An LC-MS/MS Method for Analysis of Vitamin D Metabolites and C3 Epimers in Mice Serum: Oral Supplementation Compared to UV Irradiation
Abstract
:1. Introduction
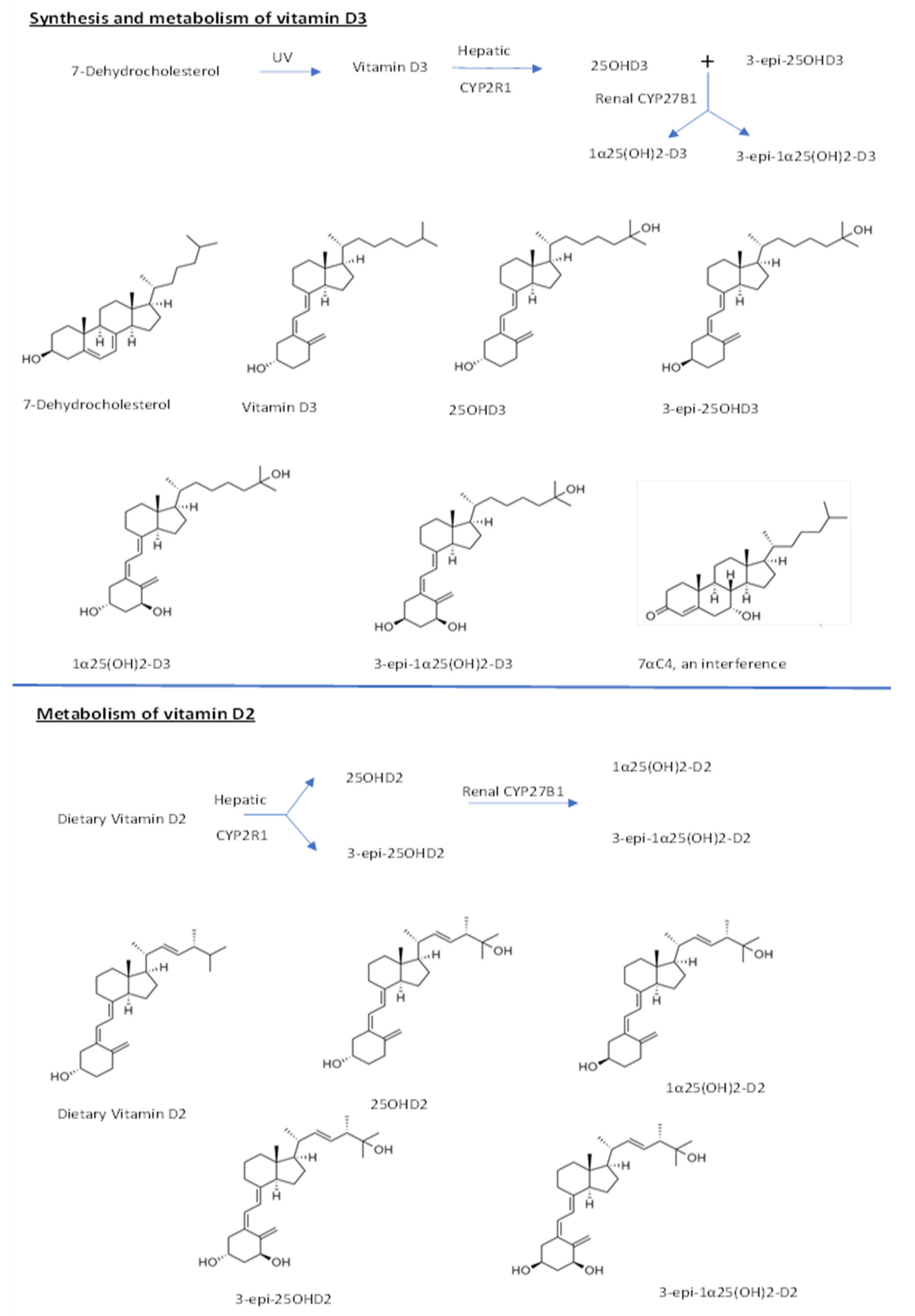
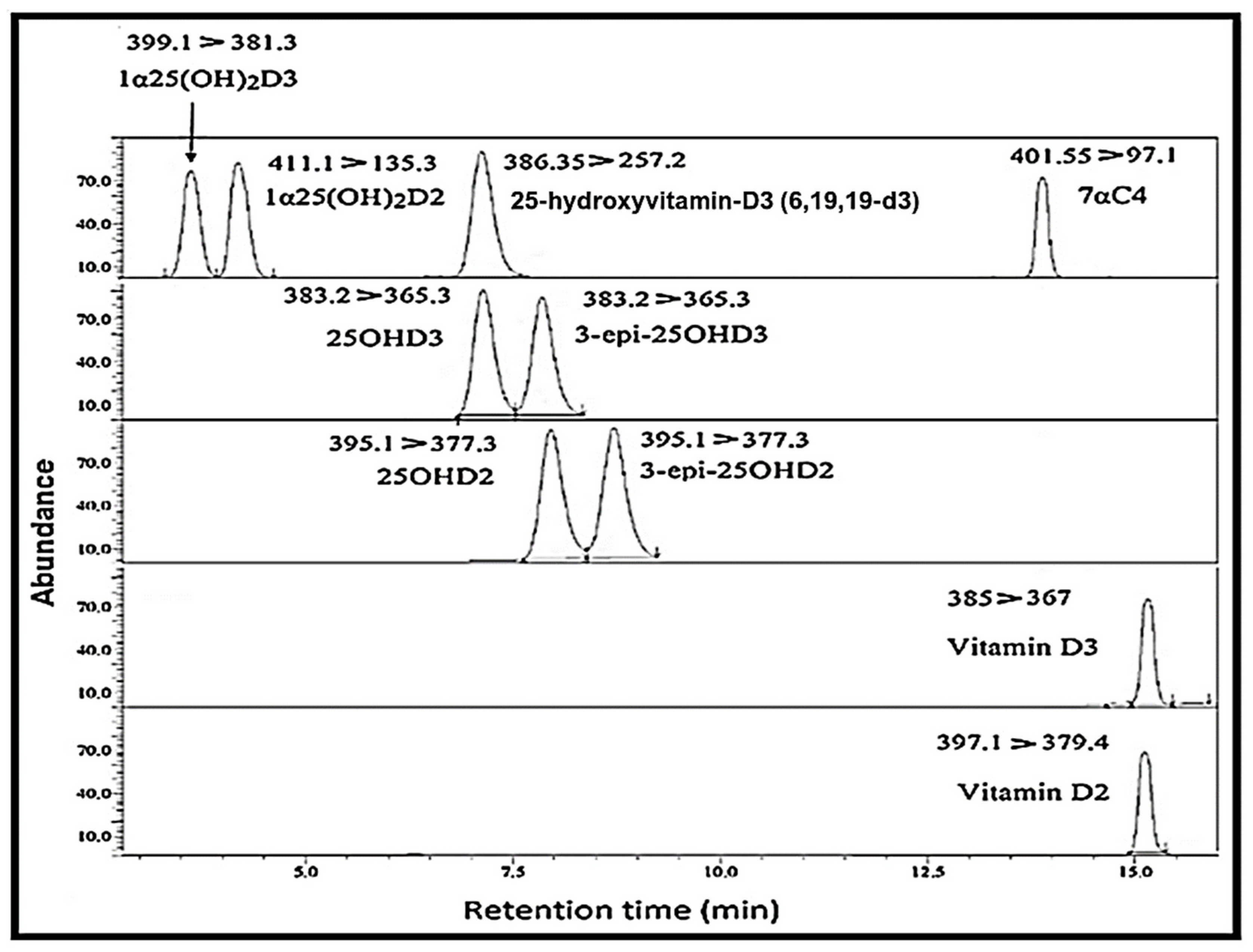
2. Results
3. Discussion
4. Materials and Methods
4.1. Mice Sample Analysis
4.2. LC-MS/MS System
4.3. Method Validation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, L.; Xiao, H. Inflammation in diabetes and cardiovascular disease: A new perspective on vitamin D. Cardiovasc. Endocrinol. Metab. 2015, 4, 127–131. [Google Scholar] [CrossRef]
- Manucha, W.; Juncos, L.I. The protective role of vitamin D on the heart and the kidney. Ther. Adv. Cardiovasc. Dis. 2017, 11, 12–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeLuca, G.; Kimball, S.; Kolasinski, J.; Ramagopalan, S.; Ebers, G. The role of vitamin D in nervous system health and disease. Neuropathol. Appl. Neurobiol. 2013, 39, 458–484. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M.F. Vitamin D—effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 2013, 5, 111–148. [Google Scholar] [CrossRef] [Green Version]
- Khazai, N.; Judd, S.E.; Tangpricha, V. Calcium and vitamin D: Skeletal and extraskeletal health. Curr. Rheumatol. Rep. 2008, 10, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Anagnostis, P.; Karras, S.; Goulis, D.G. Vitamin D in human reproduction: A narrative review. Int. J. Clin. Pract. 2013, 67, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Kamr, A.M.; Dembek, K.A.; Reed, S.M.; Slovis, N.M.; Zaghawa, A.A.; Rosol, T.J.; Toribio, R.E. Vitamin D metabolites and their association with calcium, phosphorus, and PTH concentrations, severity of illness, and mortality in hospitalized equine neonates. PLoS ONE 2015, 10, e0127684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, J.G.; Bushinsky, D.A. Calcium and phosphorus homeostasis. Blood Purif. 2009, 27, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Bellavia, D.; Costa, V.; de Luca, A.; Maglio, M.; Pagani, S.; Fini, M.; Giavaresi, G. Vitamin D level between calcium-phosphorus homeostasis and immune system: New perspective in osteoporosis. Curr. Osteoporos. Rep. 2016. [Google Scholar] [CrossRef]
