Cinnamal Sensing and Luminescence Color Tuning in a Series of Rare-Earth Metal−Organic Frameworks with Trans-1,4-cyclohexanedicarboxylate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Crystal Structure
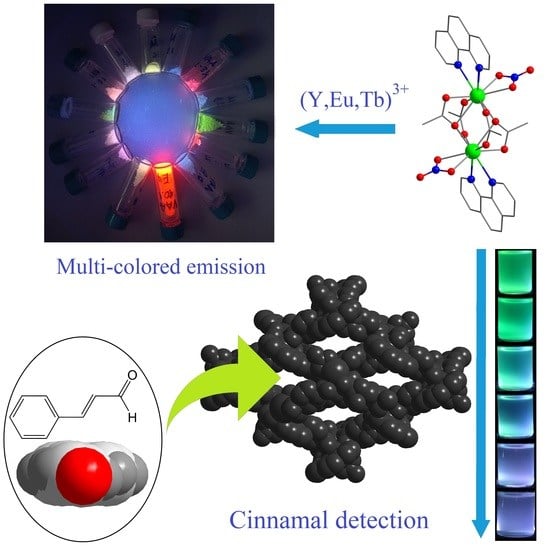
2.2. Luminescent Properties
2.3. Luminescent Sensing
3. Experimental Section
3.1. Reagents
3.2. Instruments
3.3. Synthetic Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Hanna, S.L.; Redfern, L.R.; Alezi, D.; Islamoglu, T.; Farha, O.K. Reticular chemistry in the rational synthesis of functional zirconium cluster-based MOFs. Coord. Chem. Rev. 2019, 386, 32–49. [Google Scholar] [CrossRef]
- Yaghi, O.M. Reticular chemistry in all dimensions. ACS Cent. Sci. 2019, 5, 1295–1300. [Google Scholar] [CrossRef] [Green Version]
- Ali Akbar Razavi, S.; Morsali, A. Linker functionalized metal-organic frameworks. Coord. Chem. Rev. 2019, 399, 213023. [Google Scholar] [CrossRef]
- Samanidou, V.F.; Deliyanni, E.A. Metal organic frameworks: Synthesis and application. Molecules 2020, 25, 960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, S.L.; Champness, N.R. A periodic table of metal-organic frameworks. Coord. Chem. Rev. 2020, 414, 213295. [Google Scholar] [CrossRef]
- Raptopoulou, C.P. Metal-organic frameworks: Synthetic methods and potential applications. Materials 2021, 14, 310. [Google Scholar] [CrossRef]
- Velasco, E.; Osumi, Y.; Teat, S.J.; Jensen, S.; Tan, K.; Thonhauser, T.; Li, J. fluorescent detection of carbon disulfide by a highly emissive and robust isoreticular series of zr-based luminescent metal organic frameworks (LMOFs). Chemistry 2021, 3, 327–337. [Google Scholar] [CrossRef]
- Barsukova, M.O.; Sapchenko, S.A.; Dybtsev, D.N.; Fedin, V.P. Scandium-organic frameworks: Progress and prospects. Russ. Chem. Rev. 2018, 87, 1139–1167. [Google Scholar] [CrossRef]
- Thorarinsdottir, A.E.; Harris, T.D. Metal–organic framework magnets. Chem. Rev. 2020, 120, 8716–8789. [Google Scholar] [CrossRef] [PubMed]
- Garai, B.; Bon, V.; Krause, S.; Schwotzer, F.; Gerlach, M.; Senkovska, I.; Kaskel, S. Tunable flexibility and porosity of the metal–organic framework dut-49 through postsynthetic metal exchange. Chem. Mater. 2020, 32, 889–896. [Google Scholar] [CrossRef]
- Demakov, P.A.; Bogomyakov, A.S.; Urlukov, A.S.; Andreeva, A.Y.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Transitio metal coordination polymers with trans-1,4-cyclohexanedicarboxylate: Acidity-controlled synthesis, structures and properties. Materials 2020, 13, 486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubskikh, V.A.; Lysova, A.A.; Samsonenko, D.G.; Lavrov, A.N.; Kovalenko, K.A.; Dybtsev, D.N.; Fedin, V.P. 3D metal-organic frameworks based on Co(II) and bithiophendicarboxylate: Synthesis, crystal structures, gas adsorption, and magnetic properties. Molecules 2021, 526, 1269. [Google Scholar] [CrossRef] [PubMed]
- Nonat, A.M.; Charbonnière, L. Upconversion of light with molecular and supramolecular lanthanide complexes. Coord. Chem. Rev. 2020, 409, 213192. [Google Scholar] [CrossRef]
- Saraci, F.; Quezada-Novoa, V.; Rafael Donnarumma, P.; Howarth, A.J. Rare-earth metal–organic frameworks: From structure to applications. Chem. Soc. Rev. 2020, 49, 7949–7977. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-P.; Li, D.-S.; Xia, W.; Guo, S.-S.; Dong, W.-W. Three novel lanthanide metal-organic frameworks (Ln-MOFs) constructed by unsymmetrical aromatic dicarboxylatic tectonics: Synthesis, crystal structures and luminescent properties. Molecules 2014, 19, 14352–14365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mironova, O.A.; Ryadun, A.A.; Sukhikh, T.S.; Konchenko, S.N.; Pushkarevsky, N.A. Synthesis and luminescence studies of lanthanide complexes (Gd, Tb, Dy) with phenyl- and 2-pyridylthiolates supported by a bulky β-diketiminate ligand. Impact of the ligand environment on terbium(iii) emission. New J. Chem. 2020, 44, 19769–19779. [Google Scholar] [CrossRef]
