Mexican Oregano (Lippia graveolens Kunth) as Source of Bioactive Compounds: A Review
Abstract
:1. Introduction
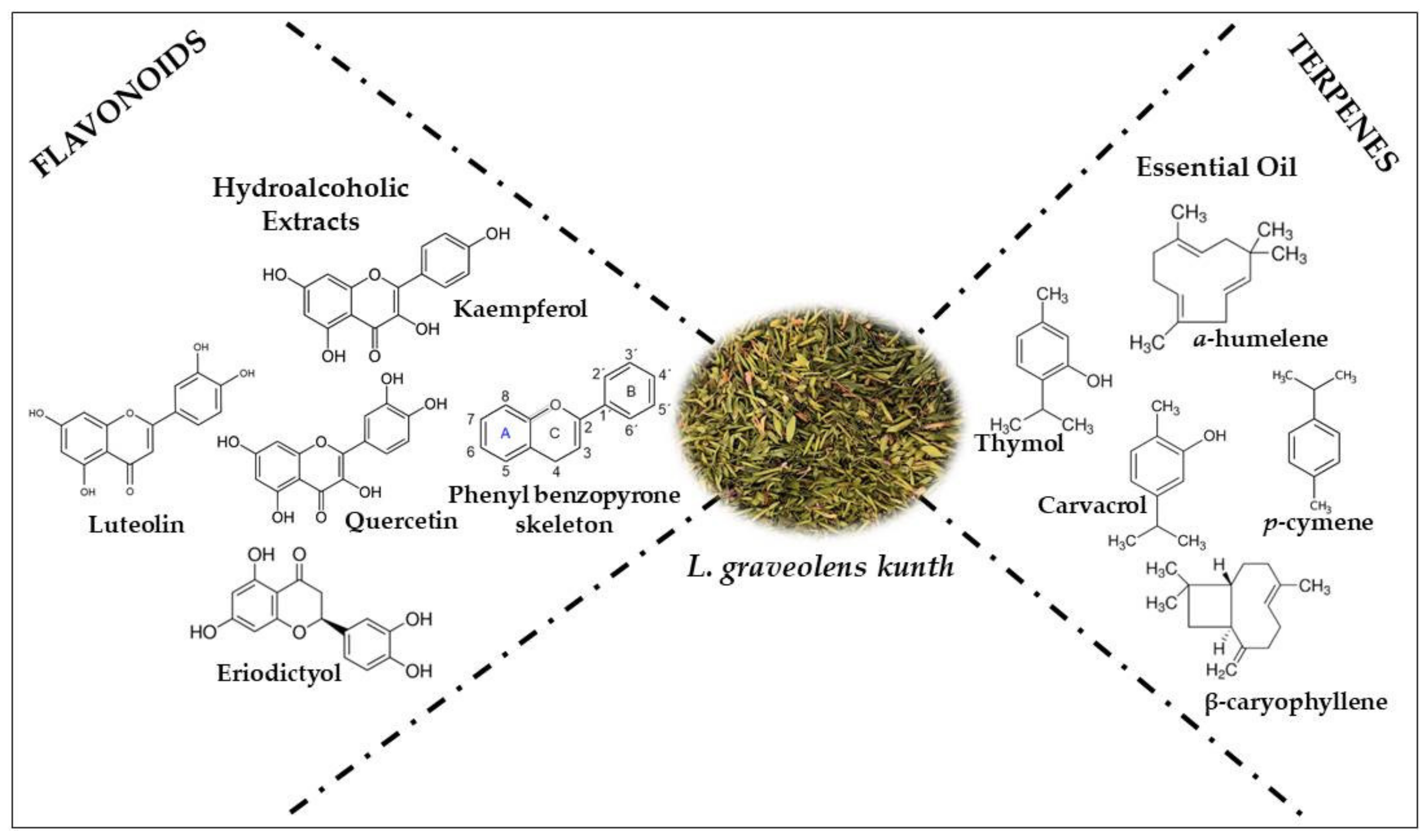
2. Chemical Composition
2.1. Essential Oils (EOs)
2.2. Polyphenolic Compounds (PCs)
2.3. Oregano by-Products a Source of Polyphenolic Compounds
3. Extraction Techniques
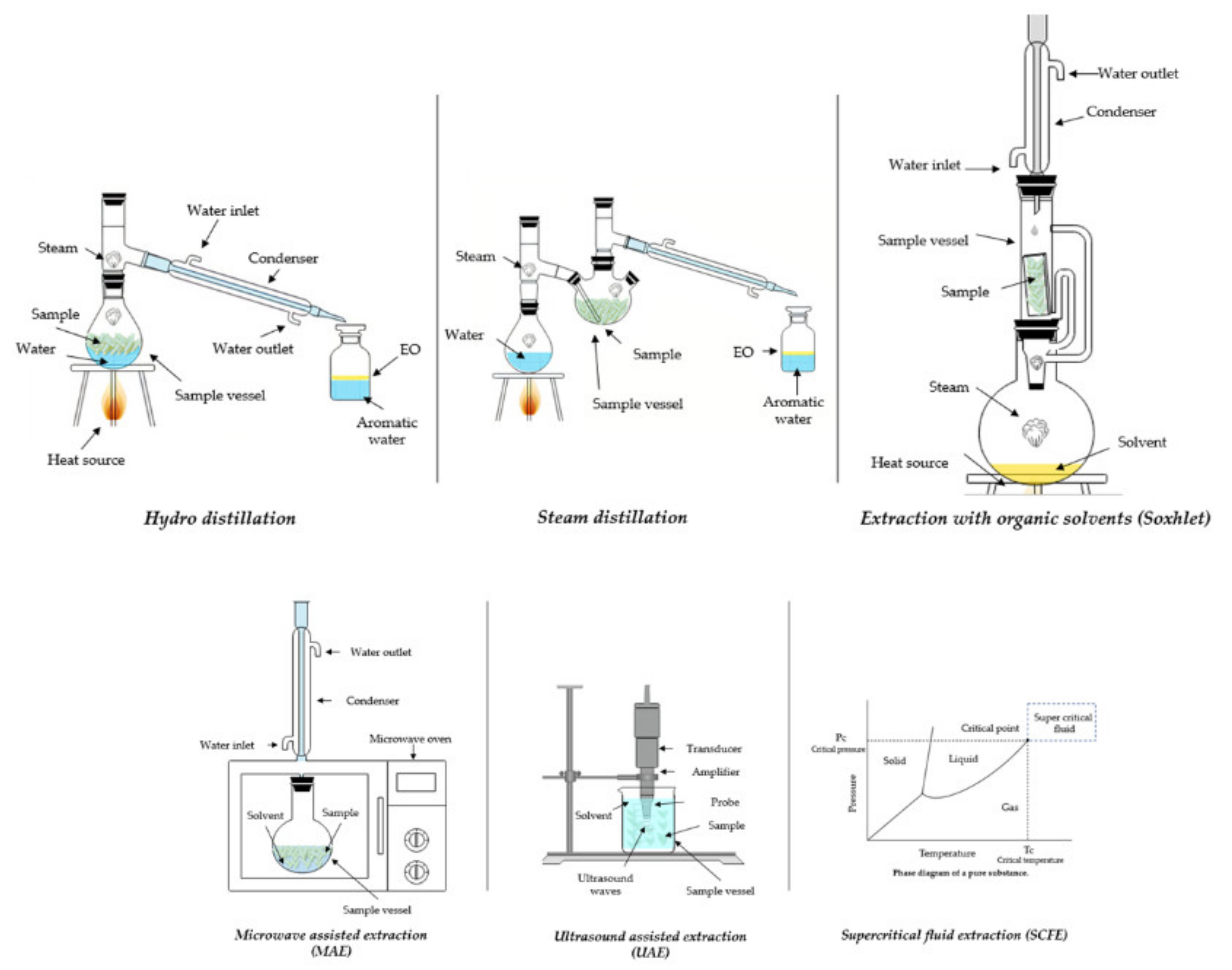
3.1. Conventional
3.1.1. Hydrodistillation
3.1.2. Steam Distillation
3.1.3. Extraction with Organic Solvents
3.2. Emerging Technologies
3.2.1. Ultrasound-Assisted Extraction (UAE)
3.2.2. Microwave-Assisted Extraction (MAE)
3.2.3. Supercritical Fluid Extraction (SCFE)
3.3. Chemical Variation of Extraction Techniques
4. Bioactive Potential of Mexican Oregano Fractions
4.1. Food
4.2. Health
4.3. Agronomic
5. Research, Innovation, and Technological Perspectives
5.1. Intellectual Property
5.2. Post Pandemic Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Calvo-Irabién, L.M.; Parra-Tabla, V.; Acosta-Arriola, V.; Escalante-Erosa, F.; Díaz-Vera, L.; Dziba, G.R.; Peña-Rodríjuez, L.M. Essential Oils of Mexican Oregano (Lippia graveolens Kunth) Populations along an Edapho-Climatic Gradient. J. Chem. Biodivers. 2014, 11, 1010–1021. [Google Scholar] [CrossRef]
- Soto-Armenta, L.C.; Sacramento-Rivero, J.C.; Acereto-Escoffié, P.O.; Peraza-González, E.E.; Reyes-Sosa, C.F.; Rocha-Uribe, J.A. Extraction Yield of Essential Oil from Lippia graveolens Leaves by Steam Distillation at Laboratory and Pilot Scales. Har Krishan Bhalla Sons 2017, 20, 610–621. [Google Scholar] [CrossRef]
- Cheikhyoussef, A.; Cheikhyoussef, N.; Ramadan, M. Cold pressed oregano (Origanum vulgare) oil. In Cold Pressed Oils; Ramadan, M.F., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 289–293. [Google Scholar]
