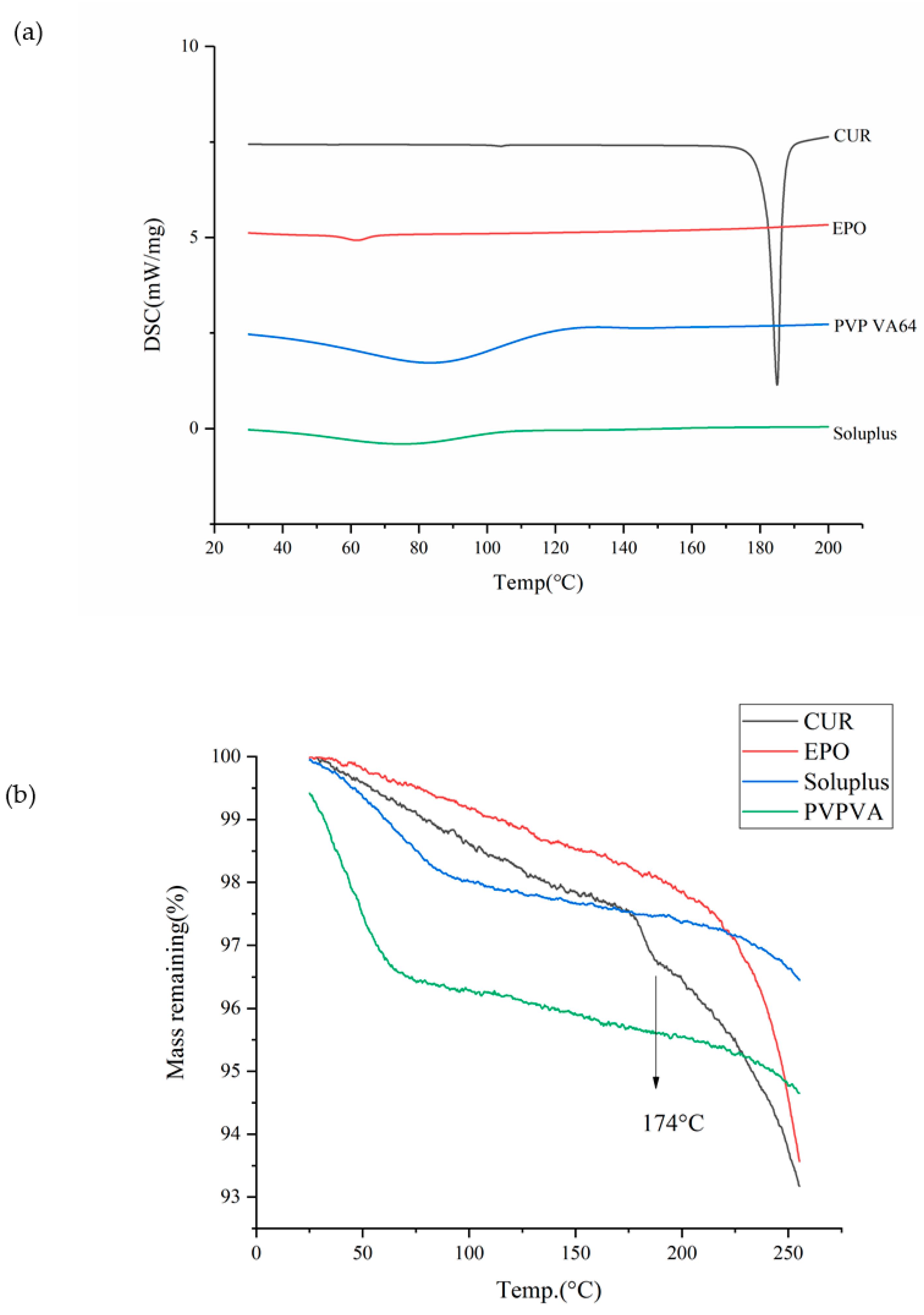
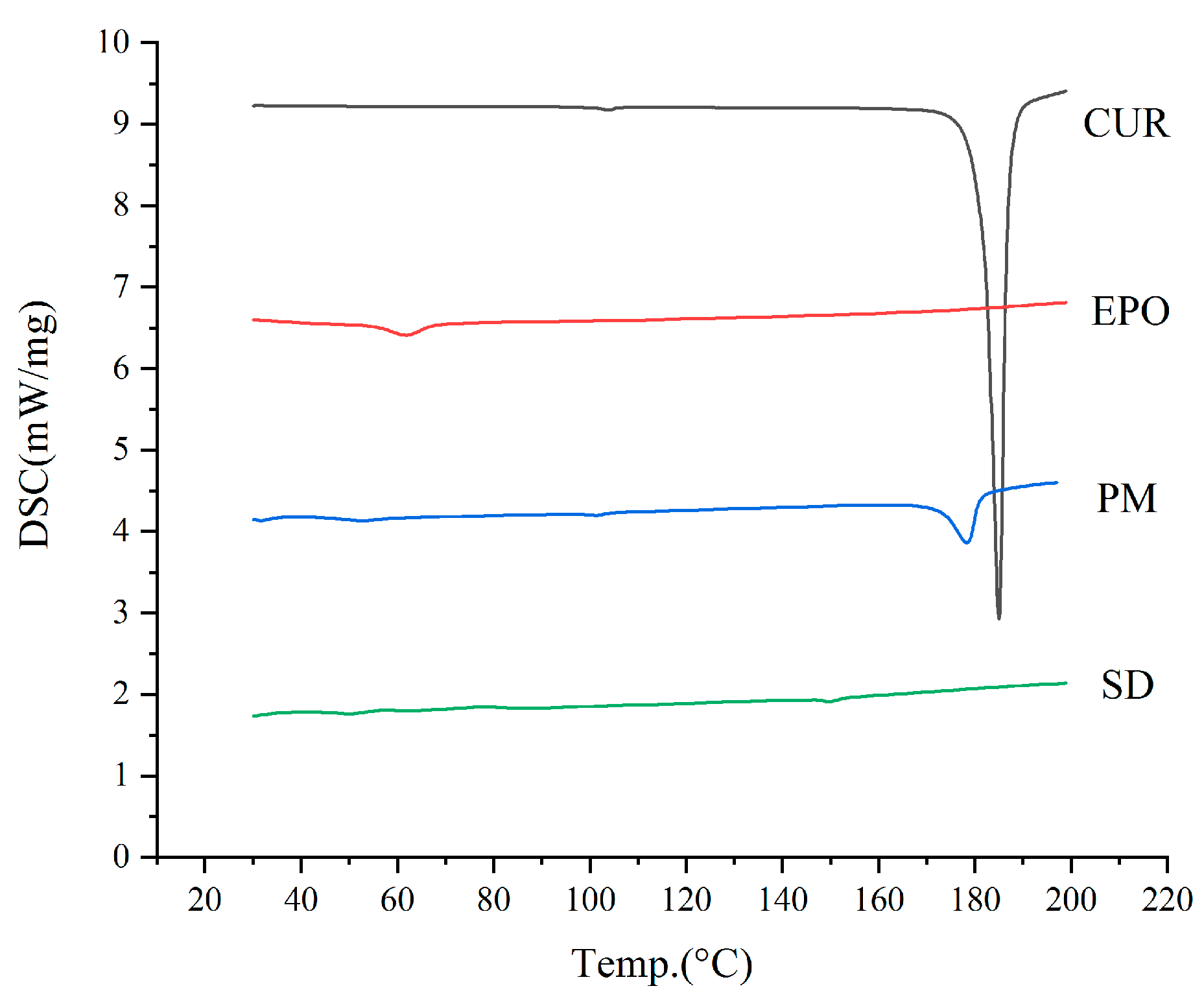
3.1. Thermal Analysis
3.2. Miscibility Study
- Solubility Parameters
3.3. Preparation of Cur-EPO SD
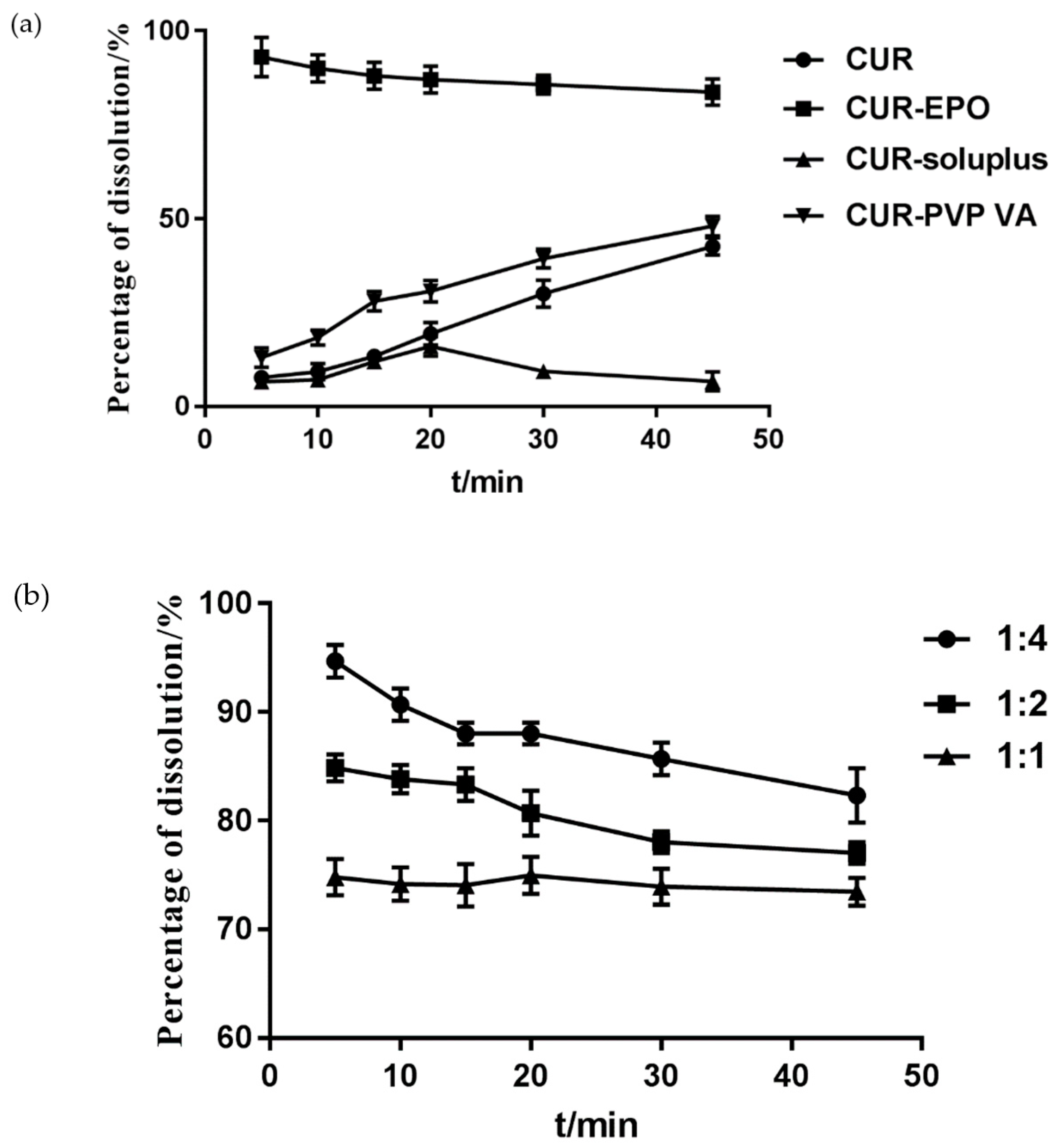
3.3.1. The Determination of the Carrier and the Ratio of the Carrier and the Drug
3.3.2. Single Factor Test for Preparation Process
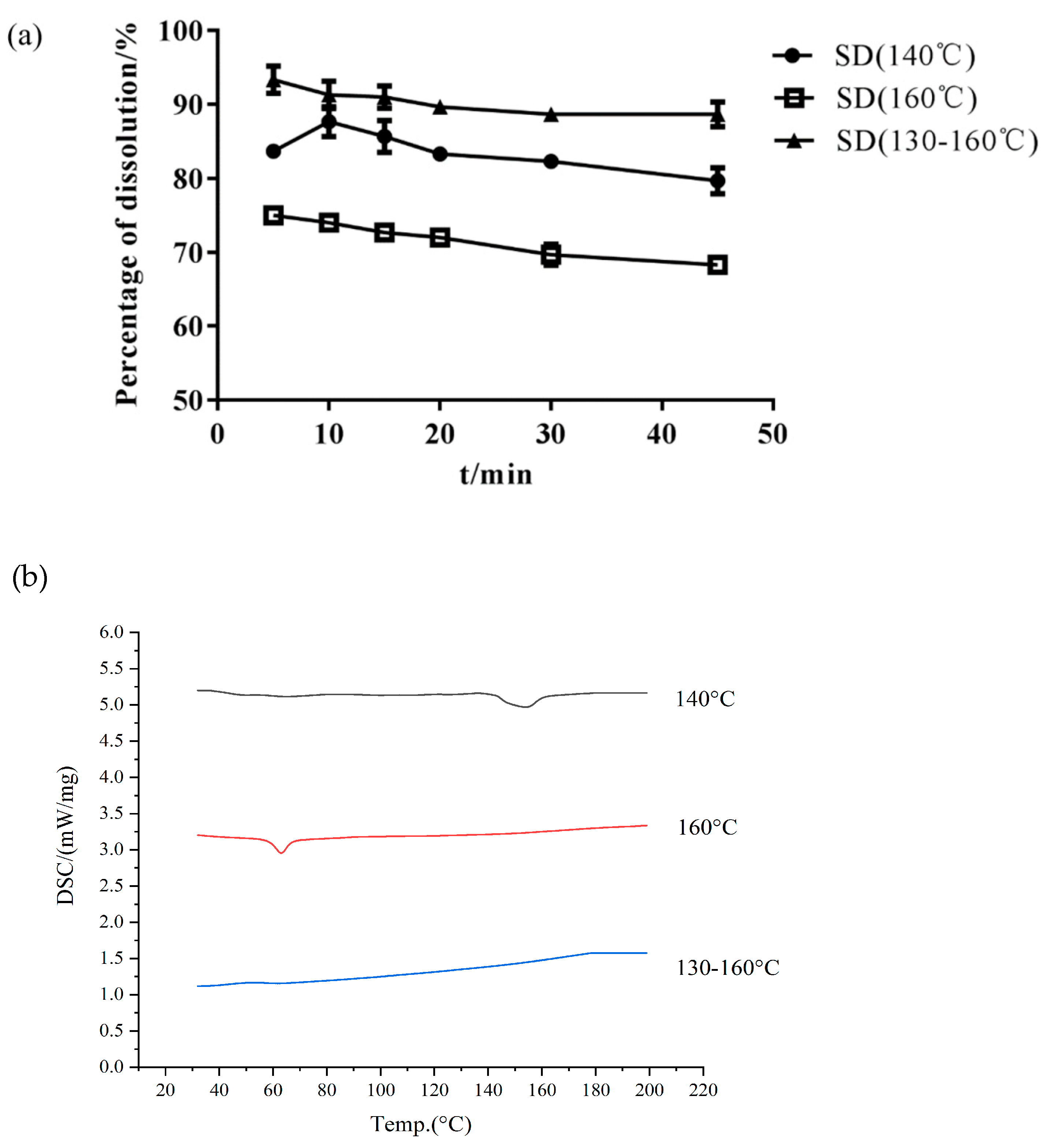
- Effect of Barrel Temperature
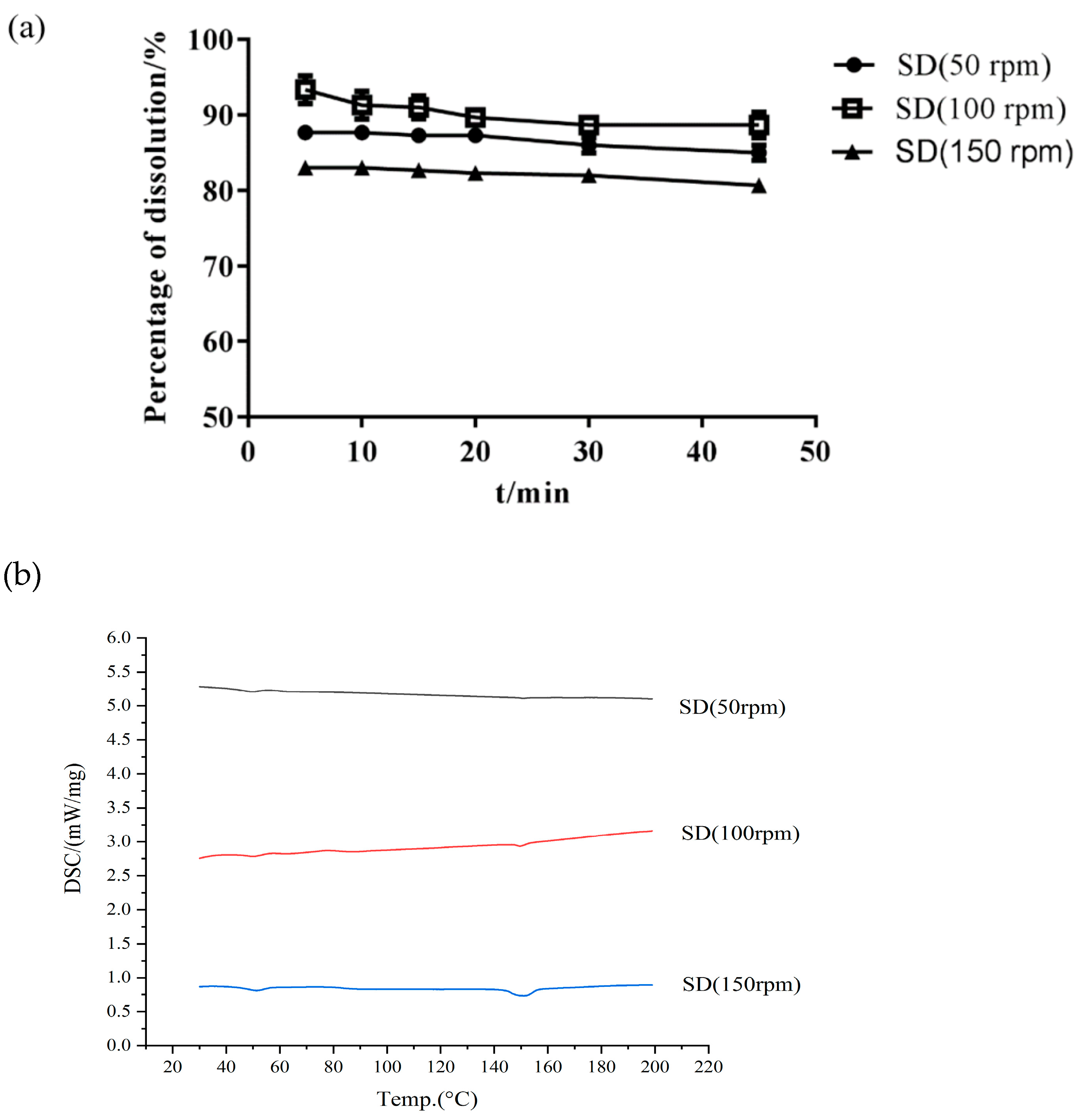
- Effect of Screw Speed
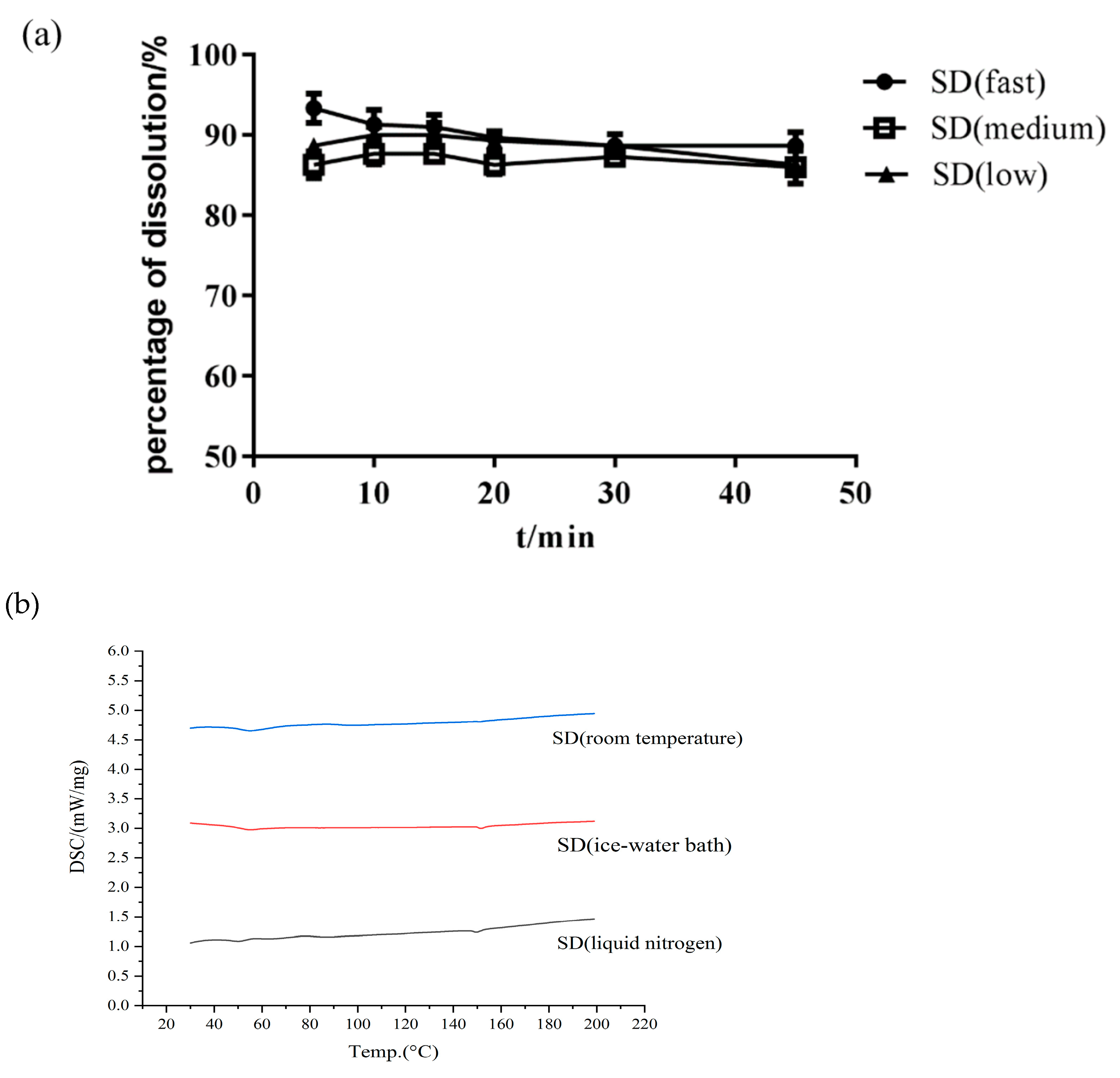
- Effect of Cooling Method
3.4. The Characterization of the SD
3.4.1. DSC
3.4.2. XRD
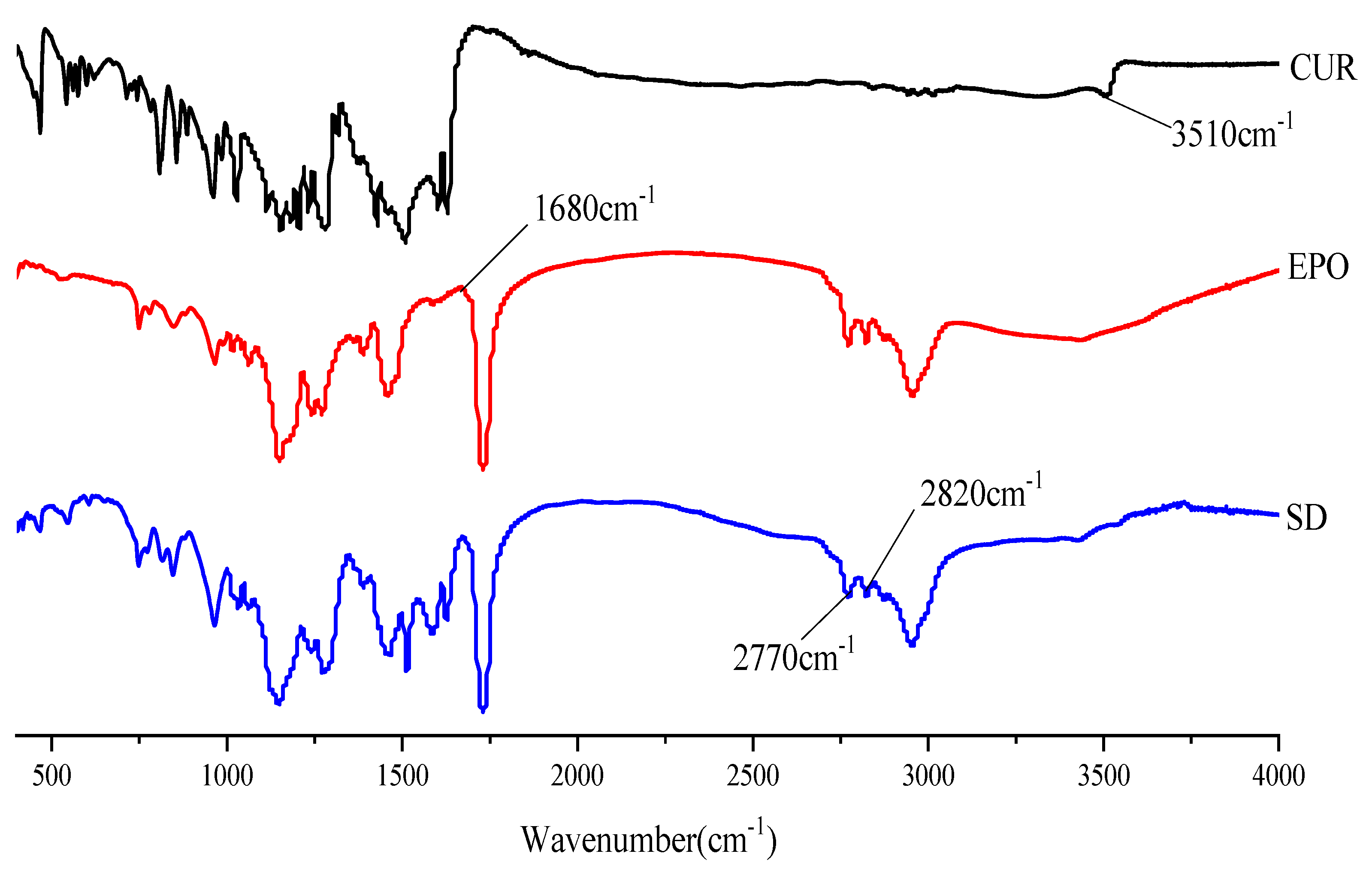
3.4.3. FTIR
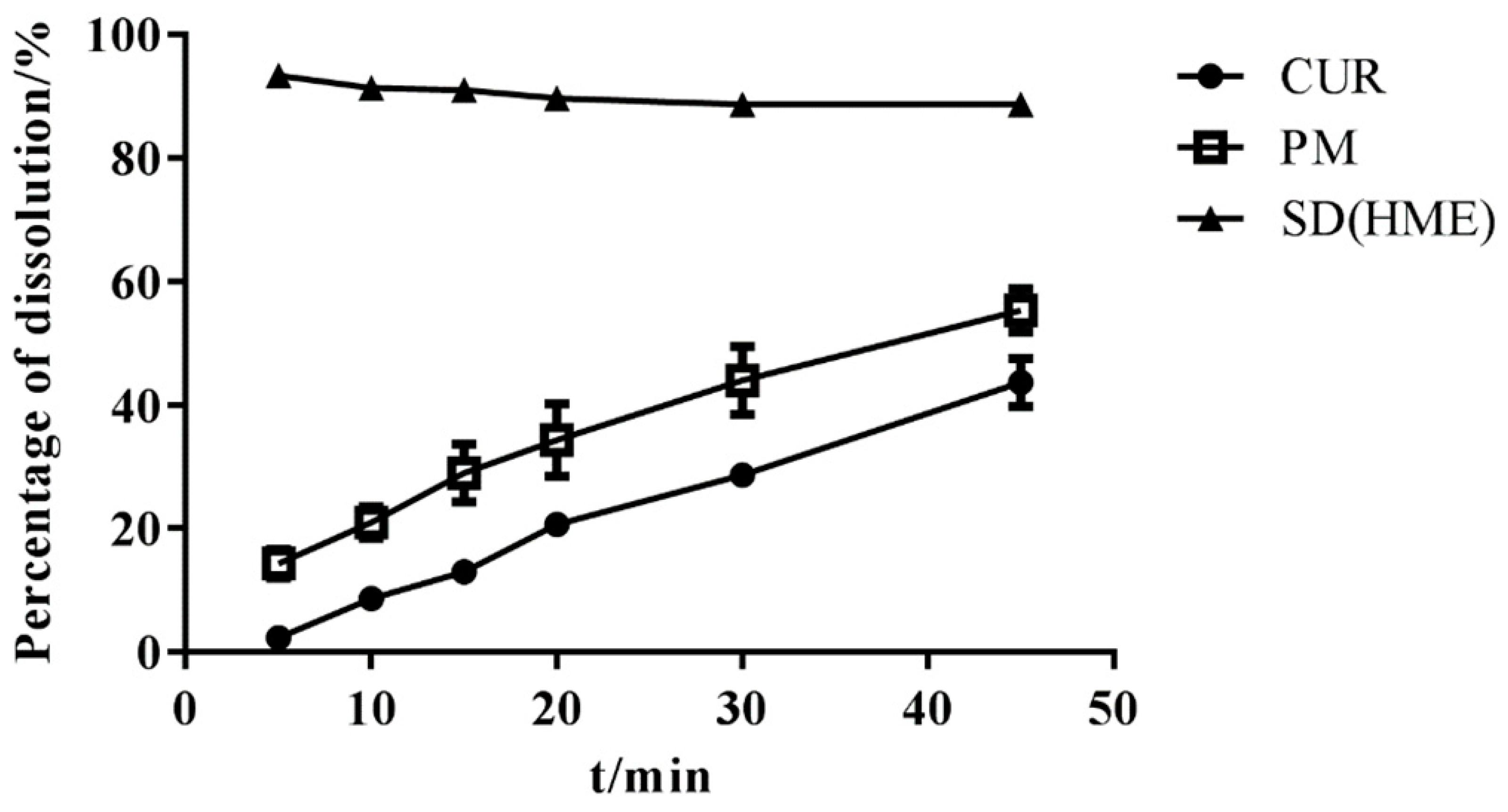
3.5. In Vitro Dissolution Study
3.6. Equilibrium Solubility Studies
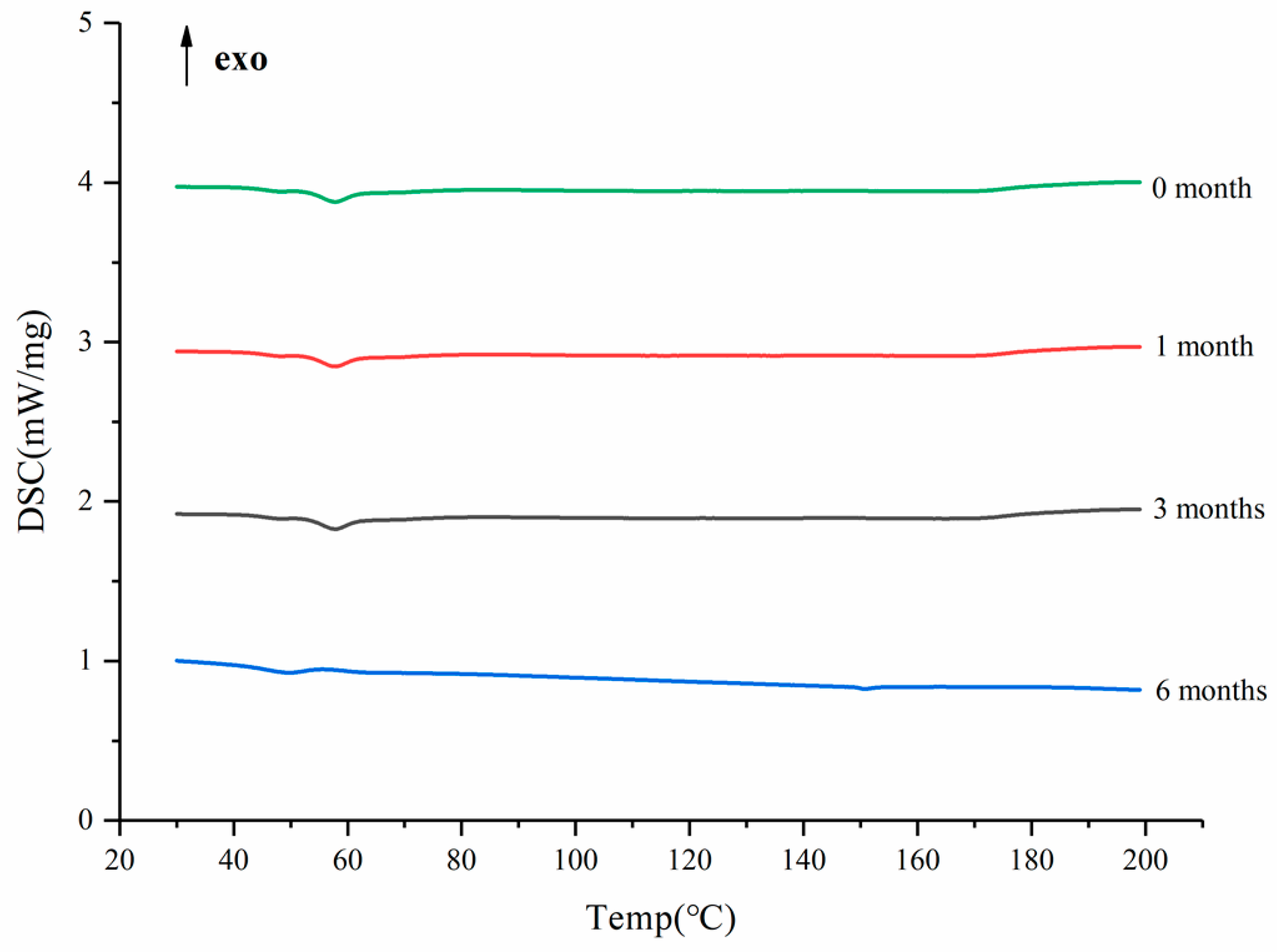
3.7. Acceleration of Stability Study
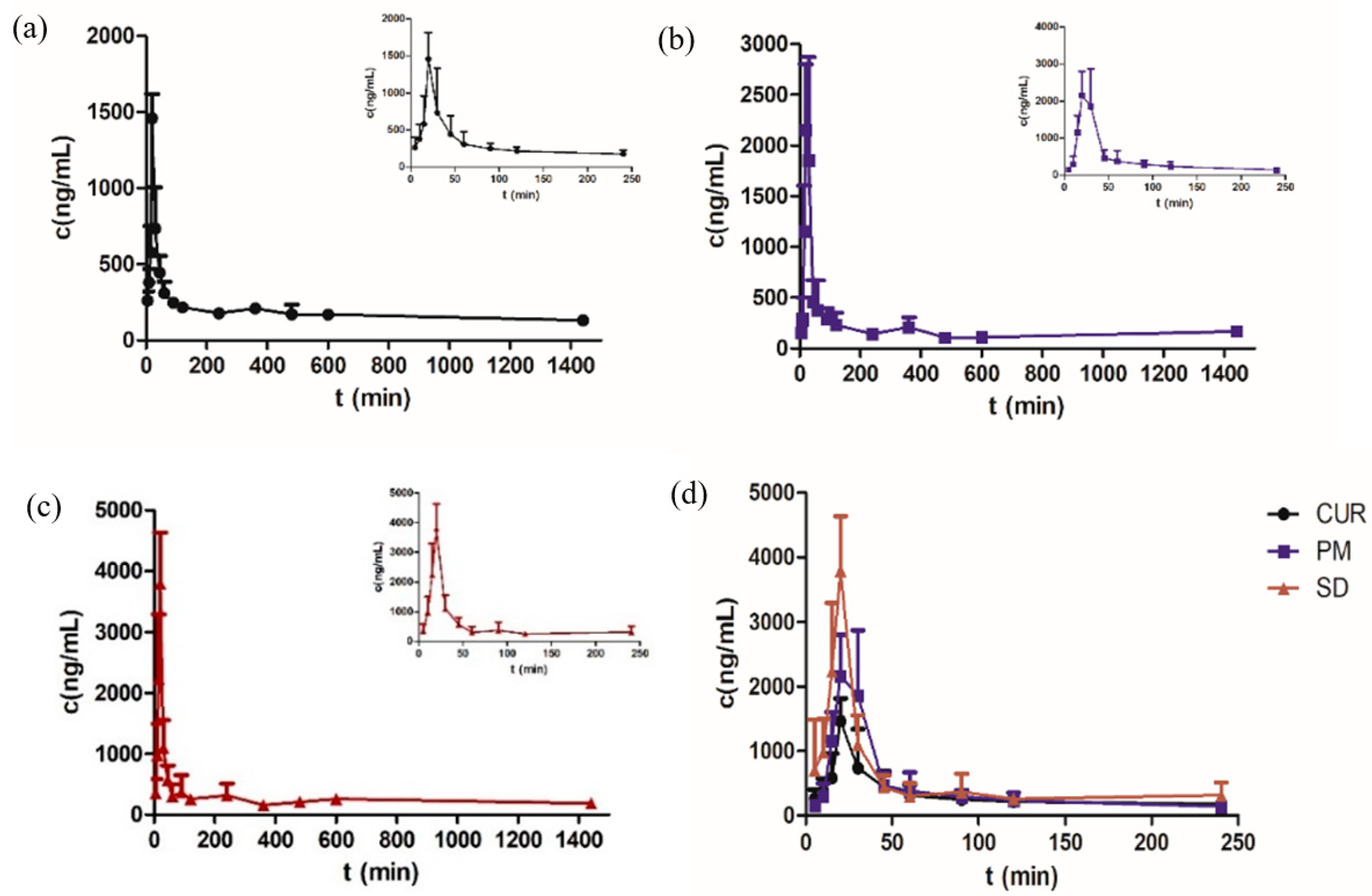
3.8. Pharmacokinetic Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shakeri, A.; Ward, N.; Panahi, Y.; Sahebkar, A. Anti-Angiogenic Activity of Curcumin in Cancer Therapy: A Narrative Review. Curr. Vasc. Pharmacol. 2019, 17, 262–269. [Google Scholar] [CrossRef]
- Kareem, S.M.; Mahmood, S.S.; Hindi, N.K. Effects of Curcumin and Silymarin on the Shigella dysenteriae and Campylobacter jejuni In vitro. J. Gastrointest. Cancer 2020, 51, 824–828. [Google Scholar] [CrossRef]
- Zeng, L.; Yu, G.; Hao, W.; Yang, K.; Chen, H. The efficacy and safety of Curcuma longa extract and curcumin supplements on osteoarthritis: A systematic review and meta-analysis. Biosci. Rep. 2021, 41, BSR20210817. [Google Scholar] [CrossRef]
- Pancholi, V.; Smina, T.P.; Kunnumakkara, A.B.; Maliakel, B.; Krishnakumar, I.M. Safety assessment of a highly bioavailable curcumin-galactomannoside complex (CurQfen) in healthy volunteers, with a special reference to the recent hepatotoxic reports of curcumin supplements: A 90-days prospective study. Toxicol. Rep. 2021, 8, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, H.; Bian, W.; Liu, Y.; Liu, X.; Ma, S.; Zheng, X.; Du, Z.; Zhang, K.; Ouyang, D. Molecular Interactions for the Curcumin-Polymer Complex with Enhanced Anti-Inflammatory Effects. Pharmaceutics 2019, 11, 442. [Google Scholar] [CrossRef] [Green Version]
