Egg Case Protein 3: A Constituent of Black Widow Spider Tubuliform Silk
Abstract
:1. Introduction
2. Results and Discussion
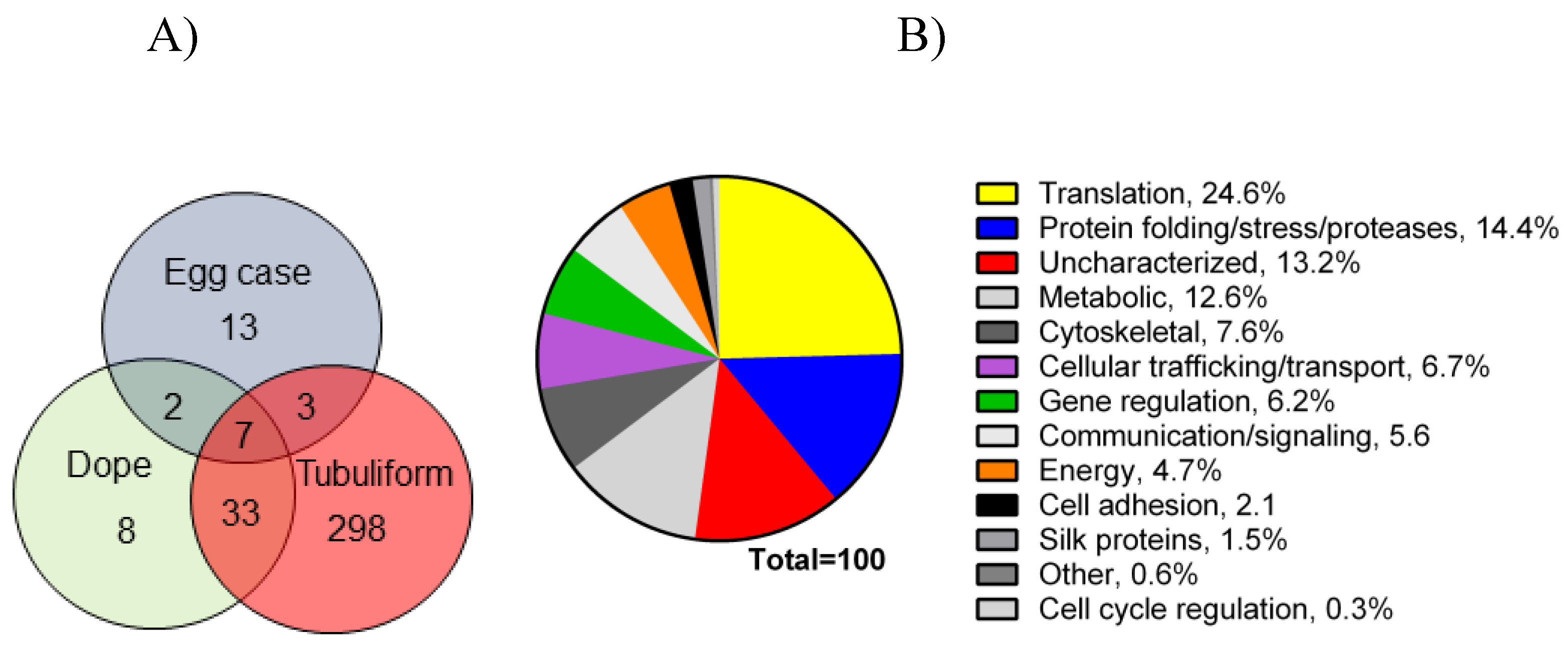
2.1. Proteomic Analysis of Tubuliform Glands, Spinning Dope, and Egg Sacs
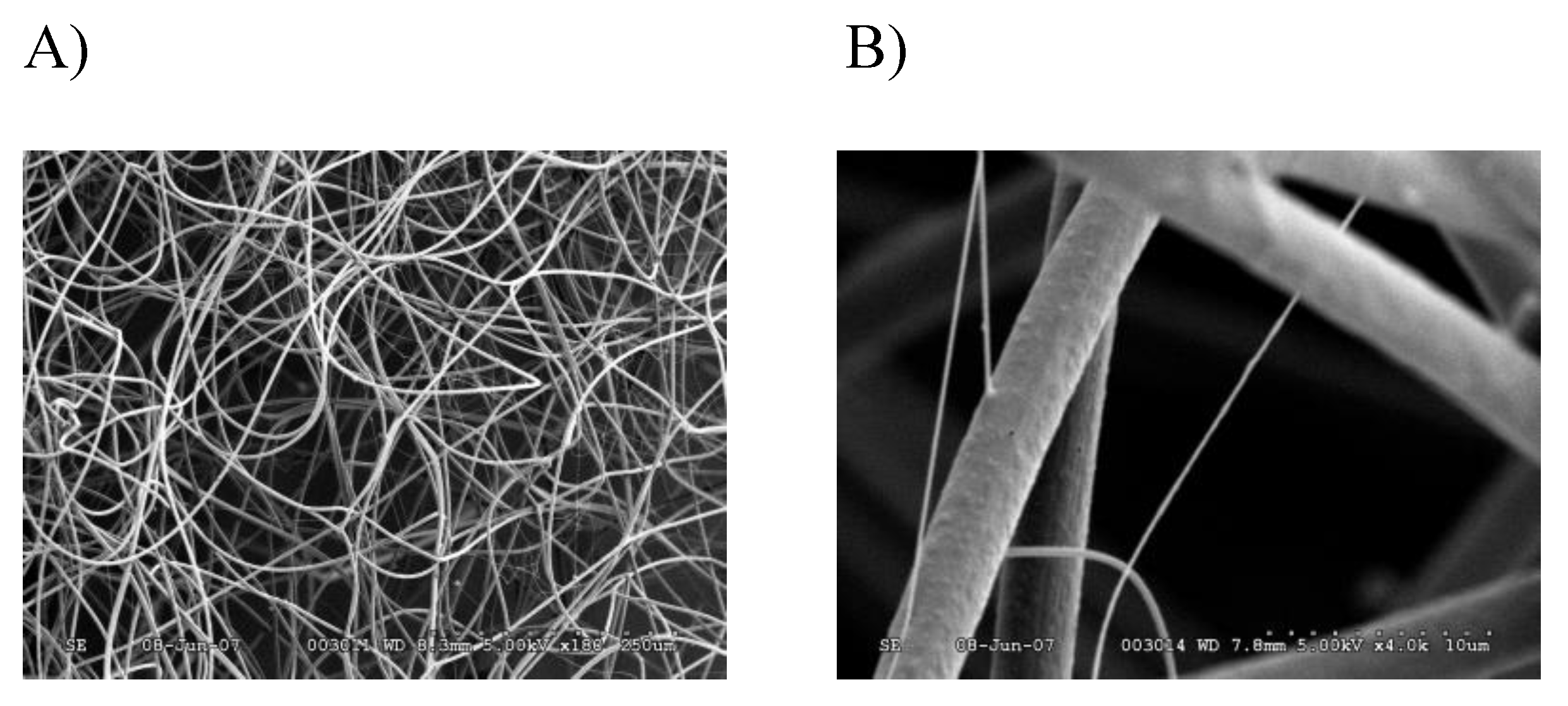
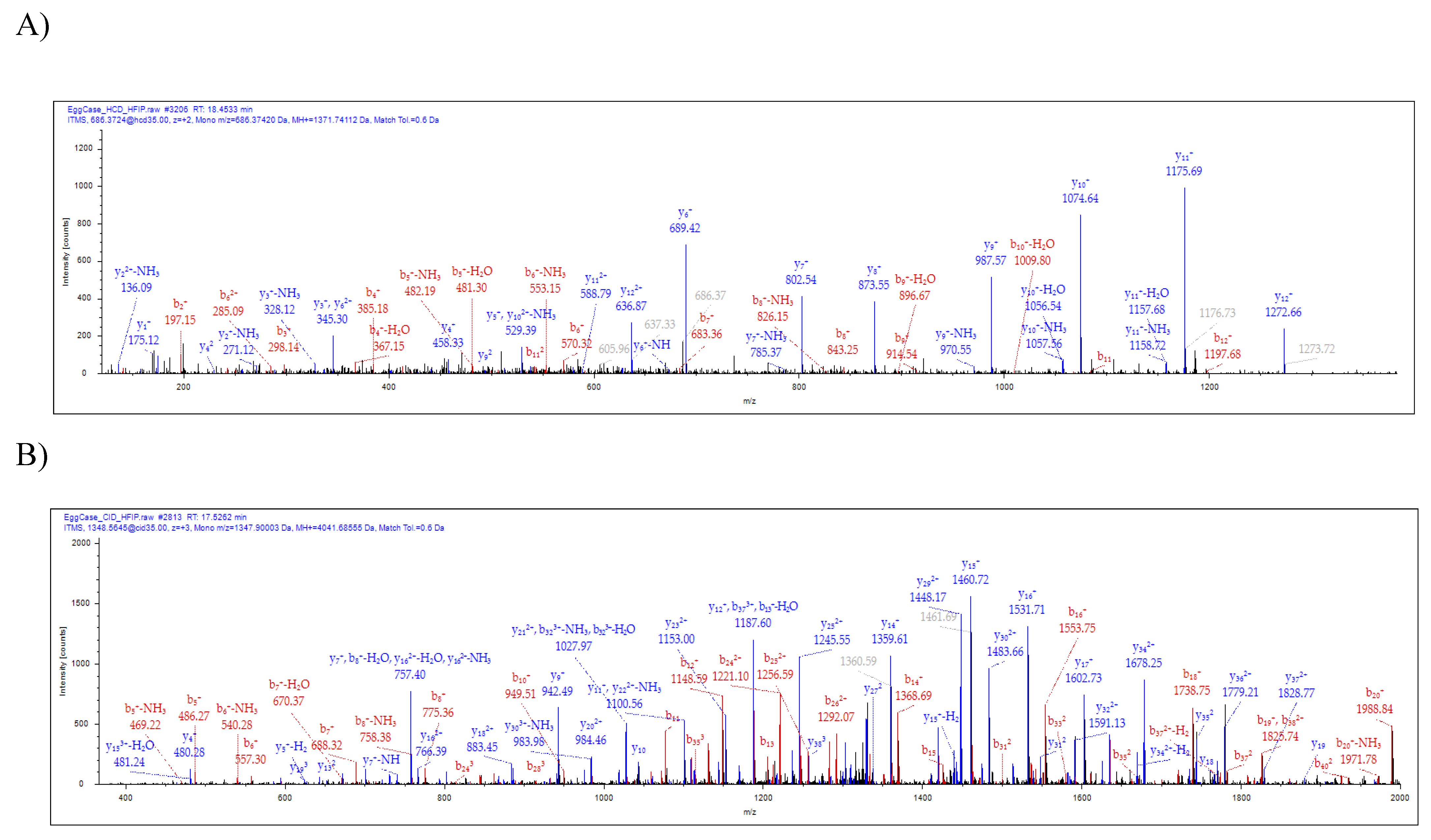
2.2. Characterization of a Novel Protein Identified in Tubuliform Silk
3. Materials and Methods
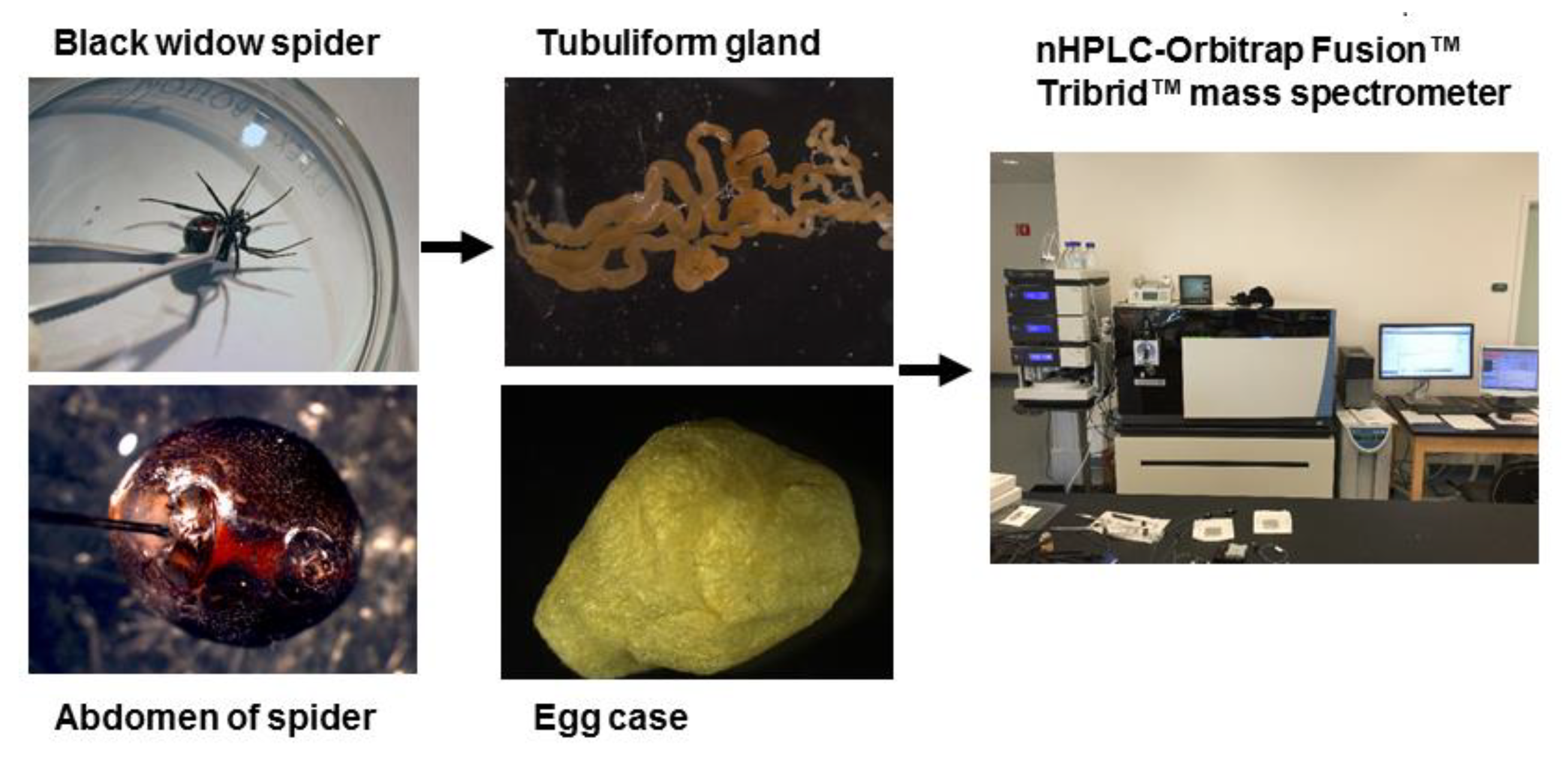
3.1. Spider Care
3.2. Sample Preparation for Proteomics
3.3. nLC-ESI-MS/MS Analysis
3.4. Proteomic Data Analysis
3.5. Cloning of ECP3 cDNA
3.6. Real-Time Quantitative PCR Analysis (RT-qPCR)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gosline, J.M.; Guerette, P.A.; Ortlepp, C.S.; Savage, K.N. The mechanical design of spider silks: From fibroin sequence to mechanical function. J. Exp. Biol. 1999, 202, 3295–3303. [Google Scholar] [CrossRef]
- Moore, A.M.; Tran, K. Material properties of cobweb silk from the black widow spider Latrodectus hesperus. Int. J. Biol. Macromol. 1999, 24, 277–282. [Google Scholar] [CrossRef]
- Lane, A.K.; Hayashi, C.Y.; Whitworth, G.B.; Ayoub, N.A. Complex gene expression in the dragline silk producing glands of the western black widow (Latrodectus hesperus). BMC Genomics 2013, 14, 846. [Google Scholar] [CrossRef] [Green Version]
