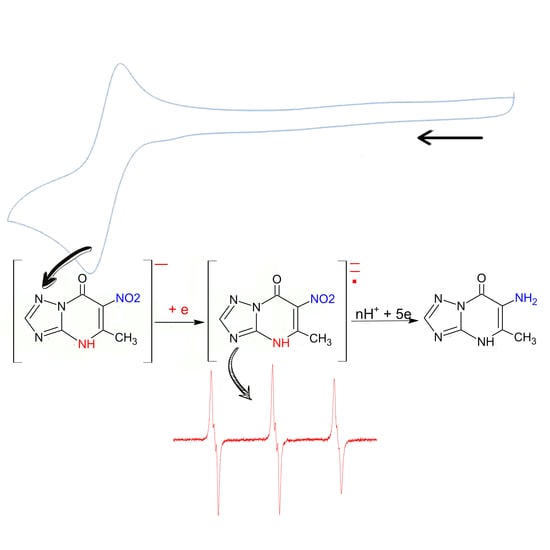
Redox Conversions of 5-Methyl-6-nitro-7-oxo-4,7-dihydro-1,2,4triazolo[1,5-a]pyrimidinide L-Arginine Monohydrate as a Promising Antiviral Drug
Abstract
Share and Cite
Ivoilova, A.; Mikhalchenko, L.V.; Tsmokalyuk, A.; Leonova, M.; Lalov, A.; Mozharovskaia, P.; Kozitsina, A.N.; Ivanova, A.V.; Rusinov, V.L. Redox Conversions of 5-Methyl-6-nitro-7-oxo-4,7-dihydro-1,2,4triazolo[1,5-a]pyrimidinide L-Arginine Monohydrate as a Promising Antiviral Drug. Molecules 2021, 26, 5087. https://doi.org/10.3390/molecules26165087
Ivoilova A, Mikhalchenko LV, Tsmokalyuk A, Leonova M, Lalov A, Mozharovskaia P, Kozitsina AN, Ivanova AV, Rusinov VL. Redox Conversions of 5-Methyl-6-nitro-7-oxo-4,7-dihydro-1,2,4triazolo[1,5-a]pyrimidinide L-Arginine Monohydrate as a Promising Antiviral Drug. Molecules. 2021; 26(16):5087. https://doi.org/10.3390/molecules26165087
Chicago/Turabian StyleIvoilova, Alexandra, Ludmila V. Mikhalchenko, Anton Tsmokalyuk, Marina Leonova, Andrey Lalov, Polina Mozharovskaia, Alisa N. Kozitsina, Alla V. Ivanova, and Vladimir L. Rusinov. 2021. "Redox Conversions of 5-Methyl-6-nitro-7-oxo-4,7-dihydro-1,2,4triazolo[1,5-a]pyrimidinide L-Arginine Monohydrate as a Promising Antiviral Drug" Molecules 26, no. 16: 5087. https://doi.org/10.3390/molecules26165087
APA StyleIvoilova, A., Mikhalchenko, L. V., Tsmokalyuk, A., Leonova, M., Lalov, A., Mozharovskaia, P., Kozitsina, A. N., Ivanova, A. V., & Rusinov, V. L. (2021). Redox Conversions of 5-Methyl-6-nitro-7-oxo-4,7-dihydro-1,2,4triazolo[1,5-a]pyrimidinide L-Arginine Monohydrate as a Promising Antiviral Drug. Molecules, 26(16), 5087. https://doi.org/10.3390/molecules26165087