Apigenin Ameliorates Scopolamine-Induced Cognitive Dysfunction and Neuronal Damage in Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Apigenin on the Body Weight Change and Organs Weight in Scopolamine-Injected Mice
2.2. Effect of Apigenin on the T-Maze Test in Scopolamine-Injected Mice
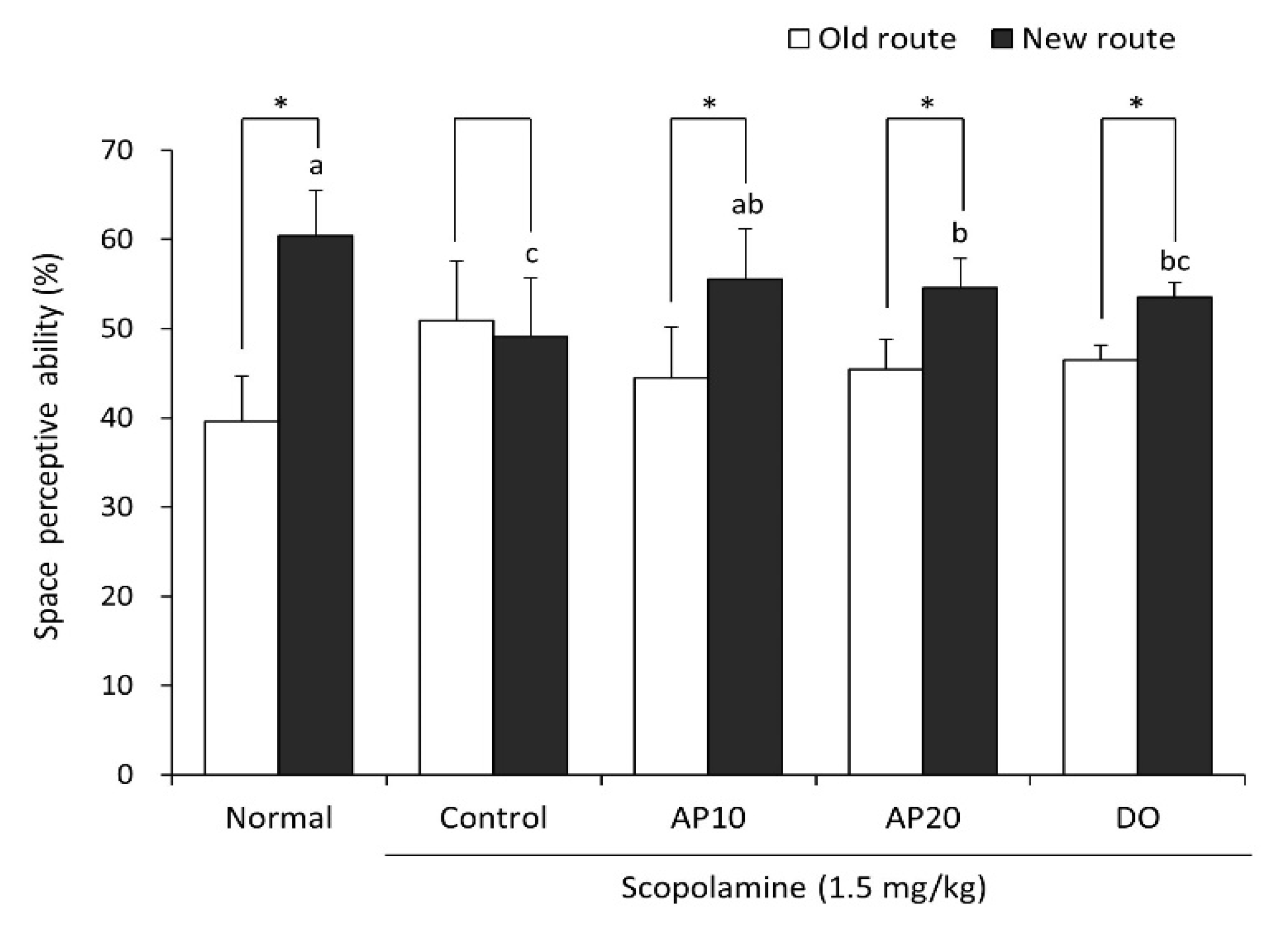
2.3. Effect of Apigenin on Novel Object Recognition Test in Scopolamine-Injected Mice
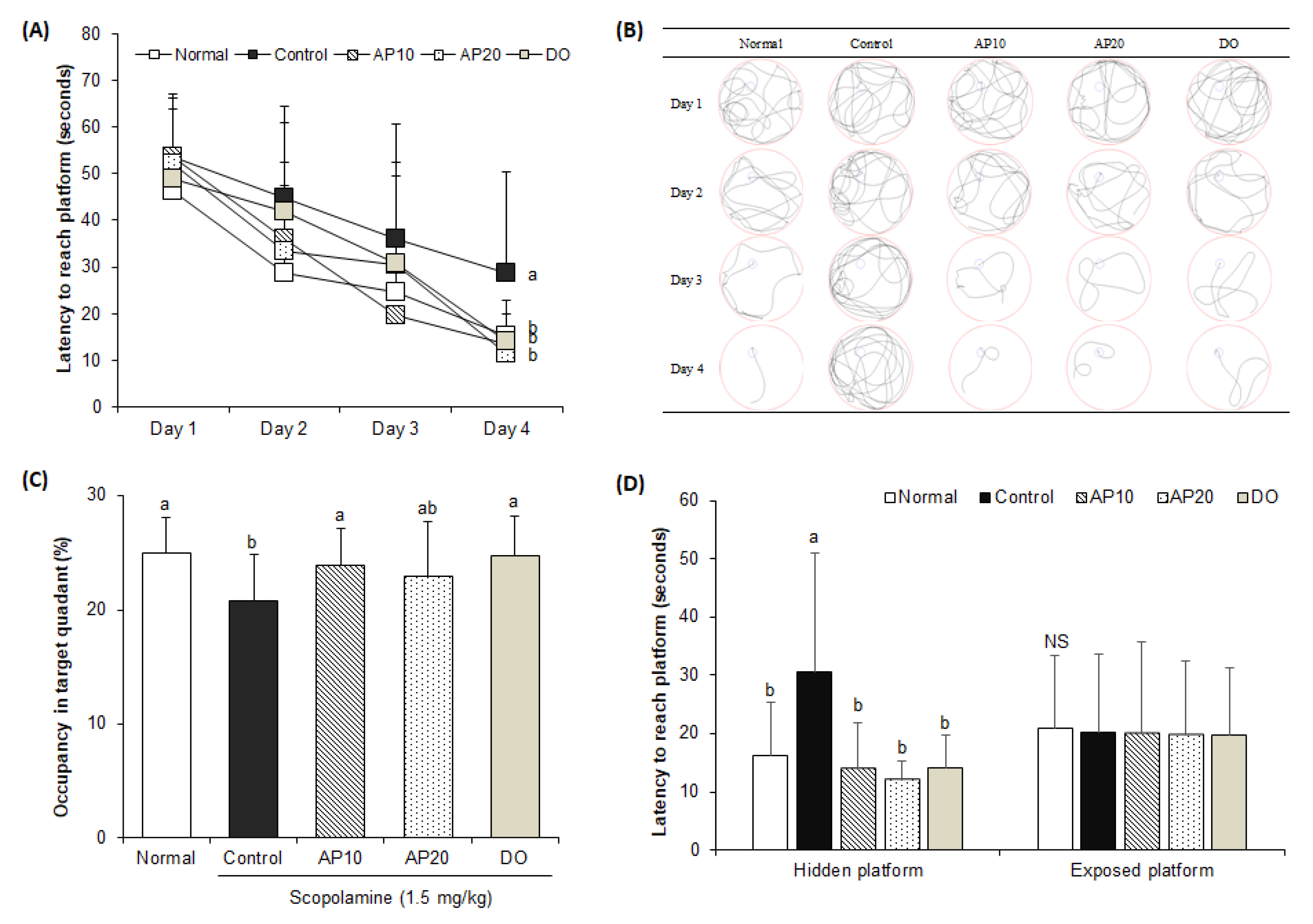
2.4. Effect of Apigenin on Morris Water Maze Test in Scopolamine-Injected Mice
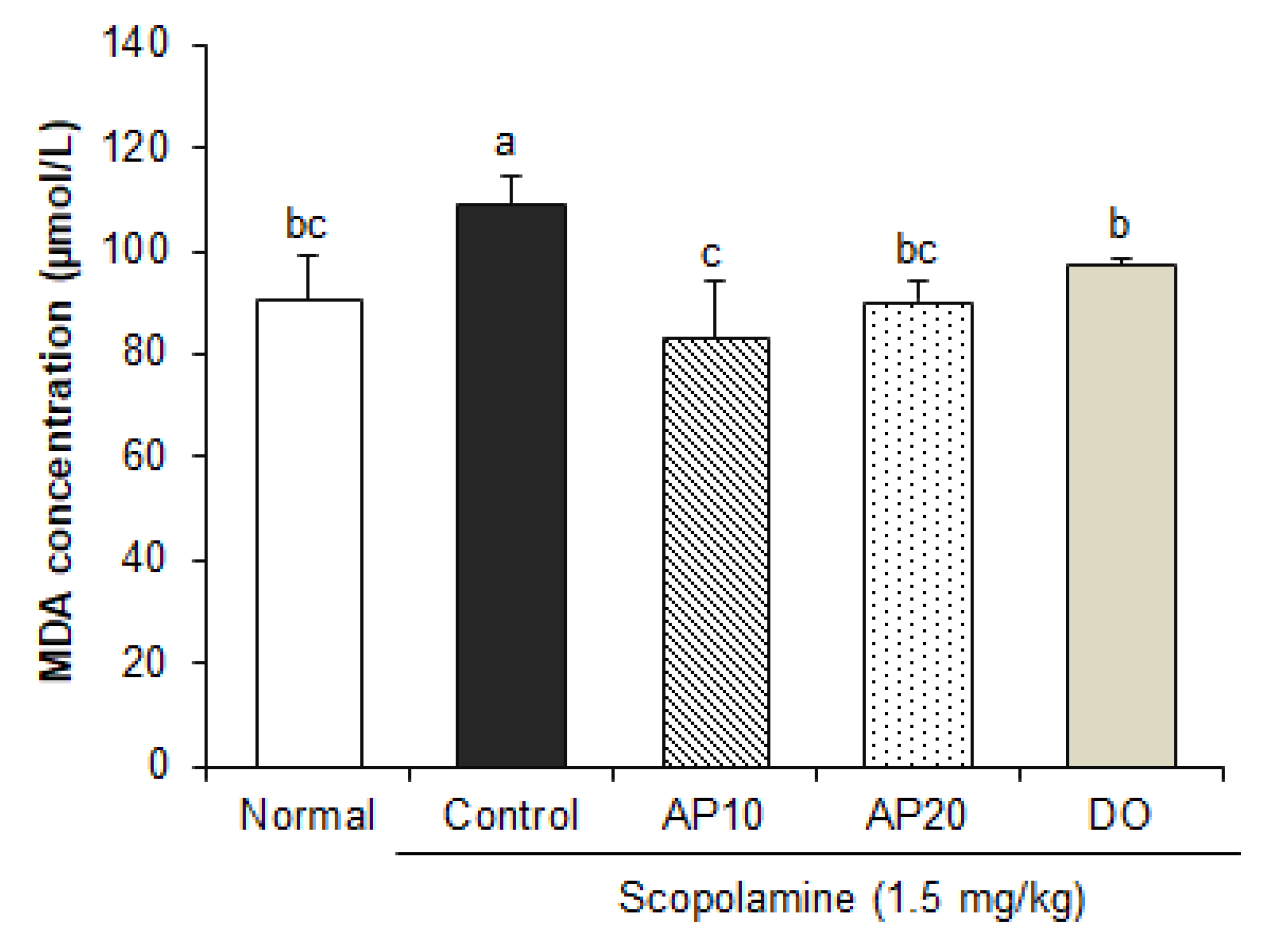
2.5. Effect of Apigenin on Lipid Peroxidation in the Brain of Scopolamine-Injected Mice
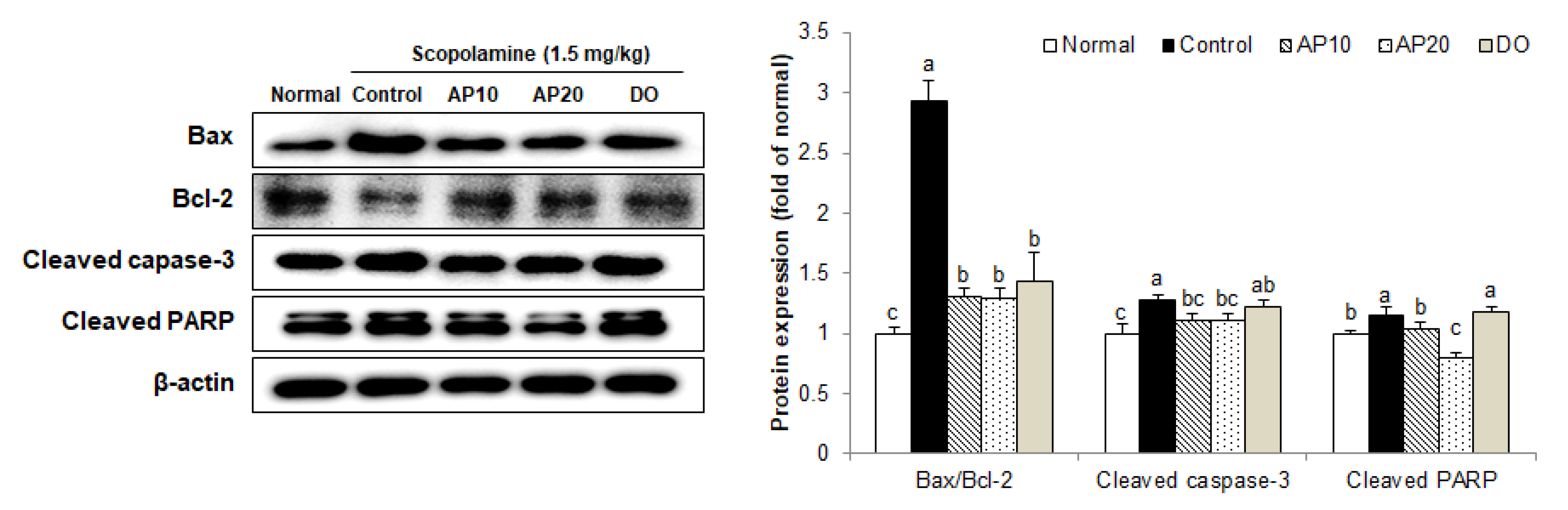
2.6. Effect of Apigenin on Apoptosis-Related Protein Expressions in the Brains of Scopolamine-Injected Mice
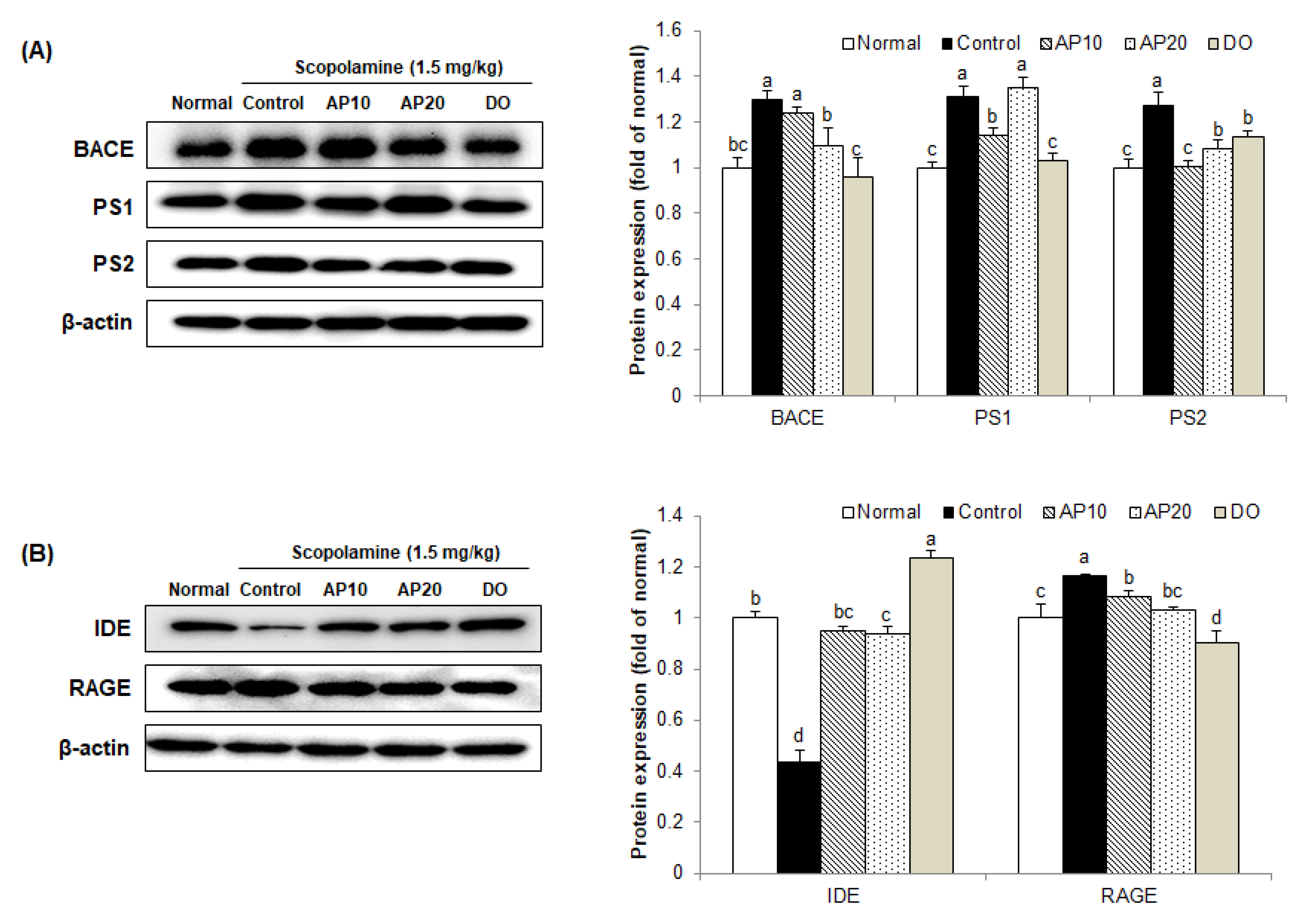
2.7. Effect of Apigenin on Amyloidogenic Pathway in the Brains of Scopolamine-Injected Mice
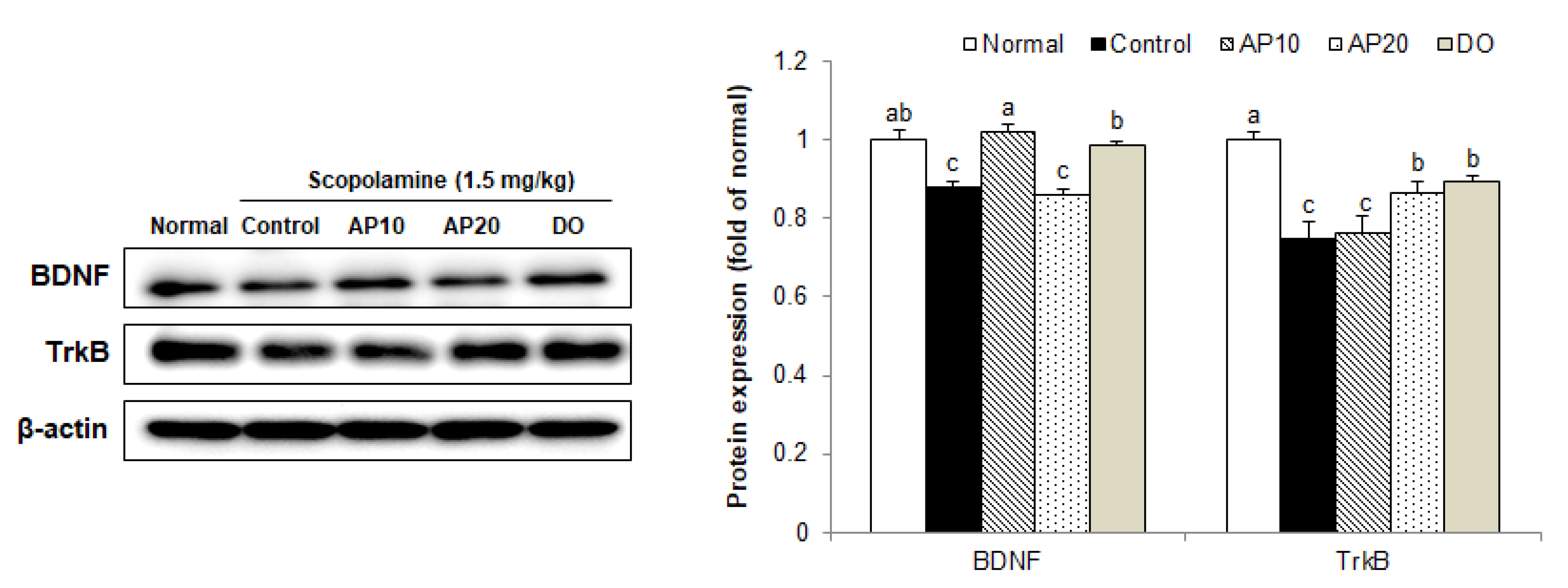
2.8. Effect of Apigenin on TrkB/BDNF Pathway Protein Expressions in the Brains of Scopolamine-Injected Mice
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Animals and Experimental Protocols
