Tributyltin(IV) Butyrate: A Novel Epigenetic Modifier with ER Stress- and Apoptosis-Inducing Properties in Colon Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
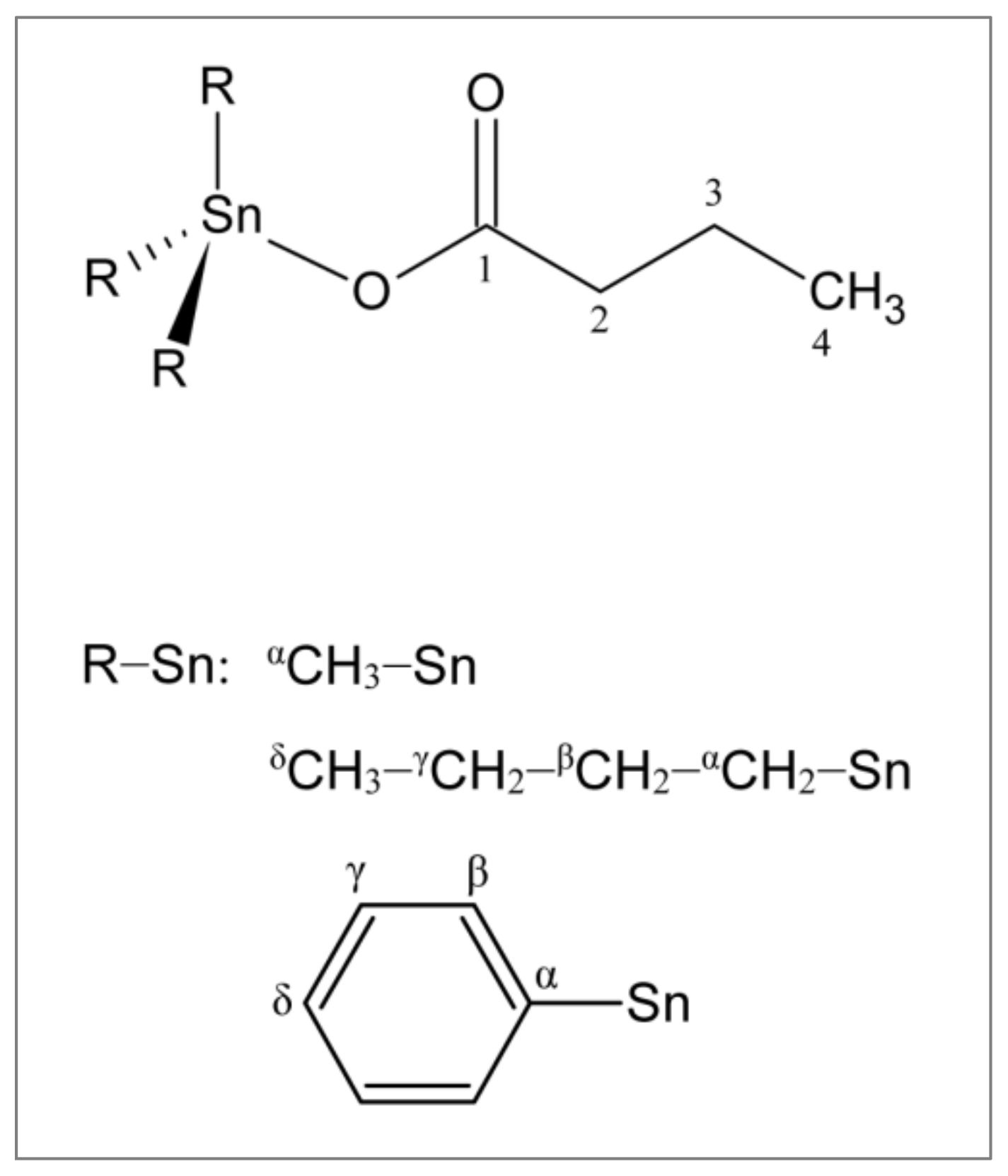
2.1. Synthesis and Characterization of Triorganotin(IV) Butyrates
2.2. Biological Study
2.2.1. The Effects of Different Triorganotin(IV) Butyrates on Colon Cancer Cells’ Viability
2.2.2. The Effect of BT2 on Cell Cycle and ER Stress
2.2.3. BT2 Promotes Apoptotic Cell Death
2.2.4. BT2 Induces Histone Deacetylation
3. Materials and Methods
3.1. Materials
3.2. Synthetic Procedures of Triorganotin(IV) Complexes
3.2.1. Trimethyltin(IV) butyrate (BT1)
3.2.2. Tributyltin(IV) butyrate (BT2)
3.2.3. Triphenyltin(IV) butyrate (BT3)
3.3. Instrumentation
3.4. Cell Cultures
3.5. Cell Viability MTT Assay Evaluation
3.6. Cell Cycle Evaluation
3.7. Evaluation of Cell Death
3.8. Western Blotting Analysis
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pellerito, L. Organotin(IV) n+ complexes formed with biologically active ligands: Equilibrium and structural studies, and some biological aspects. Coord. Chem. Rev. 2002, 224, 111–150. [Google Scholar] [CrossRef]
- Gielen, M. Review: Organotin compounds and their therapeutic potential: A report from the Organometallic Chemistry Department of the Free University of Brussels. Appl. Organometal. Chem. 2002, 16, 481–494. [Google Scholar] [CrossRef]
- Sirajuddin, M.; Ali, S. Organotin(IV) Carboxylates as Promising Potential Drug Candidates in the Field of Cancer Chemotherapy. CPD 2017, 22, 6665–6681. [Google Scholar] [CrossRef] [PubMed]
- Bajka, B.H.; Clarke, J.M.; Cobiac, L.; Topping, D.L. Butyrylated starch protects colonocyte DNA against dietary protein-induced damage in rats. Carcinogenesis 2008, 29, 2169–2174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corfe, B.M.; Williams, E.A.; Bury, J.P.; Riley, S.A.; Croucher, L.J.; Lai, D.Y.; Evans, C.A. A study protocol to investigate the relationship between dietary fibre intake and fermentation, colon cell turnover, global protein acetylation and early carcinogenesis: The FACT study. BMC Cancer 2009, 9, 332. [Google Scholar] [CrossRef] [Green Version]
- Le Leu, R.K.; Hu, Y.; Brown, I.L.; Young, G.P. Effect of high amylose maize starches on colonic fermentation and apoptotic response to DNA-damage in the colon of rats. Nutr. Metab. 2009, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.-J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2007, 27, 104–119. [Google Scholar] [CrossRef]
- Cahours, A. Untersuchungen über die metallhaltigen organischen Radicale. Ann. Chem. Pharm. 1860, 114, 227–255. [Google Scholar] [CrossRef] [Green Version]
- Caseri, W. Initial organotin chemistry. J. Organomet. Chem. 2014, 751, 20–24. [Google Scholar] [CrossRef]