- Dudhipala, N.; Veerabrahma, K. Candesartan cilexetil loaded solid lipid nanoparticles for oral delivery: Characterization, pharmacokinetic and pharmacodynamic evaluation. Drug Deliv. 2016, 23, 395–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudhipala, N.; Janga, K.Y.; Gorre, T. Comparative study of nisoldipine-loaded nanostructured lipid carriers and solid lipid nanoparticles for oral delivery: Preparation, characterization, permeation and pharmacokinetic evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.G.; Dong, L.C.; Song, Y.Q. Nanosizing of a drug/carrageenan complex to increase solubility and dissolution rate. Int. J. Pharm. 2007, 342, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Tiwary, A.K.; Bedi, N. Canagliflozin loaded SMEDDS: Formulation optimization for improved solubility, permeability and pharmacokinetic performance. J. Pharm. Investig. 2019, 49, 67–85. [Google Scholar] [CrossRef]
- Hadipour Moghaddam, S.P.; Farhat, S.; Vatanara, A. Porous Microparticles Containing Raloxifene Hydrochloride Tailored by Spray Freeze Drying for Solubility Enhancement. Adv. Pharm. Bull. 2018, 8, 217–223. [Google Scholar] [CrossRef]
- Hong, W.; Guo, F.; Yu, N.; Ying, S.; Lou, B.; Wu, J.; Gao, Y.; Ji, X.; Wang, H.; Li, A.; et al. A Novel Folic Acid Receptor-Targeted Drug Delivery System Based on Curcumin-Loaded β-Cyclodextrin Nanoparticles for Cancer Treatment. Drug Des. Devel. Ther. 2021, 15, 2843–2855. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Feldmann, G.; Soni, S.; Ravi, R.; Karikar, C.; Maitra, A.; Maitra, A. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): A novel strategy for human cancer therapy. J. Nanobiotechnol. 2007, 5, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Ngo, H.; Ngo, V.T.-K.; Vo, V.T.; Nguyen, P.K.; Vo Van, T.; Tran, P.H.-L.; Tran, T.T.-D. Effects of Absorbent on the Dissolution Rate of PEG-Based Solid Dispersions Containing Poorly Water-Soluble Drug; Springer: Singapore, 2018; pp. 515–518. [Google Scholar]
