Black Garlic and Its Bioactive Compounds on Human Health Diseases: A Review
Abstract
:1. Introduction
2. Data Collection
3. Thermal Processing of Black Garlic
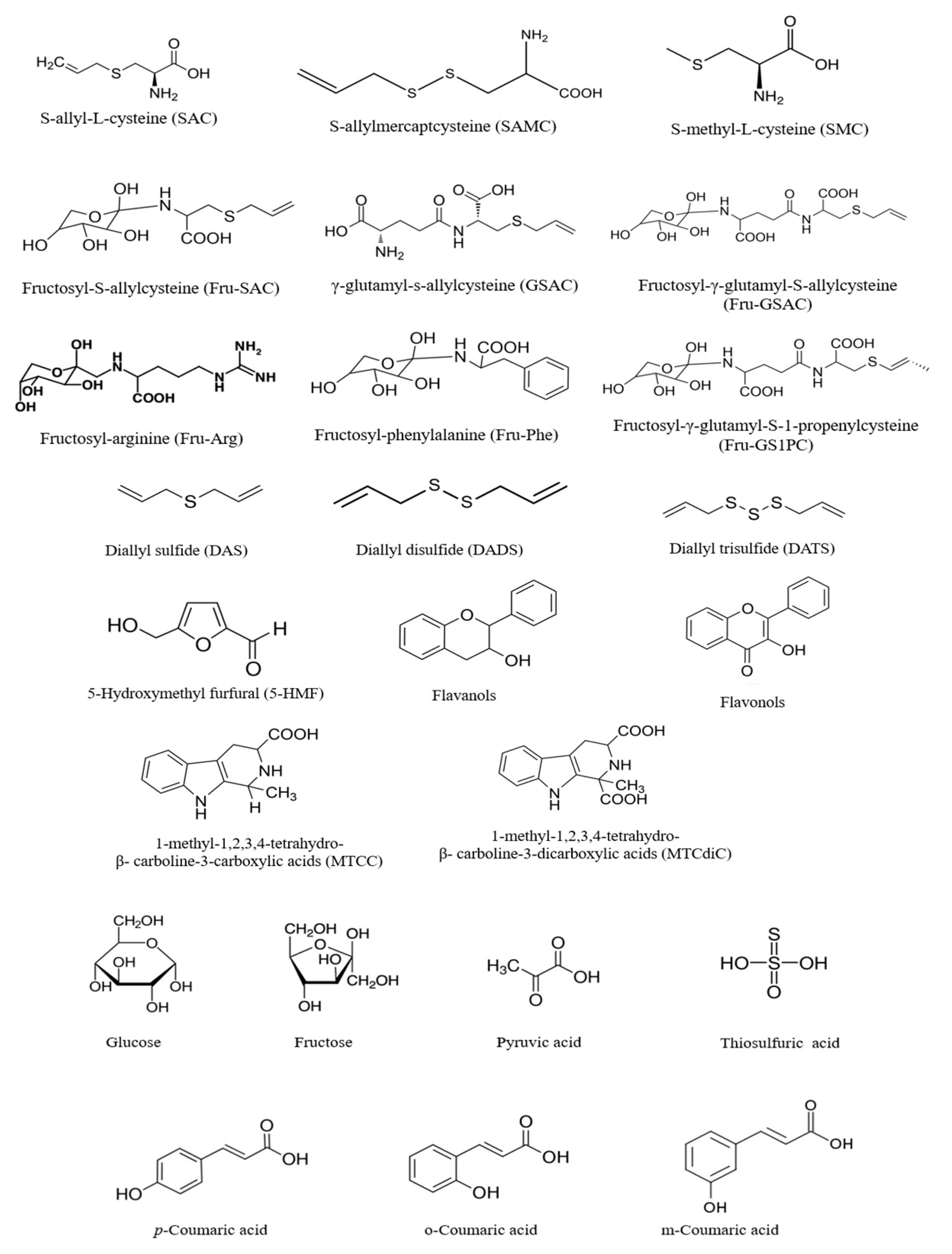
4. Composition of Black Garlic
5. Formation of Phytochemicals of Black Garlic during Millard Reaction
6. Impact of Black Garlic on Health Promotion and Diseases Treatment
6.1. Effects of Black Garlic on Metabolic Disorders
6.1.1. Black Garlic and Diabetes Mellitus
6.1.2. Black Garlic and Obesity
6.2. Effects of Black Garlic on Genitourinary Tract Diseases
6.3. Effects of Black Garlic on Digestive Diseases
6.3.1. Black Garlic and Liver Diseases
6.3.2. Black Garlic and Inflammatory Diseases
6.3.3. Black Garlic and Other Gastrointestinal Diseases
6.4. Effects of Black Garlic on Cardiovascular System Diseases
6.4.1. Black Garlic and Platelet Aggregation
6.4.2. Black Garlic and Arterial Hypertension
6.4.3. Black Garlic and Atherosclerosis
6.5. Effects of Black Garlic on Neurodegenerative Diseases
6.6. Effects of Black Garlic on Cancer Diseases
7. Conclusions and Future Perspectives
Funding
Conflicts of Interest
Abbreviations
| BG | black garlic |
| Temp. | temperature |
| 5-HMF | 5-Hydroxymethylfurfural |
| SAC | s-allyl cysteine |
| FG | fresh garlic |
| BG | black garlic |
| DM | dry matter |
| DW | dry weight |
| FM | fresh matter |
| GAE | gallic acid equivalent |
| QE | quercetin equivalent |
| RE | rutin equivalent |
| CAE | catechin equivalents |
| OD | optical density |
| SAC | s-allyl cysteine |
| NF-κβ | nuclear factor-κβ |
| HDL-C | high-density lipoprotein cholesterol |
| GOT | glutamic oxaloacetic transaminase |
| GPT | glutamic pyruvic transaminase |
| γ-GTP | γ-glutamyl transpeptidase |
| SOD | superoxide dismutase |
| GSH-Px | glutathione peroxidase |
| CAT | catalase |
| GHb | glycated hemoglobin |
| STZ | streptozotocin |
| TBARS | thiobarbituric acid reactive substances |
| TNF-α | tumor necrosis factor-α |
| IL-6 | interleukin-6 |
| LDL | low-density lipoprotein |
| TG | triglyceride |
| TC | total cholesterol |
| C/EBP α | CCAAT/enhancer-binding protein α |
| UCP1 | uncoupling protein 1 |
| CPT1 | carnitine palmitoyl transferase 1 |
| ACO | acyl-coenzyme A oxidase |
| HSL | hormone sensitive lipase |
| ATGL | adipose triglyceride lipase |
| Sirt1 | sirtuin 1 |
| FOXO1 | forkhead box O1 |
| PPAR α | peroxisome proliferator-activated receptor α |
| AMPK | AMP-activated protein kinase |
| SREBP-1c | sterol regulatory element binding protein-1c |
| ACC | acetyl-CoA carboxylase |
| FAS | fatty acid synthase |
| SCD1 | stearoyl-CoA desaturase-1 |
| HFD | high-fat diet |
| LPO | lipid peroxidation |
| GSSG | glutathione disulfide |
| ADP | adenosine diphosphate |
| RAS | renin-angiotensin system |
| OFRs | oxygen free radicals |
| PVN | paraventricular nucleus |
| CSAR | cardiac sympathetic afferent reflex |
| ACE | angiotensin-converting enzyme |
| Fru-Arg | N-(1-deoxy-D-fructos-1-yl)-l-arginine |
| Fru-Met | N-(1-deoxy-D-fructos-1-yl)-l-methionine |
| CAC | coronary artery calcification |
| CRP | c-reactive protein |
| EAT | epicardial adipose tissue |
| PAT | pericardial adipose tissue |
| PaAT | periaortic adipose tissue |
| SAT | subcutaneous adipose tissue |
| IgG | immunoglobulin G |
| IgM | immunoglobulin M |
| MDA-LDL | malondialdehyde-low-density lipoprotein |
| HDL | high-density lipoprotein |
| OxPL/apoB | oxidized phospholipids/apolipoprotein B |
| LP | lipoprotein |
| GAD | glutamate decarboxylase |
| VGLUT1 | vesicular glutamate transporter 1 |
| GSK-3β | glycogen synthase kinase 3 beta |
| sAPP α | soluble amyloid precursor protein α |
| Aβ | β-amyloid |
| TEAC | trolox equivalent antioxidant capacity |
| GSH | glutathione |
| GRd | glutathione reductase |
| GPx | glutathione peroxidase |
| PPARγ | proliferator activated receptor γ |
| Ser-pHSL | serum-phosphorylated HSL |
| Mn-SOD | manganese superoxide dismutase |
| GR | glutathione reductase |
| Nrf2 | nuclear factor-erythroid factor 2-related factor 2 |
| NO | nitric oxide |
| COX-2 | cyclooxygenase-2 |
| TGF-β1 | transforming growth factor beta 1 |
| AST | aspartate transaminase |
| ALT | alanine transaminase |
| LDL-C | low-density lipoprotein-cholesterol |
| LDH | lactate dehydrogenase |
| ALP | alkaline phosphatase |
| ATP | adenosine triphosphate |
| IL-1β | interleukin-1β |
| BCL-2 | B-cell lymphoma 2 |
| Bax | BCL2-associated X protein |
| MDA | malondialdehyde |
| MAPK | mitogen-activated protein kinase |
| LDL/V-LDL | low density lipoprotein/very-low-density lipoprotein |
| GSH-Rd | glutathione reductase |
| ERK | extracellular-signal-regulated kinase |
| JNK | c-Jun N-terminal kinase |
| LPS | lipopolysaccharides |
| VCAM-1 | vascular cell adhesion protein-1 |
| ICAM-1 | intercellular adhesion molecule-1 |
| iNOS | inducible nitric oxide synthase |
| AP-1 | activator protein-1 |
| PGE2 | prostaglandin E2 |
| MPO | myeloperoxidase |
| tGSH | total glutathione |
| BW | body weight |
| BMI | body mass index |
| LDL-C/apo-B | low-density lipoprotein cholesterol/apolipoprotein B |
| TXB2 | Thromboxane B2 |
| SERBP-2 | sterol regulatory element binding protein-2 |
| ACAT-2 | acetyltransferase-2 |
| HMG-CoA | 3-hydroxy-3-methylglutaryl coenzyme A |
| MPP+ | 1-methyl-4-phenylpyridinium ion |
| GLUT3 | glucose transporter 3 |
| GCLC | glutamate cysteine ligase catalytic subunit |
| G6-PD | glucose 6-phosphate dehydrogenase |
| PC | Protein carbonyl |
| 8-OHdG | 8-hydroxy-2-deoxyguanosine |
| mRNA | messenger ribonucleic acid |
| NMDA | N-methyl-D-aspartate |
| cdk1 | cyclin-dependent kinase 1 |
| MMP-2 | matrix metalloproteinase-2 |
| MMP-9 | matrix metalloproteinase-9 |
| DOX | doxorubicin |
| P-gp | p-glycoprotein |
| TGF-β1 | transforming growth factor beta 1 |
| TβRII | type II TGF-β receptor |
| p-samd 2/3 | phosphorylated-suppressor of mothers against decapentaplegic 2/3 |
| smad 4 | suppressor of mothers against decapentaplegic homolog 4 |
| smad7 | suppressor of mothers against decapentaplegic homolog 7 |
| Id-1 | inhibitor of differentiation-1 |
| SNAP25 | synaptosomal associated protein of 25 kDa |
| ABTS | 2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid |
References
- Doré, J.; Blottière, H. The influence of diet on the gut microbiota and its consequences for health. Curr. Opin. Biotechnol. 2015, 32, 195–199. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: A comprehensive review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaparapu, J.; Pragada, P.M.; Geddada, M.N.R. Fruits and Vegetables and its Nutritional Benefits. In Functional Foods Nutraceuticals; Springer: Berlin/Heidelberg, Germany, 2020; pp. 241–260. [Google Scholar]
- Del Rio-Celestino, M.; Font, R. The health benefits of fruits and vegetables. Foods 2020, 9, 369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diretto, G.; Rubio-Moraga, A.; Argandoña, J.; Castillo, P.; Gómez-Gómez, L.; Ahrazem, O. Tissue-specific accumulation of sulfur compounds and saponins in different parts of garlic cloves from purple and white ecotypes. Molecules 2017, 22, 1359. [Google Scholar] [CrossRef]
- Liyanagamage, D.; Jayasinghe, S.; Attanayake, A.P.; Karunaratne, V. Medicinal plants in management of diabetes mellitus: An overview. Ceylon J. Sci. 2020, 49, 3–11. [Google Scholar] [CrossRef]
- Bose, S.; Laha, B.; Banerjee, S. Quantification of allicin by high performance liquid chromatography-ultraviolet analysis with effect of post-ultrasonic sound and microwave radiation on fresh garlic cloves. Pharmacogn. Mag. 2014, 10, S288–S293. [Google Scholar] [CrossRef] [Green Version]
- Qu, Z.; Mossine, V.V.; Cui, J.; Sun, G.Y.; Gu, Z. Protective effects of AGE and its components on neuroinflammation and neurodegeneration. Neuromolecular Med. 2016, 18, 474–482. [Google Scholar] [CrossRef]
- Szychowski, K.; Rybczynska-Tkaczyk, K.; Gawel-Beben, K.; Swieca, M.; Karas, M.; Jakubczyk, A.; Matysiak-Kucharek, M.; Binduga, U.; Gminski, J. Characterization of active compounds of different garlic (Allium sativum L.) cultivars. Pol. J. Food Nutr. Sci. 2018, 68, 73–81. [Google Scholar] [CrossRef]
- Ryu, J.H.; Kang, D. Physicochemical properties, biological activity, health benefits, and general limitations of aged black garlic: A review. Molecules 2017, 22, 919. [Google Scholar] [CrossRef] [Green Version]
- Tanamai, J.; Veeramanomai, S.; Indrakosas, N.; Indrakosas, N. The efficacy of cholesterol-lowering action and side effects of garlic enteric coated tablets in man. J. Med. Assoc. Thail. 2004, 87, 1156–1161. [Google Scholar]
- Mathew, B.C.; Biju, R.S. Neuroprotective effects of garlic a review. Libyan J. Med. 2008, 3, 23–33. [Google Scholar] [PubMed]
- Santos, F.C.C.; Carvalho, N.U.M. Tintura alcoólica de alho (Allium sativum) sobre endoparasitas gastrintestinais de ovinos. Ciência Anim. Bras. 2014, 15, 115–118. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Yan, Y.; Yu, Q.; Deng, Y.; Wu, D.; Wang, Y.; Ge, Y.; Li, S.; Zhao, J. Comparison of immunomodulatory effects of fresh garlic and black garlic polysaccharides on RAW 264.7 Macrophages. J. Food Sci. 2017, 82, 765–771. [Google Scholar] [CrossRef]
- Bae, S.E.; Cho, S.Y.; Won, Y.D.; Lee, S.H.; Park, H.J. Changes in S-allyl cysteine contents and physicochemical properties of black garlic during heat treatment. LWT-Food Sci. Technol. 2015, 55, 397–402. [Google Scholar] [CrossRef]