- Nath, M.; Vats, M.; Roy, P. Mode of action of tin-based anti-proliferative agents: Biological studies of organotin(IV) derivatives of fatty acids. J. Photochem. Photobiol. B Biol. 2015, 148, 88–100. [Google Scholar] [CrossRef]
- Di Stefano, R.; Scopelliti, M.; Pellerito, C.; Casella, G.; Fiore, T.; Stocco, G.C.; Vitturi, R.; Colomba, M.; Ronconi, L.; Sciacca, I.D. Organometallic complexes with biological molecules. XVIII. Alkyltin(IV) cephalexinate complexes: Synthesis, solid state and solution phase investigations. J. Inorg. Biochem. 2004, 98, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Pellerito, C.; D’Agati, P.; Fiore, T.; Mansueto, C.; Mansueto, V.; Stocco, G.; Nagy, L.; Pellerito, L. Synthesis, structural investigations on organotin(IV) chlorin-e6 complexes, their effect on sea urchin embryonic development and induced apoptosis. J. Inorg. Biochem. 2005, 99, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Pellerito, O.; Prinzivalli, C.; Foresti, E.; Sabatino, P.; Abbate, M.; Casella, G.; Fiore, T.; Scopelliti, M.; Pellerito, C.; Giuliano, M.; et al. Synthesis, chemical characterization and biological activity of new histone acetylation/deacetylation specific inhibitors: A novel and potential approach to cancer therapy. J. Inorg. Biochem. 2013, 125, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Abbate, M.; Casella, G.; Fiore, T.; Grasso, G.; Pellerito, C.; Scopelliti, M.; Spinella, A.; Pellerito, L. Structural characterization of triorganotin(IV) complexes with sodium fusidate and DFT calculations. J. Organomet. Chem. 2010, 695, 1405–1413. [Google Scholar] [CrossRef]
- Pellerito, C.; Morana, O.; Ferrante, F.; Calvaruso, G.; Notaro, A.; Sabella, S.; Fiore, T. Synthesis, chemical characterization, computational studies and biological activity of new DNA methyltransferases (DNMTs) specific inhibitor. Epigenetic regulation as a new and potential approach to cancer therapy. J. Inorg. Biochem. 2015, 150, 18–27. [Google Scholar] [CrossRef]
- Pellerito, C.; Emanuele, S.; Ferrante, F.; Celesia, A.; Giuliano, M.; Fiore, T. Tributyltin(IV) ferulate, a novel synthetic ferulic acid derivative, induces autophagic cell death in colon cancer cells: From chemical synthesis to biochemical effects. J. Inorg. Biochem. 2020, 205, 110999. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Shin, H.; Kim, J.-H. Change in gene expression profiles of secreted frizzled-related proteins (SFRPs) by sodium butyrate in gastric cancers: Induction of promoter demethylation and histone modification causing inhibition of Wnt signaling. Int. J. Oncol. 2012, 40, 1533–1542. [Google Scholar] [CrossRef] [Green Version]
- Frew, A.J.; Johnstone, R.W.; Bolden, J.E. Enhancing the apoptotic and therapeutic effects of HDAC inhibitors. Cancer Lett. 2009, 280, 125–133. [Google Scholar] [CrossRef]