- Shin, M.S.; Yu, J.S.; Lee, J.; Ji, Y.S.; Joung, H.J.; Han, Y.M.; Yoo, H.H.; Kang, K.S. A Hydroxypropyl Methylcellulose-Based Solid Dispersion of Curcumin with Enhanced Bioavailability and its Hepatoprotective Activity. Biomolecules 2019, 9, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, I.S.; Cha, J.S.; Choi, M.K. Characterization, in Vivo and in Vitro Evaluation of Solid Dispersion of Curcumin Containing d-α-Tocopheryl Polyethylene Glycol 1000 Succinate and Mannitol. Molecules 2016, 21, 1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulthe, V.V.; Chaudhari, P.D.; Aboul-Enein, H.Y. Freeze-dried amorphous dispersions for solubility enhancement of thermosensitive API having low molecular lipophilicity. Drug Res. 2014, 64, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.Q.; Feng, X.; Morott, J.T.; Pimparade, M.B.; Tiwari, R.V.; Zhang, F.; Repka, M.A. A novel floating controlled release drug delivery system prepared by hot-melt extrusion. Eur. J. Pharm. Biopharm. 2016, 98, 108–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almotairy, A.; Almutairi, M.; Althobaiti, A.; Alyahya, M.; Sarabu, S.; Alzahrani, A.; Zhang, F.; Bandari, S.; Repka, M.A. Effect of pH modifiers on the solubility, dissolution rate, and stability of telmisartan solid dispersions produced by hot-melt extrusion technology. J. Drug Deliv. Sci. Technol. 2021, 65, 102674. [Google Scholar] [CrossRef]
- Malaquias, L.F.B.; Sá-Barreto, L.C.L.; Freire, D.O.; Silva, I.C.R.; Karan, K.; Durig, T.; Lima, E.M.; Marreto, R.N.; Gelfuso, G.M.; Gratieri, T.; et al. Taste masking and rheology improvement of drug complexed with beta-cyclodextrin and hydroxypropyl-β-cyclodextrin by hot-melt extrusion. Carbohydr. Polym. 2018, 185, 19–26. [Google Scholar] [CrossRef]
