Electrophysiological Responses of Bactrocera kraussi (Hardy) (Tephritidae) to Rectal Gland Secretions and Headspace Volatiles Emitted by Conspecific Males and Females
Abstract
:1. Introduction
2. Results
2.1. Analysis of Rectal Gland Extracts and Headspace Collections
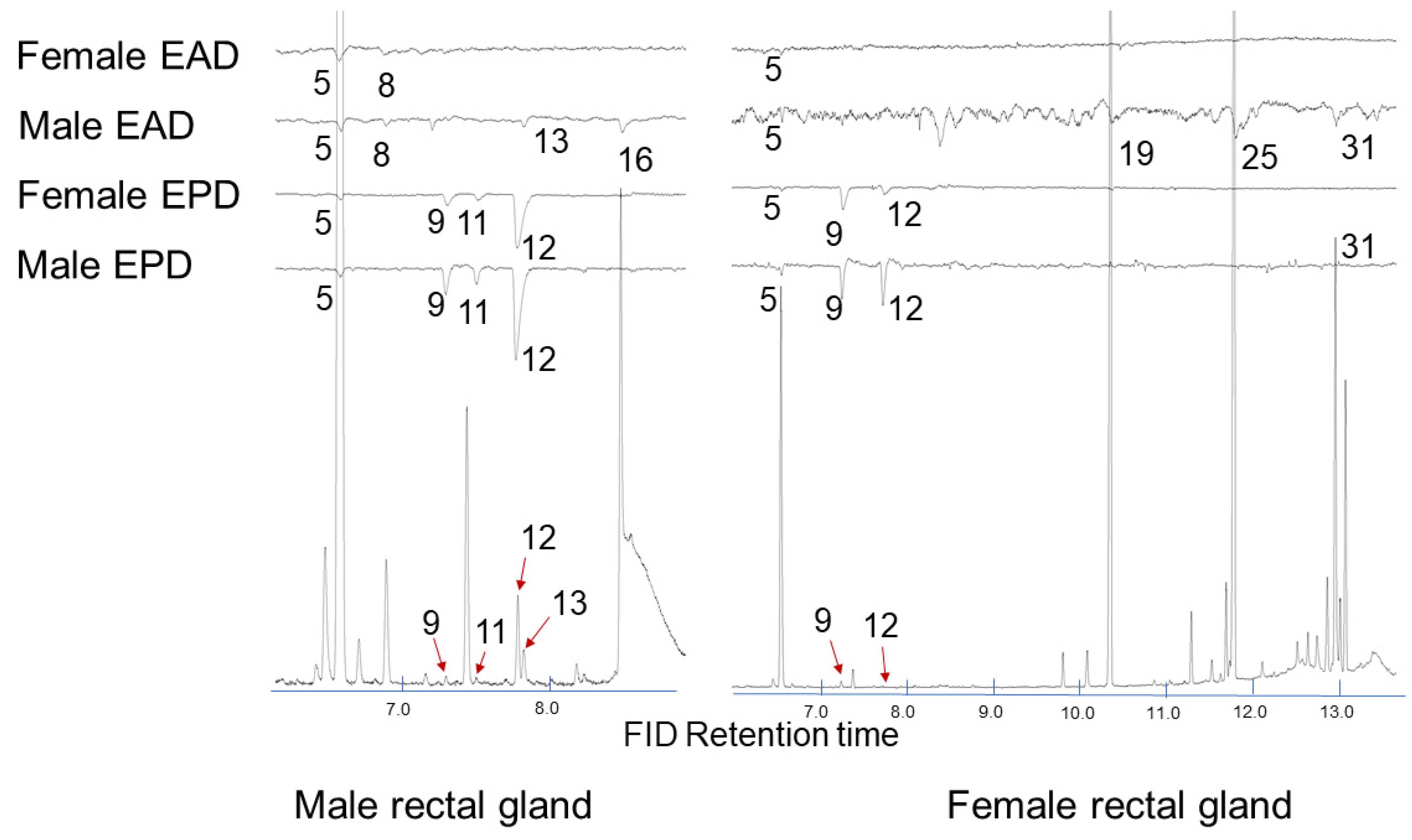
2.2. Electrophysiology
3. Discussion
4. Materials and Methods
4.1. Insects
4.2. Rectal Gland Extraction
4.3. Headspace Collection
4.4. Analysis of Rectal Gland Extracts and Headspace Collections
4.5. Synthesis
4.5.1. General Procedure
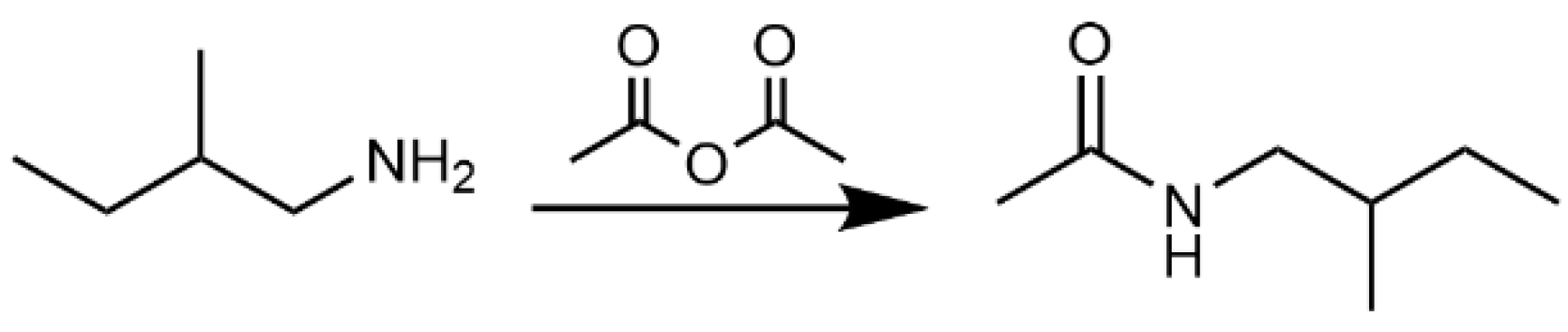
4.5.2. Synthesis of N-(2-Methylbutyl)acetamide (3)
4.5.3. Synthesis of N-(3-Methylbutyl)acetamide (4)
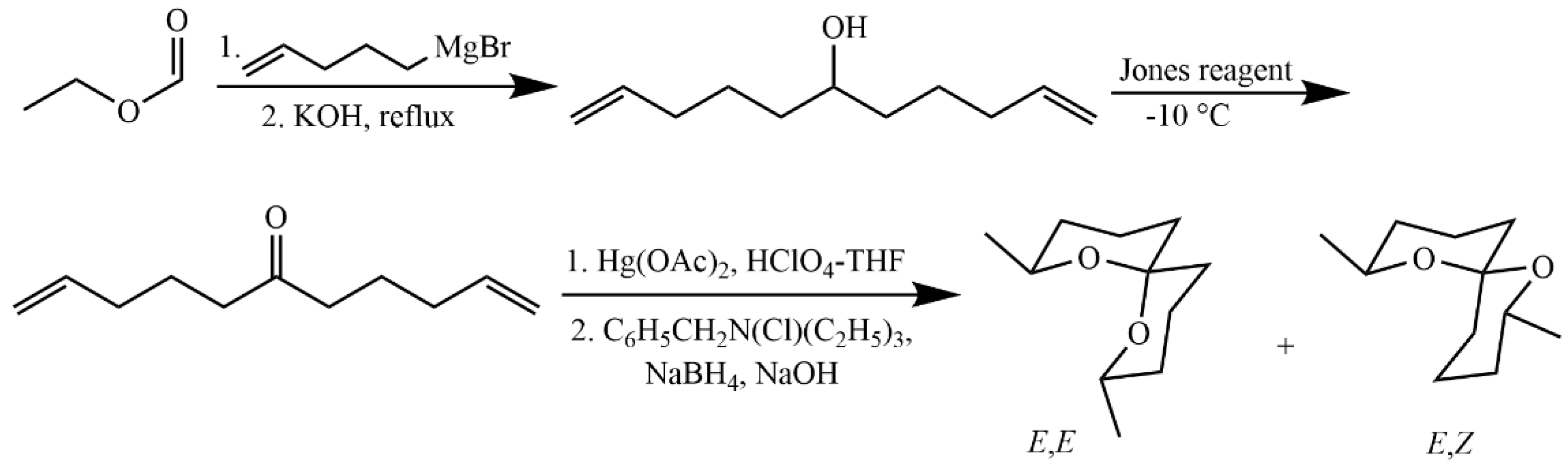
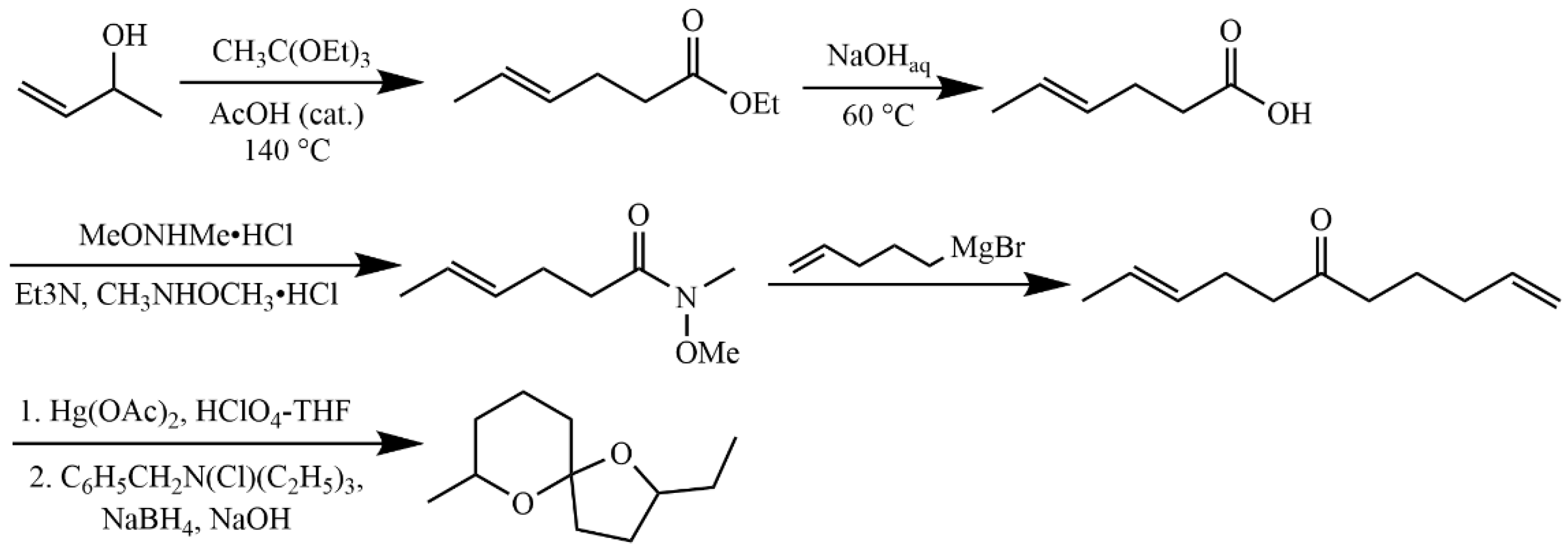
4.5.4. Synthesis of 2,8-Dimethyl-1,7-dioxaspiro[5.5]undecane
4.5.5. Synthesis of 2-Ethyl-7-methyl-1,6-dioxaspiro[4.5]undecane (6)
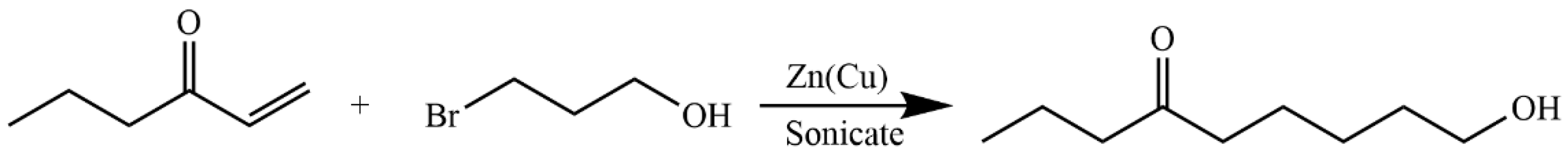
4.5.6. Synthesis of 6-Oxanon-1-ol (11)
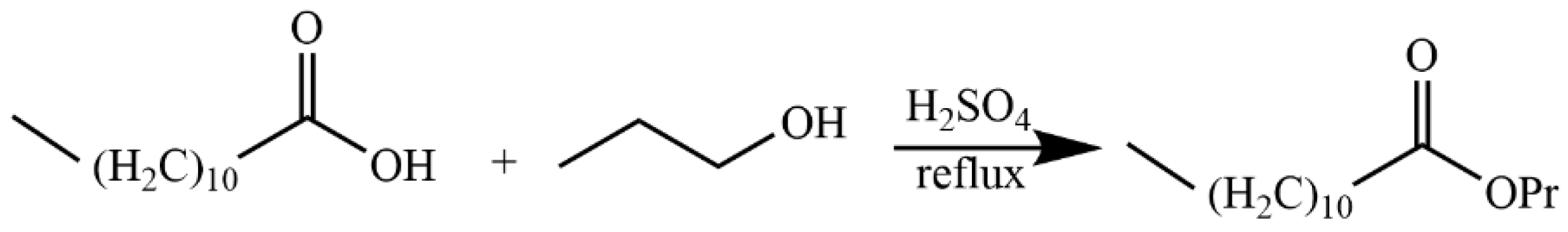
4.5.7. Synthesis of Propyl Dodecanoiate (15)
4.5.8. Synthesis of Ethyl (Z)-Hexadec-9-enoate (31)
4.5.9. Synthesis of Ethyl (E)-Octadec-9-enoate (34)
4.6. Electrophysiology
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Witzgall, P.; Kirsch, P.; Cork, A. Sex pheromones and their impact on pest management. J. Chem. Ecol. 2010, 36, 80–100. [Google Scholar] [CrossRef]
- Benelli, G.; Daane, K.M.; Canale, A.; Niu, C.-Y.; Messing, R.H.; Vargas, R.I. Sexual communication and related behaviours in Tephritidae: Current knowledge and potential applications for integrated pest management. J. Pest. Sci. 2014, 87, 385–405. [Google Scholar] [CrossRef]
- Nation, J.L. Courtship behavior and evidence for a sex attractant in the male Caribbean fruit fly, Anastrepha suspensa. Ann. Entomol. Soc. Am. 1972, 65, 1364–1367. [Google Scholar] [CrossRef]
- Perkins, M.V. Characterisation and Synthesis of Bactrocera Fruit fly Pheromones. Ph.D. Thesis, University of Queensland, Brisbane, Australia, 1990. [Google Scholar]
- Cruz-López, L.; Malo, E.A.; Rojas, J.C. Sex Pheromone of Anastrepha striata. J. Chem. Ecol. 2015, 41, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Sivinski, J.M.; Calkins, C. Pheromones and parapheromones in the control of tephritids. Fla. Entomol. 1986, 69, 157–168. [Google Scholar] [CrossRef]
- Hendrichs, J.; Robinson, A.S.; Cayol, J.P.; Enkerlin, W. Medfly areawide sterile insect technique programmes for prevention, suppression or eradication: The importance of mating behavior studies. Fla. Entomol. 2002, 85, 1–13. [Google Scholar] [CrossRef]
