Structure and Properties of Electrochemically Synthesized Silver Nanoparticles in Aqueous Solution by High-Resolution Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. AgNPs Preparation
2.2. Zeta Potential and Oxidation Reduction Potential (ORP) Measurements
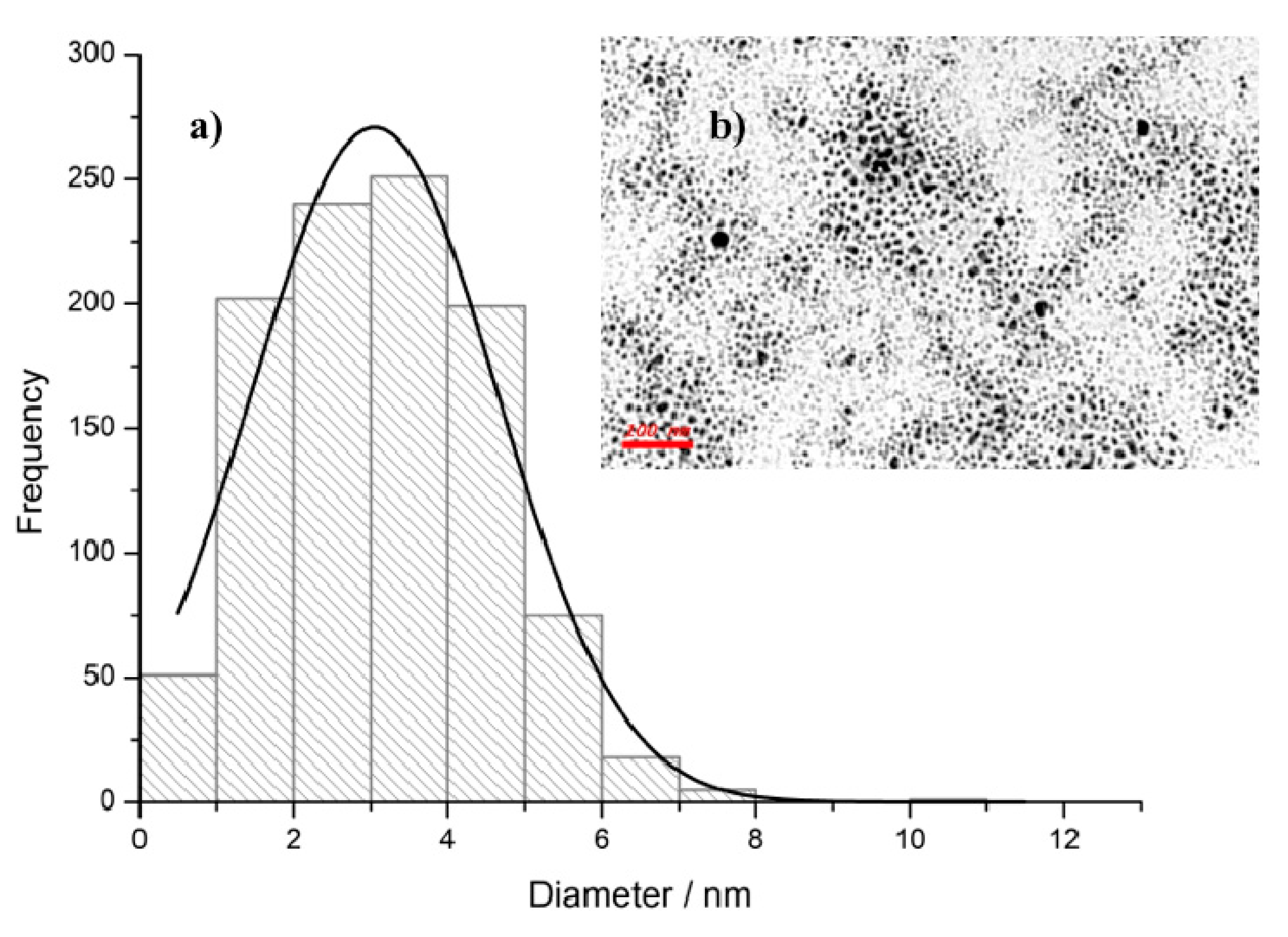
2.3. TEM and SEM Analysis
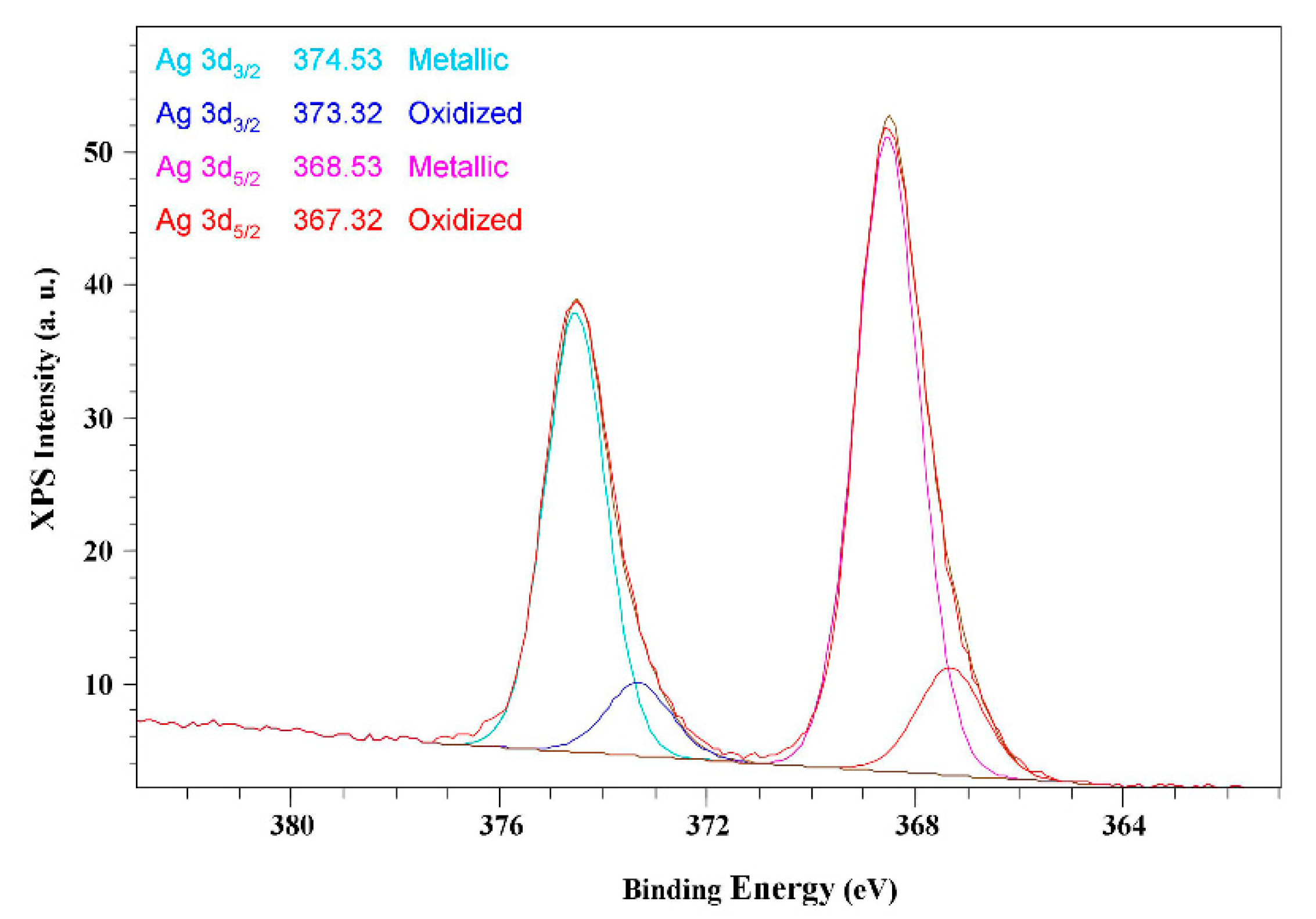
2.4. XPS and XRPD Analysis
2.5. MALDI-TOF Measurements
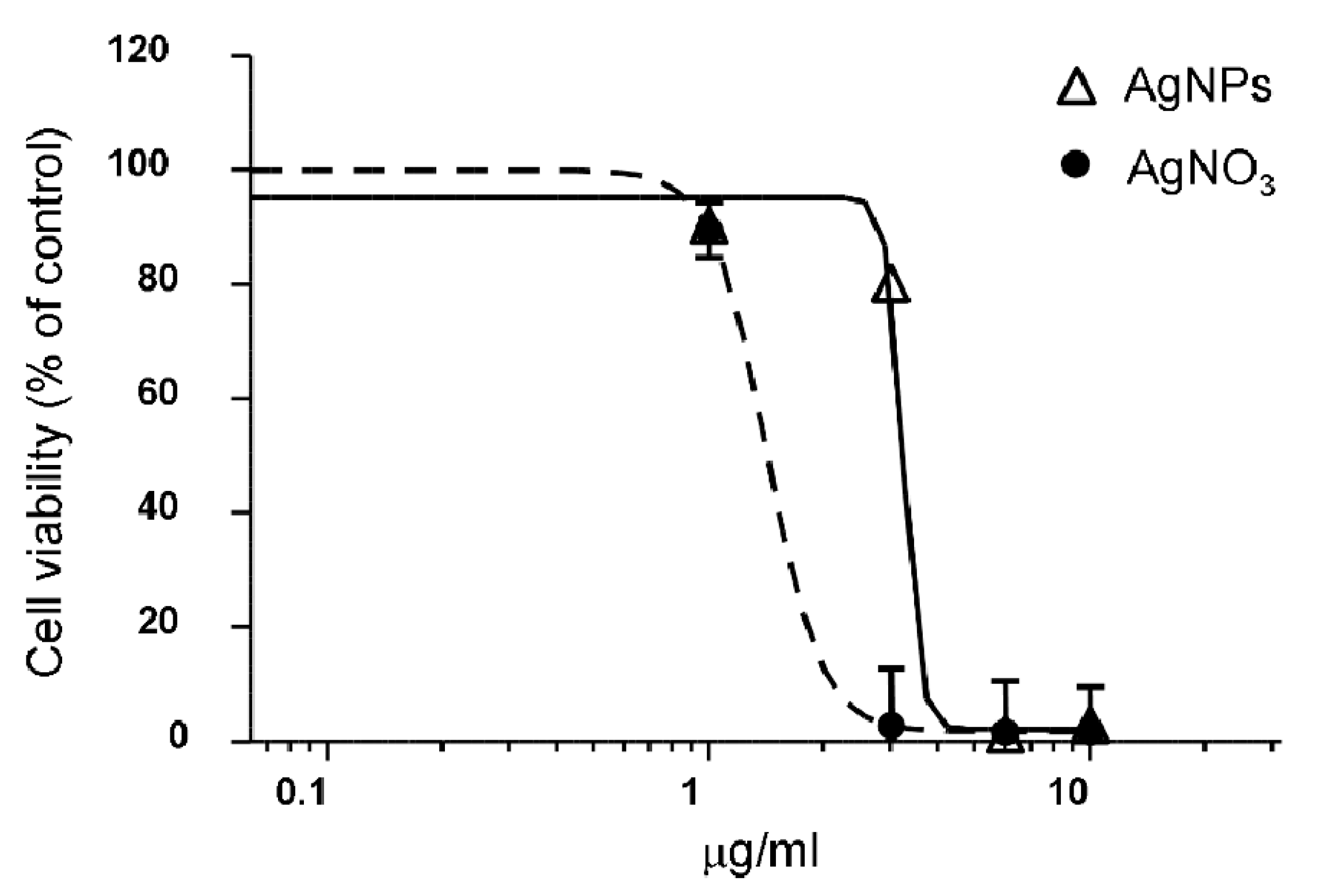
2.6. Cytotoxicity Assay
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Nowack, B.; Krug, H.F.; Height, M. 120 years of nanosilver history: Implications for policy makers. Environ. Sci. Technol. 2011, 45, 1177–1183. [Google Scholar] [CrossRef]
- Ghosh, K.; Maiti, S.N. Mechanical properties of silver-powder-filled polypropylene composites. J. Appl. Polym. Sci. 1996, 60, 323–331. [Google Scholar] [CrossRef]
- Krutyakov, Y.A.; Kudrinskiy, A.A.; Olenin, A.Y.; Lisichkin, G.V. Synthesis and properties of silver nanoparticles: Advances and prospects. Russ. Chem. Rev. 2008, 77, 233–257. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Khan, A.U.; Wei, Y.; Ahmad, A.; Khan, Z.U.H.; Tahir, K.; Khan, S.U.; Muhammad, N.; Khan, F.U.; Yuan, Q. Enzymatic browning reduction in white cabbage, potent antibacterial and antioxidant activities of biogenic silver nanoparticles. J. Mol. Liq. 2016, 215, 39–46. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle and metal. Angew. Chem. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Emam, H.E.; Ahamed, H.B. Polysaccharides templetes for assembly of nanosilver. Carbohydr. Polym. 2016, 135, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Ju, J.; Liu, W.; Perlaki, C.; Chen, K.; Feng, C.; Liu, Q. Sustained and Cost Effective Silver Substrate for Surface Enhanced Raman Spectroscopy Based Biosensing. Sci. Rep. 2017, 7, 6917. [Google Scholar] [CrossRef] [Green Version]