- Kimura, S.; Tung, Y.-C.; Pan, M.-H.; Su, N.-W.; Lai, Y.-J.; Cheng, K.-C. Black garlic: A critical review of its production, bioactivity, and application. J. Food Drug Anal. 2017, 25, 62–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Casas, L.; Lage-Yusty, M.; López-Hernández, J. Changes in the aromatic profile, sugars, and bioactive compounds when purple garlic is transformed into black garlic. J. Agric. Food Chem. 2017, 65, 10804–10811. [Google Scholar] [CrossRef]
- Amagase, H.; Petesch, B.L.; Matsuura, H.; Kasuga, S.; Itakura, Y. Intake of garlic and its bioactive components. J. Nutr. 2001, 131, 955S–962S. [Google Scholar] [CrossRef]
- Jeong, Y.; Ryu, J.; Shin, J.; Kang, M.; Kang, J.; Han, J.; Kang, D. Comparison of anti-oxidant and anti-inflammatory effects between fresh and aged black garlic extracts. Molecules 2016, 21, 430. [Google Scholar] [CrossRef] [Green Version]
- Tak, H.-M.; Kang, M.-J.; Kim, K.M.; Kang, D.; Han, S.; Shin, J.-H. Anti-inflammatory activities of fermented black garlic. J. Korean Soc. Food Sci. Nutr. 2014, 43, 1527–1534. [Google Scholar] [CrossRef]
- Kim, D.; Kang, M.J.; Hong, S.S.; Choi, Y.-H.; Shin, J.H. Antiinflammatory effects of functionally active compounds isolated from aged black garlic. Phyther. Res. 2017, 31, 53–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Jilg, H. Antioxidant and anti-inflammatory activities of black garlic extracts. J. Food Saf. Qual. 2017, 8, 2635–2643. [Google Scholar]
- Wu, J.; Liu, Y.; Dou, Z.; Wu, T.; Liu, R.; Sui, W.; Jin, Y.; Zhang, M. Black garlic melanoidins prevent obesity, reduce serum LPS levels and modulate the gut microbiota composition in high-fat diet-induced obese C57BL/6J mice. Food Funct. 2020, 11, 9585–9598. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Shiau, D.; Cheng, M.; Tseng, C.; Chen, C.; Wu, M.; Hsu, C. Black garlic ameliorates obesity induced by a high-fat diet in rats. J. Food Nutr. Res. 2017, 5, 736–741. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.Y.; Han, K.-H.; Bae, S.-H.; Han, S.H.; Lee, Y.K. Effects of the Fermented Black Garlic Extract on Lipid Metabolism and Hepatoprotection in Mice. Korean J. Food Nutr. 2020, 33, 17–26. [Google Scholar]
- Shin, J.; Lee, C.; Oh, S.; Yun, J.; Kang, M.; Han, S.; Park, H.; Jung, J.; Chung, Y.; Kang, J. Hepatoprotective effect of aged black garlic extract in rodents. Toxicol. Res. 2014, 30, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Tsai, J.; Chen, Y.; Wu, J.; Cheng, K.; Lai, P.; Liu, K.; Lin, Y.; Huang, Y.; Hsieh, C. Extracts from fermented black garlic exhibit a hepatoprotective effect on acute hepatic injury. Molecules 2019, 24, 1112. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.; Kim, J.; Hwang, Y.; Hwang, K.; Om, A.; Kim, J.; Cho, K. The beneficial effects of aged black garlic extract on obesity and hyperlipidemia in rats fed a high-fat diet. J. Med. Plants Res. 2011, 5, 3159–3168. [Google Scholar]
- Seo, Y.-J.; Gweon, O.-C.; Im, J.-E.; Lee, Y.-M.; Kang, M.-J.; Kim, J.-I. Effect of garlic and aged black garlic on hyperglycemia and dyslipidemia in animal model of type 2 diabetes mellitus. Prev. Nutr. Food Sci. 2009, 14, 1–7. [Google Scholar] [CrossRef]
- Alkreathy, H.M. Potential Anticancer Effects of Aged Garlic Extract and its Water-soluble Organosulfur Compounds. J. Pharm. Res. Int. 2020, 32, 108–121. [Google Scholar] [CrossRef]
- Dong, M.; Yang, G.; Liu, H.; Liu, X.; Lin, S.; Sun, D.; Wang, Y. Aged black garlic extract inhibits HT29 colon cancer cell growth via the PI3K/Akt signaling pathway. Biomed. Rep. 2014, 2, 250–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Jiao, F.; Wang, Q.; Qang, J.; Yang, K.; Hu, R.; Liu, H.; Wang, H.; Wang, Y. Aged black garlic extract induces inhibition of gastric cancer cell growth in vitro and in vivo. Mol. Med. Rep. 2012, 5, 66–72. [Google Scholar] [CrossRef]
- Yoo, J.-M.; Sok, D.-E.; Kim, M.R. Anti-allergic action of aged black garlic extract in RBL-2H3 cells and passive cutaneous anaphylaxis reaction in mice. J. Med. Food. 2014, 17, 92–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Nam, S.H.; Rico, C.W.; Kang, M.Y. A comparative study on the antioxidative and anti-allergic activities of fresh and aged black garlic extracts. Int. J. Food Sci. Technol. 2012, 47, 1176–1182. [Google Scholar] [CrossRef]
- Venkatesh, Y.P. Immunomodulatory attributes of aged garlic extract and its components. In Immunology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 203–224. [Google Scholar]
- Garcia-Villalón, A.L.; Amor, S.; Monge, L.; Fernández, N.; Prodanov, M.; Muñoz, M.; Inarejos-Garcíac, A.M.; Granado, M. In vitro studies of an aged black garlic extract enriched in S-allylcysteine and polyphenols with cardioprotective effects. J. Funct. Foods. 2016, 27, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Shi, Y.; Wang, L.; Li, X.; Zhang, S.; Wang, X.; Jin, M.; Hsiao, C.-D.; Lin, H.; Han, L.; et al. Metabolomics for Biomarker Discovery in Fermented Black Garlic and Potential Bioprotective Responses against Cardiovascular Diseases. J. Agric. Food Chem. 2019, 67, 12191–12198. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Cui, J.; Mossine, V.V.; Greenlief, C.M.; Fritsche, K.; Sun, G.Y.; Gu, Z. Bioactive components from garlic on brain resiliency against neuroinflammation and neurodegeneration. Exp. Ther. Med. 2020, 19, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.-J.; Lee, S.-J.; Kang, M.-J.; Cho, H.-S.; Sung, N.-J.; Shin, J.-H. Physicochemical characteristics of black garlic (Allium sativum L.). J. Korean Soc. Food Sci. Nutr. 2008, 37, 465–471. [Google Scholar] [CrossRef]
- Zhang, X.; Li, N.; Lu, X.; Liu, P.; Qiao, X. Effects of temperature on the quality of black garlic. J. Sci. Food Agric. 2016, 96, 2366–2372. [Google Scholar] [CrossRef]
- Sun, Y.-E.; Wang, W. Changes in nutritional and bio-functional compounds and antioxidant capacity during black garlic processing. J. Food Sci. Technol. 2018, 55, 479–488. [Google Scholar] [CrossRef]
- Shin, J.-H.; Choi, D.-J.; Lee, S.-J.; Cha, J.-Y.; Kim, J.-G.; Sung, N.-J. Changes of physicochemical components and antioxidant activity of garlic during its processing. J. Life Sci. 2008, 18, 1123–1131. [Google Scholar] [CrossRef] [Green Version]
- Toledano-Medina, M.A.; Pérez-Aparicio, J.; Moreno-Rojas, R.; Merinas-Amo, T. Evolution of some physicochemical and antioxidant properties of black garlic whole bulbs and peeled cloves. Food Chem. 2016, 199, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Feng, Y.; Liu, J.; Yan, J.; Wang, M.; Sasaki, J.-I.; Lu, C. Black garlic (Allium sativum) extracts enhance the immune system. Med. Aromat. Plant Sci. Biotechnol. 2010, 4, 37–40. [Google Scholar]
- Choi, I.S.; Cha, H.S.; Lee, Y.S. Physicochemical and antioxidant properties of black garlic. Molecules 2014, 19, 16811–16823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.-H.; Kim, I.-J.; Kang, S.-T.; Kim, Y.-H.; Lee, J.-O.; Ryu, C.-H. Development of black garlic Yakju and its antioxidant activity. Korean J. Food Sci. Technol. 2010, 42, 69–74. [Google Scholar]
- Andersen, R.; Sørensen, A. An enzymatic method for the determination of fructans in foods and food products. Eur. Food Res. Technol. 1999, 210, 148–152. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Nutritional and therapeutic potential of Allium vegetables. J Nutr Ther. 2017, 6, 18–37. [Google Scholar] [CrossRef]
- Cheong, K.L.; Yan, F.; Huang, X. Enymologic characterization of garlic fructan exohydrolase. J. Food Biochem. 2012, 36, 248–253. [Google Scholar] [CrossRef]
- Liang, T.; Wei, F.; Lu, Y.; Kodani, Y.; Nakada, M.; Miyakawa, T.; Tanokura, M. Comprehensive NMR analysis of compositional changes of black garlic during thermal processing. J. Agric. Food Chem. 2015, 63, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Sun, L.; Chen, M.; Wang, J. An analysis of the changes on intermediate products during the thermal processing of black garlic. Food Chem. 2015, 23, 56–61. [Google Scholar] [CrossRef]
- Lei, M.-M.; Xu, M.-Y.; Zhang, Z.-S.; Zhang, M.; Gao, Y.-F. The analysis of saccharide in black garlic and its antioxidant activity. Adv. J. Food Sci. Technol. 2014, 6, 755–760. [Google Scholar] [CrossRef]
- Molina-Calle, M.; de Medina, V.; Calderón-Santiago, M.; Priego-Capote, F.; de Castro, M.D. Untargeted analysis to monitor metabolic changes of garlic along heat treatment by LC-QTOF MS/MS. Electrophoresis 2017, 38, 2349–2360. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, N.; Qiao, X.; Qiu, Z. Effects of thermal treatment on polysaccharide degradation during black garlic processing. LWT-Food Sci. Technol. 2018, 95, 223–229. [Google Scholar] [CrossRef]
- Qiu, Z.; Lu, X.; Li, N.; Zhang, M.; Qiao, X. Characterization of garlic endophytes isolated from the black garlic processing. Microbiologyopen 2018, 7, 1–11. [Google Scholar] [CrossRef]
- Kamanna, V.S.; Chandrasekhara, N. Fatty acid composition of garlic (Allium sativum Linnaeus) lipids. J. Am. Oil Chem. Soc. 1980, 57, 175–176. [Google Scholar] [CrossRef]
- Lu, X. Study on Formation Mechanism and Function of Black Garlic Oligosaccharides. Doctoral Thesis, Shandong Agriculture University, Taian, China, 2017. [Google Scholar]
- Zamora, R.; Hidalgo, F.J. Coordinate contribution of lipid oxidation and Maillard reaction to the nonenzymatic food browning. Crit. Rev. Food Sci. Nutr. 2005, 45, 49–59. [Google Scholar] [CrossRef]