- Clarke, T.H.; Garb, J.E.; Hayashi, C.Y.; Haney, R.A.; Lancaster, A.K.; Corbett, S.; Ayoub, N.A. Multi-tissue transcriptomics of the black widow spider reveals expansions, co-options, and functional processes of the silk gland gene toolkit. BMC Genomics 2014, 15, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaw, R.C.; Correa-Garhwal, S.M.; Clarke, T.H.; Ayoub, N.A.; Hayashi, C.Y. Proteomic evidence for components of spider silk synthesis from black widow silk glands and fibers. J. Proteome Res. 2015, 14, 4223–4231. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Kohler, K.; Falick, A.M.; Moore, A.M.; Jones, P.R.; Vierra, C. Spider egg case core fibers: Trimeric complexes assembled from TuSp1, ECP-1, and ECP-2. Biochemistry 2006, 45, 3506–3516. [Google Scholar] [CrossRef] [PubMed]
- Larracas, C.; Hekman, R.; Dyrness, S.; Arata, A.; Williams, C.; Crawford, T.; Vierra, C.A. Comprehensive proteomic analysis of spider dragline silk from black widows: A recipe to build synthetic silk fibers. Int. J. Mol. Sci. 2016, 17, 1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasanthavada, K.; Hu, X.; Falick, A.M.; La Mattina, C.; Moore, A.M.; Jones, P.R.; Yee, R.; Reza, R.; Tuton, T.; Vierra, C. Aciniform spidroin, a constituent of egg case sacs and wrapping silk fibers from the black widow spider Latrodectus hesperus. J. Biol. Chem. 2007, 282, 35088–35097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Mattina, C.; Reza, R.; Hu, X.; Falick, A.M.; Vasanthavada, K.; McNary, S.; Yee, R.; Vierra, C.A. Spider