- El-Sayed, A.M.; Venkatesham, U.; Unelius, C.R.; Sporle, A.; Pérez, J.; Taylor, P.W.; Suckling, D.M. Chemical composition of the rectal gland and volatiles released by female Queensland fruit fly, Bactrocera tryoni (Diptera: Tephritidae). Environ. Entomol. 2019, 48, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Haniotakis, G.E. Sexual attraction in the olive fruit fly, Dacus oleae (Gmelin). Environ. Entomol. 1974, 3, 82–86. [Google Scholar] [CrossRef]
- Mazomenos, B.E.; Haniotakis, G.E. Male olive fruit fly attraction to synthetic sex pheromone components in laboratory and field tests. J. Chem. Ecol. 1985, 11, 397–405. [Google Scholar] [CrossRef]
- Carpita, A.; Canale, A.; Raffaelli, A.; Saba, A.; Benelli, G.; Raspi, A. (Z)-9-tricosene identified in rectal gland extracts of Bactrocera oleae males: First evidence of a male-produced female attractant in olive fruit fly. Naturwissenschaften 2012, 99, 77–81. [Google Scholar] [CrossRef]
- Canale, A.; Germinara, S.G.; Carpita, A.; Benelli, G.; Bonsignori, G.; Stefanini, C.; Raspi, A.; Rotundo, G. Behavioural and electrophysiological responses of the olive fruit fly, Bactrocera oleae (Rossi) (Diptera: Tephritidae), to male- and female-borne sex attractants. Chemoecology 2013, 23, 155–164. [Google Scholar] [CrossRef]
- Frías-Lasserre, D. Effects of female fruit-marking pheromones on oviposition, mating, and male behavior in the neotropical species Rhagoletis conversa Bréthes and Rhagoletis brncici Frías (Diptera: Tephritidae). Neotrop. Entomol. 2015, 44, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Bellas, T.E.; Fletcher, B.S. Identification of the major components in the secretion from the rectal pheromone glands of the queensland fruit flies Dacus tryoni and Dacus neohumeralis (Diptera: Tephritidae). J. Chem. Ecol. 1979, 5, 795–803. [Google Scholar] [CrossRef]
- Baker, R.; Herbert, R.H.; Lomer, R.A. Chemical components of the rectal gland secretions of male Dacus cucurbitae, the melon fly. Experientia 1982, 38, 232–233. [Google Scholar] [CrossRef]
- Baker, R.; Bacon, A.J. The identification of spiroacetals in the volatile secretions of two species of fruit fly (Dacus dorsalis, Dacus curcurbitae). Experientia 1985, 41, 1484–1485. [Google Scholar] [CrossRef]
- Baker, R.; Herbert, R.H. Isolation and synthesis of 1,7-dioxaspiro[5.5]undecane and 1,7-dioxaspiro[5.5]undecan-3-and -4-ols from the olive fly (Dacus oleae). J. Chem. Soc. Perkin Trans. 1987, 1, 1123–1127. [Google Scholar] [CrossRef]
- Kitching, W.; Lewis, J.A.; Perkins, M.V.; Drew, R.; Moore, C.J.; Schurig, V.; Koenig, W.A.; Francke, W. Chemistry of fruit flies. Composition of the rectal gland secretion of (male) Dacus cucumis (cucumber fly) and Dacus halfordiae. Characterization of (Z,Z)-2,8-dimethyl-1,7-dioxaspiro[5.5]undecane. J. Org. Chem. 1989, 54, 3893–3902. [Google Scholar] [CrossRef]