- Pompilio, A.; Geminiani, C.; Bosco, D.; Rana, R.; Aceto, A.; Bucciarelli, T.; Scotti, L.; di Bonaventura, G. Electrochemically synthesized silver nanoparticles are active against planktonic and biofilm cells of Pseudomonas aeruginosa and other cystic fibrosis-associated bacterial pathogens. Front. Microbiol. 2018, 9, 1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelini, G.; Scotti, L.; Aceto, A.; Gasbarri, C. Silver Nanoparticles as interactive media for the azobenzenes isomerization in aqueous solution: From linear to stretched kinetics. J. Mol. Liq. 2019, 284, 592–598. [Google Scholar] [CrossRef]
- Scott, R.W.J.; Wilson, O.M.; Crooks, R.M. Synthesis, characterization, and applications of dendrimer-encapsulated nanoparticles. J. Phys. Chem. B 2005, 109, 692–704. [Google Scholar] [CrossRef]
- Xia, Y.; Tang, Z. Monodisperse inorganic supraparticles: Formation mechanism, properties and applications. Chem. Comm. 2012, 48, 6320–6336. [Google Scholar] [CrossRef] [PubMed]
- Panacek, A.; Kvitek, L.; Prucek, R.; Kolar, M.; Vecerova, R.; Pizurova, N.; Sharma, V.K.; Nevecna, T.J.; Zboril, R. Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef] [PubMed]
- Bonnemann, H.; Richards, R.M. Nanoscopic metal particles synthetic methods and potential applications. Eur. J. Inorg. Chem. 2001, 10, 2455–2480. [Google Scholar] [CrossRef]
- Lee, I.; Han, S.W.; Kim, K. Simultaneous preparation of SERS-active metal colloids and plates by laser ablation. J. Raman Spectrosc. 2001, 32, 947–952. [Google Scholar] [CrossRef]
- Long, D.W.; Wu, G.Z.; Chen, S.M. Preparation of oligochitosan stabilized silver nanoparticles by gamma irradiation. Radiat. Phys.Chem. 2007, 76, 1126–1131. [Google Scholar] [CrossRef]
- Titova, V.N.; Kazakov, V.A.; Petrova, N.V.; Białłozor, S. The influence of the solvent on the kinetics of silver electrodeposition. J. Electroanal. Chem. 1995, 381, 227–230. [Google Scholar] [CrossRef]