3.3. T-Maze Test
3.4. Novel Object Recognition Test
3.5. Morris Water Maze Test
3.6. Measurement of MDA Levels
3.7. Western Blot Analysis
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Stuchlik, A. Dynamic learning and memory, synaptic plasticity and neurogenesis: An update. Front. Behav. Neurosci. 2014, 8, 106. [Google Scholar] [CrossRef] [Green Version]
- Salim, S. Oxidative stress and the central nervous system. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Michaelis, E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Lazarov, O.; Mattson, M.P.; Peterson, D.A.; Pimplikar, S.W.; van Praag, H. When neurogenesis encounters aging and disease. Trends. Neurosci. 2010, 33, 569–579. [Google Scholar] [CrossRef] [Green Version]
- Perrin, R.J.; Fagan, A.M.; Holtzman, D.M. Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature 2009, 461, 916–922. [Google Scholar] [CrossRef]
- Graham, W.V.; Bonito-Oliva, A.; Sakmar, T.P. Update on Alzheimer’s disease therapy and prevention strategies. Annu. Rev. Med. 2017, 68, 413–430. [Google Scholar] [CrossRef] [Green Version]
- Ariomon, M.; Takeda, S.; Post, K.L.; Svirsky, S.; Hyman, B.T.; Berezovska, O. Oxidative stress and lipid peroxidation are upstream of amyloid pathology. Neurobiol. Dis. 2015, 84, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Lauderback, C.M. Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: Potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic. Biol. Med. 2002, 32, 1050–1060. [Google Scholar] [CrossRef]
- Parfenova, H.; Basuroy, S.; Bhattacharya, S.; Techranova, D.; Qu, Y.; Regan, R.F.; Leffler, C.W. Glutamate induces oxidative stress and apoptosis in cerebral vascular endothelial cells: Contributions of HO-1 and HO-2 to cytoprotection. Am. J. Physiol. Cell Physiol. 2006, 290, C1399–C1410. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Maier, O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: Role of TNF. Oxid. Med. Cell Longev. 2015, 2015, 610813. [Google Scholar] [CrossRef] [Green Version]
- Swomley, A.M.; Butterfield, D.A. Oxidative stress in Alzheimer disease and mild cognitive impairment: Evidence from human data provided by redox proteomics. Arch. Toxicol. 2015, 89, 1669–1680. