Impacts of Hydrothermal Treatments on the Morphology, Structural Characteristics, and In Vitro Digestibility of Water Caltrop Starch
Abstract
:1. Introduction
2. Results and Discussion
2.1. Proximate Compositions
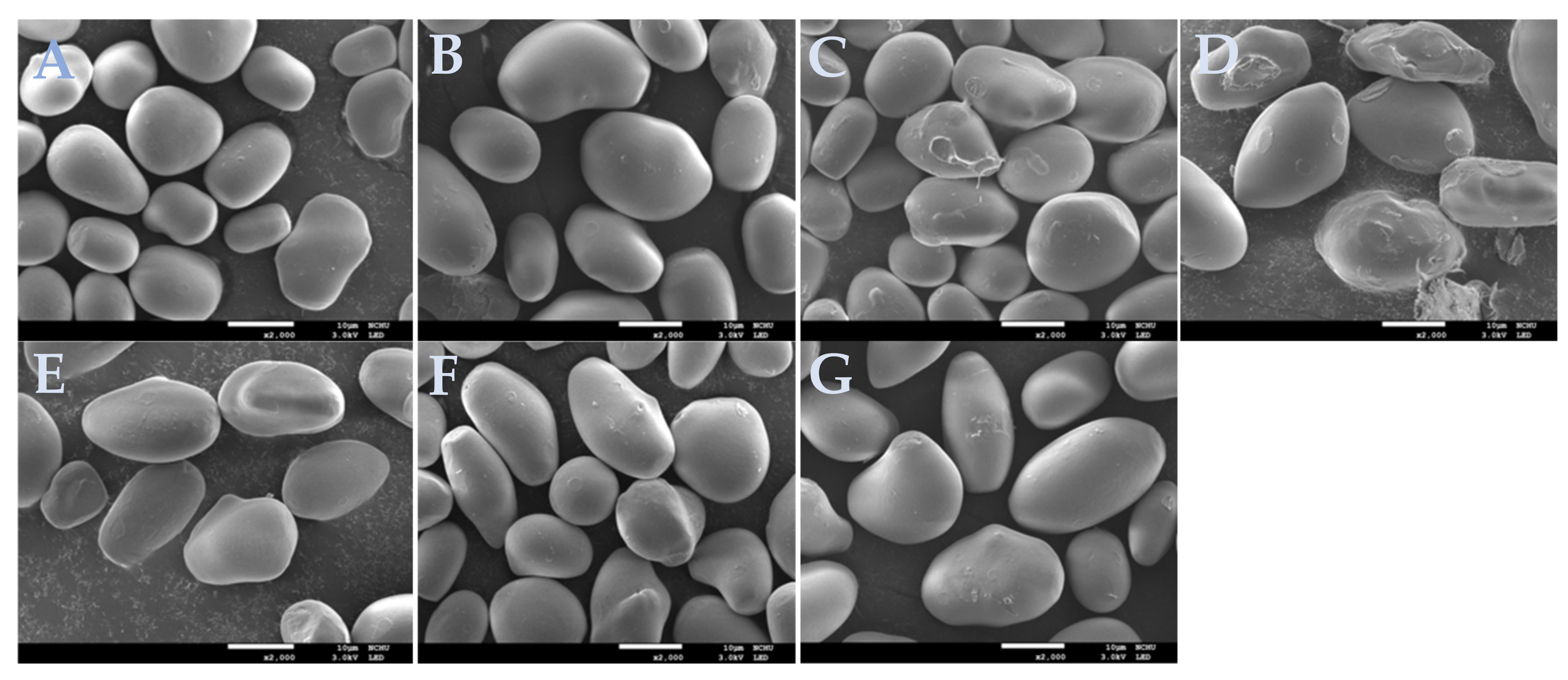
2.2. Morphology of Starch Granules
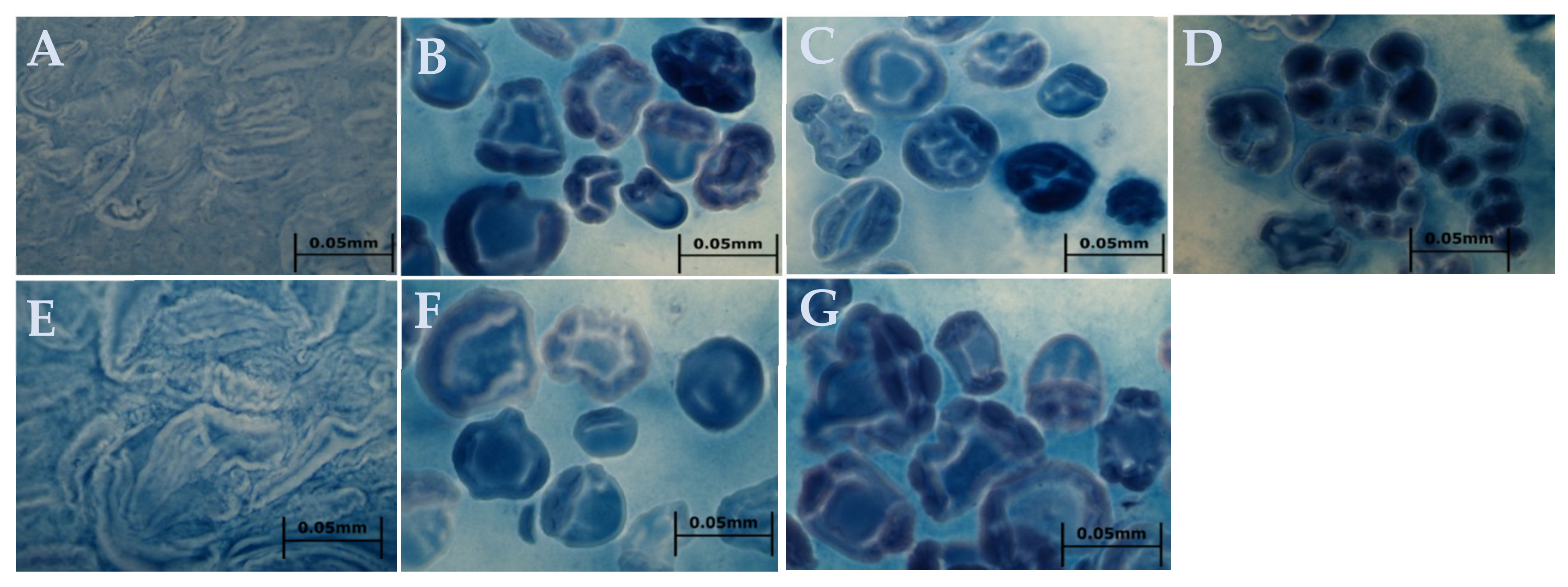
2.3. Pasting Properties and Iodine Staining Morphology
2.4. Starch Crystalline Pattern and Relative Crystallinity
2.5. In Vitro Digestibility
2.6. Amylose and Damaged Starch Content
3. Materials and Methods
3.1. Materials
3.2. Starch Isolation
3.3. Proximate Composition Analysis
3.4. Hydrothermal Treatments
3.4.1. Heat-Moisture Treatment (HMT)
3.4.2. Annealing Treatment (ANN)
3.4.3. Dual Hydrothermal Treatment
3.5. Morphological Observation of Starch Granules
3.6. Pasting Properties and Iodine Staining Observation of Starch Paste
3.7. Starch Crystalline Pattern and Relative Crystallinity
3.8. In Vitro Digestibility
3.9. Amylose and Damaged Starch Content
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhu, F. Chemical composition, health effects, and uses of water caltrop. Trends Food Sci. Technol. 2016, 49, 136–145. [Google Scholar] [CrossRef]
- Wang, J.; Liu, T.; Bian, X.; Hua, Z.; Chen, G.; Wu, X. Structural characterization and physicochemical properties of starch from four aquatic vegetable varieties in China. Int. J. Biol. Macromol. 2021, 172, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Durmus, Y.; Anil, M.; Simsek, S. Effects of hazelnut skin, cross-linked starch, and oxidized starch on wheat flour and dough quality. J. Food Process. Preserv. 2021, 45, e14919. [Google Scholar] [CrossRef]
- Altemimi, A.B. Extraction and optimization of potato starch and its application as a stabilizer in yogurt manufacturing. Foods 2018, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heydari, A.; Razavi, S.M.A.; Farahnaky, A. Effect of high pressure-treated wheat starch as a fat replacer on the physical and rheological properties of reduced-fat O/W emulsions. Innov. Food Sci. Emerg. Technol. 2021, 70, 102702. [Google Scholar] [CrossRef]
- Kaur, L.; Singh, J. Starch: Modified starches. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 152–159. [Google Scholar]
- Sandberg, J.C.; Björck, I.M.; Nilsson, A.C. Effects of whole grain rye, with and without resistant starch type 2 supplementation, on glucose tolerance, gut hormones, inflammation and appetite regulation in an 11–14.5 hour perspective; a randomized controlled study in healthy subjects. Nutr. J. 2017, 16, 25. [Google Scholar] [CrossRef]
