Heterocyclic Chemistry Applied to the Design of N-Acyl Homoserine Lactone Analogues as Bacterial Quorum Sensing Signals Mimics
Abstract
:1. Introduction
1.1. The Power of Heterocyclic Chemistry for the Design of Bioactive Molecules
1.2. Role of Acyl Homoserine Lactones in Bacterial Quorum Sensing
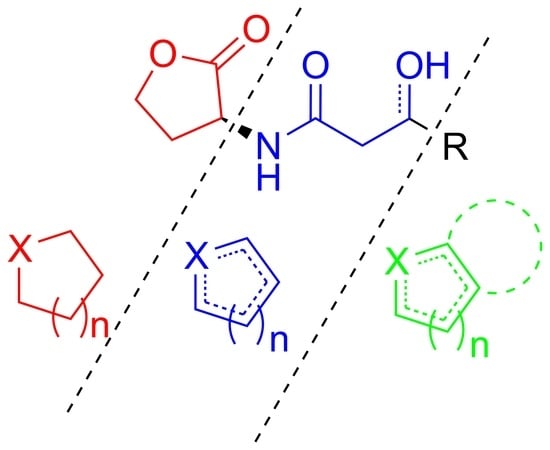
2. AHLs Structural Analogues Including a Heterocyclic Modification
2.1. Heterocycles as Replacement of the Lactone
2.1.1. Non-Aromatic Heterocycles as Lactone Mimics
2.1.2. Aromatic Heterocycles as Lactone Mimics
2.2. Heterocycles as Amide Bond Bioisosteres in AHLs
2.3. Heterocycles as Appendages Modifying the AHL Side Chain
3. Non-AHL Related Heterocyclic Compounds with AHL-Like QS Activity
3.1. Furanone Type Analogues
3.2. Other Heterocyclic Systems
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fletcher: J., T.; Boriraj, G. Benzodiazepine Synthesis and Rapid Toxicity Assay. J. Chem. Educ. 2010, 87, 631–633. [Google Scholar] [CrossRef]
- Bruno, O.; Brullo, C.; Schenone, S.; Bondavalli, F.; Ranise, A.; Tognolini, M.; Ballabeni, V.; Barocelli, E. Synthesis and pharmacological evaluation of 5H-[1]benzopyrano [4,3-d]pyrimidines effective as antiplatelet/analgesic agents. Bioorg. Med. Chem. 2004, 12, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Isloor, A.M.; Kalluraya, B.; Shetty, P. Regioselective reaction: Synthesis, characterization and pharmacological studies of some new Mannich bases derived from 1,2,4-triazoles. Eur. J. Med. Chem. 2009, 44, 3784–3787. [Google Scholar] [CrossRef]
- Kumar, R.; Nair, R.R.; Dhiman, S.S.; Sharma, J.; Prakash, O. Organoiodine (III)-mediated synthesis of 3-aryl/heteroaryl-5,7-dimethyl-1,2,4-triazolo[4,3-c]pyrimidines as antibacterial agents. Eur. J. Med. Chem. 2009, 44, 2260–2264. [Google Scholar] [CrossRef]
- Navidpour, L.; Shafaroodi, H.; Abdi, K.; Amini, M.; Ghahremani, M.H.; Dehpour, A.R.; Shafiee, A. Design, synthesis, and biological evaluation of substituted 3-alkylthio-4,5-diaryl-4H-1,2,4-triazoles as selective COX-2 inhibitors. Bioorg. Med. Chem. 2006, 14, 2507–2517. [Google Scholar] [CrossRef] [PubMed]
- Bruno, O.; Brullo, C.; Schenone, S.; Bondavalli, F.; Ranise, A.; Tognolini, M.; Impicciatore, M.; Ballabeni, V.; Barocelli, E. Synthesis, antiplatelet and antithrombotic activities of new 2-substituted benzopyrano[4,3-d]pyrimidin-4-cycloamines and 4-amino/cycloamino-benzopyrano[4,3-d]pyrimidin-5-ones. Bioorg. Med. Chem. 2006, 14, 121–130. [Google Scholar] [CrossRef]
- Savini, L.; Chiasserini, L.; Pellerano, C.; Filippelli, W.; Falcone, G. Synthesis and pharmacological activity of 1,2,4-triazolo[4,3-a]quinolines. Il Farmaco 2001, 56, 939–945. [Google Scholar] [CrossRef]
- Bekircan, O.; Gumrukcuoglu, N. Synthesis of some 3,5-biphenyl-4H-1,2,4-triazole derivatives as antitumor agents. Indian J. Chem. Sect. B 2005, 44, 2107–2113. [Google Scholar]
- Al-Soud, Y.A.; Al-Masoudi, N.A.; Ferwanah Ael, R. Synthesis and properties of new substituted 1,2,4-triazoles: Potential antitumor agents. Bioorg. Med. Chem. 2003, 11, 1701–1708. [Google Scholar] [CrossRef]