- Lee, J.; Harnly, J.M. Free amino acid and cysteine sulfoxide composition of 11 garlic (Allium sativum L.) cultivars by gas chromatography with flame ionization and mass selective detection. J. Agric. Food Chem. 2005, 53, 9100–9104. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.G.; Kim, H.Y.; Woo, K.S.; Lee, J.; Jeong, H.S. Biological activities of Maillard reaction products (MRPs) in a sugar-amino acid model system. Food Chem. 2011, 126, 221–227. [Google Scholar] [CrossRef]
- Qiu, Z.; Zheng, Z.; Zhang, B.; Sun-Waterhouse, D.; Qiao, X. Formation, nutritional value, and enhancement of characteristic components in black garlic: A review for maximizing the goodness to humans. Compr. Rev. Food Sci. Food Saf. 2020, 19, 801–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, E.; Kohno, M.; Hamano, H.; Niwano, Y. Increased anti-oxidative potency of garlic by spontaneous short-term fermentation. Plant Foods Hum. Nutr. 2006, 61, 157–160. [Google Scholar] [CrossRef]
- Kodera, Y.; Suzuki, A.; Imada, O.; Kasuga, S.; Sumioka, I.; Kanezawa, A.; Taru, N.; Fujikawa, M.; Nagae, S.; Masamoto, K. Physical, chemical, and biological properties of S-allylcysteine, an amino acid derived from garlic. J. Agric. Food Chem. 2002, 50, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lu, X.; Pei, H.; Qiao, X. Effect of freezing pretreatment on the processing time and quality of black garlic. J. Food Process Eng. 2015, 38, 329–335. [Google Scholar] [CrossRef]
- Ritota, M.; Casciani, L.; Han, B.Z.; Cozzolino, S.; Leita, L.; Sequi, P.; Valentini, M. Traceability of Italian garlic (Allium sativum L.) by means of HRMAS-NMR spectroscopy and multivariate data analysis. Food Chem. 2012, 135, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Blecker, C.; Fougnies, C.; van Herck, J.-C.; Chevalier, J.-P.; Paquot, M. Kinetic study of the acid hydrolysis of various oligofructose samples. J. Agric. Food Chem. 2002, 50, 1602–1607. [Google Scholar] [CrossRef]
- Rahman, M.S. Handbook of Food Preservation; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Limacher, A.; Kerler, J.; Davidek, T.; Schmalzried, F.; Blank, I. Formation of furan and methylfuran by Maillard-type reactions in model systems and food. J. Agric. Food Chem. 2008, 56, 3639–3647. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kang, O.-J.; Gweon, O.-C. Changes in the content of fat-and water-soluble vitamins in black garlic at the different thermal processing steps. Food Sci. Biotechnol. 2013, 22, 283–287. [Google Scholar] [CrossRef]
- Rayman, M.P. Food-chain selenium and human health: Emphasis on intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef] [Green Version]
- Houston, M.C.; Harper, K.J. Potassium, magnesium, and calcium: Their role in both the cause and treatment of hypertension. J. Clin. Hypertens. 2008, 10, 3–11. [Google Scholar] [CrossRef]
- Kang, O.-J. Physicochemical characteristics of black garlic after different thermal processing steps. Prev. Nutr. Food Sci. 2016, 21, 348–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinkawa, H.; Takemura, S.; Minamiyama, Y.; Kodai, S.; Tsukioka, T.; Osada-Oka, M.; Kubo, S.; Okada, S.; Suehiro, S. S-allylcysteine is effective as a chemopreventive agent against porcine serum-induced hepatic fibrosis in rats. Osaka City Med. J. 2009, 55, 61–69. [Google Scholar] [PubMed]
- Yang, P.; Song, H.; Wang, L.; Jing, H. Characterization of key aroma-active compounds in black garlic by sensory-directed flavor analysis. J. Agric. Food Chem. 2019, 67, 7926–7934. [Google Scholar] [CrossRef]
- Kang, O.-J. Evaluation of melanoidins formed from black garlic after different thermal processing steps. Prev. Nutr. Food Sci. 2016, 21, 398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rios-Rios, K.L.; Montilla, A.; Olano, A.; Villamiel, M. Physicochemical changes and sensorial properties during black garlic elaboration: A review. Trends Food Sci. Technol. 2019, 88, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.L.; Jiang, N.T.; Jiang, X.M. Colour, Taste, and Odor Chemistry of Food; China Light Industry Press: Beijing, China, 1984. [Google Scholar]
- Xiaoming, L.; Li, N.; Qiao, X.; Qiu, Z.; Liu, P. Composition analysis and antioxidant properties of black garlic extract. J. Food Drug Anal. 2016, 10, 340–349. [Google Scholar]
- Kim, I.-D.; Park, Y.-S.; Park, J.-J.; Dhungana, S.K.; Shin, D.-H. Physicochemical and antioxidant properties of garlic (A. sativum) prepared by different heat treatment conditions. Korean J. Food Sci. Technol. 2019, 51, 452–458. [Google Scholar]
- Liu, J.; Zhang, G.; Cong, X.; Wen, C. Black garlic improves heart function in patients with coronary heart disease by improving circulating antioxidant levels. Front. Physiol. 2018, 9, 1435–1445. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-S.; Kang, O.-J.; Gweon, O.-C. Comparison of phenolic acids and flavonoids in black garlic at different thermal processing steps. J. Funct. Foods. 2013, 5, 80–86. [Google Scholar] [CrossRef]
- Setiyoningrum, F.; Priadi, G.; Afiati, F.; Herlina, N.; Solikhin, A. Composition of spontaneous black garlic fermentation in a water bath. Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Shin, J.-H.; Choi, D.-J.; Lee, S.-J.; Cha, J.-Y.; Sung, N.-J. Antioxidant activity of black garlic (Allium sativum L.). J. Korean Soc. Food Sci. Nutr. 2008, 37, 965–971. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kim, M.-J.; Bang, W.-S.; Kim, K.-S.; Park, S.-S. Determination of s-allyl-l-cystein, diallyl disulfide, and total amino acids of black garlic after spontaneous short-term fermentation. J. Korean Soc. Food Sci. Nutr. 2012, 41, 661–665. [Google Scholar] [CrossRef]
- Al-Shehri, S.A. Efficacy of black garlic extract on anti-tumor and anti-oxidant activity enhancement in rats. Clin. Nutr. Open Sci. 2021, 36, 126–139. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, H.; Kim, H.S.; Kim, Y.-R.; Noh, S.H. Optimum conditions for S-allyl-(L)-cysteine accumulation in aged garlic by RSM. Food Sci. Biotechnol. 2014, 23, 717–722. [Google Scholar] [CrossRef]
- Thao, H.P.; Tuan, N.D.; Zemann, A. Quantitative analysis of S-Allylcysteine in black garlic via Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry. Syst. Rev. Pharm. 2019, 10, 161–166. [Google Scholar]
- Kim, J.H.; Yu, S.H.; Cho, Y.J.; Pan, J.H.; Cho, H.T.; Kim, J.H.; Bong, H.; Lee, Y.; Chang, M.H.; Jeong, Y.J. Preparation of S-allylcysteine-enriched black garlic juice and its antidiabetic effects in streptozotocin-induced insulin-deficient mice. J. Agric. Food Chem. 2017, 65, 358–363. [Google Scholar] [CrossRef]
- Sasaki, J.I.; Lu, C.; Machiya, E.; Tanahashi, M.; Hamada, K. Processed black garlic (Allium sativum) extracts enhance anti-tumor potency against mouse tumors. Energy 2007, 227, 278–281. [Google Scholar]
- Sato, E.; Kohno, M.; Niwano, Y. Increased level of tetrahydro-β-carboline derivatives in short-term fermented garlic. Plant Foods Hum. Nutr. 2006, 61, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lei, M.; Liu, R.; Gao, Y.; Xu, M.; Zhang, M. Evaluation of alliin, saccharide contents and antioxidant activities of black garlic during thermal processing. J. Food Biochem. 2015, 39, 39–47. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, M.; Wang, C.; Zhou, H.; Fan, L.; Huang, X. Thermolysis kinetics and thermal degradation compounds of alliin. Food Chem. 2017, 223, 25–30. [Google Scholar] [CrossRef]
- Xu, X.; Miao, Y.; Chen, J.Y.; Zhang, Q.; Wang, J. Effective production of S-allyl-L-cysteine through a homogeneous reaction with activated endogenous -γ-glutamyltranspeptidase in garlic (Allium Sativum). J. Food Sci. Technol. 2015, 52, 1724–1729. [Google Scholar] [CrossRef] [Green Version]
- Alberti, K.G.M.M.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Thomson, M.; Al-Qattan, K.K.; Divya, J.S.; Ali, M. Anti-diabetic and anti-oxidant potential of aged garlic extract (AGE) in streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2015, 16, 1–9. [Google Scholar]
- Thomson, M.; Al-Qattan, K.; Jayasree, D.; Ali, M. Oral intake of aged garlic extract (AGE) ameliorates oxidative stress and other streptozotocin-induced diabetic complications in rats. Int. J. Pharmacol. 2017, 13, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.-J.; Lee, S.J.; Sung, N.J.; Shin, J.-H. The effect of extract powder from fresh and black garlic on main components in serum and organs of streptozotocin-induced diabetic rats. J. Life Sci. 2013, 23, 432–442. [Google Scholar] [CrossRef]
- Lee, Y.M.; Gweon, O.C.; Seo, Y.J.; Im, J.; Kang, M.J.; Kim, M.J.; Kim, J.I. Antioxidant effect of garlic and aged black garlic in animal model of type 2 diabetes mellitus. Nutr. Res. Pract. 2009, 3, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si, L.; Lin, R.; Jia, Y.; Jian, W.; Yu, Q.; Wang, M.; Yang, S. Lactobacillus bulgaricus improves antioxidant capacity of black garlic in the prevention of gestational diabetes mellitus: A randomized control trial. Biosci. Rep. 2019, 39, BSR2018225. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-M.; Seo, D.-Y.; Lee, S.-H.; Baek, Y.-H. Effects of exhaustive exercise and aged garlic extract supplementation on weight, adipose tissue mass, lipid profiles and oxidative stress in high fat diet induced obese rats. J. Life Sci. 2010, 20, 1889–1895. [Google Scholar] [CrossRef]
- Lee, H.-S.; Lim, W.-C.; Lee, S.-J.; Lee, S.-H.; Lee, J.-H.; Cho, H.-Y. Antiobesity effect of garlic extract fermented by Lactobacillus plantarum BL2 in diet-induced obese mice. J. Med. Food. 2016, 19, 823–829. [Google Scholar] [CrossRef]