- Yang, F.; Su, Y.; Zhang, J.; Dinunzio, J.; Leone, A.; Huang, C.; Brown, C.D. Rheology Guided Rational Selection of Processing Temperature To Prepare Copovidone-Nifedipine Amorphous Solid Dispersions via Hot Melt Extrusion (HME). Mol. Pharm. 2016, 13, 3494–3505. [Google Scholar] [CrossRef] [PubMed]
- Mendonsa, N.S.; Murthy, S.N.; Hashemnejad, S.M.; Kundu, S.; Zhang, F.; Repka, M.A. Development of poloxamer gel formulations via hot-melt extrusion technology. Int. J. Pharm. 2018, 537, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Chuah, A.M.; Jacob, B.; Jie, Z.; Ramesh, S.; Mandal, S.; Puthan, J.K.; Deshpande, P.; Vaidyanathan, V.V.; Gelling, R.W.; Patel, G.; et al. Enhanced bioavailability and bioefficacy of an amorphous solid dispersion of curcumin. Food Chem. 2014, 156, 227–233. [Google Scholar] [CrossRef]
- Stefanis, E.; Panayiotou, C. Prediction of Hansen Solubility Parameters with a New Group-Contribution Method. Int. J. Thermophys. 2008, 29, 568–585. [Google Scholar] [CrossRef]
- Leuner, C.; Dressman, J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000, 50, 47–60. [Google Scholar] [CrossRef]
- Van Krevelen, D.W. Cohesive Properties and Solubility. Chapter 7. Prop. Polym. 2009, 189–227. [Google Scholar] [CrossRef]
- Karimpour, M.; Feizi, M.A.H.; Mahdavi, M.; Krammer, B.; Verwanger, T.; Najafi, F.; Babaei, E. Development of curcumin-loaded gemini surfactant nanoparticles: Synthesis, characterization and evaluation of anticancer activity against human breast cancer cell lines. Phytomedicine 2019, 57, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Zhang, X.; Zhu, W.; Di, L. The Preparation of Curcumin Sustained-Release Solid Dispersion by Hot-Melt Extrusion-II. Optimization of Preparation Process and Evaluation In Vitro and In Vivo. J. Pharm. Sci. 2020, 109, 1253–1260. [Google Scholar] [CrossRef]