- Remya, R.; Jyothi, A.N.; Sreekumar, J. Effect of chemical modification with citric acid on the physicochemical properties and resistant starch formation in different starches. Carbohydr. Polym. 2018, 202, 29–38. [Google Scholar] [CrossRef]
- Su, C.; Zhao, K.; Zhang, B.; Liu, Y.; Jing, L.; Wu, H.; Gou, M.; Jiang, H.; Zhang, G.; Li, W. The molecular mechanism for morphological, crystal, physicochemical and digestible property modification of wheat starch after repeated versus continuous heat-moisture treatment. LWT 2020, 129, 109399. [Google Scholar] [CrossRef]
- Raigond, P.; Ezekiel, R.; Raigond, B. Resistant starch in food: A review. J. Sci. Food Agric. 2015, 95, 1968–1978. [Google Scholar] [CrossRef]
- Jiang, F.; Du, C.; Jiang, W.; Wang, L.; Du, S.-k. The preparation, formation, fermentability, and applications of resistant starch. Int. J. Biol. Macromol. 2020, 150, 1155–1161. [Google Scholar] [CrossRef]
- Kaur, B.; Ariffin, F.; Bhat, R.; Karim, A.A. Progress in starch modification in the last decade. Food Hydrocoll. 2012, 26, 398–404. [Google Scholar] [CrossRef]
- Schafranski, K.; Ito, V.C.; Lacerda, L.G. Impacts and potential applications: A review of the modification of starches by heat-moisture treatment (HMT). Food Hydrocoll. 2021, 117, 106690. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, S. Influence of heat-moisture treatment (HMT) on physicochemical and functional properties of starches from different Indian oat (Avena sativa L.) cultivars. Int. J. Biol. Macromol. 2019, 122, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Molavi, H.; Razavi, S.M.A.; Farhoosh, R. Impact of hydrothermal modifications on the physicochemical, morphology, crystallinity, pasting and thermal properties of acorn starch. Food Chem. 2018, 245, 385–393. [Google Scholar] [CrossRef]
- Yassaroh, Y.; Woortman, A.J.J.; Loos, K. A new way to improve physicochemical properties of potato starch. Carbohydr. Polym. 2019, 204, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Li, X.; Chen, L.; Xie, F.; Li, L.; Huang, J. Effect of heat-moisture treatment on multi-scale structures and physicochemical properties of breadfruit starch. Carbohydr. Polym. 2017, 161, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.-J.; Liu, Q.; Hoover, R. Impact of annealing and heat-moisture treatment on rapidly digestible, slowly digestible and resistant starch levels in native and gelatinized corn, pea and lentil starches. Carbohydr. Polym. 2009, 75, 436–447. [Google Scholar] [CrossRef]