- Fletcher, M.T.; Wells, J.A.; Jacobs, M.F.; Krohn, S.; Kitching, W.; Drew, R.A.I.; Moore, C.J.; Francke, W. Chemistry of fruit-flies. Spiroacetal-rich secretions in several Bactrocera species from the South-West Pacific region. J. Chem. Soc. Perkin Trans. 1992, 1, 2827–2831. [Google Scholar] [CrossRef]
- Booth, Y.K.; Schwartz, B.D.; Fletcher, M.T.; Lambert, L.K.; Kitching, W.; De Voss, J.J. A diverse suite of spiroacetals, including a novel branched representative, is released by female Bactrocera tryoni (Queensland fruit fly). Chem. Commun. 2006, 42, 3975–3977. [Google Scholar] [CrossRef]
- Benelli, G.; Bonsignori, G.; Stefanini, C.; Raspi, A.; Canale, A. The production of female sex pheromone in Bactrocera oleae (Rossi) young males does not influence their mating chances. Entomol. Sci. 2013, 16, 47–53. [Google Scholar] [CrossRef]
- Noushini, S.; Perez, J.; Park, S.J.; Holgate, D.; Jamie, I.; Jamie, J.; Taylor, P. Rectal gland chemistry, volatile emissions, and antennal responses of male and female banana fruit fly, Bactrocera musae. Insects 2019, 11, 32. [Google Scholar] [CrossRef] [Green Version]
- Noushini, S.; Park, S.J.; Jamie, I.; Jamie, J.; Taylor, P. Sampling technique biases in the analysis of fruit fly volatiles: A case study of Queensland fruit fly. Sci. Rep. 2020, 10, 19799. [Google Scholar] [CrossRef]
- Fletcher, M.T.; Kitching, W. Chemistry of fruit flies. Chem. Rev. 1995, 95, 789–828. [Google Scholar] [CrossRef]
- Symonds, M.R.E.; Moussalli, A.; Elgar, M.A. The evolution of sex pheromones in an ecologically diverse genus of flies. Biol. J. Linn. Soc. 2009, 97, 594–603. [Google Scholar] [CrossRef]
- Noushini, S.; Park, S.J.; Jamie, I.; Jamie, J.; Taylor, P. Rectal gland exudates and emissions of Bactrocera bryoniae: Chemical identification, electrophysiological and pheromonal functions. Chemoecology 2021, 31, 137–148. [Google Scholar] [CrossRef]
- Zhang, H.; Fletcher, M.T.; Dettner, K.; Francke, W.; Kitching, W. Synthesis and absolute stereochemistry of spiroacetals in rove beetles (Coleoptera: Staphylinidae). Tetrahedron Lett. 1999, 40, 7851–7854. [Google Scholar] [CrossRef]
- Bruschini, C.; Cervo, R.; Protti, I.; Turillazzi, S. Caste differences in venom volatiles and their effect on alarm behaviour in the paper wasp Polistes dominulus (Christ). J. Exp. Biol. 2008, 211, 2442–2449. [Google Scholar] [CrossRef] [Green Version]
- Schultz, M.; McMahon, J.; Krosch, M.N.; Royer, J.E.; Bottrill, M.; Woods, N.; Woods, B.; Blacket, M. The Australian Handbook for the Identification of Fruit Flies. Version 3.1; Plant Health Australia: Canberra, Australia, 2018. [Google Scholar]
- Hancock, D.L.; Hamacek, E.L.; Lloyd, A.C.; Elson-Harris, M.M. The Distribution and Host Plants of Fruit Flies (Diptera: Tephritidae) in Australia; DPI Publications: Brisbane, Australia, 2000. [Google Scholar]
- Advanced Chemistry Development Inc. ACD/Labs Virsion V11.02; Advanced Chemistry Development Inc.: Toronto, ON, Canada, 2020. [Google Scholar]