minor ampullate silk proteins are constituents of prey wrapping silk in the cob weaver Latrodectus hesperus. Biochemistry 2008, 47, 4692–4700. [Google Scholar] [CrossRef]
- He, Q.; Duan, Z.; Yu, Y.; Liu, Z.; Liang, S. The venom gland transcriptome of Latrodectus tredecimguttatus revealed by deep sequencing and cdna library analysis. PLoS ONE 2013, 8, e81357. [Google Scholar] [CrossRef] [Green Version]
- Casem, M.L.; Collin, M.A.; Ayoub, N.A.; Hayashi, C.Y. Silk gene transcripts in the developing tubuliform glands of the western black widow, Latrodectus hesperus. J. Arachnol. 2010, 38, 99–103. [Google Scholar] [CrossRef]
- Jeffery, F.; La Mattina, C.; Tuton-Blasingame, T.; Hsia, Y.; Gnesa, E.; Zhao, L.; Franz, A.; Vierra, C. Microdissection of black widow spider silk-producing glands. J. Vis. Exp. 2011, 47, e2382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nentwig, W. Ecophysiology of Spiders; Springer: Berlin, Germany, 1987. [Google Scholar]
- Foelix, R. Biology of Spiders; Oxford University Press: New York, NY, USA, 1996. [Google Scholar]
- Guerette, P.A.; Ginzinger, D.G.; Weber, B.H.; Gosline, J.M. Silk properties determined by gland-specific expression of a spider fibroin gene family. Science 1996, 272, 112–115. [Google Scholar] [CrossRef]
