Dissipation Dynamics of Doxycycline and Gatifloxacin and Accumulation of Heavy Metals during Broiler Manure Aerobic Composting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Preparation and Collection of Broiler Manure
2.3. Composting Experimental Design
2.4. Sample Preparation
2.5. Analytical Methods
2.6. Modeling of Dissipation Kinetics
2.7. Statistical Analysis
3. Results
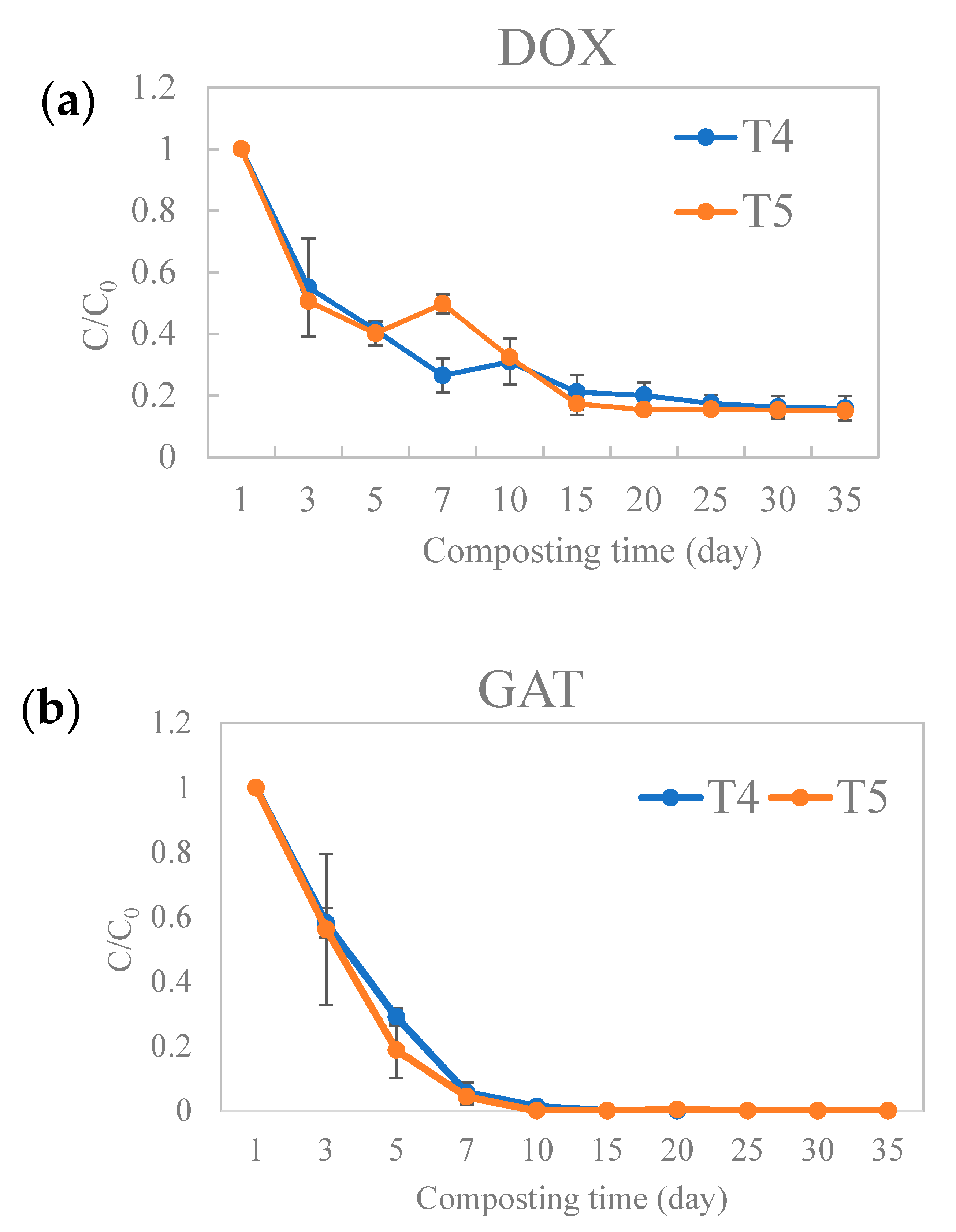
3.1. Persistence of Antibiotics (DOX and GAT) during the Composting Process
3.2. Dissipation Kinetics of DOX and GAT during Broiler Manure Composting
3.3. Changes in the Total Amount of Heavy Metals during Composting
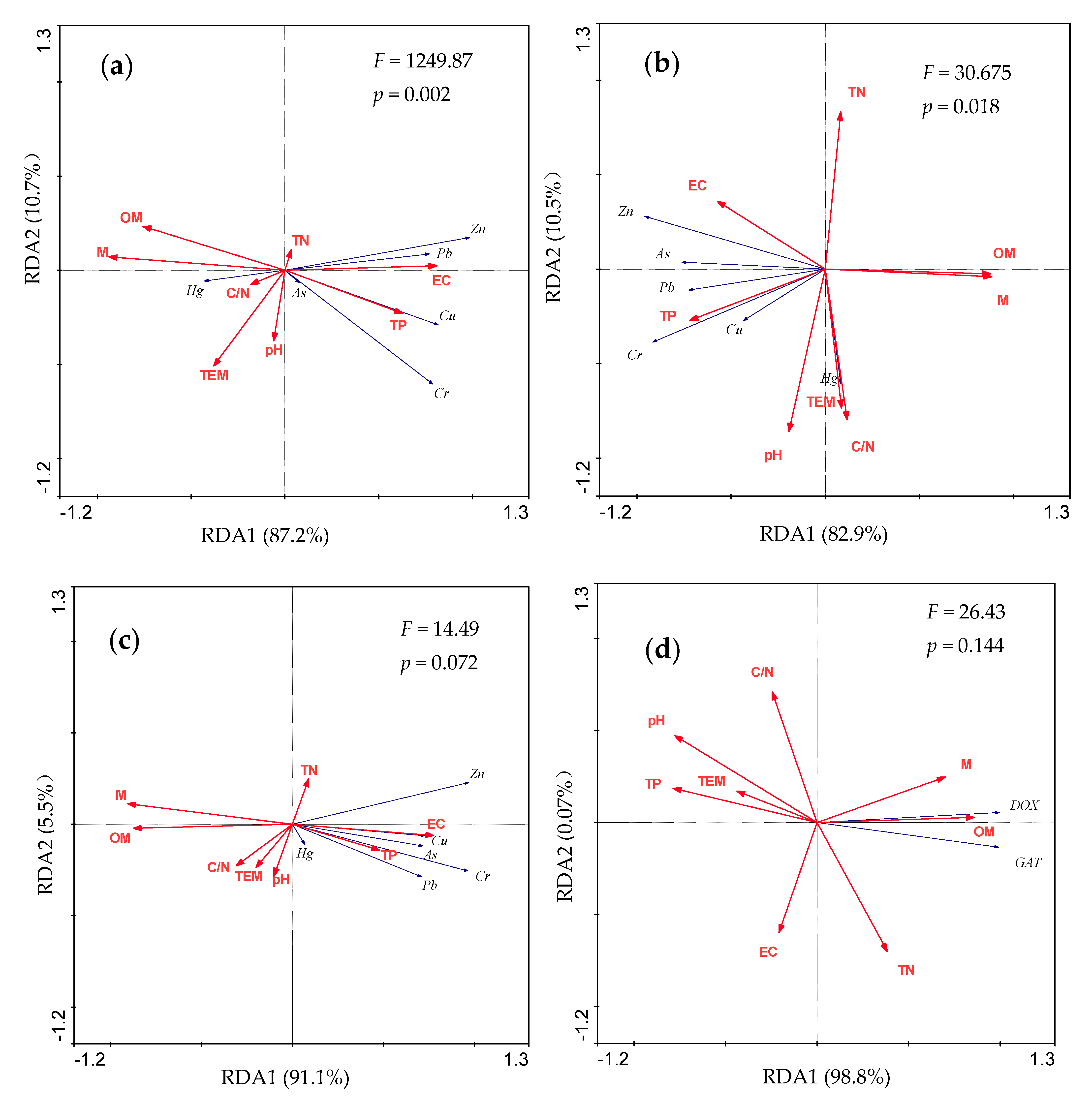
3.4. Redundancy Analysis (RDA) of Antibiotics and Heavy Metals Levels and Physicochemical Properties during Composting
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Larson, C. China’s lakes of pig manure spawn antibiotic resistance. Science 2015, 347, 704. [Google Scholar] [CrossRef]
- Rusu, A.; Hancu, G.; Uivarosi, V. Fluoroquinolone pollution of food, water and soil, and bacterial resistance. Environ. Chem. Lett. 2015, 13, 21–36. [Google Scholar] [CrossRef]
- Van Epps, A.; Blaney, L. Antibiotic residues in animal waste: Occurrence and degradation in conventional agricultural waste management practices. Curr. Pollut. Rep. 2016, 2, 135–155. [Google Scholar] [CrossRef] [Green Version]