- Schwartz, B.D.; Booth, Y.K.; Fletcher, M.T.; Kitching, W.; De Voss, J.J. Spiroacetal biosynthesis in fruit flies is complex: Distinguishable origins of the same major spiroacetal released by different Bactrocera spp. Chem. Commun. 2010, 46, 1526–1528. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.D.; Moore, C.J.; Rahm, F.; Hayes, P.Y.; Kitching, W.; De Voss, J.J. Spiroacetal biosynthesis in insects from diptera to hymenoptera: The giant ichneumon wasp Megarhyssa nortoni nortoni cresson. J. Am. Chem. Soc. 2008, 130, 14853–14860. [Google Scholar] [CrossRef] [PubMed]
- Noushini, S.; Perez, J.; Park, S.J.; Holgate, D.; Mendez Alvarez, V.; Jamie, I.; Jamie, J.; Taylor, P. Attraction and electrophysiological response to identified rectal gland volatiles in Bactrocera frauenfeldi (Schiner). Molecules 2020, 25, 1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canale, A.; Benelli, G.; Germinara, G.S.; Fusini, G.; Romano, D.; Rapalini, F.; Desneux, N.; Rotundo, G.; Raspi, A.; Carpita, A. Behavioural and electrophysiological responses to overlooked female pheromone components in the olive fruit fly, Bactrocera oleae (Diptera: Tephritidae). Chemoecology 2015, 25, 147–157. [Google Scholar] [CrossRef]
- Levi-Zada, A.; Levy, A.; Rempoulakis, P.; Fefer, D.; Steiner, S.; Gazit, Y.; Nestel, D.; Yuval, B.; Byers, J.A. Diel rhythm of volatile emissions of males and females of the peach fruit fly Bactrocera zonata. J. Insect Physiol. 2020, 120, 103970. [Google Scholar] [CrossRef]
- Verschut, T.A.; Farnier, K.; Cunningham, J.P.; Carlsson, M.A. Behavioral and physiological evidence for palp detection of the male-specific attractant cuelure in the Queensland fruit fly (Bactrocera tryoni). Front. Physiol. 2018, 9. [Google Scholar] [CrossRef]
- Biswas, M.J.H.; Mainali, B.; Park, S.J.; Taylor, P.; Rempoulakis, P. Electrophysiological responses to cuelure of raspberry ketone-fed Queensland fruit flies. J. Econ. Entomol. 2020, 113, 2832–2839. [Google Scholar] [CrossRef]
- Oh, H.-W.; Jeong, S.A.; Kim, J.; Park, K.C. Morphological and functional heterogeneity in olfactory perception between antennae and maxillary palps in the pumpkin fruit fly, Bactrocera depressa. Arch. Insect Biochem. Physiol. 2019, 101, e21560. [Google Scholar] [CrossRef]
- Steiner, L.F.; Mitchell, S. Tephritid Fruit Flies. In Insect Colonization and Mass Production; Smith, C.N., Ed.; Academic Press: New York, NY, USA, 1966; pp. 555–583. [Google Scholar] [CrossRef]