- Gatesy, J.; Hayashi, C.; Motriuk, D.; Woods, J.; Lewis, R. Extreme diversity, conservation, and convergence of spider silk fibroin sequences. Science 2001, 291, 2603–2605. [Google Scholar] [CrossRef]
- Ayoub, N.A.; Garb, J.E.; Tinghitella, R.M.; Collin, M.A.; Hayashi, C.Y. Blueprint for a high-performance biomaterial: Full-length spider dragline silk genes. PLoS ONE 2007, 2, e514. [Google Scholar] [CrossRef] [PubMed]
- Beckwitt, R.; Arcidiacono, S. Sequence conservation in the C-terminal region of spider silk proteins (spidroin) from Nephila clavipes (tetragnathidae) and Araneus bicentenarius (araneidae). J. Biol. Chem. 1994, 269, 6661–6663. [Google Scholar] [CrossRef]
- Hayashi, C.Y.; Blackledge, T.A.; Lewis, R.V. Molecular and mechanical characterization of aciniform silk: Uniformity of iterated sequence modules in a novel member of the spider silk fibroin gene family. Mol. Biol. Evol. 2004, 21, 1950–1959. [Google Scholar] [CrossRef] [Green Version]
- Trancik, J.E.; Czernuszka, J.T.; Bell, F.I.; Viney, C. Nanostructural features of a spider dragline silk as revealed by electron and X-ray diffraction studies. Polymer 2006, 47, 5633–5642. [Google Scholar] [CrossRef]
- Jenkins, J.E.; Holland, G.P.; Yarger, J.L. High resolution magic angle spinning NMR investigation of silk protein structure within major ampullate glands of orb weaving spiders. Soft Matter 2012, 8, 1947–1954. [Google Scholar] [CrossRef]