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Dimayuga, E.; Keller, J.N. Oxidative damage, protein synthesis, and protein degradation in Alzheimer’s disease. Curr. Alzheimer Res. 2007, 4, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.H.; He, Z.; Peng, Y.L.; Jin, W.D.; Wang, Z.; Mu, L.Y.; Chang, M.; Wang, R. Phoenixin-14 enhances memory and mitigates memory impairment induced by Aβ1-42 and scopolamine in mice. Brain Res. 2015, 1629, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.S. The cellular and molecular processes associated with scopolamine-induced memory deficit: A model of Alzheimer’s biomarkers. Life Sci. 2019, 233, 116695. [Google Scholar] [CrossRef]
- Aydin, E.; Hritcu, L.; Dogan, G.; Hayta, S.; Bagci, E. The effects of inhaled Pimipinella peregrina essential oil on scopolamine-induced memory impairment, anxiety, and depression in laboratory rats. Mol. Neurobiol. 2016, 53, 6557–6567. [Google Scholar] [CrossRef] [PubMed]
- Falsafi, S.K.; Deli, A.; Hoger, H.; Pollak, A.; Lubec, G. Scopolamine administration modulates muscarinic, nicotinic and NMDA receptor systems. PLoS ONE 2012, 7, e32082. [Google Scholar] [CrossRef]
- Jeong, E.J.; Lee, K.Y.; Kim, S.H.; Sung, S.H.; Kim, Y.C. Cognitive-enhancing and antioxidant activities of iridoid glycosides from Scrophularia buergeriana in scopolamine-treated mice. Eur. J. Pharmacol. 2008, 588, 78–84. [Google Scholar] [CrossRef]