- Hermann, M.; Ruschitzka, F. Vitamin D and hypertension. Curr. Hypertens. Rep. 2008, 10, 49–51. [Google Scholar] [CrossRef]
- Legarth, C.; Grimm, D.; Wehland, M.; Bauer, J.; Krüger, M. The impact of vitamin D in the treatment of essential hypertension. Int. J. Mol. Sci. 2018, 19, 455. [Google Scholar] [CrossRef] [Green Version]
- Szeto, C.-C.; Li, P.K.-T. The use of vitamin D analogues in chronic kidney diseases: Possible mechanisms beyond bone and mineral metabolism. NDT Plus 2009, 2, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Negrea, L. Active vitamin D in chronic kidney disease: Getting right back where we started from? Kidney Dis. 2019, 5, 59–68. [Google Scholar] [CrossRef]
- Trémezaygues, L.; Reichrath, J. Vitamin D analogs in the treatment of psoriasis: Where are we standing and where will we be going? Derm.-Endocrinol. 2011, 3, 180–186. [Google Scholar] [CrossRef]
- Ma, Y.; Trump, D.L.; Johnson, C.S. Vitamin D in combination cancer treatment. J. Cancer 2010, 1, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dankers, W.; Colin, E.M.; van Hamburg, J.P.; Lubberts, E. Vitamin D in autoimmunity: Molecular mechanisms and therapeutic potential. Front. Immunol. 2017, 7, 697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, S.R.; Li, D.; Jeffery, L.E.; Raza, K.; Hewison, M. Vitamin D, autoimmune disease and rheumatoid arthritis. Calcif. Tissue Int. 2019, 106, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.; Frommer, J.; McNeill, S.; Richtand, N.; Henley, J.; Potts, J., Jr. Photometabolism of 7-dehydrocholesterol to previtamin D3 in skin. Biochem. Biophys. Res. Commun. 1977, 76, 107–114. [Google Scholar] [CrossRef]
- Anderson, P.; O’Loughlin, P.; May, B.; Morris, H. Quantification of mRNA for the vitamin D metabolizing enzymes CYP27B1 and CYP24 and vitamin D receptor in kidney using real-time reverse transcriptase-polymerase chain reaction. J. Mol. Endocrinol. 2003, 31, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Kamao, M.; Tatematsu, S.; Hatakeyama, S.; Sakaki, T.; Sawada, N.; Inouye, K.; Ozono, K.; Kubodera, N.; Reddy, G.S.; Okano, T. C-3 epimerization of vitamin D3 metabolites and further metabolism of C-3 epimers: 25-hydroxyvitamin D3 is metabolized to 3-epi-25-hydroxyvitamin D3 and subsequently metabolized through C-1α or C-24 hydroxylation. J. Biol. Chem. 2004, 279, 15897–15907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jäpelt, R.B.; Jakobsen, J. Vitamin D in plants: A review of occurrence, analysis, and biosynthesis. Front. Plant Sci. 2013, 4, 136. [Google Scholar] [CrossRef] [Green Version]