- Zaky, A.M.; Assaf, F.H.; el Rehim, S.S.A.; Mohamed, B.M. Electrochemical behaviour of silver in borate buffer solutions. Appl. Surf. Sci. 2004, 221, 349–357. [Google Scholar] [CrossRef]
- Rodrigues-Sanchez, L.; Blanko, M.L.; Lopez-Quintela, M.A. Electrochemical synthesis of silver nanoparticles. J. Phys. Chem. 2000, 104, 9683–9688. [Google Scholar] [CrossRef]
- Gracheva, E.; Kreizberg, G.N.; Golikov, I.V. Electrochemical synthesis of silver nanoparticles. FEN-Nauka 2011, 1, 7–9. [Google Scholar]
- Shmigel, A.V.; Tikhonov, P.A.; Arsent’ev, M.Y.; Pugachev, K.E. Electrochemical fabrication and studies of metal silver nanoparticles. Glass Phys. Chem. 2015, 41, 329–333. [Google Scholar] [CrossRef]
- Khaydarov, R.A.; Khaydarov, R.R.; Gapurova, O.; Estrin, Y.; Scheper, T. Electrochemical method for the synthesis of silver nanoparticles. J. Nanopart. Res. 2009, 11, 1193–1200. [Google Scholar] [CrossRef]
- Yin, B.S.; Ma, H.Y.; Wang, S.Y.; Chen, S.H. Electrochemical synthesis of silver nanoparticles under protection of poly(Nvinylpyrrolidone). J. Phys. Chem. B 2003, 107, 8898–8904. [Google Scholar] [CrossRef]
- He, B.; Tan, J.J.; Liew, K.Y.; Liu, H. Synthesis of size controlled Ag nanoparticles. J. Mol. Catal. A Chem. 2004, 221, 121–126. [Google Scholar] [CrossRef]
- Cáceres-Vélez, P.R.; Fascineli, M.L.; Sousa, M.H.; Grisolia, C.K.; Yate, L.; de Souza, P.E.N.; Estrela-Lopis, I.; Moya, S.; Azevedo, R.B. Humic acid attenuation of silver nanoparticle toxicity by ion complexation and the formation of a Ag3+ coating. J. Hazard. Mat. 2018, 353, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Kolwas, K.; Derkachova, A.; Shopa, M. Size characteristics of surface plasmons and their manifestation in scattering properties of metal particles. J. Quant. Spectrosc. Radiat. Transf. 2009, 110, 1490–1501. [Google Scholar] [CrossRef]