- Bing, C.; Xuhong, Q.; Song, C.; Haidong, L.; Gonghua, S. Synthesis and insecticidal activity of 1,2,4-triazole derivatives. ARKIVOC 2003, 2, 141–145. [Google Scholar]
- Abdelli, A.; Gharsa, H.; Jmai, M.; Gaucher, A.; Efrit, M.L.; M’Rabet, H.; Prim, D. Versatile approach to densely substituted isoxazolines and pyrazolines: Focus on a quaternary carbon center as a constitutive feature. Tetrahedron Lett. 2020, 61, 4. [Google Scholar] [CrossRef]
- Jabli, D.; Milad, R.; Abderrabba, M.; Efrit, M.L. Synthesis, Antibacterial Activity and DFT Calculation of Naphtopyrano, Furo and Pyrazolo [3,2,e] [1,2,4]Triazolo-[1,5-c]Pyrimidine Derivatives. Chem. Afr. 2019, 2, 597–613. [Google Scholar] [CrossRef] [Green Version]
- Jabli, D.; Lahbib, K.; Dridi, K.; Lotfi Efrit, M. Antibacterial Study Novel 2-cyanomethylthieno [3,2-e] [1,2,4]- triazolo[1,5-c]pyrimidine. J. Pharm. Chem. Biol. Sci. 2015, 3, 178–187. [Google Scholar]
- Sabbah, M.; Fontaine, F.; Grand, L.; Boukraa, M.; Efrit, M.L.; Doutheau, A.; Soulère, L.; Queneau, Y. Synthesis and biological evaluation of new N-acyl-homoserine-lactone analogues, based on triazole and tetrazole scaffolds, acting as LuxR-dependent quorum sensing modulators. Bioorg. Med. Chem. 2012, 20, 4727–4736. [Google Scholar] [CrossRef]
- Sabbah, M.; Bernollin, M.; Doutheau, A.; Soulere, L.; Queneau, Y. A new route towards fimbrolide analogues: Importance of the exomethylene motif in LuxR dependent quorum sensing inhibition. MedChemComm 2013, 4, 363–366. [Google Scholar] [CrossRef]
- Meanwell, N.A. Synopsis of some recent tactical application of bioisosteres in drug design. J. Med. Chem. 2011, 54, 2529–2591. [Google Scholar] [CrossRef] [PubMed]
- Hansch, C.; Kiehs, K.; Lawrence, G.L. The role of substituents in the hydrophobic bonding of phenols by serum and mitochondrial proteins. J. Am. Chem. Soc. 1965, 87, 5770–5773. [Google Scholar] [CrossRef]
- Reed, C.W.; Washecheck, J.P.; Quitlag, M.C.; Jenkins, M.T.; Rodriguez, A.L.; Engers, D.W.; Blobaum, A.L.; Conn, P.J.; Niswender, C.M.; Lindsley, C.W. Surveying heterocycles as amide bioisosteres within a series of mGlu(7) NAMs: Discovery of VU6019278. Bioorg. Med. Chem. Lett. 2019, 29, 1211–1214. [Google Scholar] [CrossRef] [PubMed]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef]
- Fuqua, W.C.; Winans, S.C.; Greenberg, E.P. Quorum sensing in bacteria: The LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 1994, 176, 269–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, M.; Sexton, D.J.; Diggle, S.P.; Greenberg, E.P. Acyl-homoserine lactone quorum sensing: From evolution to application. Annu. Rev. Microbiol. 2013, 67, 43–63. [Google Scholar] [CrossRef]
- Papenfort, K.; Vogel, J. Regulatory RNA in bacterial pathogens. Cell Host Microbe 2010, 8, 116–127. [Google Scholar] [CrossRef] [Green Version]