- Kheiri Manjili, H.; Ghasemi, P.; Malvandi, H.; Mousavi, M.S.; Attari, E.; Danafar, H. Pharmacokinetics and in vivo delivery of curcumin by copolymeric mPEG-PCL micelles. Eur. J. Pharm. Biopharm. 2017, 116, 17–30. [Google Scholar] [CrossRef]
- Pi, C.; Yuan, J.; Liu, H.; Zuo, Y.; Feng, T.; Zhan, C.; Wu, J.; Ye, Y.; Zhao, L.; Wei, Y. In vitro and in vivo evaluation of curcumin loaded hollow microspheres prepared with ethyl cellulose and citric acid. Int. J. Biol. Macromol. 2018, 115, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.A.; Taylor, L.S. Evaluation of amorphous solid dispersion properties using thermal analysis techniques. Adv. Drug Del. Rev. 2012, 64, 396–421. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, D.J.; Williams, A.C.; Timmins, P.; York, P. Solubility parameters as predictors of miscibility in solid dispersions. J. Pharm. Sci. 1999, 88, 1182–1190. [Google Scholar] [CrossRef]
- Chokshi, R.J.; Zia, H.; Sandhu, H.K.; Shah, N.H.; Malick, W.A. Improving the Dissolution Rate of Poorly Water Soluble Drug by Solid Dispersion and Solid Solution. Drug Deliv. 2007, 14, 33–45. [Google Scholar] [CrossRef]
- Liu, H.; Wang, P.; Zhang, X.; Shen, F.; Gogos, C.G. Effects of extrusion process parameters on the dissolution behavior of indomethacin in Eudragit® E PO solid dispersions. Int. J. Pharm. 2010, 383, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Maddineni, S.; Lu, J.; Repka, M.A. Melt extrusion with poorly soluble drugs. Int. J. Pharm. 2013, 453, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Reitz, E.; Podhaisky, H.; Ely, D.; Thommes, M. Residence time modeling of hot melt extrusion processes. Eur. J. Pharm. Biopharm. 2013, 85, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Lang, B.; Mcginity, J.W.; Williams, R.O., 3rd. Dissolution enhancement of itraconazole by hot-melt extrusion alone and the combination of hot-melt extrusion and rapid freezing--effect of formulation and processing variables. Mol. Pharm. 2014, 11, 186–196. [Google Scholar] [CrossRef]