- Seo, D.Y.; Lee, S.; Figueroa, A.; Kwak, Y.S.; Kim, N.; Rhee, B.D.; Ko, K.S.; Bang, H.S.; Beak, Y.H.; Han, J. Aged garlic extract enhances exercise-mediated improvement of metabolic parameters in high fat diet-induced obese rats. Nutr. Res. Pract. 2016, 6, 513. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Mathews, A.E.; Rodrigues, C.; Eudy, B.J.; Rowe, C.A.; O’Donoughue, A.; Percival, S.S. Aged garlic extract supplementation modifies inflammation and immunity of adults with obesity: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. ESPEN 2019, 24, 148–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-C.; Kao, T.-H.; Tseng, C.-Y.; Chang, W.-T.; Hsu, C.-L. Methanolic extract of black garlic ameliorates diet-induced obesity via regulating adipogenesis, adipokine biosynthesis, and lipolysis. J. Funct. Foods. 2014, 9, 98–108. [Google Scholar] [CrossRef]
- Nam, H.; Jung, H.; Kim, Y.; Kim, B.; Kim, K.H.; Park, S.J.; Suh, J.G. Aged black garlic extract regulates lipid metabolism by inhibiting lipogenesis and promoting lipolysis in mature 3T3-L1 adipocytes. Food Sci. Biotechnol. 2018, 27, 575–579. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Pischetsrieder, M.; Ahmed, N. Aged garlic extract and S-allyl cysteine prevent formation of advanced glycation endproducts. Eur. J. Pharmacol. 2007, 561, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, P.D.; Barrera, D.; Medina-Campos, O.N.; Hernández-Pando, R.; Ibarra-Rubio, M.E.; Pedraza-Chaverri, J. Aged garlic extract attenuates gentamicin induced renal damage and oxidative stress in rats. Life Sci. 2003, 73, 2543–2556. [Google Scholar] [CrossRef]
- Shiju, T.M.; Rajesh, N.G.; Viswanathan, P. Renoprotective effect of aged garlic extract in streptozotocin-induced diabetic rats. Indian J. Pharmacol. 2013, 45, 18. [Google Scholar] [CrossRef] [PubMed]
- Albrakati, A. Aged garlic extract rescues ethephon-induced kidney damage by modulating oxidative stress, apoptosis, inflammation, and histopathological changes in rats. Environ. Sci. Pollut. Res. 2021, 28, 6818–6829. [Google Scholar] [CrossRef]
- Lee, T.W.; Bae, E.; Kim, J.H.; Jang, H.N.; Cho, H.S.; Chang, S.H.; Park, D.J. The aqueous extract of aged black garlic ameliorates colistin-induced acute kidney injury in rats. Ren. Fail. 2019, 41, 24–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, A.G.; Shaikh, A.A.; Memon, Z.A. Hepatoprotective Effect of Black Garlic Extract against Carbon Tetrachloride Induced Liver Injury in Rats. J. Peoples Univ. Med. Health Sci. 2014, 4, 86–91. [Google Scholar]
- Kim, M.H.; Kim, M.J.; Lee, J.H.; Han, J.I.; Kim, J.H.; Sok, D.E.; Kim, M.R. Hepatoprotective effect of aged black garlic on chronic alcohol-induced liver injury in rats. J. Med. Food. 2011, 14, 732–738. [Google Scholar] [CrossRef]
- Jiang, G.; Ramachandraiah, K.; Murtaza, M.A.; Wang, L.; Li, S.; Ameer, K. Synergistic effects of black ginseng and aged garlic extracts for the amelioration of nonalcoholic fatty liver disease (NAFLD) in mice. Food Sci. Nutr. 2021, 9, 3091–3099. [Google Scholar] [CrossRef]
- Lee, H.S.; Lim, W.C.; Lee, S.J.; Lee, S.H.; Yu, H.J.; Lee, J.H.; Cho, H.Y. Hepatoprotective effects of lactic acid-fermented garlic extract against acetaminophen-induced acute liver injury in rats. Food Sci. Biotechnol. 2016, 25, 867–873. [Google Scholar] [CrossRef]
- Park, C.; Gweon, O.-C.; Choi, Y.H.; Kim, J.-I. Aged black garlic inhibits cyclooxygenase-2 expression and prostaglandin E2 production by phorbol 12-myristate-13-acetate through inactivation of nuclear factor-kappab. Cancer Prev. Res. 2009, 14, 161–170. [Google Scholar]
- Kim, H.K.; Choi, Y.W.; Lee, E.N.; Park, J.K.; Kim, S.G.; Park, D.J.; Kim, B.S.; Lim, Y.T.; Yoon, S. 5-Hydroxymethylfurfural from black garlic extract prevents TNFα-induced monocytic cell adhesion to HUVECs by suppression of vascular cell adhesion molecule-1 expression, reactive oxygen species generation and NF-κB activation. Phyther. Res. 2011, 25, 965–974. [Google Scholar] [CrossRef]
- Kong, F.; Lee, B.H.; Wei, K. 5-hydroxymethylfurfural mitigates lipopolysaccharide-stimulated inflammation via suppression of MAPK, NF-κB and mTOR activation in RAW 264.7 cells. Molecules 2019, 24, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.H.; Park, J.K.; Choi, Y.W.; Kim, Y.H.; Lee, E.N.; Lee, J.R.; Kim, H.S.; Baek, S.Y.; Kim, B.S.; Lee, K.S. Hexane extract of aged black garlic reduces cell proliferation and attenuates the expression of ICAM-1 and VCAM-1 in TNF–α–activated human endometrial stromal cells. Int. J. Mol. Med. 2013, 32, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Yoo, Y.C.; Kim, H.J.; Shin, S.K.; Sohn, E.J.; Min, A.Y.; Sung, N.Y.; Kim, M.R. Aged black garlic exerts anti-inflammatory effects by decreasing no and proinflammatory cytokine production with less cytoxicity in LPS-stimulated raw 264.7 macrophages and LPS-induced septicemia mice. J. Med. Food. 2014, 17, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-A.; Tsai, J.-C.; Cheng, K.-C.; Liu, K.-F.; Chang, C.-K.; Hsieh, C.-W. Extracts of black garlic exhibits gastrointestinal motility effect. Food Res. Int. 2018, 107, 102–109. [Google Scholar] [CrossRef]
- Li, X.; Liu, R.; Zhang, M. Laxative effects of mixed black garlic beverage on BALB/c mice. China Food Addit. 2014, 6, 49–53. [Google Scholar]
- Kim, K.J.; Kim, S.H.; Shin, M.-R.; Kim, Y.J.; Park, H.-J.; Roh, S.-S. Protective effect of S-allyl cysteine-enriched black garlic on reflux esophagitis in rats via NF-kB signaling pathway. J. Funct. Foods. 2019, 58, 199–206. [Google Scholar] [CrossRef]
- El-Ashmawy, N.E.; Khedr, E.G.; El-Bahrawy, H.A.; Selim, H.M. Gastroprotective effect of garlic in indomethacin induced gastric ulcer in rats. Nutrition 2016, 32, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Badr, G.M.; Al-Mulhim, J.A. The protective effect of aged garlic extract on nonsteroidal anti-inflammatory drug-induced gastric inflammations in male albino rats. Evidence-Based Complement. Altern. Med. 2014. [Google Scholar] [CrossRef] [Green Version]
- Yüncü, M.; Eralp, A.; Celõk, A. Effect of aged garlic extract against methotrexate-induced damage to the small intestine in rats. Phyther. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006, 20, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Blann, A.D. Platelets: The universal killer? Biochim. Biophys. Acta BBA Mol. Basis Dis. 2007, 1772, 715–717. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.K.; Maulik, S.K. Effect of garlic on cardiovascular disorders: A review. Nutr. J. 2002, 1, 1–14. [Google Scholar] [CrossRef]
- Morihara, N.; Hino, A. Aged garlic extract suppresses platelet aggregation by changing the functional property of platelets. J. Nat. Med. 2017, 71, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-K. The Inhibiton Effects of Hypercholesterolemia and Platelet in Fermented and Non-Fermented Preparation of Garlic. Int. J. Internet Broadcast. Commun. 2019, 11, 1–10. [Google Scholar]
- Irfan, M.; Kim, M.; Kim, K.S.; Kim, T.H.; Kim, S.D.; Hong, S.B.; Kim, H.K.; Rhee, M.H. Fermented garlic ameliorates hypercholesterolemia and inhibits platelet activation. Evidence-Based Complement. Altern. Med. 2019. [Google Scholar] [CrossRef]
- Seo, D.Y.; Lee, S.R.; Kim, H.K.; Baek, Y.H.; Kwak, Y.S.; Ko, T.H.; Kim, N.; Rhee, B.D.; Ko, K.S.; Park, B.J.; et al. Independent beneficial effects of aged garlic extract intake with regular exercise on cardiovascular risk in postmenopausal women. Nutr. Res. Pract. 2012, 6, 226. [Google Scholar] [CrossRef] [Green Version]
- Jung, E.S.; Park, S.H.; Choi, E.K.; Ryu, B.H.; Park, B.H.; Kim, D.S.; Kim, Y.G.; Chae, S.W. Reduction of blood lipid parameters by a 12-wk supplementation of aged black garlic: A randomized controlled trial. Nutrition 2014, 30, 1034–1039. [Google Scholar] [CrossRef]
- Steiner, M.; Li, W. Aged garlic extract, a modulator of cardiovascular risk factors: A dose-finding study on the effects of AGE on platelet functions. J. Nutr. 2001, 131, 980S–984S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, K.; Billington, D. Dietary supplementation with aged garlic extract inhibits ADP-induced platelet aggregation in humans. J. Nutr. 2000, 130, 2662–2665. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Arbeláez, D.; Lahera, V.; Oubiña, P.; Valero-Muñoz, M.; Heras, N.D.L.; Rodríguez, Y.; García, R.G.; Camacho, P.A.; López-Jaramillo, P. Aged garlic extract improves adiponectin levels in subjects with metabolic syndrome: A double-blind, placebo-controlled, randomized, crossover study. Mediat. Inflamm. 2013. [Google Scholar] [CrossRef] [PubMed]
- Wlosinska, M.; Nilsson, A.-C.; Hlebowicz, J.; Fakhro, M.; Malmsjö, M.; Lindstedt, S. Aged garlic extract reduces IL-6: A double-blind placebo-controlled trial in females with a low risk of cardiovascular disease. Evidence-Based Complement. Altern. Med. 2021. [Google Scholar] [CrossRef]
- O’Rourke, M. Arterial stiffness, systolic blood pressure, and logical treatment of arterial hypertension. Hypertension 1990, 15, 339–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Te Riet, L.; van Esch, J.H.M.; Roks, A.J.M.; van den Meiracker, A.H.; Danser, A.H.J. Hypertension: Renin-angiotensin-aldosterone system alterations. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef] [PubMed]