- Welsh, M.A.; Eibergen, N.R.; Moore, J.D.; Blackwell, H.E. Small molecule disruption of quorum sensing cross-regulation in pseudomonas aeruginosa causes major and unexpected alterations to virulence phenotypes. J. Am. Chem. Soc. 2015, 137, 1510–1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, P. Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiology 2007, 153, 3923–3938. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.H.; Wang, L.H.; Xu, J.L.; Zhang, H.B.; Zhang, X.F.; Zhang, L.H. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 2001, 411, 813–817. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Givskov, M. Quorum-sensing blockade as a strategy for enhancing host defences against bacterial pathogens. Philos. Trans. R Soc. Lond. B Biol. Sci. 2007, 362, 1213–1222. [Google Scholar] [CrossRef]
- Kaufmann, G.F.; Sartorio, R.; Lee, S.H.; Rogers, C.J.; Meijler, M.M.; Moss, J.A.; Clapham, B.; Brogan, A.P.; Dickerson, T.J.; Janda, K.D. Revisiting quorum sensing: Discovery of additional chemical and biological functions for 3-oxo-N-acylhomoserine lactones. Proc. Natl. Acad. Sci. USA 2005, 102, 309–314. [Google Scholar] [CrossRef] [Green Version]
- De Lamo Marin, S.; Xu, Y.; Meijler, M.M.; Janda, K.D. Antibody catalyzed hydrolysis of a quorum sensing signal found in Gram-negative bacteria. Bioorg. Med. Chem. Lett. 2007, 17, 1549–1552. [Google Scholar] [CrossRef]
- Piletska, E.V.; Stavroulakis, G.; Larcombe, L.D.; Whitcombe, M.J.; Sharma, A.; Primrose, S.; Robinson, G.K.; Piletsky, S.A. Passive control of quorum sensing: Prevention of Pseudomonas aeruginosa biofilm formation by imprinted polymers. Biomacromolecules 2011, 12, 1067–1071. [Google Scholar] [CrossRef]
- Magennis, E.P.; Fernandez-Trillo, F.; Sui, C.; Spain, S.G.; Bradshaw, D.J.; Churchley, D.; Mantovani, G.; Winzer, K.; Alexander, C. Bacteria-instructed synthesis of polymers for self-selective microbial binding and labelling. Nat. Mater. 2014, 13, 748–755. [Google Scholar] [CrossRef] [Green Version]
- Motib, A.; Guerreiro, A.; Al-Bayati, F.; Piletska, E.; Manzoor, I.; Shafeeq, S.; Kadam, A.; Kuipers, O.; Hiller, L.; Cowen, T.; et al. Modulation of Quorum Sensing in a Gram-Positive Pathogen by Linear Molecularly Imprinted Polymers with Anti-infective Properties. Ang. Chem. Int. Ed. 2017, 56, 16555–16558. [Google Scholar] [CrossRef] [PubMed]
- Geske, G.D.; O’Neill, J.C.; Blackwell, H.E. Expanding dialogues: From natural autoinducers to non-natural analogues that modulate quorum sensing in Gram-negative bacteria. Chem. Soc. Rev. 2008, 37, 1432–1447. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.M.; Queneau, Y.; Soulère, L.; von Bodman, S.; Doutheau, A. Mechanisms and synthetic modulators of AHL-dependent gene regulation. Chem. Rev. 2011, 111, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Majik, M.S.; Gawas, U.B.; Mandrekar, V.K. Next generation quorum sensing inhibitors: Accounts on structure activity relationship studies and biological activities. Bioorg. Med. Chem. 2020, 28, 115728. [Google Scholar] [CrossRef] [PubMed]
- Chbib, C. Impact of the structure-activity relationship of AHL analogues on quorum sensing in Gram-negative bacteria. Bioorg. Med. Chem. 2020, 28, 115282. [Google Scholar] [CrossRef]