- Black, L.J.; Lucas, R.M.; Sherriff, J.L.; Björn, L.O.; Bornman, J.F. In pursuit of vitamin D in plants. Nutrients 2017, 9, 136. [Google Scholar] [CrossRef] [Green Version]
- Al-Zohily, B.; Al-Menhali, A.; Gariballa, S.; Haq, A.; Shah, I. Epimers of vitamin D: A review. Int. J. Mol. Sci. 2020, 21, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djekic-Ivankovic, M.; Lavery, P.; Agellon, S.; Weiler, H.A. The C-3α Epimer of 25-hydroxycholecalciferol from endogenous and exogenous sources supports normal growth and bone mineral density in weanling rats. J. Nutr. 2017, 147, 141–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teegarden, M.D.; Campbell, A.R.; Cooperstone, J.L.; Tober, K.L.; Schwartz, S.J.; Oberyszyn, T.M. 25-Hydroxyvitamin D3 and its C-3 epimer are elevated in the skin and serum of Skh-1 mice supplemented with dietary vitamin D3. Mol. Nutr. Food Res. 2017, 61, 1700293. [Google Scholar] [CrossRef] [PubMed]
- Messerlian, S.; Gao, X.; St-Arnaud, R. The 3-epi-and 24-oxo-derivatives of 1α, 25 dihydroxyvitamin D3 stimulate transcription through the vitamin D receptor. J. Steroid Biochem. Mol. Biol. 2000, 72, 29–34. [Google Scholar] [CrossRef]
- Singh, R.J.; Taylor, R.L.; Reddy, G.S.; Grebe, S.K. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J. Clin. Endocrinol. Metab. 2006, 91, 3055–3061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macova, L.; Šimková, M.; Bicikova, M. The Importance of Determining Vitamin D Epimers, 22nd ed.; European Congress of Endocrinology; BioScientifica: Bristol, UK, 2020. [Google Scholar]
- Aronov, P.A.; Hall, L.M.; Dettmer, K.; Stephensen, C.B.; Hammock, B.D. Metabolic profiling of major vitamin D metabolites using Diels–Alder derivatization and ultra-performance liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2008, 391, 1917. [Google Scholar] [CrossRef] [Green Version]
- Shah, I.; Al-Dabbagh, B.; Gariballa, S.; Al-Menhali, A.; Muhammad, N.; Yasin, J.; Ashraf, S.S. Application of a new vitamin D blood test on the Emirati population. J. Steroid Biochem. Mol. Biol. 2018, 180, 118–128. [Google Scholar] [CrossRef]