- Safar, M.M.; Arab, H.H.; Rizk, S.M.; EI-Maraghy, S.A. Bone marrow-derived endothelial progenitor cells protect against scopolamine-induced Alzheimer-like pathological aberrations. Mol. Neurobiol. 2016, 53, 1403–1418. [Google Scholar] [CrossRef]
- Chen, B.H.; Park, J.H.; Lee, T.K.; Song, M.; Kim, H.; Lee, J.C.; Kim, Y.M.; Lee, C.H.; Hwang, I.W.; Kang, I.J.; et al. Melatonin attenuates scopolamine-induced cognitive impairment via protecting against demyelination through BDNF-TrkB signaling in the mouse dentate gyrus. Chem.-Biol. Interact. 2018, 285, 8–13. [Google Scholar] [CrossRef]
- Craig, L.A.; Hong, N.S.; McDonald, R.J. Revisiting the cholinergic hypothesis in the development of Alzheimer’s disease. Neurosci. Biobehav. Rev. 2011, 35, 1397–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doody, R.S. Refining treatment guidelines in Alzheimer’s disease. Geriatrics 2005, 2005, S14–S20. [Google Scholar]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods Release 3.1. 2014. Available online: https://www.ars.usda.gov/ARSUserFiles/80400525/Data/Flav/Flav_R03-1.pdf (accessed on 10 July 2017).