- Jayakody, L.; Hoover, R. Effect of annealing on the molecular structure and physicochemical properties of starches from different botanical origins—A review. Carbohydr. Polym. 2008, 74, 691–703. [Google Scholar] [CrossRef]
- Chung, H.-J.; Hoover, R.; Liu, Q. The impact of single and dual hydrothermal modifications on the molecular structure and physicochemical properties of normal corn starch. Int. J. Biol. Macromol. 2009, 44, 203–210. [Google Scholar] [CrossRef]
- Wei, H.-X.; Liang, B.-D.; Chai, Y.-R.; Xue, L.-P.; Wang, X.-Q.; Yin, X.-M. Effect of different heat treatments on physicochemical properties and structural and digestibility of water caltrop starch. Starch Stärke 2020, 72, 1900275. [Google Scholar] [CrossRef]
- Bharti, I.; Singh, S.; Saxena, D.C. Exploring the influence of heat moisture treatment on physicochemical, pasting, structural and morphological properties of mango kernel starches from Indian cultivars. LWT Food Sci. Technol. 2019, 110, 197–206. [Google Scholar] [CrossRef]
- Liu, H.; Lv, M.; Wang, L.; Li, Y.; Fan, H.; Wang, M. Comparative study: How annealing and heat-moisture treatment affect the digestibility, textural, and physicochemical properties of maize starch. Starch Stärke 2016, 68, 1158–1168. [Google Scholar] [CrossRef]
- Yadav, B.S.; Guleria, P.; Yadav, R.B. Hydrothermal modification of Indian water chestnut starch: Influence of heat-moisture treatment and annealing on the physicochemical, gelatinization and pasting characteristics. LWT Food Sci. Technol. 2013, 53, 211–217. [Google Scholar] [CrossRef]
- da Rosa Zavareze, E.; Dias, A.R.G. Impact of heat-moisture treatment and annealing in starches: A review. Carbohydr. Polym. 2011, 83, 317–328. [Google Scholar] [CrossRef]
- Singh, H.; Chang, Y.H.; Lin, J.-H.; Singh, N.; Singh, N. Influence of heat–moisture treatment and annealing on functional properties of sorghum starch. Food Res. Int. 2011, 44, 2949–2954. [Google Scholar] [CrossRef]
- Ali, N.A.; Dash, K.K.; Routray, W. Physicochemical characterization of modified lotus seed starch obtained through acid and heat moisture treatment. Food Chem. 2020, 319, 126513. [Google Scholar] [CrossRef]
- Chung, H.J.; Liu, Q.; Hoover, R. Effect of single and dual hydrothermal treatments on the crystalline structure, thermal properties, and nutritional fractions of pea, lentil, and navy bean starches. Food Res. Int. 2010, 43, 501–508. [Google Scholar] [CrossRef]
- Kawabata, A.; Takase, N.; Miyoshi, E.; Sawayama, S.; Kimura, T.; Kudo, K. Microscopic observation and X-ray diffractometry of heat/moisture-treated starch granules. Starch Stärke 1994, 46, 463–469. [Google Scholar] [CrossRef]