- Belousov, Y.A.; Drozdov, A.A.; Taydakov, I.V.; Marchetti, F.; Pettinari, R.; Pettinari, C. Lanthanide azolecarboxylate compounds: Structure, luminescent properties and applications. Coord. Chem. Rev. 2021, 445, 214084. [Google Scholar] [CrossRef]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal–organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef]
- Zhao, S.-N.; Wang, G.; Poelman, D.; Van Der Voort, P. Luminescent lanthanide MOFs: A unique platform for chemical sensing. Materials 2018, 11, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, Y.; Chen, S.; Zhou, Y.; Zhang, Y.; Xu, M. Recent progress in metal–organic framework (MOF) based luminescent chemodosimeters. Nanomaterials 2019, 9, 974. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.-J.; Liu, W.-S. Effective luminescence sensing of Fe3+, Cr2O72−, MnO4− and 4-nitrophenol by lanthanide metal–organic frameworks with a new topology type. Dalton Trans. 2019, 48, 12287–12295. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Z.Z.; Liang, X.Y.; Zhang, X.L.; Jia, Y.J.; Hu, M. A water-stable europium-MOF as a multifunctional luminescent sensor for some trivalent metal ions (Fe3+, Cr3+, Al3+), PO43− ions, and nitroaromatic explosives. Dalton Trans. 2019, 48, 1786–1794. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Y.; Zhu, M.; Kosinova, M.; Fedin, V.P.; Gao, E. Three coordination polymers with regulated coordination interactions as fluorescent sensors for monitoring purine metabolite uric acid. Dalton Trans. 2020, 49, 4343–4351. [Google Scholar] [CrossRef]
- Wang, T.T.; Liu, J.-Y.; Guo, R.; An, J.-D.; Huo, J.-Z.; Liu, Y.-Y.; Shi, W.; Ding, B. Solvothermal Preparation of a Lanthanide Metal-Organic Framework for Highly Sensitive Discrimination of Nitrofurantoin and l-Tyrosine. Molecules 2021, 26, 3673. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, M.; Chen, L.; Li, Z.; Li, S.; Jiang, F.; Hong, M. Construction of a stable lanthanide metal-organic framework as a luminescent probe for rapid naked-eye recognition of Fe3+ and acetone. Molecules 2021, 26, 1695. [Google Scholar] [CrossRef]
- Gill, A.O.; Holley, R.A. Mechanisms of bactericidal action of cinnamaldehyde against listeria monocytogenes and of eugenol against l. monocytogenes and lactobacillus sakei. Appl. Environ. Microbiol. 2004, 70, 5750–5755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Bayati, F.A.; Mohammed, M.J. Isolation, identification, and purification of cinnamaldehyde from Cinnamomum zeylanicum bark oil. An antibacterial study. Pharm. Biol. 2009, 47, 61–66. [Google Scholar] [CrossRef]
- Zaio, Y.P.; Gatti, G.; Ponce, A.A.; Saavedra Larralde, N.A.; Martinez, M.J.; Zunino, M.P.; Zygadlo, J.A. Cinnamaldehyde and related phenylpropanoids, natural repellents, and insecticides against Sitophilus zeamais (Motsch.). A chemical structure-bioactivity relationship. J. Sci. Food Agric. 2015, 98, 5822–5831. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, K.; Yang, H.; Yuan, Y.; Yue, T. Antifungal mechanism of cinnamaldehyde and citral combination against Penicillium expansum based on FT-IR fingerprint, plasma membrane, oxidative stress and volatile profile. RSC Adv. 2018, 8, 5806–5815. [Google Scholar] [CrossRef] [Green Version]