- Gutiérrez-Grijalva, E.P.; Antunes-Ricardo, M.; Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Heredia, J.B. Cellular antioxidant activity and in vitro inhibition of α-glucosidase, α- amylase and pancreatic lipase of oregano polyphenols under simulated gastrointestinal digestion. Int. Food Res. J. 2019, 116, 676–686. [Google Scholar] [CrossRef]
- Ortega-Ramirez, L.A.; Rodríguez-Garcia, I.; Silva-Espinoza, B.A.; Ayala-Zavala, J.F. Oregano (Origanum spp.) Oils. In Essential Oils in Food Preservation; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 625–629. [Google Scholar]
- Díaz-de León, C.I.; Gónzález-Álvarez, M.A.; Guzmán-Lucio, M.A.; Nuñez-Gúzman, G.R.; Moreno-limón, S. The oregano of the genus Lippia (Verbenaceae) and Poliomintha (Lamiaceae) in the state of Nuevo León, Mexico. Poliboténica 2020, 50, 1–18. [Google Scholar] [CrossRef]
- Torres-León, C.; Ventura-Sobrevilla, J.; Serna-Cock, L.; Ascacio-Valdés, J.A.; Contreras-Esquivel, J.; Aguilar, C.N. Pentagalloylglucose (PGG): A valuable phenolic compound with functional properties. J. Funct. Foods 2017, 37, 176–189. [Google Scholar] [CrossRef]
- Cui, H.; Zhanga, C.; Lib, C.; Lina, L. Antibacterial mechanism of oregano essential oil. J. Ind. Crop. Prod. 2019, 139. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. F. 2013, 12. [Google Scholar] [CrossRef]
- Ren, M. Targeting Open Market with Strategic Business Innovations: A Case Study of Growth Dynamics in Essential Oil and Aromatherapy Industry. J. Open Innov. Technol. Mark. Complex 2019, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Codex Alimentarius Commission. Food Standards Programme Codex Committee on Spices and Culinary Herb, Thiruvananthapuram, India, 21–25 January 2019. Available online: http://www.fao.org/fao-who-codexalimentarius/shproxy/zh/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-736-04%252FCRDS%252Fsc04_crd04x.pdf (accessed on 5 March 2021).