- Gasbarri, C.; Angelini, G. Polarizability over dipolarity for the spectroscopic behavior of azobenzenes in room-temperature ionic liquids and organic solvents. J. Mol. Liq. 2017, 229, 185–188. [Google Scholar] [CrossRef]
- Angelini, G.; Gasbarri, C. Solvent scales comparison by using α-nitrocyclohexanone as probe in ionic liquids, organic solvents and CH3CN/CHCl3 mixtures. Tetrahedron 2017, 73, 3036–3039. [Google Scholar] [CrossRef]
- Gasbarri, C.; Angelini, G. Spectroscopic investigation of fluorinated phenols as pH-sensitive probes in mixed liposomal systems. RSC Adv. 2014, 4, 17840–17845. [Google Scholar] [CrossRef]
- Angelini, G.; Pisani, M.; Mobbili, G.; Marini, M.; Gasbarri, C. Neutral liposomes containing crown ether-lipids as potential DNA vectors. Biochim. Biophys. Acta 2013, 1828, 2506–2512. [Google Scholar] [CrossRef] [Green Version]
- Gasbarri, C.; Croce, F.; Meschini, I.; Bowen, C.H.; Marinelli, L.; di Stefano, A.; Angelini, G. Single-Walled Carbon Nanotubes in Highly Viscous Media: A Comparison between the Dispersive Agents [BMIM][BF4], L121, and Triton X-100. Chem. Eur. J. 2016, 22, 546–549. [Google Scholar] [CrossRef]
- Angelini, G.; Gasbarri, C.; Kazarian, S.G. Pluronic L121, BMIM BF4 and PEG-400 comparison to identify the best solvent for CO2 sorption. J. Mol. Liq. 2018, 258, 85–88. [Google Scholar] [CrossRef]
- Angelini, G.; Gasbarri, C. Polymeric Aggregates in Ionic Liquids: The Green Future of the Delivery Systems. Curr. Drug Targets 2015, 16, 1606–1611. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.; Viale, M.; Guernelli, S.; Gasbarri, C.; Rizzato, E.; Maccagno, M.; Petrillo, G.; Aiello, C.; Ferrini, S.; Spinelli, D. Strategies for improving the water solubility of new antitumour nitronaphthylbutadiene derivatives. Org. Biomol. Chem. 2010, 8, 5674–5681. [Google Scholar] [CrossRef] [PubMed]