- Xiao, J.F.; Zhou, B.; Ressom, H.W. Metabolite identification and quantitation in LC-MS/MS-based metabolomics. TrAC Trends Anal. Chem. 2012, 32, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Shah, I.; James, R.; Barker, J.; Petroczi, A.; Naughton, D.P. Misleading measures in Vitamin D analysis: A novel LC-MS/MS assay to account for epimers and isobars. Nutr. J. 2011, 10, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, I.; Petroczi, A.; Naughton, D.P. Method for simultaneous analysis of eight analogues of vitamin D using liquid chromatography tandem mass spectrometry. Chem. Cent. J. 2012, 6, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cashman, K.D.; Kinsella, M.; Walton, J.; Flynn, A.; Hayes, A.; Lucey, A.J.; Seamans, K.M.; Kiely, M. The 3 epimer of 25-hydroxycholecalciferol is present in the circulation of the majority of adults in a nationally representative sample and has endogenous origins. J. Nutr. 2014, 144, 1050–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, I.; Akhtar, M.K.; Hisaindee, S.; Rauf, M.A.; Sadig, M.; Ashraf, S.S. Clinical diagnostic tools for vitamin D assessment. J. Steroid Biochem. Mol. Biol. 2018, 180, 105–117. [Google Scholar] [CrossRef]
- Chailurkit, L.; Aekplakorn, W.; Ongphiphadhanakul, B. Serum C3 epimer of 25-hydroxyvitamin D and its determinants in adults: A national health examination survey in Thais. Osteoporos. Int. 2015, 26, 2339–2344. [Google Scholar] [CrossRef]
- Lensmeyer, G.; Poquette, M.; Wiebe, D.; Binkley, N. The C-3 epimer of 25-hydroxyvitamin D3 is present in adult serum. J. Clin. Endocrinol. Metab. 2012, 97, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Baur, A.C.; Kühn, J.; Brandsch, C.; Hirche, F.; Stangl, G.I. Intake of ergosterol increases the vitamin D concentrations in serum and liver of mice. J. Steroid Biochem. Mol. Biol. 2019, 194, 105435. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.; Lim, Y. Vitamin D3 improves lipophagy associated renal lipid metabolism and tissue damage in diabetic mice. Nutr. Res. 2020, 80, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Zhao, L.; Ren, F.; Guo, H. Lactoferrin stimulates the expression of vitamin D receptor in vitamin D deficient mice. J. Funct. Foods 2019, 55, 48–56. [Google Scholar] [CrossRef]