- Ried, K.; Travica, N.; Sali, A. The effect of aged garlic extract on blood pressure and other cardiovascular risk factors in uncontrolled hypertensives: The AGE at Heart trial. Integr. Blood Press. Control 2019, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Ried, K.; Travica, N.; Sali, A. The effect of Kyolic aged garlic extract on gut microbiota, inflammation, and cardiovascular markers in hypertensives: The GarGIC Trial. Front. Nutr. 2018, 7, 122. [Google Scholar] [CrossRef]
- Ried, K.; Frank, O.R.; Stocks, N.P. Aged garlic extract lowers blood pressure in patients with treated but uncontrolled hypertension: A randomised controlled trial. Maturitas 2010, 67, 144–150. [Google Scholar] [CrossRef]
- Castro, C.; Lorenzo, A.G.; González, A.; Cruzado, M. Garlic components inhibit angiotensin II-induced cell-cycle progression and migration: Involvement of cell-cycle inhibitor p27Kip1 and mitogen-activated protein kinase. Mol. Nutr. Food Res. 2010, 54, 781–787. [Google Scholar] [CrossRef]
- Yu, J.; Shan, Y.; Li, S.; Zhang, L. Potential contribution of Amadori compounds to antioxidant and angiotensin I converting enzyme inhibitory activities of raw and black garlic. LWT-Food Sci. Technol. 2020, 129, 109553. [Google Scholar] [CrossRef]
- Jang, E.-K.; Seo, J.-H.; Lee, S.-P. Physiological activity and antioxidative effects of aged black garlic (Allium sativum L.) extract. Korean J. Food Sci. Technol. 2008, 40, 443–448. [Google Scholar]
- Han, Y.; Fan, Z.D.; Yuan, N.; Xie, G.Q.; Gao, J.; De, W.; Gao, X.Y.; Zhu, G.Q. Superoxide anions in the paraventricular nucleus mediate the enhanced cardiac sympathetic afferent reflex and sympathetic activity in renovascular hypertensive rats. J. Appl. Physiol. 2011, 110, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Chen, J.; Zhou, G.; Xu, X.; Zhang, Q.; Wang, J. The antihypertensive effect of black garlic (Allium sativum) in spontaneously hypertensive rats via Scavenging of free radicals. Res. Health Nutr. 2014, 2, 5–12. [Google Scholar]
- Peters, E.B.; Kibbe, M.R. Nanomaterials to Resolve Atherosclerosis. ACS Biomater. Sci. Eng. 2020, 6, 3693–3712. [Google Scholar] [CrossRef]
- Morihara, N.; Hino, A.; Yamaguchi, T.; Suzuki, J. Aged Garlic Extract Suppresses the Development of Atherosclerosis in Apolipoprotein E-Knockout Mice. J. Nutr. 2016, 146, 460S–463S. [Google Scholar] [CrossRef] [PubMed]
- Efendy, J.L.; Simmons, D.L.; Campbell, G.R.; Campbell, J.H. The effect of the aged garlic extract, Kyolic’, on the development of experimental atherosclerosis. Atherosclerosis 1997, 132, 37–42. [Google Scholar] [CrossRef]
- Wlosinska, M.; Nilsson, A.C.; Hlebowicz, J.; Hauggaard, A.; Kjellin, M.; Fakhro, M.; Lindstedt, S. The effect of aged garlic extract on the atherosclerotic process—A randomized double-blind placebo-controlled trial. BMC Complement. Med. Ther. 2020, 20, 1–10. [Google Scholar]
- Zeb, I.; Ahmadi, N.; Kadakia, J.; Larijani, V.N.; Flores, F.; Li, D.; Budoff, M.J.; Nasir, K. Aged garlic extract and coenzyme Q10 have favorable effect on inflammatory markers and coronary atherosclerosis progression: A randomized clinical trial. J. Cardiovasc. Dis. Res. 2012, 3, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Budoff, M.J.; Ahmadi, N.; Gul, K.M.; Liu, S.T.; Flores, F.R.; Tiano, J.; Takasu, J.; Miller, E.; Tsimikas, S. Aged garlic extract supplemented with B vitamins, folic acid and L-arginine retards the progression of subclinical atherosclerosis: A randomized clinical trial. Prev. Med. 2009, 49, 101–107. [Google Scholar] [CrossRef]
- Zeb, I.; Ahmadi, N.; Flores, F.; Budoff, M.J. Randomized trial evaluating the effect of aged garlic extract with supplements versus placebo on adipose tissue surrogates for coronary atherosclerosis progression. Coron. Artery Dis. 2018, 29, 325–328. [Google Scholar] [CrossRef] [Green Version]
- Ried, K.; Frank, O.R.; Stocks, N.P. Aged garlic extract reduces blood pressure in hypertensives: A dose-response trial. Eur. J. Clin. Nutr. 2013, 67, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, N.B. Effect of aged garlic extract on APP processing and tau phosphorylation in Alzheimer’s transgenic model Tg2576. J. Ethnopharmacol. 2006, 108, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Jeong, H.R.; Jo, Y.N.; Kim, H.J.; Shin, J.H.; Heo, H.J. Ameliorating effects of aged garlic extracts against Aβ-induced neurotoxicity and cognitive impairment. BMC Complement. Altern. Med. 2013, 13, 1–11. [Google Scholar]
- Wichai, T.; Pannangrong, W.; Welbat, J.; Chaichun, A.; Sripanidkulchai, K.; Sripanidkulchai, B. Effects of aged garlic extract on spatial memory and oxidative damage in the brain of amyloid-βinduced rats. Songklanakarin J. Sci. Technol. 2019, 41, 311–318. [Google Scholar]
- Thorajak, P.; Pannangrong, W.; Welbat, J.U.; Chaijaroonkhanarak, W.; Sripanidkulchai, K.; Sripanidkulchai, B. Effects of aged garlic extract on cholinergic, glutamatergic and GABAergic systems with regard to cognitive impairment in Aβ-induced rats. Nutrients 2017, 9, 686. [Google Scholar] [CrossRef] [Green Version]
- Ray, B.; Chauhan, N.B.; Lahiri, D.K. Oxidative insults to neurons and synapse are prevented by aged garlic extract and S-allyl-l-cysteine treatment in the neuronal culture and APP-Tg mouse model. J. Neurochem. 2011, 117, 388–402. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Qu, Z.; Mossine, V.V.; Nknolise, D.L.; Li, J.; Chen, Z.; Cheng, J.; Greenlief, C.M.; Mawhinney, T.P.; Brown, P.N.; et al. Proteomic analysis of the effects of aged garlic extract and its FruArg component on lipopolysaccharide-induced neuroinflammatory response in microglial cells. PLoS ONE 2014, 9, e113531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas, P.; Serrano-Garcia, N.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Maldonado, P.D.; Ruiz-Sánchez, E. S-Allylcysteine, a garlic compound, protects against oxidative stress in 1-methyl-4-phenylpyridinium-induced parkinsonism in mice. J. Nutr. Biochem. 2011, 22, 937–944. [Google Scholar] [CrossRef]
- Ashafaq, M.; Khan, M.M.; Raza, S.; Ahmad, A.; Khuwaja, G.; Javed, H.; Khan, A.; Islam, F.; Siddiqui, M.S.; Safhi, M.M.; et al. S-allyl cysteine mitigates oxidative damage and improves neurologic deficit in a rat model of focal cerebral ischemia. Nutr. Res. 2010, 32, 133–143. [Google Scholar] [CrossRef]
- Cervantes, M.I.; Balderas, P.M.D.O.; Gutiérrez-Baños, J.D.J.; Orozco-Ibarra, M.; Rojas, B.F.; Medina-Campos, O.N.; Espinoza-Rojo, M.; Ruiz-Tachiquín, M.; Ortiz-Plata, A.; Salazar, M.I.; et al. Comparison of antioxidant activity of hydroethanolic fresh and aged garlic extracts and their effects on cerebral ischemia. Food Chem. 2013, 140, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Atif, F.; Yousuf, S.; Agrawal, S.K. S-Allyl L-cysteine diminishes cerebral ischemia-induced mitochondrial dysfunctions in hippocampus. Brain Res. 2009, 1265, 128–137. [Google Scholar] [CrossRef]
- Colin-González, A.L.; Ortiz-Plata, A.; Villeda-Hernández, J.; Barrera, D.; Molina-Jijón, E.; Pedraza-Chaverrí, J.; Maldonado, P.D. Aged garlic extract attenuates cerebral damage and cyclooxygenase-2 induction after ischemia and reperfusion in rats. Plant Foods Hum. Nutr. 2011, 66, 348–354. [Google Scholar] [CrossRef]
- Gomez, C.D.; Aguilera, P.; Ortiz Plata, A.; Nares López, F.; Chanez Cardenas, M.E.; Flores Alfaro, E.; Ruiz-Tachiquín, M.E.; Espinoza-Rojo, M. Aged garlic extract and S-allylcysteine increase the GLUT3 and GCLC expression levels in cerebral ischemia. Adv. Clin. Med. Exp. 2019, 28, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, J.; Han, X.; Hu, W. Garlic-derived compound S-allylmercaptocysteine (SAMC) is active against anaplastic thyroid cancer cell line 8305C (HPACC). Technol. Health Care 2015, 23, S89–S93. [Google Scholar] [CrossRef]
- Yang, G.Q.; Wang, D.; Wang, Y.S.; Wang, Y.Y.; Yang, K. Radiosensitization effect of black garlic extract on lung cancer cell line Lewis cells. [Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi.] Chin. J. Integr. Tradit. West. Med. 2013, 33, 1093–1097. [Google Scholar]
- Jikihara, H.; Qi, G.; Nozoe, K.; Hirokawa, M.; Sato, H.; Sugihara, Y.; Shimamoto, F. Aged garlic extract inhibits 1, 2-dimethylhydrazine-induced colon tumor development by suppressing cell proliferation. Oncol. Rep. 2015, 33, 1131–1140. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.Y.; Yoon, M.K.; Choi, Y.W.; Gweon, O.C.; Kim, J.I.; Choi, T.H.; Choi, Y.H. Effects of aged black garlic extracts on the tight junction permeability and cell invasion in human gastric cancer cells. J. Life Sci. 2010, 20, 528–534. [Google Scholar] [CrossRef] [Green Version]
- Alkreathy, H.M.; AlShehri, N.F.; Kamel, F.O.; Alghamdi, A.K.; Esmat, A.; Karim, S. Aged garlic extract potentiates doxorubicin cytotoxicity in human breast cancer cells. Trop. J. Pharm. Res. 2020, 19, 1669–1676. [Google Scholar] [CrossRef]
- Sigounas, G.; Hooker, J.; Anagnostou, A.; Steiner, M. S-allylmercaptocysteine inhibits cell proliferation and reduces the viability of erythroleukemia, breast, and prostate cancer cell lines. Nutr. Cancer 1997, 27, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Ling, M.T.; Feng, H.; Cheung, H.W.; Tsao, S.W.; Wang, X.; Wong, Y.C. A novel anticancer effect of garlic derivatives: Inhibition of cancer cell invasion through restoration of E-cadherin expression. Carcinogenesis 2006, 27, 2180–2189. [Google Scholar] [CrossRef]