- Ng, C.L.; Lee, S.-E.; Lee, J.-K.; Kim, T.-H.; Jang, W.S.; Choi, J.-S.; Kim, Y.-H.; Kim, J.-K.; Park, J.-S. Solubilization and formulation of chrysosplenol C in solid dispersion with hydrophilic carriers. Int. J. Pharm. 2016, 512, 314–321. [Google Scholar] [CrossRef]
- Dong, L.; Mai, Y.; Liu, Q.; Zhang, W.; Yang, J. Mechanism and Improved Dissolution of Glycyrrhetinic Acid Solid Dispersion by Alkalizers. Pharmaceutics 2020, 12, 82. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, N.; Malakzadeh, S. Antioxidant, antibacterial and anti-cancer activities of β-and γ-CDs/curcumin loaded in chitosan nanoparticles. Int. J. Biol. Macromol. 2020, 147, 778–791. [Google Scholar] [CrossRef]
- Bhatia, M.; Saini, M. Formulation and evaluation of curcumin microsponges for oral and topical drug delivery. Prog. Biomater. 2018, 7, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Lee, I.W.; Shin, G.H.; Chen, X.; Park, H.J. Curcumin-Eudragit® E PO solid dispersion: A simple and potent method to solve the problems of curcumin. Eur. J. Pharm. Biopharm. 2015, 94, 322–332. [Google Scholar] [CrossRef]
- Li, L.; Yao, P. High dispersity, stability and bioaccessibility of curcumin by assembling with deamidated zein peptide. Food Chem. 2020, 319, 126577. [Google Scholar] [CrossRef]
- Bukhovets, A.V.; Fotaki, N.; Khutoryanskiy, V.V.; Moustafine, R.I. Interpolymer Complexes of Eudragit(®) Copolymers as Novel Carriers for Colon-Specific Drug Delivery. Polymers 2020, 12, 1459. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential Therapeutic Effects of Curcumin, the Anti-inflammatory Agent, Against Neurodegenerative, Cardiovascular, Pulmonary, Metabolic, Autoimmune and Neoplastic Diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [Green Version]
- Meng, F.; Trivino, A.; Prasad, D.; Chauhan, H. Investigation and correlation of drug polymer miscibility and molecular interactions by various approaches for the preparation of amorphous solid dispersions. Eur. J. Pharm. Sci. 2015, 71, 12–24. [Google Scholar] [CrossRef]