- Vermeylen, R.; Goderis, B.; Delcour, J.A. An X-ray study of hydrothermally treated potato starch. Carbohydr. Polym. 2006, 64, 364–375. [Google Scholar] [CrossRef]
- Watcharatewinkul, Y.; Puttanlek, C.; Rungsardthong, V.; Uttapap, D. Pasting properties of a heat-moisture treated canna starch in relation to its structural characteristics. Carbohydr. Polym. 2009, 75, 505–511. [Google Scholar] [CrossRef]
- Stute, R. Hydrothermal modification of starches: The difference between annealing and heat/moisture-treatment. Starch Stärke 1992, 44, 205–214. [Google Scholar] [CrossRef]
- Li, H.; Wang, R.; Liu, J.; Zhang, Q.; Li, G.; Shan, Y.; Ding, S. Effects of heat-moisture and acid treatments on the structural, physicochemical, and in vitro digestibility properties of lily starch. Int. J. Biol. Macromol. 2020, 148, 956–968. [Google Scholar] [CrossRef]
- Bet, C.D.; de Oliveira, C.S.; Colman, T.A.D.; Marinho, M.T.; Lacerda, L.G.; Ramos, A.P.; Schnitzler, E. Organic amaranth starch: A study of its technological properties after heat-moisture treatment. Food Chem. 2018, 264, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ward, R.; Gao, Q. Effect of heat-moisture treatment on the formation and physicochemical properties of resistant starch from mung bean (Phaseolus radiatus) starch. Food Hydrocolloids 2011, 25, 1702–1709. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.; Cummings, J. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar]
- Van Hung, P.; Binh, V.T.; Nhi, P.H.Y.; Phi, N.T.L. Effect of heat-moisture treatment of unpolished red rice on its starch properties and in vitro and in vivo digestibility. Int. J. Biol. Macromol. 2020, 154, 1–8. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Q.; Xu, X.; Qi, L.; Dong, Z.; Luo, Z.; Lu, X.; Peng, X. Structural changes of waxy and normal maize starches modified by heat moisture treatment and their relationship with starch digestibility. Carbohydr. Polym. 2017, 177, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.-Y. Effect of Heat-Moisture Treatments on the Digestibility and Physicochemical Properties of Tainung No.57 and Tainung No.66 Sweet Potato Starches. Master’s Thesis, National Chung Hsing University, Taichung, Taiwan, 2018. [Google Scholar]