- Scotti, L.; Angelini, G.; Gasbarri, C.; Bucciarelli, T. Uncoated negatively charged silver nanoparticles: Speeding up the electrochemical synthesis. Mater. Res. Express 2017, 4, 105001. [Google Scholar] [CrossRef] [Green Version]
- Muralia, G.; Vattikuti, S.V.P.; Kshetri, Y.K.; Lee, H.; Modigunta, J.K.R.; Reddy, C.S.; Park, S.; Lee, S.; Poornaprakash, B.; Lee, H.; et al. Near-infrared-activated Z-scheme NaYF4:Yb/Tm@Ag3PO4/Ag@g-C3N4 photocatalyst for enhanced H2 evolution under simulated solar light irradiation. Chem. Eng. J. 2021, 421, 129687. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Reddy, L.V.; Devarayapalli, K.C.; Vattikuti, S.V.P.; Wee, Y.J.; Shim, J. Environmentally Friendly Synthesis: Photocatalytic Dye Degradation and Bacteria Inactivation Using Ag/f-MWCNTs Composite. J. Clust. Sci. 2021, 32, 711–718. [Google Scholar] [CrossRef]
- Barbieri, F.; Würth, R.; Pattarozzi, A.; Verduci, I.; Mazzola, C.; Cattaneo, M.G.; Tonelli, M.; Solari, A.; Bajetto, A.; Daga, A.; et al. Inhibition of Chloride Intracellular Channel 1 (CLIC1) as Biguanide Class-Effect to Impair Human Glioblastoma Stem Cell Viability. Front. Pharmacol. 2018. [Google Scholar] [CrossRef] [Green Version]
- Saeb, A.T.; Alshammari, A.S.; Al-Brahim, H.; Al-Rubeaan, K.A. Production of silver nanoparticles with strong and stable antimicrobial activity against highly pathogenic and multidrug resistant bacteria. Sci. World J. 2014, 2014, 704708. [Google Scholar] [CrossRef]
- Shabatina, T.I.; Mascetti, J.; Ogden, J.S.; Sergeev, G.B. Competitive cryochemical reactions of transitino metal atoms, clusters and nanosized particles. Russ. Chem. Rev. 2007, 76, 1123–1137. [Google Scholar] [CrossRef]