- Chhabra, S.R.; Stead, P.; Bainton, N.J.; Salmond, G.P.; Stewart, G.S.; Williams, P.; Bycroft, B.W. Autoregulation of carbapenem biosynthesis in Erwinia carotovora by analogues of N-(3-oxohexanoyl)-L-homoserine lactone. J. Antibiot. 1993, 46, 441–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McInnis, C.E.; Blackwell, H.E. Thiolactone modulators of quorum sensing revealed through library design and screening. Bioorg. Med. Chem. 2011, 19, 4820–4828. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.; Gorgulla, C.; Boursier, M.E.; Rexrode, N.; Brown, E.C.; Arthanari, H.; Blackwell, H.E.; Nagarajan, R. N-Acyl Homoserine Lactone Analog Modulators of the Pseudomonas aeruginosa Rhll Quorum Sensing Signal Synthase. ACS Chem. Biol. 2019, 14, 2305–2314. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, C.T.; Miller, L.C.; Siryaporn, A.; Drescher, K.; Semmelhack, M.F.; Bassler, B.L. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc. Natl. Acad. Sci. USA 2013, 110, 17981–17986. [Google Scholar] [CrossRef] [Green Version]
- Boursier, M.E.; Combs, J.B.; Blackwell, H.E. N-Acyl l-Homocysteine Thiolactones Are Potent and Stable Synthetic Modulators of the RhlR Quorum Sensing Receptor in Pseudomonas aeruginosa. ACS Chem. Biol. 2019, 14, 186–191. [Google Scholar] [CrossRef]
- Smith, K.M.; Bu, Y.; Suga, H. Library screening for synthetic agonists and antagonists of a Pseudomonas aeruginosa autoinducer. Chem. Biol. 2003, 10, 563–571. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.; Zheng, Y.; Starks, R.; Opoku-Temeng, C.; Ma, X.; Sintim, H.O. 3-Aminooxazolidinone AHL analogs as hydrolytically-stable quorum sensing agonists in Gram-negative bacteria. MedChemComm 2015, 6, 1086–1092. [Google Scholar] [CrossRef] [Green Version]
- Biswas, N.N.; Kutty, S.K.; Barraud, N.; Iskander, G.M.; Griffith, R.; Rice, S.A.; Willcox, M.; Black, D.S.; Kumar, N. Indole-based novel small molecules for the modulation of bacterial signalling pathways. Org. Biomol. Chem. 2015, 13, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Manson, D.E.; O’Reilly, M.C.; Nyffeler, K.E.; Blackwell, H.E. Design, Synthesis, and Biochemical Characterization of Non-Native Antagonists of the Pseudomonas aeruginosa Quorum Sensing Receptor LasR with Nanomolar IC(50) Values. ACS Infect. Dis. 2020, 6, 649–661. [Google Scholar] [CrossRef]
- Fuentes-Gutiérrez, A.; Curiel-Quesada, E.; Correa-Basurto, J.; Martínez-Muñoz, A.; Reyes-Arellano, A. N-Heterocycles Scaffolds as Quorum Sensing Inhibitors. Design, Synthesis, Biological and Docking Studies. Int. J. Mol. Sci. 2020, 21, 9512. [Google Scholar] [CrossRef]
- Bucio-Cano, A.; Reyes-Arellano, A.; Correa-Basurto, J.; Bello, M.; Torres-Jaramillo, J.; Salgado-Zamora, H.; Curiel-Quesada, E.; Peralta-Cruz, J.; Avila-Sorrosa, A. Targeting quorum sensing by designing azoline derivatives to inhibit the N-hexanoyl homoserine lactone-receptor CviR: Synthesis as well as biological and theoretical evaluations. Bioorg. Med. Chem. 2015, 23, 7565–7577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes-Arellano, A.; Bucio-Cano, A.; Montenegro-Sustaita, M.; Curiel-Quesada, E.; Salgado-Zamora, H. Imidazolines as non-classical bioisosteres of N-acyl homoserine lactones and quorum sensing inhibitors. Int. J. Mol. Sci. 2012, 13, 1284–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deryabin, D.G.; Galadzhieva, A.A.; Duskaev, G.K. Screening of N-Hexanamide and 2H-1,3-Benzodioxol Derivatives for Quorum Sensing Modulation in Chromobacterium violaceum. Microbiology 2020, 89, 733–739. [Google Scholar] [CrossRef]