- Geng, H.-W.; Yin, F.-Y.; Zhang, Z.-F.; Gong, X.; Yang, Y. Butyrate Suppresses Glucose Metabolism of Colorectal Cancer Cells via GPR109a-AKT Signaling Pathway and Enhances Chemotherapy. Front. Mol. Biosci. 2021, 8, 634874. [Google Scholar] [CrossRef] [PubMed]
- Mrkvicova, A.; Chmelarova, M.; Peterova, E.; Havelek, R.; Baranova, I.; Kazimirova, P.; Rudolf, E.; Rezacova, M. The effect of sodium butyrate and cisplatin on expression of EMT markers. PLoS ONE 2019, 14, e0210889. [Google Scholar] [CrossRef]
- Ramos, M.G.; Rabelo, F.L.A.; Brumatti, G.; Bueno-da-Silva, A.E.; Amarante-Mendes, G.P.; Alvarez-Leite, J.I. Butyrate Increases Apoptosis Induced by Different Antineoplastic Drugs in Monocytic Leukemia Cells. Chemotherapy 2004, 50, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Shetty, M.G.; Pai, P.; Deaver, R.E.; Satyamoorthy, K.; Babitha, K.S. Histone deacetylase 2 selective inhibitors: A versatile therapeutic strategy as next generation drug target in cancer therapy. Pharmacol. Res. 2021, 170, 105695. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Feng, F.; Li, H. Research advances on epigenetics and cancer metabolism. J. Zhejiang Univ. Med. Sci. 2021, 50, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, H.; Gao, P. Metabolic reprogramming and epigenetic modifications on the path to cancer. Protein Cell 2021. [Google Scholar] [CrossRef]
- Osada, S.; Nishikawa, J.; Nakanishi, T.; Tanaka, K.; Nishihara, T. Some organotin compounds enhance histone acetyltransferase activity. Toxicol. Lett. 2005, 155, 329–335. [Google Scholar] [CrossRef]
- Hanaoka, S.; Ishida, K.; Tanaka, S.; Sakamoto, S.; Okuda, K.; Sanoh, S.; Ohta, S.; Kotake, Y. Tributyltin induces epigenetic changes and decreases the expression of nuclear respiratory factor-1. Metallomics 2018, 10, 337–345. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Zhang, J.; Chen, Y.; Zuo, Z. DNA hypomethylation induced by tributyltin, triphenyltin, and a mixture of these in Sebastiscus marmoratus liver. Aquat. Toxicol. 2009, 95, 93–98. [Google Scholar] [CrossRef]
- Kristeleit, R.; Stimson, L.; Workman, P.; Aherne, W. Histone modification enzymes: Novel targets for cancer drugs. Expert Opin. Emerg. Drugs 2004, 9, 135–154. [Google Scholar] [CrossRef]
- Vigushin, D.M.; Coombes, R.C. Histone deacetylase inhibitors in cancer treatment. Anti Cancer Drugs 2002, 13, 1–13. [Google Scholar] [CrossRef]
- Andrade, F.D.O.; Furtado, K.S.; Heidor, R.; Sandri, S.; Hebeda, C.B.; Miranda, M.L.P.; Fernandes, L.H.G.; Yamamoto, R.C.; Horst, M.A.; Farsky, S.H.P.; et al. Antiangiogenic effects of the chemopreventive agent tributyrin, a butyric acid prodrug, during the promotion phase of hepatocarcinogenesis. Carcinogenesis 2019, 40, 979–988. [Google Scholar] [CrossRef]
- Deacon, G. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Deacon, G.B.; Huber, F.; Phillips, R.J. Diagnosis of the nature of carboxylate coordination from the direction of shifts of carbon oxygen stretching frequencies. Inorg. Chim. Acta 1985, 104, 41–45. [Google Scholar] [CrossRef]