- OuYang, Q.; Duan, X.; Li, L.; Tao, N. Cinnamaldehyde exerts its antifungal activity by disrupting the cell wall integrity of geotrichum citri-aurantii. Front. Microbiol. 2019, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Yang, K.; Chen, L.; Liu, M.; Geng, Q.; He, X.; Li, Y.; Liu, Y.; Tian, J. Cinnamaldehyde, a promising natural preservative against aspergillus flavus. Front. Microbiol. 2019, 10, 2895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaac-Renton, M.; Li, M.K.; Parsons, L.M. Cinnamon spice and everything not nice. Dermatitis 2015, 26, 116–121. [Google Scholar] [CrossRef]
- Friedman, M.; Kozukue, N.; Harden, L.A. Cinnamaldehyde content in foods determined by gas chromatography−mass spectrometry. J. Agric. Food Chem. 2000, 48, 5702–5709. [Google Scholar] [CrossRef]
- Wang, Y.; Ocariz, J.; Hammersand, J.; MacDonald, E.; Bartczak, A.; Kero, F.; Young, V.Y.; Williams, K.R. Determination of cinnamaldehyde in cinnamon by SPME–GC–MS. An instrumental analysis experiment. J. Chem. Educ. 2008, 85, 957–958. [Google Scholar] [CrossRef]
- Gopu, C.L.; Aher, S.; Mehta, H.; Paradkar, A.R.; Mahadik, K.R. Simultaneous determination of cinnamaldehyde, eugenol and piperine by HPTLC densitometric method. Phytochem. Anal. 2008, 19, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Gursale, A.; Dighe, V.; Parekh, G. simultaneous quantitative determination of cinnamaldehyde and methyl eugenol from stem bark of cinnamomum zeylanicum blume using RP-HPLC. J. Chromatogr. Sci. 2010, 48, 59–62. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.-J.; Lee, J.-H.; Kim, J.-H.; Choi, G.-H.; Cho, N.-J.; Park, B.-J. Quantitative analysis of cinnamaldehyde, cinnamylalcohol and salicylaldehyde in commercial biopesticides containing cinnamon extract using gas chromatography-flame ionization detector. Korean J. Environ. Agric. 2014, 33, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Hoi, J.K.; Lieder, B.; Pignitter, M.; Hans, J.; Ley, J.P.; Lietard, J.; Hoelz, K.; Somoza, M.; Somoza, V. Identification of cinnamaldehyde as most effective fatty acid uptake reducing cinnamon-derived compound in differentiated caco-2 cells compared to its structural analogues cinnamyl alcohol, cinnamic acid, and cinnamyl isobutyrate. J. Agric. Food Chem. 2019, 67, 11638–11649. [Google Scholar] [CrossRef]
- Foudah, A.I.; Shakeel, F.; Alqarni, M.H.; Ross, S.A.; Salkini, M.A.; Alam, P. Simultaneous estimation of cinnamaldehyde and eugenol in essential oils and traditional and ultrasound-assisted extracts of different species of cinnamon using a sustainable/green HPTLC technique. Molecules 2021, 26, 2054. [Google Scholar] [CrossRef]
- Konar, S.; Samanta, D.; Mandal, S.; Das, S.; Kr Mahto, M.; Shaw, M.; Mandald, M.; Pathak, A. selective and sensitive detection of cinnamaldehyde by nitrogen and sulphur co-doped carbon dots: A detailed systematic study. RSC Adv. 2018, 8, 42361–42373. [Google Scholar] [CrossRef] [Green Version]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The cambridge structural database. Acta Crystallogr. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Huang, X.; Sun, H.; Dou, J.; Li, D.; Wang, D.; Liu, G. A dimeric luminescent lanthanide complex [Eu(PAA)2(phen)(NO3)]2: Hydrothermal synthesis, crystal structure and fluorescence. J. Coord. Chem. 2007, 60, 2045–2050. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Fan, J.; Zhang, W.-G. Studies of radii-dependent lanthanide coordination behavior with 4-acetamidobenzoate and 1,10-phenanthroline. Z. Anorg. Allg. Chem. 2009, 635, 2333–2339. [Google Scholar] [CrossRef]