- Lin, F.-Y.; Lin, Y.-F.; Lin, Y.-S.; Yang, C.-M.; Wang, C.-C.; Hsiao, Y.-H. Relative D3 vitamin deficiency and consequent cognitive impairment in an animal model of Alzheimer’s disease: Potential involvement of collapsin response mediator protein-2. Neuropharmacology 2020, 164, 107910. [Google Scholar] [CrossRef]
- Ghaly, S.; Bliuc, D.; Center, J.R.; Clarke, M.W.; Jones, A.P.; Trend, S.; Kermode, A.G.; Neale, R.E.; Hart, P.H. Vitamin D C3-epimer levels are proportionally higher with oral vitamin D supplementation compared to ultraviolet irradiation of skin in mice but not humans. J. Steroid Biochem. Mol. Biol. 2019, 186, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Momeni, S.N.; Masoud, S.A.; Banafshe, H.R. Inhibitory effects of chronic administration of vitamin D3 on pentylenetetrazole-induced seizures in mice. Epilepsy Res. 2019, 149, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.G.; Ochalek, J.T.; Kaufmann, M.; Jones, G.; DeLuca, H.F. CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. Proc. Natl. Acad. Sci. USA 2013, 110, 15650–15655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeLuca, H.F. Is there more to learn about functional vitamin D metabolism? J. Steroid Biochem. Mol. Biol. 2015, 148, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Jamali, N.; Sorenson, C.M.; Sheibani, N. Vitamin D and regulation of vascular cell function. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H753–H765. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.T.; Kim, T.K.; Shehabi, H.Z.; Semak, I.; Tang, E.K.; Nguyen, M.N.; Benson, H.A.; Korik, E.; Janjetovic, Z.; Chen, J. In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012, 26, 3901–3915. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.T.; Zmijewski, M.A.; Plonka, P.M.; Szaflarski, J.P.; Paus, R. How UV light touches the brain and endocrine system through skin, and why. Endocrinology 2018, 159, 1992–2007. [Google Scholar] [CrossRef] [Green Version]
- Dimitrov, V.; White, J.H. Species-specific regulation of innate immunity by vitamin D signaling. J. Steroid Biochem. Mol. Biol. 2016, 164, 246–253. [Google Scholar] [CrossRef]