- Pérez, J.; Park, S.J.; Taylor, P.W. Domestication modifies the volatile emissions produced by male Queensland fruit flies during sexual advertisement. Sci. Rep. 2018, 8, 16503. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.M.; Byers, J.A.; Manning, L.M.; Jürgens, A.; Mitchell, V.J.; Suckling, D.M. Floral scent of Canada thistle and tts potential as a generic insect attractant. J. Econ. Entomol. 2008, 101, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.; Bhattacharjya, G.; Talukdar, B.; Patel, B.K. Chemoselective acylation of amines in aqueous media. Eur. J. Org. Chem. 2004, 2004, 1254–1260. [Google Scholar] [CrossRef]
- ASTM E1782-08. Standard Test Method for Determing Vapour Pressure by Thermal Analysis; ASTM International: West Conshohocken, PA, USA, 2008. [Google Scholar]
- Tay, G.C.; Sizemore, N.; Rychnovsky, S.D. Stereoselection in Intramolecular Diels–Alder Reactions of 2-Cyano-1-azadienes: Indolizidine and Quinolizidine Synthesis. Org. Lett. 2016, 18, 3050–3053. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Kamei, N.; Yokoshima, S.; Fukuyama, T. Total Synthesis of (−)-Histrionicotoxin. Org. Lett. 2011, 13, 4446–4449. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.R.; Waetzig, S.R.; Woerpel, K.A. Palladium(II)-Catalyzed Cyclization of Unsaturated Hydroperoxides for the Synthesis of 1,2-Dioxanes. Org. Lett. 2009, 11, 3290–3293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, J.; Kaur, J.; Nayyar, S.; Bhandari, M.; Kad, G.L. Ultrasound Mediated Synthesis of a Few Naturally Occurring Compounds. ChemInform 2000, 32, 386–390. [Google Scholar] [CrossRef]
- Perkins, M.V.; Fletcher, M.T.; Kitching, W.; Drew, R.A.I.; Moore, C.J. Chemical studies of rectal gland secretions of some species of Bactrocera dorsalis complex of fruit flies (diptera: Tephritidae). J. Chem. Ecol. 1990, 16, 2475–2487. [Google Scholar] [CrossRef] [PubMed]
- Denton, R.M.; Tang, X.; Przeslak, A. Catalysis of Phosphorus(V)-Mediated Transformations: Dichlorination Reactions of Epoxides Under Appel Conditions. Org. Lett. 2010, 12, 4678–4681. [Google Scholar] [CrossRef] [PubMed]
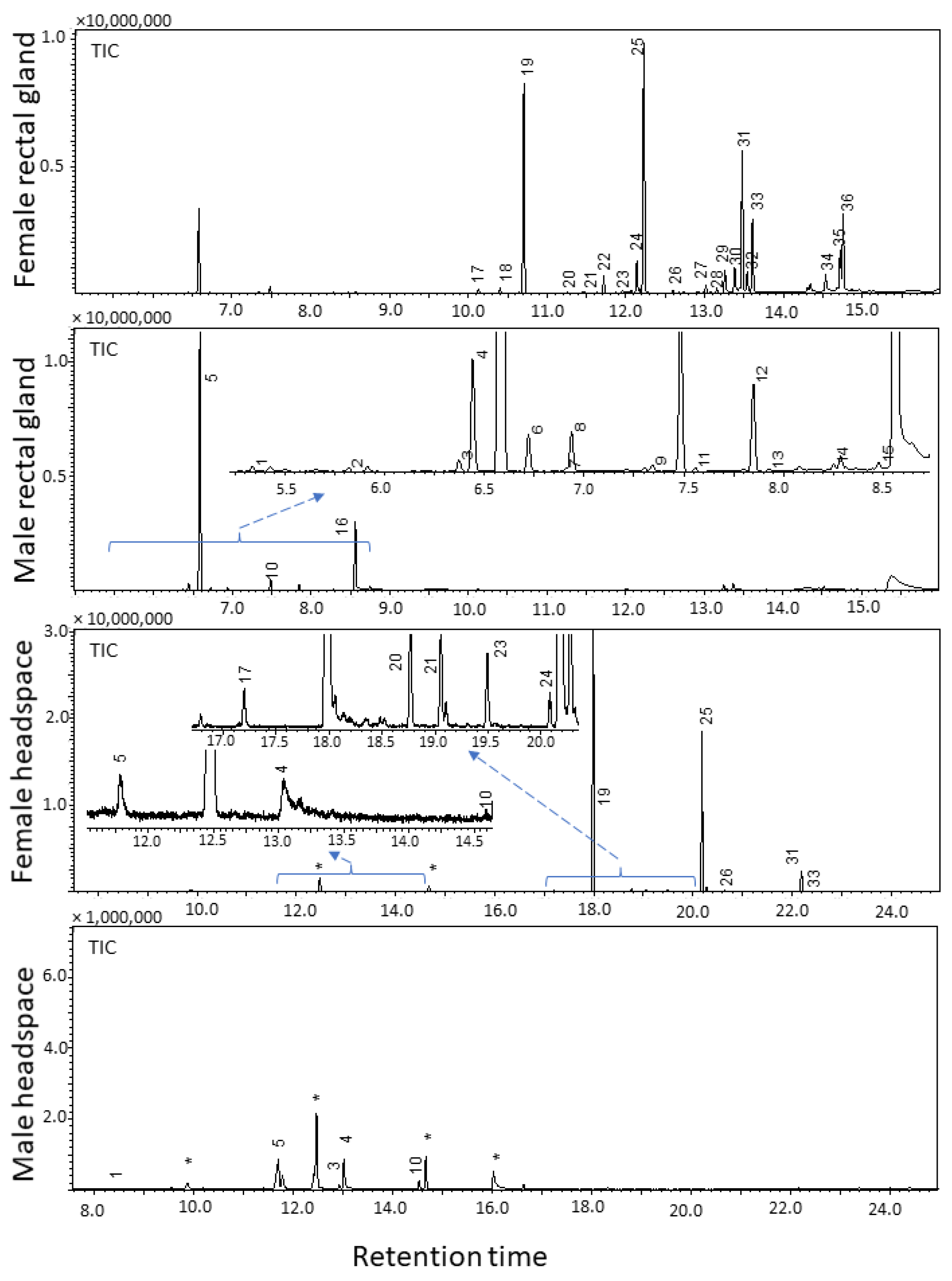



| RI | Name | MW | Characteristic EI ions m/z (%) | FRG (%) | FHS (%) | MRG (%) | MHS (%) |
|---|---|---|---|---|---|---|---|
| 1027 | 2-Ethyl-1-hexanol (1) | 130.2 | 112 (M–H2O, 2.1), 99 (β-cleavage product, 1.1), 98 (6.9), 83 (25.8), 70 (25.9), 69 (10.1), 57 (100), 56 (CH3CH2CH=CH2, 25.3) | ND | ND | 2.3 | 3.4 |
| 1073 | 2,7-Dimethyl-1,6-dioxaspiro[4.5]decane (2) | 170.1 | 170 (M+, 5.9), 126 (M–C2H4O,18.2), 101 ((C5H7O)=OH, 100), 98 ((C5H7O)=CH2, 90.5), 83 (33.6), 69 (23.6), 57 (28.1), 55 (44.1), 43 (43.0), 41 (25.5) | <1 | ND | <1 | ND |
| 1124 | N-(2-Methylbutyl)acetamide (3) | 129.1 | 129 (M+, 9.7), 100 (M–C2H5, 34.7), 73 (β-cleavage product, 43.2), 72 (β-cleavage product, 95.6), 60 (CH3C(OH)NH+, 61.3), 43 (100) | ND | ND | <1 | 5.1 |
| 1131 | N-(3-methylbutyl)acetamide (4) | 129.1 | 129 (M+, 4.5), 114 (M–CH3, 9.7), 86 (M–C3H7, 25.2), 73 (β-cleavage product, 85.7), 72 (β-cleavage product, 72.2), 60 (CH3C(OH)NH+, 36.5), 43 (100) | <1 | <1 | 2.2 | 44.3 |