- Jenkins, J.E.; Creager, M.S.; Butler, E.B.; Lewis, R.V.; Yarger, J.L.; Holland, G.P. Solid-state NMR evidence for elastin-like beta-turn structure in spider dragline silk. Chem. Commun. 2010, 46, 6714–6716. [Google Scholar] [CrossRef]
- Hieber, C.S. Spider cocoons and their suspension systems as barriers to generalist and specialist predators. Oecologia 1992, 91, 530–535. [Google Scholar] [CrossRef]
- Tian, M.; Lewis, R.V. Molecular characterization and evolutionary study of spider tubuliform (eggcase) silk protein. Biochemistry 2005, 44, 8006–8012. [Google Scholar] [CrossRef]
- Hu, X.; Kohler, K.; Falick, A.M.; Moore, A.M.; Jones, P.R.; Sparkman, O.D.; Vierra, C. Egg case protein-1. A new class of silk proteins with fibroin-like properties from the spider Latrodectus hesperus. J. Biol. Chem. 2005, 280, 21220–21230. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Lawrence, B.; Kohler, K.; Falick, A.M.; Moore, A.M.; McMullen, E.; Jones, P.R.; Vierra, C. Araneoid egg case silk: A fibroin with novel ensemble repeat units from the black widow spider, Latrodectus hesperus. Biochemistry 2005, 44, 10020–10027. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.H.; Garb, J.E.; Haney, R.A.; Chaw, R.C.; Hayashi, C.Y.; Ayoub, N.A. Evolutionary shifts in gene expression decoupled from gene duplication across functionally distinct spider silk glands. Sci. Rep. 2017, 7, 8393. [Google Scholar] [CrossRef]