- Jeganathan, B.; Prakya, V. Interpolyelectrolyte complexes of Eudragit® EPO with hypromellose acetate succinate and Eudragit® EPO with hypromellose phthalate as potential carriers for oral controlled drug delivery. AAPS PharmSciTech 2015, 16, 878–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butreddy, A.; Sarabu, S.; Bandari, S.; Batra, A.; Lawal, K.; Chen, N.N.; Bi, V.; Durig, T.; Repka, M.A. Influence of Plasdone™ S630 Ultra—an Improved Copovidone on the Processability and Oxidative Degradation of Quetiapine Fumarate Amorphous Solid Dispersions Prepared via Hot-Melt Extrusion Technique. AAPS PharmSciTech 2021, 22, 196. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, H.; Rahmawati, R.A.; Syamsur Rijal, M.A.; Isadiartuti, D. Curcumin micelles entrapped in eudragit S-100 matrix: A synergistic strategy for enhanced oral delivery. Future Sci. OA 2021, 7, FSO677. [Google Scholar] [CrossRef] [PubMed]
- Chivate, A.; Garkal, A.; Hariharan, K.; Mehta, T. Exploring novel carrier for improving bioavailability of Itraconazole: Solid dispersion through hot-melt extrusion. J. Drug Deliv. Sci. Technol. 2021, 63, 102541. [Google Scholar] [CrossRef]
- Huang, Y.; Cao, S.; Zhang, Q.; Zhang, H.; Fan, Y.; Qiu, F.; Kang, N. Biological and pharmacological effects of hexahydrocurcumin, a metabolite of curcumin. Arch. Biochem. Biophys. 2018, 646, 31–37. [Google Scholar] [CrossRef]
- Attia, Y.A.; Alharthi, M.A.; Hassan, S.S. Turmeric (Curcuma longa Linn.) as a phytogenic growth promoter alternative for antibiotic and comparable to mannan oligosaccharides for broiler chicks. Rev. Mex. Cienc. Pecu. 2017, 8, 11–21. [Google Scholar] [CrossRef]
- Guo, Z.; Lu, M.; Li, Y.; Pang, H.; Lin, L.; Liu, X.; Wu, C. The utilization of drug-polymer interactions for improving the chemical stability of hot-melt extruded solid dispersions. J. Pharm. Pharmacol. 2014, 66, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.C.; Cho, C.W. Physicochemical characterizations of piroxicam-poloxamer solid dispersion. Pharm. Dev. Technol. 1997, 2, 403–407. [Google Scholar] [CrossRef] [PubMed]