- Granados-Sánchez, D.; Martínez-Salvador, M.; López-Ríos, G.; Borja-De la Rosa, A.; Rodríguez-Yam, G. Ecology, harvesting and marketing of oregano (Lippia graveolens H. B. K.) in Mapimí, Durango. Rev. Chapingo. Ser. Cienc. For. y Ambiente 2013, 19, 305–322. [Google Scholar]
- Reyes-Jurado, F.; Cervantes-Rincón, T.; Bach, H.; López-Malo, A.; Palou, E. Antimicrobial activity of Mexican oregano (Lippia berlandieri), thyme (Thymus vulgaris), and mustard (Brassica nigra) essential oils in gaseous phase. Ind. Crop. Prod. 2019, 131, 90–95. [Google Scholar] [CrossRef]
- Bayir, A.G.; Kiziltan, H.S.; Kocyigit, A. Plant Family, Carvacrol, and Putative Protection in Gastric Cancer. In Dietary Interventions in Gastrointestinal Diseases Foods, Nutrients, and Dietary Supplements; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 3–18. [Google Scholar]
- Arias, J.; Mejía, J.; Córdoba, Y.; Martínez, J.R.; Stashenko, E. Optimization of flavonoids extraction from Lippia graveolens and Lippia origanoides chemotypes with ethanol-modified supercritical CO2 after steam distillation. Ind. Crop. Prod. 2020, 146, 112170. [Google Scholar] [CrossRef]
- Rassem, H.; Nour, A.; Yunus, R. Biological activities of essential oils—A review. J. Pac. Int. 2018, 2, 63–76. [Google Scholar]
- Rehman, R.; Hanif, M.; Mushtaq, Z.; Al-Sadi, A. Biosynthesis of essential oils in aromatic plants: A review. Food Rev. Int. 2016, 32, 117–160. [Google Scholar] [CrossRef]
- Ludwiczuk, A.; Skalicka-Wozniak, K.; Georgiev, M.I. Terpenoids. In Pharmacognosy: Fundamentals, Applications and Strategies; Badal, S., Delgoda, R., Eds.; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar] [CrossRef]
- Cid-Pérez, T.S.; Ávila-Sosa, R.; Ochoa-Velasco, C.E.; Rivera-Chavira, B.E.; Nevárez-Moorillón, G.V. Antioxidant and Antimicrobial Activity of Mexican Oregano (Poliomintha longiflora) Essential Oil, Hydrosol and Extracts fromWaste Solid Residues. Plants 2019, 8, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayramoglu, B.; Sahin, S.; Summu, G. Solvent-free microwave extraction of essential oil from oregano. J. Food Eng. 2008, 88, 535–540. [Google Scholar] [CrossRef]
- Figiel, A.; Szumny, A.; Gutiérrez-Ortíz, A.; Carbonell-Barrachina, Á.A. Composition of oregano essential oil (Origanum vulgare) as affected by drying method. J. Food Eng. 2010, 98, 240–247. [Google Scholar] [CrossRef]
- Gerami, F.; Moghaddam, P.R.; Ghorbani, R.; Hassani, A. Effects of irrigation intervals and organic manure on morphological traits, essential oil content and yield of oregano (Origanum vulgare L.). Anais da Academia Brasileira de Ciências 2016, 88, 2375–2385. [Google Scholar] [CrossRef] [Green Version]
- Morshedloo, M.R.; Mumivand, H.; Craker, L.E.; Maggi, F. Chemical composition and antioxidant activity of essential oils in Origanum vulgare subsp. gracile at different phenological stages and plant parts. J. Food Process. Preserv. 2017, 42. [Google Scholar] [CrossRef]
- Toncer, O.; Karaman, S.; Kizil, S.; Diraz, E. Changes in Essential Oil Composition of Oregano (Origanum onites L.) due to Diurnal Variations at Different Development Stages. Not. Bot. Horti Agrobot. Cluj-Napoca 2009, 37, 177–181. [Google Scholar]
- Leyva-López, N.; Nair, V.; Banh, W.Y.; Cisneros-Zevallos, L.; Basilio Heredia, J. Protective role of terpenes and polyphenols from three species of Oregano (Lippia graveolens, Lippia palmeri and Hedeoma patens) on the suppression of lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells. J. Ethnopharmacol. 2016, 187, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Chital, M.C.; Flores-Martínez, H.; Orozco-Ávila, I.; León-Campos, C.; Suárez-Jacobo, Á.; Estarrón-Espinosa, M. Identification and Quantification of Phenolic Compounds from Mexican Oregano (Lippia graveolens HBK) Hydroethanolic Extracts and Evaluation of Its Antioxidant Capacity. Molecules 2021, 26, 702. [Google Scholar] [CrossRef]
- Barbieri, N.; Sanchez-Contreras, A.; Canto, A.; Cauich-Rodriguez, J.V.; Vargas-Coronado, R.; Calvo-Irabien, L.M. Effect of cyclodextrins and Mexican oregano (Lippia graveolens Kunth) chemotypes on the microencapsulation of essential oil. Ind. Crop. Prod. 2018, 121, 114–123. [Google Scholar] [CrossRef]
- Herrera-Rodríguez, S.E.; López-Rivera, R.J.; García-Márquez, E.; Estarrón-Espinosa, M.; Espinosa-Andrews, H. Mexican oregano (Lippia graveolens) essential oil-in-water emulsions: Impact of emulsifier type on the antifungal activity of Candida albicans. Food Sci. Biotechnol. 2019, 28, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Cid-Pérez, T.S.; Nevárez-Moorillón, G.V.; Torres-Muñoz, J.V.; Palou, E.; López-Malo, A. Mexican oregano (Lippia berlandieri and Poliomintha longiflora) oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 551–560. [Google Scholar] [CrossRef]
- Irrera, N.; D’ascola, A.; Pallio, G.; Bitto, A.; Mannino, F.; Arcoraci, V. β-caryophyllene inhibits cell proliferation through a direct modulation of CB2 receptors in glioblastoma cells. Cancers 2020, 12, 1038. [Google Scholar] [CrossRef] [Green Version]
- De Meireles, A.L.P.; da Silva Rocha, K.A.; Kozhevnikova, E.F.; Kozhevnikov, I.V.; Gusevskaya, E.V. Heteropoly acid catalysts for the valorization of biorenewables: Isomerization of caryophyllene oxide in green solvents. Mol. Catal. 2018, 458, 213–222. [Google Scholar] [CrossRef]
- Ngamprasertsith, S.; Menwa, J.; Sawangkeaw, R. Caryophyllene oxide extraction from lemon basil (Ocimum citriodorum Vis.) straw by hydrodistillation and supercritical CO2. J. Supercrit. Fluids 2018, 138, 1–6. [Google Scholar] [CrossRef]