- Yin, X.; Fan, J.; Wang, Z.H.; Zheng, S.R.; Tan, J.B.; Zhang, W.G. Luminescent lanthanide complexes with 4-acetamidobenzoate: Synthesis, supramolecular assembly via hydrogen bonds, crystal structures and photoluminescence. J. Solid State Chem. 2011, 184, 1850–1857. [Google Scholar] [CrossRef]
- Lu, Y.-B.; Jiang, X.-M.; Zhu, S.-D.; Du, Z.-Y.; Liu, C.-M.; Xie, Y.-R.; Liu, L.-X. Anion effects on lanthanide(III) tetrazole-1-acetate dinuclear complexes showing slow magnetic relaxation and photofluorescent emission. Inorg. Chem. 2016, 55, 3738–3749. [Google Scholar] [CrossRef] [PubMed]
- Casanovas, B.; Font-Bardía, M.; Speed, S.; Salah El Fallah, M.; Vicente, R. Field-induced SMM and visible/NIR-luminescence behaviour of dinuclear lnIII complexes with 2-fluorobenzoate. Eur. J. Inorg. Chem. 2018, 2018, 1928–1937. [Google Scholar] [CrossRef]
- Demakov, P.A.; Ryadun, A.A.; Dorovatovskii, P.V.; Lazarenko, V.A.; Samsonenko, D.G.; Brylev, K.A.; Fedin, V.P.; Dybtsev, D.N. Intense multi-colored luminescence in a series of rare-earth metal-organic frameworks with aliphatic linkers. Dalton Trans. 2021. [Google Scholar] [CrossRef] [PubMed]
- Demakov, P.A.; Ryadun, A.A.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Structure and luminescent properties of europium(iii) coordination polymers with thiophene ligands. J. Struct. Chem. 2020, 61, 1965–1974. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef]
- Pan, M.; Liao, W.-M.; Yin, S.-Y.; Sun, S.-S.; Su, C.-Y. Single-phase white-light-emitting and photoluminescent color-tuning coordination assemblies. Chem. Rev. 2018, 118, 8889–8935. [Google Scholar] [CrossRef]
- Margariti, A.; Pournara, A.D.; Manos, M.J.; Lazarides, T.; Papaefstathiou, G.S. Towards white-light emission by Tb3+/Eu3+ substitution in a Ca2+ framework. Polyhedron 2018, 153, 24–30. [Google Scholar] [CrossRef]
- Barsukova, M.O.; Cherezova, S.V.; Sapianik, A.A.; Lundovskaya, O.V.; Samsonenko, D.G.; Fedin, V.P. Lanthanide contraction effect and white-emitting luminescence in a series of metal–organic frameworks based on 2,5-pyrazinedicarboxylic acid. RSC Adv. 2020, 10, 38252–38259. [Google Scholar] [CrossRef]
- Vidyakina, A.A.; Kolesnikov, I.E.; Bogachev, N.A.; Skripkin, M.Y.; Tumkin, I.I.; Lähderanta, E.; Mereshchenko, A.S. Gd3+-Doping Effect on Upconversion Emission of NaYF4: Yb3+, Er3+/Tm3+ Microparticles. Materials 2020, 13, 3397. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.-W.; Dong, G.; Cui, R.; Li, X. 3D lanthanide-coordination frameworks constructed by a ternary mixed-ligand: Crystal structure, luminescence and luminescence sensing. CrystEngComm 2020, 22, 740–750. [Google Scholar] [CrossRef]
- Gontcharenko, V.E.; Kiskin, M.A.; Dolzhenko, V.D.; Korshunov, V.M.; Taydakov, I.V.; Belousov, Y.A. Mono- and mixed metal complexes of Eu3+, Gd3+, and Tb3+ with a diketone, bearing pyrazole moiety and CHF2-group: Structure, color tuning, and kinetics of energy transfer between lanthanide ions. Molecules 2021, 26, 2655. [Google Scholar] [CrossRef]
- Green, A.P.; Buckley, A.R. Solid state concentration quenching of organic fluorophores in PMMA. Phys. Chem. Chem. Phys. 2015, 17, 1435–1440. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Park, S.-R.; Suh, M.C. Concentration quenching behavior of thermally activated delayed fluorescence in a solid film. J. Phys. Chem. C 2017, 121, 13986–13997. [Google Scholar] [CrossRef]