- Nath, M.; Vats, M.; Roy, P. Tri- and diorganotin(IV) complexes of biologically important orotic acid: Synthesis, spectroscopic studies, in vitro anti-cancer, DNA fragmentation, enzyme assays and in vivo anti-inflammatory activities. Eur. J. Med. Chem. 2013, 59, 310–321. [Google Scholar] [CrossRef]
- Nath, M.; Vats, M.; Roy, P. Design, spectral characterization, anti-tumor and anti-inflammatory activity of triorganotin(IV) hydroxycarboxylates, apoptosis inducers: In vitro assessment of induction of apoptosis by enzyme, DNA-fragmentation, acridine orange and comet assays. Inorg. Chim. Acta 2014, 423, 70–82. [Google Scholar] [CrossRef]
- Whiffen, D.H. 273. Vibrational frequencies and thermodynamic properties of fluoro-, chloro-, bromo-, and iodo-benzene. J. Chem. Soc. 1956, 1350–1356. [Google Scholar] [CrossRef]
- Lockhart, T.P.; Manders, W.F. Structure determination by NMR spectroscopy. Dependence of |2J(119Sn,1H)| on the Me-Sn-Me angle in methyltin(IV) compounds. Inorg. Chem. 1986, 25, 892–895. [Google Scholar] [CrossRef]
- Lockhart, T.P.; Manders, W.F. Solid-state carbon-13 NMR investigation of methyltin(IV) compounds. Correlation of NMR parameters with molecular structure. J. Am. Chem. Soc. 1987, 109, 7015–7020. [Google Scholar] [CrossRef]
- Holeček, J.; Lyčka, A. Dependence of [1J(119Sn13C)] on the C—Sn—C angle in n-butyltin(IV) compounds. Inorg. Chim. Acta 1986, 118, L15–L16. [Google Scholar] [CrossRef]
- Holeček, J.; Nádvorník, M.; Handlíř, K.; Lyčka, A. 13C and 119Sn NMR Study of some four- and five-coordinate triphenyltin(IV) compounds. J. Organomet. Chem. 1983, 241, 177–184. [Google Scholar] [CrossRef]
- Baul, T.S.B.; Masharing, C.; Basu, S.; Rivarola, E.; Holčapek, M.; Jirásko, R.; Lyčka, A.; de Vos, D.; Linden, A. Synthesis, characterization, cytotoxic activity and crystal structures of tri- and di-organotin(IV) complexes constructed from the β-{[(E)-1-(2-hydroxyaryl)alkylidene]amino}propionate and β-{[(2Z)-(3-hydroxy-1-methyl-2-butenylidene)]amino}propionate skeletons. J. Organomet. Chem. 2006, 691, 952–965. [Google Scholar] [CrossRef]
- Baul, T.S.B.; Singh, K.S.; Holčapek, M.; Jirásko, R.; Rivarola, E.; Linden, A. Synthesis, characterization and crystal structures of polymeric and dimeric triphenyltin(IV) complexes of 4-[((E)-1-{2-hydroxy-5-[(E)-2-(2-carboxyphenyl)-1-diazenyl]phenyl} methylidene)amino]aryls. J. Organomet. Chem. 2005, 690, 4232–4242. [Google Scholar] [CrossRef]
- Cao, M.; Zhang, Z.; Han, S.; Lu, X. Butyrate inhibits the proliferation and induces the apoptosis of colorectal cancer HCT116 cells via the deactivation of mTOR/S6K1 signaling mediated partly by SIRT1 downregulation. Mol. Med. Rep. 2019, 19, 3941–3947. [Google Scholar] [CrossRef] [PubMed]