- Chavan, M.J.; Wakte, P.S.; Shinde, D.B. Analgesic and anti-inflammatory activity of Caryophyllene oxide from Annona squamosa L. bark. Phytomedicine 2010, 17, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Hartsel, J.A.; Eades, J.; Hickory, B.; Makriyannis, A. Cannabis sativa and Hemp. In Nutraceuticals: Efficacy, Safety and Toxicity; Gupta, R.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 735–754. [Google Scholar] [CrossRef]
- Russo, E.B.; Marcu, J. The Usual Suspects and a Few Promising Leads. In Cannabis Pharmacology; Advances in Pharmacology, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 80, pp. 67–134. [Google Scholar] [CrossRef]
- Oliveira, R.; Balbino, E.; Carvalho, R.; Antunes, A.; Gomes, T.; Fernandes, R.; Coelho, G.B.; Carvalho, G.; Mendes, K.; Formiga, M.F.; et al. Effect of p-cymene and rosmarinic acid on gastric ulcer healing—Involvement of multiple endogenous curative mechanisms. Phytomedicine 2021, 86, 153497. [Google Scholar] [CrossRef]
- Pilau, M.R.; Alves, S.H.; Weiblen, R.; Arenhart, S.; Cueto, A.P.; Lovato, L.T. Antiviral activity of the Lippia graoveolens (Mexican oregano) essential oil and its main compound carvacrol against human and animal viruses. Braz. J. Microbiol. 2011, 42, 1616–1624. [Google Scholar] [CrossRef] [Green Version]
- Mediouni, S.; Jablonski, J.A.; Tsida, S.; Barsamian, A.; Kessing, C.; Richard, A.; Biswas, A.; Toledo, F.; Andrade, V.M.; Even, Y.; et al. Oregano Oil and Its Principal Component, Carvacrol, Inhibit HIV-1 Fusion into Target Cells. J. Virol. 2020, 94, 15. [Google Scholar] [CrossRef]
- Asif, M.; Saleem, M.; Saadullah, M.; Yaseen, H.S.; Zarzour, R.A. COVID-19 and therapy with essential oils having antiviral anti-inflammatory, and immunomodulatory properties. Inflammopharmacology 2020, 28, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Muturi, E.; Ramirez, J.; Doll, M.K.; Bowman, M.J. Combined Toxicity of Three Essential Oils Against Aedes aegypti (Diptera: Culicidae) Larvae. J. Med. Entomol. 2017, 1–8. [Google Scholar] [CrossRef]
- Rosa, D.S.; Vargas, B.P.; Silveira, M.V.; Rosa, C.H.; Martins, M.L.; Rosa, G.R. On the Use of Calcined Agro-Industrial Waste as Palladium Supports in the Production of Eco-Friendly Catalysts: Rice Husks and Banana Peels Tested in the Suzuki–Miyaura Reaction. Waste Biomass Valorization 2019, 10, 2285–2296. [Google Scholar] [CrossRef]
- Gan, R.-Y.; Chan, C.-L.; Yang, Q.-Q.; Li, H.-B.; Zhang, D.; Ge1, Y.-Y.; Gunaratne, A.; Ge1, J.; Corke, H. Bioactive compounds and beneficial functions of sprouted grains. In Sprouted Grains: Nutritional Value, Production and Applications; Feng, H., Nemzer, B., DeVries, J.W., Eds.; AACC International Press: Cambridge, MA, USA, 2019. [Google Scholar] [CrossRef]
- Babu, P.V.A.; Liu, D. Flavonoids and Cardiovascular Health. In Complementary and Alternative Therapies and the Aging Population; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Picos-Salas, M.A.; Leyva-López, N.; Criollo-Mendoza, M.S.; Vazquez-Olivo, G.; Heredia, J.B. Flavonoids and Phenolic Acids from Oregano: Occurrence, Biological Activity and Health Benefits. Plants 2017, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.-Y.; Li, Q.; Bi, K.-S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Cálinoiu, L.F.; Vodnar, D.C. Thermal Processing for the Release of Phenolic Compounds from Wheat and Oat Bran. Biomolecules 2019, 10, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacanli, M.; Başaran, A.A.; Başaran, N. Galangin as a plant phenolic and usage in health and disease. In Polyphenols Prevention and Treatment of Human Disease; Academic Press: Cambridge, MA, USA, 2018; pp. 433–438. [Google Scholar]
- Ramos-Tovar, E.; Muriel, P. Phytotherapy for the Liver. In Dietary Interventions in Liver Disease: Foods, Nutrients, and Dietary Supplements; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 101–121. [Google Scholar] [CrossRef]
- Hasanein, P.; Emamjomeh, A. Beneficial Effects of Natural Compounds on Heavy Metal-Induced Hepatotoxicity. In Dietary Interventions in Liver Disease: Foods, Nutrients, and Dietary Supplements; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 345–355. [Google Scholar] [CrossRef]
- Bekut, M.; Brkić, S.; Kladar, N.; Dragović, G.; Gavarić, N.; Božin, B. Potential of selected Lamiaceae plants in anti(retro)viral therapy. Pharmacol. Res. 2018, 133, 301–314. [Google Scholar] [CrossRef]
- Tiwari, N.; Kumar, A.; Singh, A.K.; Bajpai, S.; Agrahari, A.K.; Kishore, D. Leishmaniasis control: Limitations of current drugs and prospects of natural products. In Discovery and Development of Therapeutics from Natural Products against Neglected Tropical Diseases; Brahmachari, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 293–350. [Google Scholar] [CrossRef]
- Torres-León, C.; Chávez-González, M.L.; Hernández-Almanza, A.; Martínez-Medina, G.A.; Ramírez-Gúzman, N.; Londoño-Hernández, L.; Aguilar, C.N. Recent advances on the microbiological and enzymatic processing for conversion of food wastes to valuable bioproducts. Curr. Opin. Food Sci. 2020, 35, 40–45. [Google Scholar] [CrossRef]
- Méndez-Tovar, I.; Herrero, B.; Pérez-Magariño, S.; Pereira, J.A.; Asensio-S-Manzanera, M.C. By-product of Lavandula latifolia essential oil distillation as source of antioxidants. J. Food Drug Anal. 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Oreopoulou, A.; Goussias, G.; Tsimogiannis, D.; Oreopoulou, V. Hydro-alcoholic extraction kinetics of phenolicsfrom oregano: Optimization of the extractionparameters. Food Bioprod. Process. 2020, 123, 378–389. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Yoga, L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngoc-Minh, T.; Dang-Xuan, T.; Hoang-Dung, T.; Mai-Van, T.; Andriana, Y.; Dnag-Khanh, T.; Van-Quan, N.; Ahmad, A. Isolation and Purification of Bioactive Compounds from the Stem Bark of Jatropha podagrica. Molecules 2019, 24, 889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manouchehria, R.; Saharkhiza, M.J.; Karamia, A.; Niakousarib, M. Extraction of essential oils from damask rose using green and conventional techniques: Microwave and ohmic assisted hydrodistillation versus hydrodistillation. J. Sustain. Chem. Pharm. 2018, 8, 76–81. [Google Scholar] [CrossRef]