- Gunaratne, A.; Hoover, R. Effect of heat–moisture treatment on the structure and physicochemical properties of tuber and root starches. Carbohydr. Polym. 2002, 49, 425–437. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, B.; Chen, L.; Li, X.; Zheng, B. Hierarchical structure and physicochemical properties of highland barley starch following heat moisture treatment. Food Chem. 2019, 271, 102–108. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, B.; Chen, L.; Li, X. Understanding the structure and digestibility of heat-moisture treated starch. Int. J. Biol. Macromol. 2016, 88, 1–8. [Google Scholar] [CrossRef]
- Kohyama, K.; Sasaki, T. Differential scanning calorimetry and a model calculation of starches annealed at 20 and 50 C. Carbohydr. Polym. 2006, 63, 82–88. [Google Scholar] [CrossRef]
- Wang, S.; Yu, J.; Xin, Q.; Wang, S.; Copeland, L. Effects of starch damage and yeast fermentation on acrylamide formation in bread. Food Control 2017, 73, 230–236. [Google Scholar] [CrossRef]
- Onyango, C.; Mewa, E.A.; Mutahi, A.W.; Okoth, M.W. Effect of heat-moisture-treated cassava starch and amaranth malt on the quality of sorghum-cassava-amaranth bread. Afr. J. Food Sci. 2013, 7, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Jin, F.; Yu, J. Pea starch annealing: New insights. Food Bioprocess Technol. 2013, 6, 3564–3575. [Google Scholar] [CrossRef]
- Sun, Y.-Y. Effects of Different Sugar/Salt Solutions on the Physical Properties of Purple Jade Sweet Potato Starch. Master’s Thesis, National Chung Hsing University, Taichung, Taiwan, 2009. [Google Scholar]
- Latimer, G. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2021; ISBN 978-0-935584-83-7. [Google Scholar]
- Yeh, Y. Effect of Single and Dual Hydrothermal Treatments on the Physicochemical Properties and Digestibility of Lotus Rhizome Starches. Master’s Thesis, National Chung Hsing University, Taichung, Taiwan, 2019. [Google Scholar]
- Boonna, S.; Tongta, S. Structural transformation of crystallized debranched cassava starch during dual hydrothermal treatment in relation to enzyme digestibility. Carbohydr. Polym. 2018, 191, 1–7. [Google Scholar] [CrossRef] [PubMed]


| Samples | Pasting Temperature (°C) | Peak Time (min) | Peak Viscosity | Holding Strength | Final Viscosity | Setback |
|---|---|---|---|---|---|---|
| (cP) | ||||||
| Native | 82.93 ± 0.25 a | 4.32 ± 0.04 a | 976.00 ± 4.36 f | 919.67 ± 7.37 g | 1366.67 ± 16.50 g | 447.00 ± 10.54 f |
| HMT20 | 88.30 ± 0.33 d | 4.60 ± 0.03 b | 88.00 ± 3.61 d | 314.33 ± 5.13 e | 438.67 ± 5.03 e | 124.33 ± 3.51 d |
| HMT25 | 94.40 ± 0.22 e | 4.93 ± 0.04 c | 64.33 ± 0.58 b | 95.00 ± 1.73 b | 145.67 ± 5.77 b | 50.67 ± 4.04 b |