- Lallemand, F.; Courilleau, D.; Buquet-Fagot, C.; Atfi, A.; Montagne, M.-N.; Mester, J. Sodium Butyrate Induces G2 Arrest in the Human Breast Cancer Cells MDA-MB-231 and Renders Them Competent for DNA Rereplication. Exp. Cell Res. 1999, 247, 432–440. [Google Scholar] [CrossRef]
- Lee, D.; Hokinson, D.; Park, S.; Elvira, R.; Kusuma, F.; Lee, J.-M.; Yun, M.; Lee, S.-G.; Han, J. ER Stress Induces Cell Cycle Arrest at the G2/M Phase Through eIF2α Phosphorylation and GADD45α. Int. J. Mol. Sci. 2019, 20, 6309. [Google Scholar] [CrossRef] [Green Version]
- Chaitanya, G.; Alexander, J.S.; Babu, P. PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell Commun. Signal. 2010, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Donohoe, D.R.; Collins, L.B.; Wali, A.; Bigler, R.; Sun, W.; Bultman, S.J. The Warburg Effect Dictates the Mechanism of Butyrate-Mediated Histone Acetylation and Cell Proliferation. Mol. Cell 2012, 48, 612–626. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, F.; Chen, X.; Wang, J.; Zhao, Y.; Li, Y.; He, B. Zinc-dependent Deacetylase (HDAC) Inhibitors with Different Zinc Binding Groups. Curr. Top. Med. Chem. 2019, 19, 223–241. [Google Scholar] [CrossRef]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef]
- Neumann, W.P. The Organic Chemistry of Tin; The Chemistry of Organometallic Compounds: London, UK; Interscience: New York, NY, USA, 1970; ISBN 978-0-471-63237-5. [Google Scholar]
- Notaro, A.; Emanuele, S.; Geraci, F.; D’Anneo, A.; Lauricella, M.; Calvaruso, G.; Giuliano, M. WIN55, 212-2-Induced Expression of Mir-29b1 Favours the Suppression of Osteosarcoma Cell Migration in a SPARC-Independent Manner. Int. J. Mol. Sci. 2019, 20, 5235. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuliano, M.; Pellerito, C.; Celesia, A.; Fiore, T.; Emanuele, S. Tributyltin(IV) Butyrate: A Novel Epigenetic Modifier with ER Stress- and Apoptosis-Inducing Properties in Colon Cancer Cells. Molecules 2021, 26, 5010. https://doi.org/10.3390/molecules26165010
Giuliano M, Pellerito C, Celesia A, Fiore T, Emanuele S. Tributyltin(IV) Butyrate: A Novel Epigenetic Modifier with ER Stress- and Apoptosis-Inducing Properties in Colon Cancer Cells. Molecules. 2021; 26(16):5010. https://doi.org/10.3390/molecules26165010
Chicago/Turabian StyleGiuliano, Michela, Claudia Pellerito, Adriana Celesia, Tiziana Fiore, and Sonia Emanuele. 2021. "Tributyltin(IV) Butyrate: A Novel Epigenetic Modifier with ER Stress- and Apoptosis-Inducing Properties in Colon Cancer Cells" Molecules 26, no. 16: 5010. https://doi.org/10.3390/molecules26165010
APA StyleGiuliano, M., Pellerito, C., Celesia, A., Fiore, T., & Emanuele, S. (2021). Tributyltin(IV) Butyrate: A Novel Epigenetic Modifier with ER Stress- and Apoptosis-Inducing Properties in Colon Cancer Cells. Molecules, 26(16), 5010. https://doi.org/10.3390/molecules26165010