- Valderrama, F.; Ruiz, F. An optimal control approach to steam distillation of essential oils from aromatic plants. J. Comput. Chem. Eng. 2018, 117, 25–31. [Google Scholar] [CrossRef]
- Saucedo-Pompaa, S.; Torres-Castilloc, J.A.; Castro-López, C.; Rojas, R.; Sánchez-Alejo, E.J.; Ngangyo-Heya, M.; Martínez-Ávilaa, G.C.G. Moringa plants: Bioactive compounds and promising applications in Food products. J. Food Res. Int. 2018, 111, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Perovic, A.; Stankovic, M.Z.; Veljkovic, E.B.M.; Kostic, M.D.; Stamenkovic, O.S. A further study of the kinetics and optimization of the essential oil hydrodistillation from lavender flowers. Chin. J. Chem. Eng. 2020, 29, 126–130. [Google Scholar] [CrossRef]
- Cinbilgel, I.; Kurt, Y. Oregano and/or Marjoram: Traditional Oil Production and Ethnomedical Utilization of Origanum Species in southern Turkey. J. Herb. Med. 2019, 16, 100257. [Google Scholar] [CrossRef]
- Mahanta, B.P.; Sarma, N.; Kemprai, P.; Begum, T.; Saikia, L.; Lal, M.; Haldar, S. Hydrodistillation based multifaceted value addition to Kaempferia galanga L. leaves, an agricultural residue. Ind. Crop. Prod. 2020, 154, 112642. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Nikmaram, N.; Esteghlal, S.; Khaneghah, A.M.; Niakousari, M.; Barba, F.J.; Roohinejad, S.; Koubaa, M. Efficiency of Ohmic assisted hydrodistillation for the extraction of essential oils from oregano (Origanum vulgare subsp. viride) spices. Innov. Food Sci. Emerg. Tech. 2017, 41, 172–178. [Google Scholar] [CrossRef]
- Rassem, H.H.A.; Nour, A.H.; Yunus, R.M. Techniques For Extraction of Essential Oils From Plants: A Review. Aust. J. Basic Appl. Sci. 2016, 10, 117–127. [Google Scholar]
- Khalil, A.A.; Rahman, U.; Khan, M.; Sahar, A.; Mehmoodac, T.; Khana, M. Essential oil eugenol: Sources, extraction techniques and nutraceutical perspectives. J. R. Soc. Chem. 2017, 7, 32669–32681. [Google Scholar] [CrossRef] [Green Version]
- Prado, J.M.; Vardanega, R.; Debien, I.C.N.; Meireles, M.A.A.; Gerschenson, L.N.; Sowbhagya, H.B. Conventional Extraction; Chapter 6; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Aït Addi, E.H.; Casabianca, H.; Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- González-Trujano, M.E.; Hernández-Sánchez, L.Y.; Ocotero, V.M.; Dorazco-González, A.; Fefer, P.G.; Aguirre-Hernández, E. Pharmacological evaluation of the anxiolytic-like effects of Lippia graveolens and bioactive compounds. Pharm. Biol. 2017, 55, 1569–1576. [Google Scholar] [CrossRef] [Green Version]
- Chanioti, S.; Tzia, C. Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. J. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Mohammadpour, H.; Sadrameli, S.M.; Eslami, F.; Asoodeh, A. Optimization of ultrasound-assisted extraction of Moringa peregrina oil with response surface methodology and comparison with Soxhlet method. Ind. Crop. Prod. 2019, 131, 106–116. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, V.; Kumar-Das, A. Essentials of Botanical Extraction: Principles and Applications; Academic Press: Cambridge, MA, USA, 2015; Volume 1, p. 220. [Google Scholar] [CrossRef]
- Trejo-Márquez, M.A.; Vargas-Martínez, M.G.; Sánchez-Soto, A.; Adela, L.V.A.; Pascual-Bustamante, S.; Granados, G.; Villavicencio, A.G. Extraction of bioactive compounds of plants from the Mexican desert for its applitacion in active packaging for blackberry. Rev. Iberoam. Tecnol. Postcosecha 2015, 16, 101–107. [Google Scholar]
- Rehman, M.U.; Khan, A.F.; Niaz, K. Introduction to natural products analysis. In Recent Advances in Natural Products Analysis; Silva, A.S., Nbavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–13. [Google Scholar]
- Llompart, M.; Garcia-Jares, C.; Celeiro, M. Microwave-Assisted Extraction. Reference Module in Chemistry. Mol. Sci. Chem. Eng. 2019, 67–77. [Google Scholar] [CrossRef]
- Reddy, A.V.B.; Moniruzzaman, M.; Madhavi, V.; Jaafar, J. Recent improvements in the extraction, cleanup and quantification of bioactive flavonoids. Stud. Nat. Prod. Chem. 2020, 66, 197–223. [Google Scholar] [CrossRef]
- Kataoka, H. Pharmaceutical Analysis, Sample Preparation. In Encyclopedia of Analytical Science, 2nd ed.; Wosfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Oxford, UK, 2005; pp. 107–116. ISBN 9780123693976. [Google Scholar]
- Drinić, Z.; Pljevljakušić, D.; Živković, J.; Bigović, D.; Šavikin, K. Microwave-assisted extraction of O. vulgare L. spp. hirtum essential oil: Comparison with conventional hydro-distillation. Food Bioprod. Process. 2020, 120, 158–165. [Google Scholar] [CrossRef]
- Vichi, S. Chapter 66—Extraction Techniques for the Analysis of Virgin Olive Oil Aroma. In Olives and Olive Oil in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2010; pp. 615–623. [Google Scholar] [CrossRef]
- Verma, D.K.; Dhakane, J.P.; Mahato, D.K.; Billoria, S.; Bhattacharjee, P.; Srivastav, P.P. Supercritical Fluid Extraction (SCFE) for Rice Aroma Chemicals: Recent and Advance Extraction Method. Science and Technology of Aroma, Flavour and Fragrance in Rice; Verma, D.K., Srivastav, P.P., Eds.; Apple Academic Press: San Diego, CA, USA, 2018; pp. 93–140. [Google Scholar]
- Verma, D.K.; Srivastav, P.P. A Paradigm of Volatile Aroma Compounds in Rice and Their Product with Extraction and Identification Methods: A Comprehensive Review. Food Res. Int. 2020, 130, 1–33. [Google Scholar] [CrossRef]