| 1145 | (E,E)-2,8-Dimethyl-1,7-dioxaspiro[5.5]undecane (5) | 184.1 | 184 (M+, 8.6), 169 (M–CH3, 1.6), 140 (14.1), 125 (9.7), 115 (CH3(C5H7O)=OH, 92.2), 112 (CH3(C5H7O)=CH2, 100), 97 (73.0), 69 (50.9), 55 (67.0) | 7.6 | <1 | 85.6 | 45.1 |
| 1157 | 2-Ethyl-7-methyl-1,6-dioxaspiro[4.5]decane isomer 1 (6) | 184.1 | 184 (M+, 3.1), 155 (M–C2H5, 23.1), 140 (7.1), 115 (CH3(C5H7O)=OH, 100), 112 (CH3(C5H7O)=CH2, 60.2), 97 (69.4), 85 (60.5), 69 (48.7), 55 (68.5) | <1 | ND | <1 | ND |
| 1175 | Diethyl butanedioate (7) | 174.2 | 174 (M+, 0.7), 129 (M–OC2H5, 53.5), 128 (14.1), 101 (M–COOC2H5, 100), 73 (26.0), 74 (13.4), 55(32.6), 45 (18.5), 43 (10.1) | ND | ND | <1 | ND |
| 1178 | 2-Methyl-6-pentyl-3,4-dihydro-2H-pyran (8) | 168.3 | 168 (22.5), 125 (M–C3H7, 38.2), 112 (C7H12O, 100), 97 (30.2), 84 (19.5), 83 (31.8), 70 (24.6), 55 (65.8) | ND | ND | <1 | ND |
| 1218 | (E,Z)/(Z,E)-2,8-Dimethyl-1,7-dioxaspiro[5.5]undecane (9) | 184.1 | 184 (M+, 5.8), 169 (M–CH3, 1.8), 140 (5.7), 125 (5.9), 115 (CH3(C5H7O)=OH, 100), 112 (CH3(C5H7O)=CH2, 39.2), 97 (69.8), 69 (39.1), 55 (27.3) | <1 | ND | <1 | ND |
| 1233 | (E,E)-2-Ethyl-8-methyl-1,7-dioxaspiro[5.5]undecane (10) | 198.2 | 198 (M+, 9.3), 169 (M–C2H5, 11.2), 140 (12.9), 129 (CH3CH2(C5H7O)=OH+, 40.2), 126 (CH3CH2(C5H7O)=CH2, 30.0), 115 CH3(C5H7O)=OH, 87.7), 112 (CH3(C5H7O)=CH2, 83.0), 97 (58.4), 83 (55.5), 69 (67.7), 55 (100) | <1 | <1 | 2.7 | 2.1 |
| 1240 | 2-Ethyl-7-methyl-1,6-dioxaspiro[4.5]decane isomer 2 (11) | 184.1 | 184 (M+, 5.2), 155 (M–C2H5, 26.1), 140 (9.3), 115 (CH3(C5H7O)=OH, 100), 112 (CH3(C5H7O)=CH2, 49.2), 97 (76.1), 85 (35.7), 69 (49.3), 55 (41.8) | ND | ND | <1 | ND |
| 1270 | (Z,Z)-2,8-Dimethyl-1,7-dioxaspiro[5.5]undecane (12) | 184.1 | 184 (M+, 4.3), 169 (M–CH3, 2.8), 140 (3.9), 125 (6.5), 115 (CH3(C5H7O)=OH, 100), 112 (CH3(C5H7O)=CH2, 66.5), 97 (31.7), 69 (30.3), 55 (59.0) | <1 | ND | <1 | ND |
| 1278 | 6-Hexyl-2-methyl-3,4-dihydro-2H-pyran (13) | 182.2 | 182 (M+, 16.8), 125 (M–C4H9, 42.9), 112 (C7H12O, 100), 97 (38.9), 83 (42.9), 70 (31.7), 55 (64.9) | ND | ND | <1 | ND |
| 1319 | 2-Methyl-8-propyl-1,7-dioxaspiro[5.5]undecane (14) | 212.2 | 212 (M+, 9.0), 169 (M–C3H7, 17.2), 143 (C3H7(C5H7O)=OH+, 32.6), 140 (C3H7(C5H7O)=CH2, 40.5), 125 (M–C5H11O, 47.2), 115 (CH3(C5H7O)=OH, 100), 112 (CH3(C5H7O)=CH2, 78.2) 97 (76.3), 55 (51.4), 43 (49.0) | <1 | ND | <1 | ND |
| 1336 | 2,8-Dimethyl-1,7-dioxaspiro[5.5]undecan-3-ol (15) | 200.3 | 200 (M+, 3.2), 156 (34.2), 128 (5.9), 112 (CH3(C5H7O)=CH2, 100), 97 (28.6), 83 (31.2), 55 (23.9) | <1 | ND | <1 | ND |
| 1347 | 6-Oxononan-1-ol (16) | 158.2 | 158 (M+, 0.6), 140 (M–H2O, 1.4), 115 (9.4), 112 (2.9), 99 (3.6), 97 (20.2), 86 (33.1), 79 (9.6), 73 (12.7), 71 (67.1), 69 (64.0), 58 (53.2), 55 (29.9), 43 (100), 41 (66.8) | ND | ND | 4.9 | ND |