- Müh, U.; Schuster, M.; Heim, R.; Singh, A.; Olson, E.R.; Greenberg, E.P. Novel Pseudomonas aeruginosa quorum-sensing inhibitors identified in an ultra-high-throughput screen. Antimicrob. Agents Chemother. 2006, 50, 3674–3679. [Google Scholar] [CrossRef] [Green Version]
- Mamani, S.; Moinier, D.; Denis, Y.; Soulere, L.; Queneau, Y.; Talla, E.; Bonnefoy, V.; Guiliani, N. Insights into the Quorum Sensing Regulon of the Acidophilic Acidithiobacillus ferrooxidans Revealed by Transcriptomic in the Presence of an Acyl Homoserine Lactone Superagonist Analog. Front. Microbiol. 2016, 7, 19. [Google Scholar] [CrossRef]
- Brackman, G.; Risseeuw, M.; Celen, S.; Cos, P.; Maes, L.; Nelis, H.J.; Van Calenbergh, S.; Coenye, T. Synthesis and evaluation of the quorum sensing inhibitory effect of substituted triazolyldihydrofuranones. Bioorg. Med. Chem. 2012, 20, 4737–4743. [Google Scholar] [CrossRef]
- Hansen, M.R.; Jakobsen, T.H.; Bang, C.G.; Cohrt, A.E.; Hansen, C.L.; Clausen, J.W.; Le Quement, S.T.; Tolker-Nielsen, T.; Givskov, M.; Nielsen, T.E. Triazole-containing N-acyl homoserine lactones targeting the quorum sensing system in Pseudomonas aeruginosa. Bioorg. Med. Chem. 2015, 23, 1638–1650. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.Z.; Queneau, Y.; Soulère, L. Synthesis of new 1,4- and 1,5-disubstituted N-ethyl acetate and N-α-butyro-γ-lactone alkylimidazole derivatives as N-acylhomoserine lactones analogues. J. Heterocycl. Chem. 2021, in press. [Google Scholar]
- Stacy, D.M.; Le Quement, S.T.; Hansen, C.L.; Clausen, J.W.; Tolker-Nielsen, T.; Brummond, J.W.; Givskov, M.; Nielsen, T.E.; Blackwell, H.E. Synthesis and biological evaluation of triazole-containing N-acyl homoserine lactones as quorum sensing modulators. Org. Biomol. Chem. 2013, 11, 938–954. [Google Scholar] [CrossRef] [Green Version]
- Ren, D.; Zuo, R.; Wood, T.K. Quorum-sensing antagonist (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone influences siderophore biosynthesis in Pseudomonas putida and Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2005, 66, 689–695. [Google Scholar] [CrossRef]
- Markus, V.; Golberg, K.; Teralı, K.; Ozer, N.; Kramarsky-Winter, E.; Marks, R.S.; Kushmaro, A. Assessing the Molecular Targets and Mode of Action of Furanone C-30 on Pseudomonas aeruginosa Quorum Sensing. Molecules 2021, 26, 1620. [Google Scholar] [CrossRef]
- Rossi, R.; Lessi, M.; Manzini, C.; Marianetti, G.; Bellina, F. Synthesis and Biological Properties of 2(5H)-Furanones Featuring Bromine Atoms on the Heterocyclic Ring and/or Brominated Substituents. Curr. Org. Chem. 2017, 21, 964–1018. [Google Scholar] [CrossRef]
- Chang, Y.; Wang, P.C.; Ma, H.M.; Chen, S.Y.; Fu, Y.H.; Liu, Y.Y.; Wang, X.; Yu, G.C.; Huang, T.; Hibbs, D.E.; et al. Design, synthesis and evaluation of halogenated furanone derivatives as quorum sensing inhibitors in Pseudomonas aeruginosa. Eur. J. Pharm. Sci. 2019, 140, 105058. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, P.; Qin, Y.; Zhang, N.; Teng, Y.; Venter, H.; Ma, S. Design and synthesis of aryl-substituted pyrrolidone derivatives as quorum sensing inhibitors. Bioorg. Chem. 2020, 105, 104376. [Google Scholar] [CrossRef]