- Pang, L.; Zou, S.; Shi, Y.; Mao, Q.; Chen, Y. Apigenin attenuates PM2.5-induced airway hyperresponsiveness and inflammation by down-regulating NF-κB in murine model of asthma. Int. J. Clin. Exp. Pathol. 2019, 12, 3700–3709. [Google Scholar]
- Vargo, M.A.; Voss, O.H.; Poustka, F.; Cardounel, A.J.; Grotewold, E.; Doseff, A.I. Apigenin-induced-apoptosis is mediated by the activation of PKCdelta and caspases in leukemia cells. Biochem. Pharmacol. 2006, 72, 681–692. [Google Scholar] [CrossRef]
- Horváthová, K.; Novotný, L.; Tóthová, D.; Vachálková, A. Determination of free radical scavenging activity of quercetin, rutin, luteolin and apigenin in H2O2-treated human ML cells K562. Neoplasma 2004, 51, 395–399. [Google Scholar]
- Liu, R.; Zhang, T.; Yang, H.; Lan, X.; Ying, J.; Du, G. The flavonoid apigenin protects brain neurovascular coupling against amyloid-β25-35-induced toxicity in mice. J. Alzhimers Dis. 2011, 24, 85–100. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.L.; Liu, R.; Li, X.X.; Li, J.F.; Zhang, L. Neuroprotective, anti-amyloidogenic and neurotrophic effects of apigenin in an Alzheimer’s disease mouse model. Molecules 2013, 18, 9949–9965. [Google Scholar] [CrossRef]
- Spangler, E.L.; Rigby, P.; Ingram, D.K. Scopolamine impairs learning performance of rats in a 14-unit T-maze. Pharmacol. Biochem. Behav. 1986, 25, 673–679. [Google Scholar] [CrossRef]
- Cohen, S.J.; Stackman, R.W., Jr. Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav. Brain Res. 2015, 285, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef] [Green Version]
- Nikbakht, F.; Khadem, Y.; Haghani, S.; Hoseininia, H.; Sadat, A.M.; Heshemi, P.; Jamali, N. Protective role of apigenin against Aβ 25-35 toxicity via inhibition of mitochondrial cytochrome c release. Basic Clin. Neurosci. 2019, 10, 557–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Xie, W.; Xie, W.; Zhuang, W.; Jiang, C.; Liu, N. Apigenin attenuates isoflurane-induced cognitive dysfunction via epigenetic regulation and neuroinflammation in aged rats. Arch. Gerontol. Geriatr. 2017, 73, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Castegna, A.; Lauderback, C.M.; Drake, J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer’s disease brain contribute to neuronal death. Neurobiol. Aging 2002, 23, 655–664. [Google Scholar] [CrossRef]
- Guo, C.; Shen, J.; Meng, Z.; Yang, X.; Li, F. Neuroprotective effects of polygalacic acid on scopolamine-induced memory deficits in mice. Phytomedicine 2016, 23, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Nai, Y.; Feng, L.; Chen, Y.; Li, M.; Xu, H. Walnut oil prevents scopolamine-induced memory dysfunction in a mouse model. Molecules 2020, 25, 1630. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.Y.; Yu, J.; Liu, Z.Q.; Zhou, H.H. Apigenin attenuates diabetes-associated cognitive decline in rats via suppressing oxidative stress and nitric oxide synthase pathway. Int. J. Clin. Exp. Med. 2015, 8, 15506–15513. [Google Scholar]
- Han, Y.; Zhang, T.; Su, J.; Zhao, Y.; Wang, C.; Li, X. Apigenin attenuates oxidative stress and neuronal apoptosis in early brain injury following subarachnoid hemorrhage. J. Clin. Neurosci. 2017, 40, 157–162. [Google Scholar] [CrossRef]
- Balaban, H.; Nazıroğlu, M.; Demirci, K.; Övey, S. The protective role of selenium on scopolamine-induced memory impairment, oxidative stress, and apoptosis in aged rats: The involvement of TRPM2 and TRPV1 channels. Mol. Neurobiol. 2017, 54, 2852–2868. [Google Scholar] [CrossRef]
- Muhammad, T.; Ali, T.; Ikram, M.; Khan, A.; Alam, S.I.; Kim, M.O. Melatonin rescue oxidative stress-mediated neuroinflammation / neurodegeneration and memory impairment in scopolamine-induced amnesia mice model. J. Neuroimmune Pharmacol. 2019, 14, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, R.S.; Ness, J.M.; Roth, K.A. Bcl-2 family regulation of neuronal development and neurodegeneration. Biochim. Biophys. Acta 2004, 1644, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Z.; Deng, X.H.; Bentivoglio, M. Differential response of apoptosis-regulatory Bcl-2 and Bax proteins to an inflammatory challenge in the cerebral cortex and hippocampus of aging mice. Brain Res. Bull. 2007, 74, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, S.J. Bcl-2 gene family and the regulation of programmed cell death. Cancer Res. 1999, 59, 1693s–1700s. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [Green Version]