- Verma, D.K.; Srivastav, P.P. Extraction, Identification and Quantification Methods of Rice Aroma Compounds with Emphasis on 2-Acetyl-1-Pyrroline (2-AP) and Its Relation with Rice Quality: A Comprehensive Review. Food Rev. Int. 2020. [Google Scholar] [CrossRef]
- Chávez-González, M.L.; Sepúlveda, L.; Verma, D.K.; Luna-García, H.A.; Rodríguez-Durán, L.V.; Ilina, A.; Aguilar, C.N. Conventional and Emerging Extraction Processes of Flavonoids. Processes 2020, 8, 434. [Google Scholar] [CrossRef] [Green Version]
- Abhari, K.; Mousavi, A. Alternative extraction techniques to obtain, isolate and purify proteins and bioactive from aquaculture and by-products. In Advances in Food and Nutrition Research, 1st ed.; Toldrá, F., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 92, pp. 35–52. [Google Scholar] [CrossRef]
- Wang, Q.; Sho, A.; Liu, H.; Liu, L.; Zhang, Y.; Li, N.; Gong, K.; Yu, M.; Zheng, L. Peanut by-products utilization technology. In Peanuts: Processing Technology and Product Development; Wang, Q., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 211–325. [Google Scholar]
- Peter, K.V. Handbook of Herbs and Spices; Woodhead Publishing: Sawston, UK, 2012; Volume 1, p. 607. [Google Scholar]
- Chin Chew, S. Cold-pressed rapeseed (Brassica napus) oil: Chemistry and functionality. Food Res. Int. 2020, 131, 108997. [Google Scholar] [CrossRef]
- Silva, S.G.; de Oliveira, M.S.; Cruz, J.N.; da Costa, W.A.; da Silva, S.H.M.; Barreto Maia, A.A.; de Sousa, R.L.; Carvalho Junior, R.N.; de Aguiar Andrade, E.H. Supercritical CO2 extraction to obtain Lippia thymoides Mart. & Schauer (Verbenaceae) essential oil rich in thymol and evaluation of its antimicrobial activity. J. Supercrit. Fluids 2021, 168, 105064. [Google Scholar] [CrossRef]
- De Oliveira, M.S.; Silva, S.G.; da Cruz, J.N.; Ortiz, E.; da Costa, W.A.; Bezerra, F.W.F.; Cunha, V.M.B.; Cordeiro, R.M.; de Jesus Chaves Neto, A.M.; de Aguiar Andrade, E.H.; et al. Supercritical CO2 Application in Essential Oil Extraction. In Industrial Applications of Green Solvents; Inamuddin, R.M., Asiri, A.M., Eds.; Materials Research Foundations: Millersville, PA, USA, 2019; Volume 2, pp. 1–28. [Google Scholar] [CrossRef]
- Soto-Armenta, L.C.; Rivero, J.C.S.; Ruiz-Mercado, C.A.; Lope-Navarrete, M.C.; Rocha-Uribe, J.A. Extraction yield and kinetic study of Lippia graveolens with supercritical CO2. J. Supercrit. Fluids 2018, 145, 205–210. [Google Scholar] [CrossRef]
- García-Pérez, J.S.; Robledo-Padilla, F.; Cuellar-Bermudez, S.P.; Arevalo-Gallegos, A.M.; Parras-Saldivar, R.; Zavala-Yoe, R.; Ramirez-Mendoza, R.A.; Iqbal, M.N. Thermodynamics and statistical correlation between supercritical-CO2 fluid extraction and bioactivity profile of locally available Mexican plants extracts. J. Supercrit. Fluids 2016, 122, 27–34. [Google Scholar] [CrossRef]
- Borgarello, A.V.; Mezza, G.N.; Pramparo, M.C.; Gayol, M.F. Thymol enrichment from oregano essential oil by molecular distillation. Sep. Purif. Technol. 2015, 153, 60–66. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.-H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. 2017, 16, 295–315. [Google Scholar] [CrossRef] [Green Version]
- Conde-Hernández, L.A.; Espinosa-Victoria, J.R.; Trejo, A.; Guerrero-Beltrán, J.A. CO2-Supercritical extraction, hydrodistillation and steam distillation of essential oil of rosemary (Rosmarinus officinalis). J. Food Eng. 2016, 200, 81–86. [Google Scholar] [CrossRef]
- García-Bores, A.M.; Espinosa-González, A.M.; Reyna-Campos, A.; Cruz-Toscano, S.; Benítez-Flores, J.C.; Hernández-Delgado, C.T.; Flores-Maya, S.; Urzúa-Meza, M.; Peñalosa-Castro, I.; Céspedes-Acuña, C.L.; et al. Lippia graveolens photochemopreventive effect against UVB radiation-induced skin carcinogenesis. J. Photochem. Photobiol. B Biol. 2016. [Google Scholar] [CrossRef]
- Amador, S.; Nieto-Camacho, A.; Ramírez-Apan, M.T.; Matínez, M.; Maldonado, E. Cytotoxic, anti-inflammatory, and α-glucosidase inhibitory effects of flavonoids from Lippia graveolens (Mexican oregano). Med. Chem. Res. 2020, 29, 1497–1506. [Google Scholar] [CrossRef]
- Lu-Martínez, A.A.; Báez-González, J.G.; Castillo-Hernández, S.; Amaya-Guerra, C.; Rodríguez-Rodríguez, J.; García-Márquez, E. Studied of Prunus serotine oil extracted by cold pressing and antioxidant effect of P. longiflora essential oil. J. Food Sci. Technol. 2020, 58, 1420–1429. [Google Scholar] [CrossRef]
- Boroski, M.; Giroux, H.J.; Sabik, H.; Petit, H.V.; Visentainer, J.V.; Matumoto-Pintro, P.V.; Britten, M. Use of oregano extract and oregano essential oil as antioxidants in functional dairy beverage formulations. LWT Food Sci. Tech. 2012, 47, 167–174. [Google Scholar] [CrossRef]
- Chuang, L.-T.; Tsai, T.-H.; Lien, T.-J.; Huang, W.-C.; Liu, J.-J.; Chang, H.; Chang, M.-L.; Tsai, P.-J. Ethanolic Extract of Origanum vulgare Suppresses Propionibacterium acnes-Induced Inflammatory Responses in Human Monocyte and Mouse Ear Edema Models. Molecules 2018, 23, 1987. [Google Scholar] [CrossRef] [Green Version]
- Dávila-Rodríguez, M.; López-Malo, A.; Palou, E.; Ramírez-Corona, N.; Jiménez-Munguía, M.T. Essential oils microemulsions prepared with high-frequency ultrasound: Physical properties and antimicrobial activity. J. Food Sci. Technol. 2020, 57, 4133–4142. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Abd El-Hack, M.E.; Farag, M.R.; Shaheen, H.M.; Abdel-Latif, M.A.; Noreldin, A.E. The usefulness of oregano and its derivatives in poultry nutrition. Worlds Poult. Sci. J. 2018, 74, 463–473. [Google Scholar] [CrossRef]
- Hernández-Coronado, A.C.; Silva-Vázquez, R.; Rangel-Nava, Z.E.; Hernández-Martínez, C.A.; Kawas-Garza, J.R.; Hume, M.E. Mexican oregano essential oils given in drinking water on performanc6e, carcass traits, and meat quality of broilers. Poult. Sci. 2019, 98, 3050–3058. [Google Scholar] [CrossRef]