- Pinto, J.T.; Qiao, C.; Xing, J.; Rivlin, R.S.; Protomastro, M.L.; Weissler, M.L.; Tao, Y.; Thaler, H.; Heston, W.D. Effects of garlic thioallyl derivatives on growth, glutathione concentration, and polyamine formation of human prostate carcinoma cells in culture. Am. J. Clin. Nutr. 1997, 66, 398–405. [Google Scholar] [CrossRef] [Green Version]
- Pinto, J.T.; Qiao, C.; Xing, J.; Suffoletto, B.P.; Schubert, K.B.; Rivlin, R.S.; Huryk, R.F.; Bacich, D.J.; Heston, W.D. Alterations of prostate biomarker expression and testosterone utilization in human LNCaP prostatic carcinoma cells by garlic-derived S-allylmercaptocysteine. Prostate 2020, 45, 304–314. [Google Scholar] [CrossRef]
- Howard, E.W.; Ling, M.-T.; Chua, C.W.; Cheung, H.W.; Wang, X.; Wong, Y.C. Garlic-derived S-allylmercaptocysteine is a novel in vivo antimetastatic agent for androgen-independent prostate cancer. Clin. Cancer Res. 2007, 13, 1847–1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, E.W.; Lee, D.T.; Chiu, Y.T.; Chua, C.W.; Wang, X.; Wong, Y.C. Evidence of a novel docetaxel sensitizer, garlic-derived S-allylmercaptocysteine, as a treatment option for hormone refractory prostate cancer. Int. J. Cancer. 2008, 122, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Tong, D.; Qu, H.; Meng, X.; Jiang, Y.; Liu, D.; Ye, S.; Chen, H.; Jin, Y.; Fu, S.; Geng, J. S-allylmercaptocysteine promotes MAPK inhibitor-induced apoptosis by activating the TGF-βsignaling pathway in cancer cells. Oncol. Rep. 2014, 32, 1124–1132. [Google Scholar] [CrossRef]
- Xu, K.X.; Hu, H.; Zhang, X.P.; Wang, Y.L.; Chua, C.W.; Luk, S.U.; Wong, Y.C.; Ling, M.T.; Wang, X.F. Identification of a novel function of Id-1 in mediating the anticancer responses of SAMC, a water-soluble garlic derivative, in human bladder cancer cells. Mol. Med. Rep. 2011, 4, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, S.; Zhang, J.; Qu, X.; Jiang, S.; Zhong, Z.; Zhang, F.; Wong, Y.; Chen, H. Over-expression of survivin is a factor responsible for differential responses of ovarian cancer cells to S-allylmercaptocysteine (SAMC). Exp. Mol. Pathol. 2016, 100, 294–302. [Google Scholar] [CrossRef]

| Temperature | Relative Humidity | Durations (Days) | Results | Ref. | |||
|---|---|---|---|---|---|---|---|
| 60 °C | 80% | 69 | Temp. |  |  | Moisture | [41] |
| 70 °C | 33 | Allicin | |||||
| 80 °C | 24 | ||||||
| 90 °C | 12 |  | HMF | ||||
| Total phenols | |||||||
| Total acids | |||||||
| 70 °C | 90% | 35 |  Redness Redness Brightness and yellowness Brightness and yellowness | [46] | |||
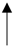 | Crude fat, crude protein, total sugar, | ||||||
| total pyruvate, glucose, amino acids | |||||||
| 70 °C | 90% | 21 |  Redness Redness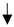 Lightness and yellowness Lightness and yellowness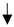 pH pH | [40] | |||
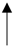 | Total polyphenol, total flavonoid, | ||||||
| total acidity, reducing sugar | |||||||
| 65 °C | 70% | 16 |  | Polyphenol content (85 °C, 70% RH) | [42] | ||
| 75 °C | 75% | ||||||
| 78 °C | 80% | 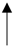 | Reducing sugar, total sugar | ||||
| 85% | total acids, 5-HMF (75 °C, 85% RH) | ||||||
| 72 °C | ~90% | 33 |  | 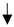 | pH | [44] | |
| Temp. | |||||||
| 75 °C | 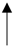 | Polyphenols, antioxidant, | |||||
| Browning intensity | |||||||
| 78 °C | |||||||
| 90 °C (1st step) | 2 |  Moisture (4th step) Moisture (4th step) | [43] | ||||
| 80 °C (2nd step) | 4 |  Total phenolic and flavonoids (4th step) Total phenolic and flavonoids (4th step) | |||||
| 60 °C (3rd step) | 4 |  Total pyruvate and thiosulfate (4th step) Total pyruvate and thiosulfate (4th step) | |||||
| 40 °C (4th step) | 1 |  Mineral content (4th step) Mineral content (4th step) | |||||
| 65–80 °C | 70–80% | 30–40 |  SAC SAC | [45] | |||
| 70 °C | 90% | 10 |  SAC (15 days) SAC (15 days) | [47] | |||
| 15 | |||||||
| 20 | |||||||
| 40 °C | 70% | 45 |  |  | Moisture | [16] | |
| pH | |||||||
| Temp. | |||||||
| 55 °C |  | Browning density | |||||
| 70 °C | SAC | ||||||
| 85 °C | Antioxidant activity | ||||||
| Phytochemical | Total Content in Fresh Garlic | Total Content in Black Garlic | Ref. |
|---|---|---|---|
| Amino acid | 843.11 ± 3.75 mg/100 g | 167.65 ± 1.08−363.10 ± 1.05 mg/100 g | [73] |
| 121.38 ± 4.72 mg/100 g | 60.84 ± 5.75−108.66 ± 12.95 mg/100 g | [46] | |
| 19.43 ± 0.01 mg/g FM | 14.86 ± 0.01 mg/g FM | [79] | |
| 57.66 mg/g sample powder | 44.01 mg/g sample powder | [80] | |
| 1943.77 ± 161.22 mg/100 g | 1486.65 ± 112.62 mg/100 g | [81] | |
| 1528.75 ± 0.83 mg/100 g | 1931.13 ± 175.48 mg/100 g | [40] | |
| Minerals | 1173.50 ± 2.43 mg/100 g | 1314.68 ± 2.76 mg/100 g | [73] |
| 15,908 mg/kg dry powder | 13,227.41 mg/kg dry powder | [80] | |
| 567.88 ± 4.48 mg/100 g | 969.12 ± 19.31 mg/100 g | [40] | |
| Reducing sugar | 58.37 ± 1.54 mg/100 g | 394.52 ± 3.29 mg/100 g | [40] |
| 1.52 ± 0.01 g/kg | 12.42 ± 0.85−16.07 ± 0.38 g/kg | [46] | |
| 295.54 ± 2.01 mg/100 g | 754.51 ± 4.05−4726.04 ± 15.74 mg/100 g | [73] | |
| 5.9 ± 0.8 g/kg DM | 472.4 ± 46.5 g/kg DM | [18] | |
| Total phenol | 1000 µg/g | 8200 µg/g | [63] |
| 18 mg GAE/kg DM | 80–140 mg GAE/kg DM | [82] | |
| 89468.55 mg QE/kg dry basis | 101,328.71–157,312.77 mg QE/kg dry basis | [83] | |
| 14 mg GAE/g | 20–60 mg GAE/g | [46] | |
| 15,200 mg GAE/kg | 24,050 mg GAE/kg | [65] | |
| 5150 mg GAE/kg DM | 14,900 mg GAE/kg DM | [44] | |
| Total flavonoid | 2348.65 mg GAE/kg dry basis | 3825.51−27,191.38 mg GAE/kg dry basis | [83] |
| 3.22 ± 0.07 mg RE/g | 5.38 ± 0.06−15.70 ± 2.11 mg RE/g | [46] | |
| 30.03 mg/kg DM | 30–105 mg/kg DM | [82] | |
| 0.25 mg/100 g | 0.70 mg/100 g | [43] | |
| 0.20 mg/100 g FG water extract | 0.50 mg/100 g BG water extract | [84] | |
| 1.40 µg CAE/mg | 1.92 µg CAE/mg | [85] | |
| SAC | 42.7 µg/g DM | 656.5 µg/g DM | [86] |
| 2.5 mg/g DW | 8.05 mg/g DW | [87] | |
| 95.07 ± 1.84−427.05 ± 3.56 µg/g | [88] | ||
| 85.46 ± 0.81−124.67 ± 1.61 µg/g | [16] | ||
| 73.5 ± 12.5 µg/g DW | 242.3 ± 6.1 µg/g DW | [89] | |
| 2.4 mg/100 g | 19.4 mg/100 g | [90] | |
| 5-HMF | 0.25 ± 0.04 g/kg FM | [65] | |
| 4.82 ± 0.06 g/kg | [41] | ||
| 6–8 g/kg | [42] | ||
| Melanoidin | ˂ 0.2 OD FM | ~2 OD FM | [75] |
| Ash | 73.59 ± 0.89 mg/100 g | 75.36 ± 0.02−114.36 ± 8.65 mg/100 g | [73] |
| 0.92 ± 0.62 % | 1.81 ± 0.05 % | [40] | |
| Volatile compounds | 49.76 µg/g | 39.04−100.46 µg/g | [83] |
| Organic acid | 16.70 ± 0.61 g/kg DM | 64.50 ± 7.55 g/kg DM | [41] |
| Alkaloid | Trace amount | 30-fold increase of FM | [91] |
| Lipid | 0.20 ± 0.01% | 0.60 ± 0.11% | [40] |
| 0.1% | 0.30% | [90] | |
| Carbohydrate | 30% | 50% | [90] |
| Protein | 0.9% | 1.2 ± 0.1% | [40] |
| 8.4% | 9.5% | [90] | |
| Vitamin | 6632.91 ± 18.62 mg/kg | 7618.24 ± 28.47–9,010.44 ± 30.61 mg/kg | [70] |
| Pyruvate | 19.01 ± 0.3 mmol/100 g | 28.05 ± 0.3 mmol/100 g | [40] |
| 49.05 ± 1.2 mmol/100 g | 246.02 ± 2.4 mmol/100 g | [20] | |
| Thiosufate | 6.50 ± 0.29 µM/g | 91.22 ± 0.54 µM/g | [20] |
| Diseases | Products | Subjects/Cell Line/Animal Model | Outcomes | Mode of Action | Ref. |
|---|---|---|---|---|---|
| Diabetes mellitus | Black garlic juice (BGJ) | Male C57BL/6J mice | SAC-enriched BGJ counteracted STZ239-induced diabetes and β-cell failure in mice. | Improved glutathione antioxidant system, increased leptin and adiponectin secretion. Inhibited hepatic gluconeogenesis and NF-κβ-mediated inflammatory signaling. | [89] |
| Aged garlic | Male Sprague-Dawley rats | Ameliorated oxidative stress and other complications of diabetes. | Decreased body weight, blood glucose, serum cholesterol, triglycerides, and fructosamine. | [97] | |
| Aged black garlic (ABG) | C57BL/KsJ-db/db mice | ABG improved insulin sensitivity and dyslipidemia in db/db mice. | Decreased serum glucose, total cholesterol, triglyceride and increased HDL-C levels. | [30] | |
| Black garlic powder (BGP) | Male Wister rats | BGP lowered blood glucose, prevented glycogen in the liver, and improved lipid metabolism. | Lowered glycosylated Hb, and total cholesterol and increased HDL-C. BGP increased the activity of GOT, GPT, γ-GTP in serum. | [98] | |
| Aged black garlic (ABG) | C57BL/KsL-db/db mice | ABG prevented diabetic complications through antioxidant activity. | Decreased TBARS levels, elevated the activities of SOD, GSH-Px, and CAT. | [99] | |
| Aged garlic (AG) and S-allyl cysteine (SAC) | BSA or lysozyme | AG + SAC prevented the formation of advanced glycation end products. | [107] | ||
| Aged garlic (AG) | Sprague-Dawley rats | AG exhibited ameliorative action on indicators of diabetes. | Decreased blood glucose, GHb, and lipid peroxidation. Markedly increased serum insulin, serum triglyceride elevation, and total cholesterol. | [96] | |
| Obesity | Aged garlic (AG) | 51 healthy adults with obesity Study period: 6 weeks | AG prevented the development of chronic diseases associated with low-grade inflammation. | Decreased TNF-α, IL-6, blood LDL levels. | [104] |
| Fermented garlic by lactic acid bacteria (FBLA) | Male C57BL/6J mice | FBLA ameliorated diet-induced obesity by inhibiting adipose tissue hypertrophy by suppressing adipogenesis. | Reduced body weight, TG, TC, retroperitoneal, epididymal, and mesenteric adipose tissue mass. Downregulated mRNA protein expression of PPARγ, C/EBPα, and lipogenic proteins, including SREBP-1c, FAS, and SCD-1. | [102] | |