| 1524 | Methyl dodecanoate (17) | 214.2 | 214 (M+, 2.6), 183 (M–OCH3, 3.7), 171 (5.0), 143 (7.0), 129 (4.7), 87 (55.9), 74 (McLafferty rearrangement product, 100), 59 (COOCH3, 10.8), 55 (29.1) | <1 | <1 | ND | ND |
| 1558 | Dodecanoic acid (18) | 200.3 | 200 (M+, 12.0), 171 (M–C2H5, 12.1), 157 (M–C3H7, 27.3), 129 (M–C5H11, 41.0), 73 (HOCOC2H4, 96.8), 60 (CH3COOH, 100) | <1 | ND | ND | ND |
| 1594 | Ethyl dodecanoate (19) | 228.4 | 228 (M+, 2.8), 199 (M–C2H5, 1.9), 183 (M–OC2H5, 5.6), 157 (7.6), 101 (35.9), 88 (McLafferty rearrangement product, 100), 73 (COOC2H5, 20.7), 70 (21.8), 61 (14.9), 60 (13.7), 55 (27.0) | 39.1 | 70.2 | ND | ND |
| 1665 | Ethyl tridecanoate (20) | 242.2 | 242 (M+, 3.4), 213 (M–C2H5, 5.1), 197 (M–OC2H5, 2.1), 199 (12.4), 157 (13.7), 101 (59.3), 88 (McLafferty rearrangement product, 100), 73 (COOC2H5, 26.5), 57 (50.2), 55 (44.7) | <1 | <1 | ND | ND |
| 1689 | Propyl dodecanoate (21) | 242.2 | 242 (M+, 1.5), 201 (21.9), 199 (M–C3H7, 3.4), 183 (M–OC3H7, 25.8), 157 (6.3), 129 (9.3), 115 (16.9), 102 (McLafferty rearrangement product, 29.3), 87 (COOC3H7, 9.8), 61 (100), 59 (6.1), 55 (33.5) | <1 | <1 | ND | ND |
| 1725 | Methyl tetradecanoate (22) | 242.2 | 242 (M+, 2.9), 211 (M–OCH3, 1.2), 199 (5.8), 143 (7.5), 125 (7.3), 111 (19.2), 101 (5.0), 97 (32.3), 87 (47.2), 74 (McLafferty rearrangement product, 100), 59 (COOCH3, 8.6), 55 (66.7) | 4.1 | <1 | ND | ND |
| 1758 | Tetradecanoic acid (23) | 228.4 | 228 (M+, 22.5), 185 (M–C3H7, 32.3), 129 (M–C7H15, 54.7), 73 (HOCOC2H4, 100), 60 (CH3COOH, 94.2) | <1 | ND | ND | ND |
| 1782 | Ethyl (Z)-tetradec-9-enoate (24) | 254.2 | 254 (M+, 2.5), 209 (M–OC2H5, 6.0), 208 (M–C2H5OH, 7.1), 166 (8.4), 124 (10.3), 88 (McLafferty rearrangement product, 32.8), 73 (COOC2H5, 14.6), 69 (45.4), 55 (100) | <1 | <1 | ND | ND |
| 1795 | Ethyl tetradecanoate (25) | 256.4 | 256 (M+, 4.4), 211 (M–OC2H5, 6.0), 213 (5.8), 157 (10.1), 101 (46.2), 88 (McLafferty rearrangement product, 100), 73 (COOC2H5, 21.8), 70 (22.7), 55 (32.2) | 25.6 | 23.7 | ND | ND |
| 1846 | 3-Methylbutyl dodecanoate (26) | 270.5 | 270 (M+, 1.0), 201 (1.5), 183 (M–OC5H11, 4.3), 115 (COOC5H11, 2.0), 70 (100), 71(34.9), 55 (18.9), 43 (46.4) | <1 | <1 | ND | ND |
| 1906 | Methyl (Z)-hexadec-9-enoate (27) | 268.4 | 268 (M+, 3.5), 237 (M–OCH3, 9.1), 236 (M–CH3OH, 12.8), 194 (12.2), 152 (11.0), 96 (33.3), 74 (McLafferty rearrangement product, 53.7), 59 (COOCH3, 19.9), 55 (100) | <1 | ND | ND | ND |
| 1926 | Methyl hexadecanoate (28) | 270.3 | 270 (M+, 1.7), 227 (1.7), 143 (9.8), 87 (36.9), 74 (McLafferty rearrangement product, 100), 59 (COOCH3, 9.3), 55 (81.1) | <1 | ND | ND | ND |
| 1944 | (Z)-Hexadec-9-enoic acid (29) | 254.4 | 254 (M+, 3.2), 237 (M–OH, 3.7), 236 (M–OH2, 18.6), 194 (6.9), 152 (9.5), 97 (54.2), 55 (100) | <1 | ND | ND | ND |