- Bauer, B.W.; Radovanovic, A.; Willson, N.-L.; Bajagai, Y.S.; Van, T.T.H.; Moore, R.J.; Stanley, D. Oregano: A potential prophylactic treatment for the intestinal microbiota. Heliyon 2019, 5, e02625. [Google Scholar] [CrossRef] [Green Version]
- Ríos, N.; Stashenko, E.E.; Duque, J.E. Evaluation of the insecticidal activity of essential oils and their mixtures against Aedes aegypti (Diptera: Culicidae). Rev. Bras. Entomol. 2017, 61, 307–311. [Google Scholar] [CrossRef]
- Govindarajan, M.; Rajeswary, M.; Hoti, S.L.; Benelli, G. Larvicidal potential of carvacrol and terpinen-4-ol from the essential oil of Origanum vulgare (Lamiaceae) against Anopheles stephensi, Anopheles subpictus, Culex quinquefasciatus and Culex tritaeniorhynchus (Diptera: Culicidae). Res. Vet. Sci. 2016, 104, 77–82. [Google Scholar] [CrossRef]
- Castilho, C.V.V.; Leitão, S.G.; Silva, V.D.; Miranda, C.O.; da Santos, M.C.S.; Bizzo, H.R. In vitro propagation of a carvacrol-producing type of Lippia origanoides Kunth: A promising oregano-like herb. Ind. Crop. Prod. 2019, 130, 491–498. [Google Scholar] [CrossRef]
- Gavin-Martin, J. Smokable Remedial Herb Blend. Spanish Patent ES2678597, 14 August 2018. [Google Scholar]
- López-Gómez, A.; Ros-Chumillas, M. Composition of Ice with Antimicrobial Activity, Manufacturing Method, and Its Applications. Spanish Patent ES2613240, 9 May 2017. [Google Scholar]
- Ninkov, D. Antimicrobial Therapeutic Compositions and Procedures for Use. Spanish Patent ES2351116, 31 January 2011. [Google Scholar]
- Lira, R.H.; Hernández, M. Natural Compounds Having Antimicrobial Activity for Preventing and Controlling Infectious Diseases in Humans and Food. Mexican Patent MXNL/A/2006/000057, 31 October 2008. [Google Scholar]
- Yanez-Reyes, J.N. Formulation Stimulating Plant Growth and Development and Resistance Inducer for the Control of Diseases Caused by Phytopatogenic Viruses and Method of Preparation. Spanish Patent ES2628278, 2 August 2017. [Google Scholar]
- Gutierrez, J.A.; García, S.; García, E. Process for the Regeneration of Mexican Oregano Plants (of the Genus Poliomintha) by Indirect Organogenesis. Mexican Patent MX365079, 28 February 2014. [Google Scholar]
- Muñoz, C.V.; Aburto, J.A. Method to Increase the Secondary Metabolites in Candelilla and Oregano. Mexican Patent MX2017013652, 24 April 2019. [Google Scholar]
- Ayseli, Y.I.; Aytekin, N.; Buyukkayhan, D.; Aslan, I.; Ayseli, M.T. Food policy, nutrition and nutraceuticals in the prevention and management of COVID-19: Advice for healthcare professionals. Trends Food Sci. Technol 2020, 105, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.; Verma, D.K.; Thakur, M.; Patel, A.R.; Srivastav, P.P.; Singh, S.; Chávez-González, M.L.; Aguilar, C.N. Encapsulated food products as a strategy to strengthen immunity against COVID-19. Front. Nutr. 2021, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Hoang, B.X. Opinions on the current pandemic of COVID-19: Use functional food to boost our immune functions. J. Infect. Public Health 2020, 13, 1811–1817. [Google Scholar] [CrossRef]
- Noor, N.; Gani, A.; Gani, A.; Shah, A. International Journal of Biological Macromolecules Exploitation of polyphenols and proteins using nanoencapsulation for anti-viral and brain boosting properties—Evoking a synergistic strategy to combat COVID-19 pandemic. Int. J. Biol. Macromol. 2021, 180, 375–384. [Google Scholar] [CrossRef] [PubMed]
- De Souza, E.L.; de Albuquerque, T.M.R.; dos Santos, A.S.; Massa, N.M.L.; de Brito Alves, J.L. Potential interactions among phenolic compounds and probiotics for mutual boosting of their health-promoting properties and food functionalities–A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1645–1659. [Google Scholar] [CrossRef]
- Zheng, K.; Wu, S.Z.; Lv, Y.W.; Pang, P.; Deng, L.; Xu, H.; Chong, X.; Yu-Cong, S.; Xiao-Yin, C. Carvacrol inhibits the excessive immune response induced by influenza virus A via suppressing viral replication and TLR/RLR pattern recognition. J. Ethnopharmacol. 2021, 268, 113555. [Google Scholar] [CrossRef]
- Torres-León, C.; Aguirre-Joya, J.A.; Czaja, A.; Aguillón-Gutiérrez, D.R. In silico Screening bioaktiver Verbindungen aus mexikanischen Wüstenpflanzen zur Vorhersage potenzieller Inhibitoren von SARSCoronavirus 2 (SARS-CoV-2). Z Arznei-Gewurzpfla 2020, 24, 153–156. [Google Scholar]

| Fraction | Species | Type of Isolation | Mainly Identified Compounds | Content | Reference | |
|---|---|---|---|---|---|---|
| Concentration (mg mL−1/μg mg−1 Extract) | % EO | |||||
| Phenolic extracts | L. graveolens | Solid: liquid (80% methanol) | Quercetin O-hexoside/ Luteolin-glucuronide-glucoside/ Lithospermic acid/Pentahydroxy dihydrochalcone derivative | - | - | [4] |
| L. graveolens | C02 SCFE (80 bar 35 °C/ atmospheric 20 °C) | Eriodyctiol /Naringenin/ Sakuranetin/ Cirsimaritin/ Chrysoeriol | 4.9 ± 0.2 B/4.6 ± 0.2 B/3.1 ± 0.29 B/2.34 ± 0.04 B/1.3 ± 0.1 B | - | [15] | |
| L. graveolens | Solid: liquid (methanol/acetone/water) (50:40:10) | Quercetin-O-hexoside/ Scutellarein 7-O-hexoside/ Phloridzin/ Trihydroxy-methoxyflavone derivative/ 6-O-Methylscutellarein | - | - | [25] | |
| L. graveolens | Solid: liquid 1:20 (58% ethanol) | Pinocembrin/ Galangin/ phlorizin/ Naringenin/Quercetin/ Hispidulin/ Taxifolin | 3.231 ± 0.390 A/ 3.231 ± 0.390 A/3.231 ± 0.390 A/3.231 ± 0.390 A/ 0.018 ± 0.008 A/0.022 ± 0.003 A/0.022 ± 0.003 A | [26] | ||
| Essential oil | L. berlandieri | Commercial | p-cymene/Carvacrol/ β-pinene/Caryophyllene/ Camphene/ α-pinene | - | 35.54/26.86/4.69/4.50/4.10/3.89 | [13] |
| P. longiflora | Clevenger-type apparatus | Carvacrol/ Thymol acetate/ Carvacrol, methyl ether/ Terpinolene/ p-cymene/ Borneol/ β-pinene | - | 23.31/17.06/7.81/6.96/6.7/4.36/ 3.57 | [19] | |