| Aged garlic | Male Sprague-Dawley rats | Modified the adipose weight and improved the oxidative stress. | Decreased Body weight gain, visceral, epididymal fat, and TBARS levels. | [101] | |
| Black garlic | Male Wister rats | Ameliorated diet-induced obesity via regulating adipogenesis, adipokine biosynthesis, and lipolysis. | Upregulated AMPK, FOXO1, Sirt1, ATGL, HSL, perilipin, ACO, CPT-1, UCP1, adiponectin, and PPAR α. Downregulated CD36, SREBP-1c, ACC, FAS, and SCD1. | [105] | |
| Aged garlic | Sprague Dawley rats | Exhibited anti-obesity, cholesterol-lowering, and anti-inflammatory effects. | Reduced body weight, visceral fat, liver weight, total cholesterol, low-density lipoprotein, and C-reactive protein. | [103] | |
| Aged black garlic | Male Sprague-Dawley rats | Improved the body weight gain and dyslipidemia through the suppression of body fat and alteration in lipid profiles and antioxidant defense system. | Decreased the body weight, adipose tissue weight, TC, TG, and increased oxidized GSH and LPO in the serum. | [29] | |
| Black garlic (BG) | Male Wister rats | BG ameliorated obesity induced by a HFD in rats. | Decreased body weight, tissue weight of liver, epididymal fat, peritoneal fat, serum triglycerides, hepatic lipid profile, GSSG, and enhanced TEAC, GSH, GRd, and GPx. | [25] | |
| Aged black garlic | 3T3-L1 preadipocytes | Exhibited anti-lipogenic and lipolytic effects. | Reduced protein expression of PPARγ, HSL, and Ser-pHSL levels. | [106] |
| Diseases | Products | Subjects/Cell Line/Animal Model | Outcomes | Mode of Action | Ref. |
|---|---|---|---|---|---|
| Nephrotoxicity | Aged garlic (AG) | Male Wister rats | AG prevented gentamicin-induced nephrotoxicity. | Decreased the oxidative stress and preserved the activities of Mn-SOD, GPx, and GR | [108] |
| Diabetic nephropathy (DNP) | Aged garlic (AG) | Albino Wistar rats | AG significantly decreased albumin levels in urine, blood urea nitrogen contents, and increased urine urea nitrogen contents. | The protective effect of AG on DNP due to its anti-glycation, hypolipidemic effects. | [109] |
| Kidney damage | Aged garlic (AG) | Albino Wistar rats | AG rescued ethephon-induced kidney damage. | Activation of Nrf2 and inhibition of inflammation and apoptotic response. | [110] |
| Kidney injury | Aged black garlic (ABG) | Male Sprague-Dawley rats | ABG ameliorated colistin-induced acute kidney injury in rats. | Reduced the levels of oxidative stress biomarkers such as 8-hydroxydeoxyguanosine and malondialdehyde. Lowered the levels of NF-κβ, inducible NO synthase, COX-2, and TGF-β1, and also restored SOD, CAT, and GSH levels. | [111] |
| Diseases | Products | Subjects/Cell Line/Animal Model | Outcomes | Mode of Action | Ref. | |
|---|---|---|---|---|---|---|
| Liver | Aged garlic | C57BL/6 mice | Modulation of glycometabolism, lipometabolism, oxidative stress, and inflammation. |  ALT, AST, TC, LDL-C, MDA ALT, AST, TC, LDL-C, MDA | [114] | |
| Black garlic (BG) | Wister rats | BG protected against oxidative damage caused by CCl4 -induced liver injury. |  | SOD, GSH-Px | [112] | |
 | ALT, AST, LDH, | |||||
| and ALP | ||||||
| Lactic acid- fermented garlic | Wister rats | Protected against oxidative liver injury. | 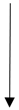 | ALP, AST, and ALT | [115] | |
| ATP depletion | ||||||
| TNF-α, IL-1β | ||||||
| Apoptosis | ||||||
| (BCL-2, Bax, Cascape-3) | ||||||
| Fermented black garlic | C57BL/6J mice | Improved the effects on fatty liver. | 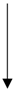 | AST, ALT | [26] | |
| Total cholesterol, | ||||||
| LDL/V-LDL-cholesterol, | ||||||
| triglyceride contents | ||||||
| Black garlic | ICR mice | Inhibited CCl4-induced hepatic injury by inhibiting lipid peroxidation and inflammation. | 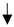 ALT, AST, ALP, and MDA ALT, AST, ALP, and MDA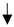 TNF-α and IL-1β TNF-α and IL-1β SOD, GSH-Px, GSH-Rd SOD, GSH-Px, GSH-Rd | [28] | ||
| Aged black garlic | Sprague-Dawley rats | Exhibited protective effects against chronic alcohol-induced liver damage. | 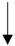 | AST, ALT, ALP, | [113] | |
| and LDH | ||||||
 GSH-Px, GR, and CAT GSH-Px, GR, and CAT | ||||||
| Aged black garlic | Sprague- Dawley rats | Showed hepatoprotective effects against liver injury. | 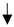 AST, and ALT AST, and ALTNo effects on ALP. | [27] | ||
| Inflammatory | Aged black garlic | RAW 264.7 cells | Suppressed the expression of classical mitogen-activated protein kinases (MAPKs) (ERK1/2 and p38 MAPK) in LPS-stimulated macrophage cells. | 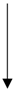 | NO, | [22] |
| prostaglandin E2 | ||||||
| IL-6, TNF-α, IL-1β | ||||||
| iNOS, COX-2 | ||||||
| Aged black garlic | U-937 cells | Inhibited expression of COX-2 and production of prostaglandin E2. | Inactivation of NF-κβ. | [116] | ||
| Aged black garlic | HES cells | Reduced cell proliferation and attenuated the expression of ICAM-1 and VCAM-1. | Inhibition of the ERK, JNK signaling pathways, ROS formation, NF-κβ, and AP-1 transcription factors. | [119] | ||
| Aged black garlic | RAW 264.7 macrophages | Exerted anti-inflammatory effects. | 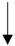 | NO, TNF-α, | [120] | |
| prostaglandin E2 | ||||||
| Fermented black garlic | RAW 264.7 cells | Exhibited anti-inflammatory effects. |  | NO, TNF-α, | [21] | |
| prostaglandin E2 | ||||||
| IL-1β, IL-6 | ||||||
| 5-HNF | RAW 264.7 cells | Exerted anti-inflammatory effects. | Inhibition of MAPK, NF-κβ and Akt/mTOR pathways. | [118] | ||
| 5-HNF | HUVE cells | Prevented TNF-α-induced monocytic cell adhesion to HUVE cells. | Suppressed vascular cell adhesion molecule-1 expression, reactive oxygen species generation and NF-κβ activation. | [117] | ||
| Gastro-intestinal motility | Black garlic | Sprague–Dawley rats | Effectively promoted gastrointestinal motility and defection. | Stimulated gastrointestinal peristalsis, enhanced gastrointestinal tract emptying, and promoted defecation. | [121] | |
| Laxative effects | Mixed black garlic beverage | BALB/c mice | Exhibited an obvious laxative effect, which improved the intestinal flora of mice. | Showed a relatively high ink-propelling rate, increased defection time, E. coli and Enterococci. | [122] | |
| Gastroesopha-geal reflux disease | Black garlic | Sprague- Dawley rats | Showed protective effect on reflux esophagitis. |  SOD, CAT SOD, CAT TNF-α, IL-6 TNF-α, IL-6 | [123] | |
| Gastric ulcer | Aged garlic | Male Wistar rats | Prevented the indomethacin-induced ulcer. | Reduced oxidative stress. Increased gastric levels of PGE2, GSH, and NO. | [124] | |
| Gastric damage | Aged garlic | Male albino rats | Heal the gastric mucosal injury induced by indomethacin. |  MDA, MPO MDA, MPO tGSH, SOD, CAT tGSH, SOD, CAT | [125] | |
| Intestinal damage | Aged garlic | Male Wistar albino rats | Reduced Intestinal damaged induced by anti- tumor drug methotrexate in the small intestine. | Aged garlic halted the MDA increase in tissue and plasma lactate elevations. Thus, protected intestinal damage by preserving cellular integrity. | [126] | |
| Diseases | Products | Subjects/Cell Line/Animal Model | Outcomes | Mode of Action | Ref. | |
|---|---|---|---|---|---|---|
| Platelet Aggregation | Aged garlic | 30 participants Study period: 12 weeks | Reduced cardiovascular risk factors |  BW, BMI, TC, LDL-C, MDA, BW, BMI, TC, LDL-C, MDA, Homocysteine Homocysteine TG TG | [132] | |
| Aged black garlic | 28 participants Study period: 12 weeks | Reduced atherogenic markers. | 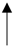 | [133] | ||
| HDL-C Ratio of LDL-C/apo-B | ||||||
| Aged garlic (AG) | 34 participants Study period: 44 weeks | AG exerted selective inhibition on platelet aggregation and adhesion, platelet functions. | [134] | |||
| Aged garlic (AG) | 23 participants Study period: 13 weeks | AG substantially suppressed the total percentage and initial platelet aggregation rate. | [135] | |||
| Aged garlic (AG) | 43 participants Study period: 24 weeks | AG increased plasma adiponectin levels. | [136] | |||
| Aged garlic (AG) | 31 participants Study period: 12 months | AG lowered IL-6 in females with a low-risk profile of the cardiovascular disease. | [137] | |||
| Aged garlic (AG) | Male Wistar rats | Suppressed the platelet aggregation by changing the functional property of the platelets. |  Extracellular ATP Extracellular ATP Extra- and intracellular TXB2 Extra- and intracellular TXB2Suppressed the phosphorylation of collagen-induced ERK, p38, and JNK. | [129] | ||
| Fermented garlic | Male Sprague-Dawley rats | Ameliorated hypercholesterolemia and inhibited platelet activation. | 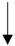 | [131] | ||
| TG, SERBP-2, ACAT-2, HMG-CoA | ||||||
| Fermented garlic (FG) | Male Sprague-Dawley rats | FG significantly inhibited platelet aggregation and granule secretion in hypercholesterolemic rats. | Inhibited collagen and ADP-induced platelet aggregation and ATP release. Downregulated the expression of SERBP, ACAT-2, and HMG-CoA. | [130] | ||
| Arterial Hypertension | Aged garlic (AG) | 88 patients with uncontrolled arterial hypertension. Study period: 12 weeks | Reduced mean blood pressure along with arterial stiffness, mean arterial pressure, central blood pressure, central pulse pressure, pulse-wave velocity, and augmentation pressure. | [140] | ||