- Sapianik, A.A.; Kiskin, M.A.; Samsonenko, D.G.; Ryadun, A.A.; Dybtsev, D.N.; Fedin, V.P. Luminescent detection by coordination polymers derived from a pre-organized heterometallic carboxylic building unit. Polyhedron 2018, 145, 147–153. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, Q.; Zhang, G. A carbazole-functionalized metal–organic framework for efficient detection of antibiotics, pesticides and nitroaromatic compounds. Dalton Trans. 2019, 48, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Sapianik, A.A.; Kiskin, M.A.; Kovalenko, K.A.; Samsonenko, D.G.; Dybtsev, D.N.; Audebrand, N.; Sun, Y.; Fedin, V.P. Rational synthesis and dimensionality tuning of MOFs from preorganized heterometallic molecular complexes. Dalton Trans. 2019, 48, 3676–3686. [Google Scholar] [CrossRef] [PubMed]
- Gao, E.; Wu, S.; Wang, J.; Zhu, M.; Zhang, Y.; Fedin, V.P. Water-stable lanthanide coordination polymers with triple luminescent centers for tunable emission and efficient self-calibration sensing wastewater pollutants. Adv. Opt. Mater. 2020, 8, 1901659. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Matveevskaya, M.; Pavlov, D.; Yakunenkov, A.; Potapov, A. Coordination polymers based on highly emissive ligands: Synthesis and functional properties. Materials 2020, 13, 2699. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, X.; Gao, E.; Wu, S.; Zhang, Y.; Zhu, M. Bifunctional luminescent Eu metal–organic framework for sensing nitroaromatic pollutants and Fe3+ ion with high sensitivity and selectivity. Appl. Organomet. Chem. 2021, 35, e6136. [Google Scholar] [CrossRef]
- Huangfu, M.; Wang, M.; Lin, C.; Wang, J.; Wu, P. Luminescent metal–organic frameworks as chemical sensors based on “mechanism–response”: A review. Dalton Trans. 2021, 50, 3429–3449. [Google Scholar] [CrossRef]
- Svetogorov, R.D.; Dorovatovskii, P.V.; Lazarenko, V.A. Belok/XSA Diffraction beamline for studying crystalline samples at kurchatov synchrotron radiation source. Cryst. Res. Technol. 2020, 55, 1900184. [Google Scholar] [CrossRef]
- Lazarenko, V.A.; Dorovatovskii, P.V.; Zubavichus, Y.V.; Burlov, A.S.; Koshchienko, Y.V.; Vlasenko, V.G.; Khrustalev, V.N. High-throughput small-molecule crystallography at the ‘belok’ beamline of the kurchatov synchrotron radiation source: Transition metal complexes with azomethine ligands as a case study. Crystals 2017, 7, 325. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demakov, P.A.; Vasileva, A.A.; Volynkin, S.S.; Ryadun, A.A.; Samsonenko, D.G.; Fedin, V.P.; Dybtsev, D.N. Cinnamal Sensing and Luminescence Color Tuning in a Series of Rare-Earth Metal−Organic Frameworks with Trans-1,4-cyclohexanedicarboxylate. Molecules 2021, 26, 5145. https://doi.org/10.3390/molecules26175145
Demakov PA, Vasileva AA, Volynkin SS, Ryadun AA, Samsonenko DG, Fedin VP, Dybtsev DN. Cinnamal Sensing and Luminescence Color Tuning in a Series of Rare-Earth Metal−Organic Frameworks with Trans-1,4-cyclohexanedicarboxylate. Molecules. 2021; 26(17):5145. https://doi.org/10.3390/molecules26175145
Chicago/Turabian StyleDemakov, Pavel A., Alena A. Vasileva, Sergey S. Volynkin, Alexey A. Ryadun, Denis G. Samsonenko, Vladimir P. Fedin, and Danil N. Dybtsev. 2021. "Cinnamal Sensing and Luminescence Color Tuning in a Series of Rare-Earth Metal−Organic Frameworks with Trans-1,4-cyclohexanedicarboxylate" Molecules 26, no. 17: 5145. https://doi.org/10.3390/molecules26175145
APA StyleDemakov, P. A., Vasileva, A. A., Volynkin, S. S., Ryadun, A. A., Samsonenko, D. G., Fedin, V. P., & Dybtsev, D. N. (2021). Cinnamal Sensing and Luminescence Color Tuning in a Series of Rare-Earth Metal−Organic Frameworks with Trans-1,4-cyclohexanedicarboxylate. Molecules, 26(17), 5145. https://doi.org/10.3390/molecules26175145