- Armenteros, J.J.A.; Tsirigos, K.D.; Sonderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. Signalp 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Jefferys, B.R.; Kelley, L.A.; Sternberg, M.J. Protein folding requires crowd control in a simulated cell. J. Mol. Biol. 2010, 397, 1329–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanafelt, M.; Larracas, C.; Dyrness, S.; Hekman, R.; La Mattina-Hawkins, C.; Rabara, T.; Wu, W.; Vierra, C.A. Egg Case Protein 3: A Constituent of Black Widow Spider Tubuliform Silk. Molecules 2021, 26, 5088. https://doi.org/10.3390/molecules26165088
Shanafelt M, Larracas C, Dyrness S, Hekman R, La Mattina-Hawkins C, Rabara T, Wu W, Vierra CA. Egg Case Protein 3: A Constituent of Black Widow Spider Tubuliform Silk. Molecules. 2021; 26(16):5088. https://doi.org/10.3390/molecules26165088
Chicago/Turabian StyleShanafelt, Mikayla, Camille Larracas, Simmone Dyrness, Ryan Hekman, Coby La Mattina-Hawkins, Taylor Rabara, Wilson Wu, and Craig A. Vierra. 2021. "Egg Case Protein 3: A Constituent of Black Widow Spider Tubuliform Silk" Molecules 26, no. 16: 5088. https://doi.org/10.3390/molecules26165088
APA StyleShanafelt, M., Larracas, C., Dyrness, S., Hekman, R., La Mattina-Hawkins, C., Rabara, T., Wu, W., & Vierra, C. A. (2021). Egg Case Protein 3: A Constituent of Black Widow Spider Tubuliform Silk. Molecules, 26(16), 5088. https://doi.org/10.3390/molecules26165088