| L. graveolens | Hydro-distillation | Different chemotypes Carvacrol/ Thymol/ β-caryophyllene | - | 53.05/70.6/27.6 | [27] | |
| L. graveolens | Commercial | Thymol/ p-cymene/ Carvacrol/ β-caryophyllene/1,8-Cineole/ γ-terpinene | - | 31.66/18.72/14.57/5.62/3.44/2.42 | [28] | |
| Specie | Analyzed Fraction | Extraction Technique | Tissue | Evaluated Bioactivity | Results | Reference | |
|---|---|---|---|---|---|---|---|
| L. berlandieri | Essential oil | - | Leaves | Antimicrobial | E. coli/MRSA/A. niger | 4/lower than 5/0.28 (MIC, μg mL−1 of air) | [13] |
| P. longiflora | Essential oil Et-OH extract Ethyl acetate extract | Hydrodistillation/Diffusion | Leaves | Antioxidant activity | DPPH● S. aureus/B. cereus | 83.70 ± 4.12 EO, 151.90 ± 6.65 E-OH, 208.60 ± 12.25 Et-Ac. (IC50, μg mL−1) | [19] |
| Antimicrobial activity | 250/250 EO, 1000/750 E-OH, 750/500 E-Ac (MIC, mg L−1) | ||||||
| L. graveolens L. palmeri | Chloroform/ methanol extracts | Agitation/Sonication | Leaves | Antiflammatory | ROS reduction COX-1 and 2 cyclooxygenases inhibition | 59.8% to 87% COX-1 78.2%/64.7%/67.8% COX-2 81.7%/74.6%/64.7% | [25] |
| L. graveolens | Essential oil | - | Leaves | Antimicrobial | Candida albicans | 6.4 to 21.5 (MLC99, μL mL−1 emulsifier agent) | [28] |
| L. graveolens | Methanolic extract | Maceration | Aerial parts | Antioxidant/UV protection | DPPH● In vivo penetration study | 21.89 ± 0.63 (IC50, μg mL−1) 20.14 ± 1.86 (μg cm−2) | [94] |
| L. graveolens | Methanol extract | Percolation | Leaves/flowers | Antiglycemic | α-glucosidase inhibition | IC50 = 37.19 μM (Hispidulin). | [95] |
| Anti-inflammatory | Antiflammatory | IC50 = 0.72–1.31 μmol/ear (Naringenin, Eriodictyol and 3-Hydroxyphloridzin). | |||||
| Cytotoxicity | U251 & SK-LU-1 human tumor cell lines. | U251 (IC50 = 37.0 µM) SK-LU-1 (IC50 = 37.5 µM) | |||||
| Patent Number | Title | Main Core | Scope | Publication Data | Country | References |
|---|---|---|---|---|---|---|
| ES2678597 | Smokable remedial herb blend | The present invention refers to a mixture of smokable herbs, which are part of a method of smoking cessation and help to clean and regenerate the lung of the tobacco smoker. | Health | 14 August 2018 | Spain | [106] |
| ES2613240 | Composition of ice with antimicrobial activity, manufacturing method, and its applications. | The invention comprises a solution of frozen drinking water and inclusion complexes formed by essential oils nano encapsulated with cyclodextrins. | Food technology | 23 May 2017 | Spain | [107] |
| ES2351116 | Antimicrobial therapeutic compositions and procedures for use. | The present invention relates to an injectable solution containing isolated carvacrol and thymol of natural origin for intramuscular or intravenous administration. | Health | 31 January 2011 | Spain | [108] |
| MXNL/A/ 2006/000057 | Natural compounds having antimicrobial activity for preventing and controlling infectious diseases in humans and food. | The present invention refers to the use of water-soluble extracts in different concentrations of Larrea tridentata added with other natural products such as extracts and essential oils of the leaves of L. graveolens and other plants known to have an antimicrobial effect | Health | 15 August 2006 | Mexico | [109] |
| ES2628278 | Biostimulant formulation of plant growth and development and inducer of resistance for the control of diseases caused by phytopathogenic viruses and method of preparation. | The biostimulant formulation is composed of extracts, vegetable oils from varieties of Chihuahuan semi-desert plants, absolute oils, and extracts from aromatic plants. | Agricultural biotechnology | 2 August 2017 | Spain | [110] |
| MX365079 | Process for the regeneration of Mexican oregano plants (of the genus Poliomintha) by indirect organogenesis. | The invention refers to a process for the in vitro regeneration of Mexican oregano (Poliomintha genus), which allows a complete plant to be obtained from an explant. | Agricultural biotechnology | 28 February 2014 | Mexico | [111] |
| MX2017013652 | Method to increase the secondary metabolites in candelilla and oregano. | The present invention relates to a method for increasing the secondary metabolites in crops such as candelilla and oregano by abiotic stressing. | Agronomic | 24 October 2019 | Mexico | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bautista-Hernández, I.; Aguilar, C.N.; Martínez-Ávila, G.C.G.; Torres-León, C.; Ilina, A.; Flores-Gallegos, A.C.; Kumar Verma, D.; Chávez-González, M.L. Mexican Oregano (Lippia graveolens Kunth) as Source of Bioactive Compounds: A Review. Molecules 2021, 26, 5156. https://doi.org/10.3390/molecules26175156
Bautista-Hernández I, Aguilar CN, Martínez-Ávila GCG, Torres-León C, Ilina A, Flores-Gallegos AC, Kumar Verma D, Chávez-González ML. Mexican Oregano (Lippia graveolens Kunth) as Source of Bioactive Compounds: A Review. Molecules. 2021; 26(17):5156. https://doi.org/10.3390/molecules26175156
Chicago/Turabian StyleBautista-Hernández, Israel, Cristóbal N. Aguilar, Guillermo C. G. Martínez-Ávila, Cristian Torres-León, Anna Ilina, Adriana C. Flores-Gallegos, Deepak Kumar Verma, and Mónica L. Chávez-González. 2021. "Mexican Oregano (Lippia graveolens Kunth) as Source of Bioactive Compounds: A Review" Molecules 26, no. 17: 5156. https://doi.org/10.3390/molecules26175156
APA StyleBautista-Hernández, I., Aguilar, C. N., Martínez-Ávila, G. C. G., Torres-León, C., Ilina, A., Flores-Gallegos, A. C., Kumar Verma, D., & Chávez-González, M. L. (2021). Mexican Oregano (Lippia graveolens Kunth) as Source of Bioactive Compounds: A Review. Molecules, 26(17), 5156. https://doi.org/10.3390/molecules26175156