| Aged garlic (AG) | 49 participants with uncontrolled arterial hypertension. Study period: 12 weeks | AG was effective in reducing blood pressure and had the potential to improve inflammation, arterial stiffness, and gut microbial profile. | [141] | |||
| Aged garlic | 9 patients Study period: 12 weeks | Mean systolic blood pressure was significantly reduced. | [155] | |||
| Aged garlic | 50 patients Study period: 12 weeks | Systolic blood pressure was reduced on an average of 10.2 ± 4.3 mm Hg. | [142] | |||
| Hypertension related to RAS | Allyl methyl Sulfide (AMS) and diallyl sulfide (DAS) | Male spontaneously hypertensive rats (SHRs) | AMS and DAS inhibited aortic smooth muscle cell angiotensin II-stimulated cell-cycle progression and migration. | The outcome was probably mediated via upregulation of the growth suppressor p27 and the attenuation of ERK 1/2 phosphorilation. | [143] | |
| Black garlic (BG) | Male spontaneously hypertensive rats (SHRs) | BG exerted a potential antihypertensive effect through OFRs in the plasma and PVN of SHRs. | Declined high blood pressure via abolishing the potentiation of angiotensin II and CSAR. | [147] | ||
| Black garlic (BG) | Rabbit lung ACE | BG was the most active in ACE inhibition with the lowest IC50 value (0.04 mg/mL). | Amadori compounds (Fru-Arg and Fru-Met) were probably attributed to ACE inhibitory activity. | [144] | ||
| Black garlic | ACE inhibitory effects of the black garlic extract were greater (88.8%) than normal garlic extract (52.7%) | [145] | ||||
| Atherosclerosis | Aged garlic (AG) | 104 patients Study period: 12 months | AG suppressed the atherosclerosis progression. | Inhibited CAC progression, lowered the levels of IL-6, glucose, and blood pressure. | [151] | |
| Aged garlic (AG) and coenzyme Q10 (CoQ10) | 65 patients Study period: 12 months | AG + CoQ10 reduced the progression of coronary atherosclerosis. | Lowered CAC progression and CRP levels. | [152] | ||
| Aged garlic | 60 patients Study period: 12 months | Reduced the progression rate of adipose tissue volumes related to CAC. | Decreased the levels of EAT, PAT, PaAT, and SAT. | [154] | ||
| Aged garlic supplemented with B vitamins, folic acid, and L-arginine | 65 patients Study period: 12 months | Improved oxidative biomarkers, vascular function, and reduced progression of atherosclerosis. | Lowered CAC progression. Decreased TG, LDL-C, homocysteine, IgG and IgM autoantibodies to MDA-LDL and apoB-immune complexes. Increased HDL, OxPL/apoB, and LP. | [153] | ||
| Aged garlic (AG) | ApoE-KO mice | AG suppressed the development of atherosclerosis. | Suppressed the increase in serum concentrations of TC, TG and reduced the relative abundance of CD11b+ cells. | [149] | ||
| Aged garlic (AG) | New Zealand white rabbit | AG protected the onset of atherosclerosis. | Reduced fatty streak development, vessel wall cholesterol accumulation, and the development of fibro-fatty plaques. | [150] | ||
| Diseases | Products | Subjects/Cell Line/Animal Model | Outcomes | Mode of Action | Ref. |
|---|---|---|---|---|---|
| Alzheimer’s | Aged garlic (AG) | Transgenic model Tg2576 | AG reduced cerebral plaques, detergent soluble and detergent resistant (fibrillar) Aβ-species with concomitantly increased α-cleaved sAPPα, reduced inflammation and conformational change in tau. | The observed change in tau phosphorylation appears to involve GSK-3β. | [156] |
| Aged garlic (AG) | PC12 cell ICR mice | AG ameliorated against Aβ-induced neurotoxicity and cognitive impairment. | AG showed ABTS radical scavenging activity, MDA inhibitory effect and reduced intracellular ROS accumulation. | [157] | |
| Aged garlic (AG) | Male Wistar rat | AG ameliorated the cognitive dysfunction in Aβ-induced neurotoxicity rats. |  SOD, GPx SOD, GPx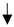 MDA levels MDA levels | [158] | |
| Aged garlic (AG) and Fru-Arg | Murine BV-2 microglial cell | AG and Fru-Arg attenuated neuroinflammatory responses. | Suppressed the production of NO. Regulated the expression of multiple protein targets associated with oxidative stress. | [161] | |
| Aged garlic (AG) | Male Wistar rats | AG significantly improved the working memory and tended to improve the reference memory in cognitively impaired rats. | Modified the cholinergic neurons, VGLUT1, and GAD in the hippocampus of Aβ-induced rats. | [159] | |
| Aged garlic (AG) and S-allyl-L- cysteine (SAC) | PC12 cells, Tg2576 transgenic mice | Both AG and SAC treatment protected neuronal cells from ROS-mediated oxidative insults and preserved the levels of pre-synaptic protein such as SNAP25. | [160] | ||
| Parkinson’s | S-allyl-L-cysteine (SAC) | C57BL/6J mice | SAC protected against oxidative stress in 1-methyl-4 phenylpyridinium-induced parkinsonism in mice. | Ameliorated MPP+-induced lipid peroxidation, ROS production, loss of dopamine in striatum, and improved locomotion deficits. | [162] |
| Cerebral ischemia | S-allyl-L- cysteine (SAC) | Male Wistar rats | SAC mitigated oxidative damage and improved neurologic deficit. | Reduced ischemic lesion volume, suppressed neuronal loss, and inhibited glial fibrillary acidic protein and inducible nitric oxide expression. | [163] |
| Aged garlic (AG) and S-allyl-L- cysteine (SAC) | Male Wistar rats | Both AG and SAC treatment induced neuroprotection. | Increased GLUT3 and GCLC mRNA expression levels. | [167] | |
| S-allyl-L-cysteine (SAC) | Male Wistar rats | SAC diminished cerebral ischemia-induced mitochondrial dysfunctions in hippocampus. | Restored GSH and G6-PD. Decreased LPO, PC, and H2O2 content as well as the brain edema. | [165] | |
| Aged garlic (AG) | Male Wistar rats | AG attenuated the cerebral ischemia-induced inflammation. | Attenuated the increase in the levels of 8-OHdG, TNF-α, and COX-2 protein. | [166] | |
| Aged garlic (AG) | Male Wistar rats | AG protected against ischemia-induced brain damage. | Decreased mRNA expression of NMDA receptor subunits after ischemia. Prevented ischemia-induced reduction in mitochondrial potential and in ATP synthesis. | [164] |
| Diseases | Products | Subjects/Cell Line/Animal Model | Outcomes | Mode of Action | Ref. |
|---|---|---|---|---|---|
| Colon cancer | Aged black garlic (ABG) | HT29 cell | ABG inhibited colon cancer cell growth | ABG reduced HT29 cell growth and promoted apoptosis by inhibiting the PI3KAkt pathway. | [32] |
| Aged garlic (AG) | DLD-1 cell and F344 rats | AG inhibited 1,2-dimethylhydrazine-induced colon tumor development. | AG delayed cell cycle progression by downregulating cyclin B1 and cdk1 expression via inactivation of NF-κB but did not induce apoptosis. | [170] | |
| Prostate cancer | SAMC | LNCaP cell, PC-3, DU 145 cells | SAMC showed positive effects against prostate cancer cells. | Rescued GSH deficits, altered prostate biomarker expression and utilized testosterone, restored the expression of E-cadherin. | [173,174,175,176,177,178] |
| Gastric cancer | Aged black garlic (ABG) | AGS cells | ABG treatment inhibited tumor metastasis and invasion. | ABG increased the tightness of tight junction. Inhibited the activities of MMP-2 and -9 in AGS cells. Repressed the levels of claudin proteins. | [171] |
| Aged black garlic (ABG) | SGC-7901 cells | ABG induced inhibition of gastric cancer cell growth. | Increased the superoxide dismutases, glutathione peroxidase, interleukin-2, and indices of spleen and thymus in Kunming mice. | [33] | |
| Breast cancer | Aged garlic (AG) | MCF-7 | Exhibited a chemosensitizing effect. | Induction of apoptosis, enhanced intracellular DOX accumulation, inhibition of P-gp activity. | [58] |
| Liver cancer | SAMC | HepG2 cells | SAMC promoted MAPK inhibitor-induced apoptosis by activating the TGF-β signaling pathway. | Activated TGF-β1, TβRII, psmad2/3, smad4 and smad7 signaling. | [179] |
| Bladder cancer | SAMC | MGH-U1 cells | SAMC inhibited the survival, invasion, and migration of bladder cancer cells. | Inactivated Id-1 pathway. | [180] |
| Thyroid cancer | SAMC | HPACC-8305C | SAMC inhibited the growth of HPACC-8305C cells. | Induction of apoptotic cell death. Inhibited telomerase activity. | [168] |
| Lung cancer | Black garlic | Lewis cells | Inhibited the growth of lung cancer cells. | Affected the expression of Bax and BCL-2 | [169] |
| Ovarian cancer | SAMC | HO8910, HO8910PM, and SKOV3 | SAMC suppressed both the proliferation and distant metastasis of epithelial ovarian cancers cells. | Down-regulated the survivin gene in HO8910PM cells with small interference RNA (siRNA). Decreased invasiveness of tumor cells. | [181] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, T.; Wang, C.-K. Black Garlic and Its Bioactive Compounds on Human Health Diseases: A Review. Molecules 2021, 26, 5028. https://doi.org/10.3390/molecules26165028
Ahmed T, Wang C-K. Black Garlic and Its Bioactive Compounds on Human Health Diseases: A Review. Molecules. 2021; 26(16):5028. https://doi.org/10.3390/molecules26165028
Chicago/Turabian StyleAhmed, Tanvir, and Chin-Kun Wang. 2021. "Black Garlic and Its Bioactive Compounds on Human Health Diseases: A Review" Molecules 26, no. 16: 5028. https://doi.org/10.3390/molecules26165028
APA StyleAhmed, T., & Wang, C.-K. (2021). Black Garlic and Its Bioactive Compounds on Human Health Diseases: A Review. Molecules, 26(16), 5028. https://doi.org/10.3390/molecules26165028