- Bower, J.; Fast, D.; Garofolo, F.; Gouty, D.; Hayes, R.; Lowes, S.; Nicholson, R.; LeLacheur, R.; Bravo, J.; Shoup, R. 8th GCC: Consolidated feedback to US FDA on the 2013 draft FDA guidance on bioanalytical method validation. Bioanalysis 2014, 6, 2957–2963. [Google Scholar] [CrossRef]
- Shah, I.; Petroczi, A.; Naughton, D.P. Exploring the role of vitamin D in type 1 diabetes, rheumatoid arthritis, and Alzheimer disease: New insights from accurate analysis of 10 forms. J. Clin. Endocrinol. Metab. 2014, 99, 808–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Time | Quality Control Concs. | 25OHD3 Conc. (ng/mL) | 25OHD2 Conc. (ng/mL) | 3-epi-25OHD3 Conc. (ng/mL) | 3-epi-25OHD2 Conc. (ng/mL) | 7αC4 Conc. (ng/mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Precision (% CV) | Accuracy % | SD | Precision (% CV) | Accuracy % | SD | Precision (% CV) | Accuracy % | SD | Precision (% CV) | Accuracy % | SD | Precision (% CV) | Accuracy % | SD | ||
| Intra-day | QCH 100 | 6.1 | 100 | 6.2 | 11.7 | 99 | 11.2 | 5.2 | 114 | 6.1 | 5.3 | 97 | 8.2 | 9.3 | 95 | 9.2 |
| QCM 50 | 9.4 | 114 | 7.5 | 6.6 | 111 | 10.1 | 8.3 | 106 | 5.0 | 10.2 | 110 | 6.0 | 11.1 | 99 | 6.4 | |
| QCL 12 | 7.3 | 103 | 1.6 | 6.4 | 93 | 1.4 | 8.1 | 108 | 1.2 | 4.8 | 104 | 6.3 | 13.6 | 85 | 1.1 | |
| 1α25(OH)2-D3 Conc. (pg/mL) | 1α25(OH)2-D2 Conc. (pg/mL) | Vitamin-D3 Conc. (ng/mL) | Vitamin-D2 Conc. (ng/mL) | |||||||||||||
| QCH 100 | 5.2 | 104 | 5.2 | 8.4 | 104 | 8.5 | 10.5 | 103 | 12.1 | 13.2 | 108 | 4.0 | ||||
| QCM 50 | 14.1 | 105 | 7.1 | 9.7 | 111 | 5.4 | 9.2 | 101 | 4.3 | 11.2 | 108 | 6.7 | ||||
| QCL 12 | 8.5 | 88 | 1.7 | 8.8 | 90 | 1.9 | 12.1 | 85 | 1.1 | 16.0 | 98 | 12.2 | ||||
| Inter-day | 25OHD3 Conc. (ng/mL) | 25OHD2 Conc. (ng/mL) | 3-epi-25OHD3 Conc. (ng/mL) | 3-epi-25OHD2 Conc. (ng/mL) | 7αC4 Conc. (ng/mL) | |||||||||||
| QCH 100 | 7.5 | 98 | 11.3 | 9.1 | 95 | 9.2 | 8.3 | 95 | 7.2 | 8.0 | 111 | 9.1 | 4.0 | 96 | 5.3 | |
| QCM 50 | 8.3 | 97 | 13.1 | 13.8 | 114 | 8.6 | 7.2 | 97 | 16.1 | 13.7 | 109 | 9.5 | 14.2 | 100 | 10.1 | |
| QCL 12 | 8.1 | 102 | 1.6 | 5.5 | 96 | 1.9 | 6.5 | 99 | 1.2 | 11.1 | 90 | 1.5 | 9.1 | 75 | 1.5 | |
| 1α25(OH)2-D3 Conc. (pg/mL) | 1α25(OH)2-D2 Conc. (pg/mL) | Vitamin-D3 Conc. (ng/mL) | Vitamin-D2 Conc. (ng/mL) | |||||||||||||