- Pradeep, A.R.; Suke, D.K.; Prasad, M.V.R.; Singh, S.P.; Martande, S.S.; Nagpal, K.; Naik, S.B.; Guruprasad, C.N.; Raju, A.P.; Singh, P.; et al. Expression of key executioner of apoptosis caspase-3 in periodontal health and disease. J. Investig. Clin. Dent. 2016, 7, 174–179. [Google Scholar] [CrossRef]
- Liu, J.; Mao, J.; Jiang, Y.; Xia, L.; Mao, L.; Wu, Y.; Ma, P.; Fang, B. AGEs induce apoptosis in rat osteoblast cells by activating the caspase-3 signaling pathway under a high-glucose environment in vitro. Appl. Biochem. Biotechnol. 2016, 178, 1015–1027. [Google Scholar] [CrossRef]
- Xu, Q.Q.; Xu, Y.J.; Yang, C.; Tang, Y.; Li, L.; Cai, H.B.; Hou, B.N.; Chen, H.F.; Wang, Q.; Shi, X.G.; et al. Sodium tanshinone IIA sulfonate attenuates scopolamine-induced cognitive dysfunctions via improving cholinergic system. Biomed. Res. Int. 2016, 2016, 9852536. [Google Scholar] [CrossRef] [Green Version]
- Demirci, K.; Nazıroğlu, M.; Övey, S.; Balaban, H. Selenium attenuates apoptosis, inflammation and oxidative stress in the blood and brain of aged rats with scopolamine-induced dementia. Metab. Brain Dis. 2017, 32, 321–329. [Google Scholar] [CrossRef]
- Kim, A.; Nam, Y.J.; Lee, M.S.; Shin, Y.K.; Sohn, D.S.; Lee, C.S. Apigenin reduces proteasome inhibition-induced neuronal apoptosis by suppressing the cell death process. Neurochem. Res. 2016, 41, 2969–2980. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [Green Version]
- Demuro, A.; Mina, E.; Kayed, R.; Milton, S.C.; Parker, I.; Glabe, C.G. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J. Biol. Chem. 2005, 280, 17294–17300. [Google Scholar] [CrossRef] [Green Version]
- Nalivaeva, N.N.; Turner, A.J. The amyloid precursor protein: A biochemical enigma in brain development, function and disease. FEBS Lett. 2013, 587, 2046–2054. [Google Scholar] [CrossRef] [Green Version]
- Scholz, D.; Chernyshova, Y.; Ückert, A.K.; Leist, M. Reduced Aβ secretion by human neurons under conditions of strongly increased BACE activity. J. Neurochem. 2018, 147, 256–274. [Google Scholar] [CrossRef] [PubMed]
- Kimberly, W.T.; Xia, W.; Rahmati, T.; Wolfe, M.S.; Selkoe, D.J. The transmembrane aspartates in presenilin 1 and 2 are obligatory for gamma-secretase activity and amyloid beta-protein generation. J. Biol. Chem. 2000, 275, 3173–3178. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Tan, J.; Mao, G.; Cui, M.Z.; Xu, X. The same gamma-secretase accounts for the multiple intramembrane cleavages of APP. J. Neurochem. 2007, 100, 1234–1246. [Google Scholar] [CrossRef]
- Deane, R.; Du Yan, S.; Submamaryan, R.K.; LaRue, B.; Jovanovic, S.; Hogg, E.; Welch, D.; Manness, L.; Lin, C.; Yu, J.; et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat. Med. 2003, 9, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Deane, R.; Singh, I.; Sagare, A.P.; Bell, R.D.; Ross, N.T.; LaRue, B.; Love, R.; Perry, S.; Paquette, N.; Deane, R.J.; et al. A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease. J. Clin. Investig. 2012, 122, 1377–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.; Xu, J.; Chen, C.; Chen, F.; Jin, P.; Zhu, K.; Hu, C.W.; You, M.; Chen, M.; Hu, F. Royal jelly reduces cholesterol levels, ameliorates Aβ pathology and enhances neuronal metabolic activities in a rabbit model of Alzheimer’s disease. Front. Aging Neurosci. 2018, 10, 50. [Google Scholar] [CrossRef] [Green Version]
- Miners, J.S.; Barua, N.; Kehoe, P.G.; Gill, S.; Love, S. Aβ-degrading enzymes: Potential for treatment of Alzheimer disease. J. Neuropathol. Exp. Neurol. 2011, 70, 944–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, D.Y.; Lee, Y.J.; Lee, S.Y.; Lee, Y.M.; Lee, H.H.; Choi, I.S.; Oh, K.W.; Han, S.B.; Nam, S.Y.; Hong, J.T. Attenuation of scopolamine-induced cognitive dysfunction by obovatol. Arch. Pharm. Res. 2012, 35, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.R.; Miller, F.D. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 2000, 10, 381–391. [Google Scholar] [CrossRef]