| Barrel Temperature/°C | Screw Speed/rmp | Cooling Method | The Content of Cur/% | |
|---|---|---|---|---|
| 1 | 140 | 100 | Room temperature | 95 |
| 2 | 130–135–140–145–150–155–160 | 100 | Room temperature | 95 |
| 3 | 160 | 100 | Room temperature | 80 |
| 4 | 130–135–140–145–150–155–160 | 50 | Room temperature | 92 |
| 5 | 130–135–140–145–150–155–160 | 150 | Room temperature | 94 |
| 6 | 130–135–140–145–150–155–160 | 100 | Ice-water bath | 95 |
| 7 | 130–135–140–145–150–155–160 | 100 | liquid nitrogen | 95 |
| Samples | Equilibrium Solubility of Cur (μg/mL) |
|---|---|
| Cur | 39.03% ± 0.63% |
| Physical mixture | 152.94% ± 1.45% |
| SD | 234.20% ± 2.33% |
| Parameters | Cur | PM * | SD ** |
|---|---|---|---|
| Cmax (ng/mL) | 1464.322 ± 24.7 | 2484.783 ± 26.8 | 3791.120 ± 22.2 |
| Tmax (min) | 22 ± 22.3 | 24 ± 22.8 | 20 ± 0 |
| AUC0–t (ng/mL min) | 267,081.009 ± 32.5 | 265,975.190 ± 21.5 | 387,231.473 ± 17.2 |
| AUC0–∞ (ng/mL min) | 533,813.494 ± 67.0 | 275,084.394 ± 15.8 | 619,877.046 ± 39.7 |
| t1/2 z (h) | 23.00 ± 53.8 | 7.03 ± 91.9 | 12.82 ± 35.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, W.; Zhang, X.; Zhu, W.; Zhang, X.; Di, L. Preparation of Curcumin-Eudragit® E PO Solid Dispersions with Gradient Temperature through Hot-Melt Extrusion. Molecules 2021, 26, 4964. https://doi.org/10.3390/molecules26164964
Fan W, Zhang X, Zhu W, Zhang X, Di L. Preparation of Curcumin-Eudragit® E PO Solid Dispersions with Gradient Temperature through Hot-Melt Extrusion. Molecules. 2021; 26(16):4964. https://doi.org/10.3390/molecules26164964
Chicago/Turabian StyleFan, Wenling, Xiaotong Zhang, Wenjing Zhu, Xinyi Zhang, and Liuqing Di. 2021. "Preparation of Curcumin-Eudragit® E PO Solid Dispersions with Gradient Temperature through Hot-Melt Extrusion" Molecules 26, no. 16: 4964. https://doi.org/10.3390/molecules26164964