- Goh, W.K.; Gardner, C.R.; Chandra Sekhar, K.V.; Biswas, N.N.; Nizalapur, S.; Rice, S.A.; Willcox, M.; Black, D.S.; Kumar, N. Synthesis, quorum sensing inhibition and docking studies of 1,5-dihydropyrrol-2-ones. Bioorg. Med. Chem. 2015, 23, 7366–7377. [Google Scholar] [CrossRef]
- Estephane, J.; Dauvergne, J.; Soulère, L.; Reverchon, S.; Queneau, Y.; Doutheau, A. N-Acyl-3-amino-5H-furanone derivatives as new inhibitors of LuxR-dependent quorum sensing: Synthesis, biological evaluation and binding mode study. Bioorg. Med. Chem. Lett. 2008, 18, 4321–4324. [Google Scholar] [CrossRef]
- Soulère, L.; Sabbah, M.; Fontaine, F.; Queneau, Y.; Doutheau, A. LuxR-dependent quorum sensing: Computer aided discovery of new inhibitors structurally unrelated to N-acylhomoserine lactones. Bioorg. Med. Chem. Lett. 2010, 20, 4355–4358. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, M.; Soulère, L.; Reverchon, S.; Queneau, Y.; Doutheau, A. LuxR dependent quorum sensing inhibition by N,N’-disubstituted imidazolium salts. Bioorg. Med. Chem. 2011, 19, 4868–4875. [Google Scholar] [CrossRef] [PubMed]
- Des Essarts, Y.R.; Sabbah, M.; Comte, A.; Soulère, L.; Queneau, Y.; Dessaux, Y.; Hélias, V.; Faure, D. N,N’-alkylated Imidazolium-derivatives act as quorum-sensing inhibitors targeting the Pectobacterium atrosepticum-induced symptoms on potato tubers. Int. J. Mol. Sci. 2013, 14, 19976–19986. [Google Scholar] [CrossRef] [PubMed] [Green Version]




























| Bacteria | AHL Signal | LuxI/R | QS Phenotype |
|---|---|---|---|
| Vibrio fischeri |  (OHHL) | LuxI/R | luminescence |
| Vibrio harveyi |  (3-OH-BHL) | LuxM/N | virulence |
| Pseudomonas aeruginosa |  (OdDHL) | LasI/R; QscR | virulence, biofilm formation |
| Agrobacteriumfabrum C58 |  (OOHL) | TraI/R | Ti plasmid transfer |
| Erwinia carotovora |  (OHHL) | ExpI/R; CarI/R | antibiotic production |
| Serratia liquefaciens |  (BHL) | SwrI/R | antibiotic production |
| Chromobacterium violaceum |  (HHL) | CviI/R | cyanide, pigment |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Li, S.; Hachicha, M.; Boukraa, M.; Soulère, L.; Efrit, M.L.; Queneau, Y. Heterocyclic Chemistry Applied to the Design of N-Acyl Homoserine Lactone Analogues as Bacterial Quorum Sensing Signals Mimics. Molecules 2021, 26, 5135. https://doi.org/10.3390/molecules26175135
Zhang Q, Li S, Hachicha M, Boukraa M, Soulère L, Efrit ML, Queneau Y. Heterocyclic Chemistry Applied to the Design of N-Acyl Homoserine Lactone Analogues as Bacterial Quorum Sensing Signals Mimics. Molecules. 2021; 26(17):5135. https://doi.org/10.3390/molecules26175135
Chicago/Turabian StyleZhang, Qiang, Sizhe Li, Maha Hachicha, Mohamed Boukraa, Laurent Soulère, Mohamed L. Efrit, and Yves Queneau. 2021. "Heterocyclic Chemistry Applied to the Design of N-Acyl Homoserine Lactone Analogues as Bacterial Quorum Sensing Signals Mimics" Molecules 26, no. 17: 5135. https://doi.org/10.3390/molecules26175135