- Björkholm, C.; Monteggia, L.M. BDNF—A key transducer of antidepressant effects. Neuropharmacology 2016, 102, 72–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, A.; Moya-Alvarado, G.; Gonzalez-Billaut, C.; Bronfman, F.C. Cellular and molecular mechanisms regulating neuronal growth by brain-derived neurotrophic factor. Cytoskeleton (Hoboken) 2016, 73, 612–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasi, M.; Vignoli, B.; Canossa, M.; Blum, R. Neurobiology of local and intercellular BDNF signaling. Pflug. Arch. 2017, 469, 593–610. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Nagappan, G.; Lu, B. Differential effects of transient and sustained activation of BDNF-TrkB signaling. Dev. Neurobiol. 2018, 78, 647–659. [Google Scholar] [CrossRef]
- Tu, F.; Pang, Q.; Huang, T.; Zhao, Y.; Liu, M.; Chen, X. Apigenin ameliorates post-stroke cognitive deficits in rats through histone acetylation-mediated neurochemical alterations. Med. Sci. Monit. 2017, 23, 4004–4013. [Google Scholar] [CrossRef] [Green Version]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A review on flavonoid apigenin: Dietary intake, ADME, antimicrobial effects, and interactions with human gut microbiota. BioMed Res. Int. 2019, 2019, 7010467. [Google Scholar] [CrossRef]
- Zhang, F.; Li, F.; Chen, G. Neuroprotective effect of apigenin in rats after contusive spinal cord injury. Neurol. Sci. 2014, 35, 583–588. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.E. Flavonoids, cognition, and dementia: Actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic. Biol. Med. 2012, 52, 35–45. [Google Scholar] [CrossRef]
- Hostetler, G.; Riedl, K.; Cardenas, H.; Diosa-Toro, M.; Arango, D.; Schwartz, S.; Doseff, A.I. Flavone deglycosylation increases their anti-inflammatory activity and absorption. Mol. Nutr. Food Res. 2012, 56, 558–569. [Google Scholar] [CrossRef]
- Meyer, H.; Bolarinwa, A.; Wolfram, G.; Linseisen, J. Bioavailability of apigenin from apiin-rich parsley in humans. Ann. Nutr. Metab. 2006, 50, 167–172. [Google Scholar] [CrossRef]
- Venigalla, M.; Gyengesi, E.; Münch, G. Curcumin and Apigenin—Novel and promising therapeutics against chronic neuroinflammation in Alzheimer’s disease. Neural Regen. Res. 2015, 10, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, K.C. A test of two explanations of spontaneous alternation. J. Comp. Physiol. Psychol. 1952, 45, 287–293. [Google Scholar] [CrossRef]
- Bevins, R.A.; Besheer, J. Object recognition in rats and mice: A one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat. Protoc. 2006, 1, 1306–1311. [Google Scholar] [CrossRef] [PubMed]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]


| Normal | Control | AP10 | AP20 | DO | |
|---|---|---|---|---|---|
| Initial body weight (g) | 34.1 ± 1.4 | 33.3 ± 1.1 | 32.9 ± 0.8 | 33.7 ± 0.9 | 33.1 ± 0.7 NS |
| Final body weight (g) | 35.4 ± 2.0 | 33.6 ± 1.4 | 34.6 ± 1.5 | 34.1 ± 0.6 | 34.2 ± 0.9 NS |
| Body weight gain (g) | 1.3 ± 1.5 | 0.3 ± 2.0 | 1.7 ± 2.2 | 0.4 ± 1.2 | 1.1 ± 1.5 NS |
| Brain (g) | 0.50 ± 0.01 | 0.49 ± 0.02 | 0.51 ± 0.01 | 0.50 ± 0.01 | 0.50 ± 0.01 NS |
| Liver (g) | 2.47 ± 0.51 | 2.45 ± 0.21 | 2.25 ± 0.23 | 2.71 ± 0.39 | 2.25 ± 0.58 NS |
| Kidney (g) | 0.53 ± 0.04 | 0.53 ± 0.06 | 0.56 ± 0.07 | 0.59 ± 0.07 | 0.56 ± 0.08 NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Kim, J.; He, M.; Lee, A.; Cho, E. Apigenin Ameliorates Scopolamine-Induced Cognitive Dysfunction and Neuronal Damage in Mice. Molecules 2021, 26, 5192. https://doi.org/10.3390/molecules26175192
Kim Y, Kim J, He M, Lee A, Cho E. Apigenin Ameliorates Scopolamine-Induced Cognitive Dysfunction and Neuronal Damage in Mice. Molecules. 2021; 26(17):5192. https://doi.org/10.3390/molecules26175192
Chicago/Turabian StyleKim, Yeojin, Jihyun Kim, Meitong He, Ahyoung Lee, and Eunju Cho. 2021. "Apigenin Ameliorates Scopolamine-Induced Cognitive Dysfunction and Neuronal Damage in Mice" Molecules 26, no. 17: 5192. https://doi.org/10.3390/molecules26175192
APA StyleKim, Y., Kim, J., He, M., Lee, A., & Cho, E. (2021). Apigenin Ameliorates Scopolamine-Induced Cognitive Dysfunction and Neuronal Damage in Mice. Molecules, 26(17), 5192. https://doi.org/10.3390/molecules26175192