| QCH 100 | 4.5 | 102 | 10.2 | 5.5 | 99 | 5.2 | 6.5 | 95 | 6.1 | 15.2 | 110 | 12.3 | ||||
| QCM 50 | 14.8 | 111 | 8.3 | 10.1 | 109 | 6.3 | 14.1 | 91 | 7.5 | 6.0 | 115 | 4.0 | ||||
| QCL 12 | 7.0 | 111 | 1.9 | 11.8 | 96 | 1.6 | 8.7 | 70 | 2.8 | 13.5 | 95 | 4.7 | ||||
| Group | Light(h/day) | Dark (h/Day) | Vitamin D Amount in Diet |
|---|---|---|---|
| SDL | 12 h/day | 12 h/day | Standard |
| SDD | 0 h/day | 24 h/day | Standard |
| DDD | 0 h/day | 24 h/day | Deficient |
| No. | Analytes | Retention Time (min) | Precursor (m/z) | Product (m/z) | Collision Energy (eV) |
|---|---|---|---|---|---|
| 1 | Vitamin-D3 | 15.104 | 385 | 367 * (quantifier) | −13 |
| 259 (qualifier) | −16 | ||||
| 91 | −56 | ||||
| 2 | Vitamin-D2 | 15.072 | 397.1 | 379.4 * | −17 |
| 69 | −19 | ||||
| 3 | 25OHD3 | 6.98 | 383.2 | 365.3 * | −15 |
| 107.1 | −30 | ||||
| 4 | 25OHD2 | 7.76 | 395.1 | 377.3 * | −17 |
| 81.1 | −38 | ||||
| 5 | 3-epi-25OHD3 | 7.69 | 383.2 | 365.3 * | −15 |
| 107.1 | −30 | ||||
| 6 | 3-epi-25OHD2 | 8.52 | 395.1 | 377.1 * | −17 |
| 81.1 | −38 | ||||
| 7 | 1α25(OH)2D3 | 3.82 | 399.1 | 381.3 * | −14 |
| 8 | 1α25(OH)2D2 | 3.948 | 411.1 | 135.3 * | −13 |
| 133.1 | −12 | ||||
| 9 | 7αC4 | 14.48 | 401.5 | 383.25 | −16 |
| 97.1 * | −29 | ||||
| 91.2 | −23 | ||||
| 10 | IS [25 hydroxyvitamin-D3 (6,19,19-d3)] | 6.97 | 386.3 | 368.2 | −15 |
| 257.2 * | −183 | ||||
| 95.2 | −35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohail, A.; Al Menhali, A.; Hisaindee, S.; Shah, I. An LC-MS/MS Method for Analysis of Vitamin D Metabolites and C3 Epimers in Mice Serum: Oral Supplementation Compared to UV Irradiation. Molecules 2021, 26, 5182. https://doi.org/10.3390/molecules26175182
Sohail A, Al Menhali A, Hisaindee S, Shah I. An LC-MS/MS Method for Analysis of Vitamin D Metabolites and C3 Epimers in Mice Serum: Oral Supplementation Compared to UV Irradiation. Molecules. 2021; 26(17):5182. https://doi.org/10.3390/molecules26175182
Chicago/Turabian StyleSohail, Amir, Asma Al Menhali, Soleiman Hisaindee, and Iltaf Shah. 2021. "An LC-MS/MS Method for Analysis of Vitamin D Metabolites and C3 Epimers in Mice Serum: Oral Supplementation Compared to UV Irradiation" Molecules 26, no. 17: 5182. https://doi.org/10.3390/molecules26175182