- Echlin, P. Handbook of Sample Preparation for Scanning Electron Microscopy and X-ray Microanalysis; Springer Science Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Gasbarri, C.; Ruggieri, F.; Foschi, M.; Aceto, A.; Scotti, L.; Angelini, G. Simple Determination of Silver Nanoparticles Concentration as Ag+ by Using ISE as Potential Alternative to ICP Optical Emission Spectrometry. ChemistrySelect 2019, 4, 9501–9504. [Google Scholar] [CrossRef]
- Luo, K.; Clair, T.P.s.; Goodman, D.W. Silver growth on TiO2(110) (1x1) and (1x2). J. Phys. Chem. B 2000, 104, 3050–3057. [Google Scholar] [CrossRef]
- Wang, P.W.; Jiang, Y.; Hsu, J.C.; Chen, Y.Y.; Lin, Y.H.; Chen, H.L. Thermal Effect on Structure of Silver in Ion-Exchanged Soda-Lime Glasses and Aluminum-Doped Zinc Oxide Films. Adv. Mat. Sci. Eng. 2011, 2011, 7. [Google Scholar] [CrossRef] [Green Version]
- Hammond, J.S.; Gaarenstroom, S.W.; Winograd, N. X-ray photoelectron spectroscopic studies of cadmium- and silver-oxygen surfaces. Anal. Chem. 1975, 47, 2193–2199. [Google Scholar] [CrossRef]
- Zheng, J.; Ding, Y.; Tian, B.; Wang, Z.L.; Zhuang, X. Luminescent and Raman Active Silver Nanoparticles with Polycrystalline Structure. J. Am. Chem. Soc. 2008, 130, 10472–10473. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Khan, F.S.T.; Rath, S.P. Silver(III)·Silver(III) Interactions that Stabilize the syn Form in a Porphyrin Dimer Upon Oxidation. Angew. Chem. Int. Ed. 2017, 56, 8849–8854. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Yan-yu, R.; Tao, W.; Chuang, W. Preparation and antibacterial activities of Ag/Ag+/Ag3+ nanoparticle composites made by pomegranate (Punica granatum) rind extract. Results Phys. 2016, 6, 299–304. [Google Scholar]
- Yang, X.; Du, Y.; Li, D.; Lv, Z.; Wang, E. One-step synthesized silver micro-dendrites used as novel separation mediums and their applications in multi-DNA analysis. Chem. Commun. 2011, 47, 10581–10583. [Google Scholar] [CrossRef]