| HMT30 | 95.25 ± 0.40 f | 4.98 ± 0.13 c | 45.33 ± 1.53 a | 53.33 ± 0.58 a | 74.67 ± 1.16 a | 21.33 ± 0.58 a |
| ANN | 84.02 ± 0.08 b | 4.27 ± 0.00 a | 715.67 ± 4.73 e | 802.00 ± 5.20 f | 1196.67 ± 4.04 f | 394.67 ± 2.08 e |
| HMT20 + ANN | 87.67 ± 0.45 c | 4.57 ± 0.04 b | 77.67 ± 1.16 c | 268.33 ± 9.87 d | 392.67 ± 9.45 d | 124.33 ± 1.16 d |
| ANN + HMT20 | 88.45 ± 0.26 d | 4.50 ± 0.00 b | 85.00 ± 3.61 d | 257.33 ± 2.89 c | 365.00 ± 2.65 c | 107.67 ± 0.58 c |
| Samples | RDS (20 min) | SDS (20–120 min) | Very-SDS (120 min–16 h) | RS (>16 h) |
|---|---|---|---|---|
| (%, d.b.) | ||||
| Native | 5.69 ± 0.16 b | 9.02 ± 0.22 b | 40.29 ± 0.43 c | 37.18 ± 0.55 c |
| HMT20 | 4.03 ± 0.09 a | 7.32 ± 0.61 a | 41.76 ± 3.05 c | 40.19 ± 0.63 d |
| HMT25 | 8.40 ± 0.12 f | 10.72 ± 0.13 c | 38.77 ± 1.69 bc | 32.44 ± 0.05 b |
| HMT30 | 26.29 ± 0.05 g | 12.37 ± 1.47 d | 36.09 ± 1.72 b | 17.01 ± 0.08 a |
| ANN | 7.71 ± 0.12 e | 8.24 ± 0.29 ab | 38.43 ± 1.48 bc | 37.74 ± 1.30 c |
| HMT20 + ANN | 6.62 ± 0.05 d | 6.85 ± 1.52 a | 32.09 ± 1.88 a | 43.90 ± 1.64 e |
| ANN + HMT20 | 5.87 ± 0.03 c | 7.28 ± 0.08 a | 35.36 ± 2.14 ab | 43.94 ± 0.84 e |
| Samples | Amylose | Damage Starch |
|---|---|---|
| (%, d.b.) | ||
| Native | 23.29 ± 0.90 ab | 1.38 ± 0.01 a |
| HMT20 | 24.20 ± 0.83 bc | 1.47 ± 0.03 a |
| HMT25 | 24.67 ± 1.14 cd | 1.87 ± 0.06 b |
| HMT30 | 25.05 ± 0.71 cd | 8.06 ± 0.22 e |
| ANN | 22.91 ± 0.17 a | 2.71 ± 0.04 c |
| HMT20 + ANN | 25.78 ± 0.28 de | 3.41 ± 0.03 d |
| ANN + HMT20 | 26.41 ± 0.09 e | 1.71 ± 0.03 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.-L.; Tsai, P.-C.; Lai, L.-S. Impacts of Hydrothermal Treatments on the Morphology, Structural Characteristics, and In Vitro Digestibility of Water Caltrop Starch. Molecules 2021, 26, 4974. https://doi.org/10.3390/molecules26164974
Liu J-L, Tsai P-C, Lai L-S. Impacts of Hydrothermal Treatments on the Morphology, Structural Characteristics, and In Vitro Digestibility of Water Caltrop Starch. Molecules. 2021; 26(16):4974. https://doi.org/10.3390/molecules26164974
Chicago/Turabian StyleLiu, Jia-Lin, Po-Ching Tsai, and Lih-Shiuh Lai. 2021. "Impacts of Hydrothermal Treatments on the Morphology, Structural Characteristics, and In Vitro Digestibility of Water Caltrop Starch" Molecules 26, no. 16: 4974. https://doi.org/10.3390/molecules26164974
APA StyleLiu, J.-L., Tsai, P.-C., & Lai, L.-S. (2021). Impacts of Hydrothermal Treatments on the Morphology, Structural Characteristics, and In Vitro Digestibility of Water Caltrop Starch. Molecules, 26(16), 4974. https://doi.org/10.3390/molecules26164974