| 1962 | Hexadecanoic acid (30) | 256.4 | 256 (M+, 22.5), 157 (M–C3H7, 30.7), 185 (M–C5H11, 22.2), 157 (M–C7H15, 22.1), 129 (M–C9H19, 52.1), 73 (HOCOC2H4, 100), 60 (CH3COOH, 91.1) | <1 | ND | ND | ND |
| 1975 | Ethyl (Z)-hexadec-9-enoate (31) | 282.3 | 282 (M+, 1.9), 237 (M–OC2H5, 10.1), 236 (M–C2H5OH, 10.7), 194 (10.0), 152 (8.8), 88 (McLafferty rearrangement product, 39.0), 73 (COOC2H5, 29.9), 69 (67.0), 55 (100) | 8.7 | <1 | ND | ND |
| 1984 | Ethyl (E)-hexadec-9-enoate (32) | 282.3 | 282 (M+, 6.8), 237 (M–OC2H5, 21.5), 236 (M–C2H5OH, 25.7), 194 (22.7), 152 (21.9), 88 (McLafferty rearrangement product, 100), 73 (COOC2H5, 15.5), 69 (26.2), 55 (100) | <1 | ND | ND | ND |
| 1994 | Ethyl hexadecanoate (33) | 284.3 | 284 (M+, 4.2), 241 (4.8), 157 (9.5), 101 (50.9), 255 (M–C2H5, 1.1), 239 (M–OC2H5, 3.9), 88 (McLafferty rearrangement product, 100), 73 (COOC2H5, 21.3), 55 (41.3), 43 (54.4) | 8.9 | <1 | ND | ND |
| 2141 | (Z)-Octadec-9-enoic acid (34) | 282.5 | 282 (M+, 2.9), 264 (M–OH, 16.7), 222 (6.7), 97 (67.1), 55 (100) | <1 | ND | ND | ND |
| 2170 | Ethyl (E)-octadec-9-enoate (35) | 310.3 | 310 (M+, 0.6), 265 (M–OC2H5, 6.8), 264 (M–C2H5OH, 10.0), 222 (7.3), 180 (7.1), 123 (9.5), 110 (16.7), 97 (38.5), 88 (McLafferty rearrangement product, 33.2), 83 (44.4), 73 (COOC2H5, 13.6), 69 (65.4), 55 (100), 43 (60.7), 41 (84.3) | 3.1 | ND | ND | ND |
| 2176 | Ethyl (Z)-octadec-9-enoate (36) | 310.3 | 310 (M+, 1.9), 265 (M–OC2H5, 5.4), 264 (M–C2H5OH, 8.3), 222 (7.1), 180 (7.2), 123 (7.9), 110 (12.5), 97 (37.4), 88 (McLafferty rearrangement product, 25.6), 83 (42.3), 73 (COOC2H5, 11.2), 69 (71.6), 55 (100), 43 (50.2), 41 (71.5) | 2.5 | ND | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noushini, S.; Park, S.J.; Perez, J.; Holgate, D.; Mendez, V.; Jamie, I.M.; Jamie, J.F.; Taylor, P.W. Electrophysiological Responses of Bactrocera kraussi (Hardy) (Tephritidae) to Rectal Gland Secretions and Headspace Volatiles Emitted by Conspecific Males and Females. Molecules 2021, 26, 5024. https://doi.org/10.3390/molecules26165024
Noushini S, Park SJ, Perez J, Holgate D, Mendez V, Jamie IM, Jamie JF, Taylor PW. Electrophysiological Responses of Bactrocera kraussi (Hardy) (Tephritidae) to Rectal Gland Secretions and Headspace Volatiles Emitted by Conspecific Males and Females. Molecules. 2021; 26(16):5024. https://doi.org/10.3390/molecules26165024
Chicago/Turabian StyleNoushini, Sally, Soo Jean Park, Jeanneth Perez, Danielle Holgate, Vivian Mendez, Ian M. Jamie, Joanne F. Jamie, and Phillip W. Taylor. 2021. "Electrophysiological Responses of Bactrocera kraussi (Hardy) (Tephritidae) to Rectal Gland Secretions and Headspace Volatiles Emitted by Conspecific Males and Females" Molecules 26, no. 16: 5024. https://doi.org/10.3390/molecules26165024