- Lanje, A.S.; Sharma, S.J.; Pode, R.B. Synthesis of silver nanoparticles: A safer alternative to conventional antimicrobial and antibacterial agents. J. Chem. Pharm. Res. 2010, 2, 478–483. [Google Scholar]
- Standke, B.; Jansen, M. Darstellung und Kristallstruktur von Ag3O4. J. Solid State 1987, 67, 278–284. [Google Scholar] [CrossRef]


| Ag Species Detection | Ag | Ag2 | Ag3 | Ag4 | Ag5 | Ag6 | Ag7 | Ag8 | Ag9 | Ag10 | Ag11 | Ag12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maldi-TOF MS Analysis | + | + | + | − | + | − | + | − | + | − | + | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasbarri, C.; Ronci, M.; Aceto, A.; Vasani, R.; Iezzi, G.; Florio, T.; Barbieri, F.; Angelini, G.; Scotti, L. Structure and Properties of Electrochemically Synthesized Silver Nanoparticles in Aqueous Solution by High-Resolution Techniques. Molecules 2021, 26, 5155. https://doi.org/10.3390/molecules26175155
Gasbarri C, Ronci M, Aceto A, Vasani R, Iezzi G, Florio T, Barbieri F, Angelini G, Scotti L. Structure and Properties of Electrochemically Synthesized Silver Nanoparticles in Aqueous Solution by High-Resolution Techniques. Molecules. 2021; 26(17):5155. https://doi.org/10.3390/molecules26175155
Chicago/Turabian StyleGasbarri, Carla, Maurizio Ronci, Antonio Aceto, Roshan Vasani, Gianluca Iezzi, Tullio Florio, Federica Barbieri, Guido Angelini, and Luca Scotti. 2021. "Structure and Properties of Electrochemically Synthesized Silver Nanoparticles in Aqueous Solution by High-Resolution Techniques" Molecules 26, no. 17: 5155. https://doi.org/10.3390/molecules26175155
APA StyleGasbarri, C., Ronci, M., Aceto, A., Vasani, R., Iezzi, G., Florio, T., Barbieri, F., Angelini, G., & Scotti, L. (2021). Structure and Properties of Electrochemically Synthesized Silver Nanoparticles in Aqueous Solution by High-Resolution Techniques. Molecules, 26(17), 5155. https://doi.org/10.3390/molecules26175155