- Collignon, P.C.; Conly, J.M.; Andremont, A.; McEwen, S.A.; Aidara-Kane, A.; for the World Health Organization Advisory Group; Bogotá Meeting on Integrated Surveillance of Antimicrobial Resistance (WHO-AGISAR); Agerso, Y.; Andremont, A.; Collignon, P.; et al. World health organization ranking of antimicrobials according to their importance in human medicine: A critical step for developing risk management strategies to control antimicrobial resistance from food animal production. Clin. Infect. Dis. 2016, 63, 1087–1093. [Google Scholar]
- Ho, Y.B.; Zakaria, M.P.; Latif, P.A.; Saari, N. Simultaneous determination of veterinary antibiotics and hormone in broiler manure, soil and manure compost by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2012, 1262, 160–168. [Google Scholar] [CrossRef]
- Ma, F.; Xu, S.; Tang, Z.; Li, Z.; Zhang, L. Use of antimicrobials in food animals and impact of transmission of antimicrobial resistance on humans. Biosaf. Health 2021, 3, 32–38. [Google Scholar] [CrossRef]
- Berendsen, B.J.A.; Lahr, J.; Nibbeling, C.; Jansen, L.J.M.; Bongers, I.E.A.; Wipfler, E.L.; van de Schans, M.G.M. The persistence of a broad range of antibiotics during calve, pig and broiler manure storage. Chemosphere 2018, 204, 267–276. [Google Scholar] [CrossRef]
- Yan, Q.F.; Li, X.Y.; Ma, B.H.; Zou, Y.D.; Wang, Y.; Liao, X.D.; Liang, J.; Mi, J.D.; Wu, Y.B. Different concentrations of doxycycline in swine manure affect the microbiome and degradation of doxycycline residue in soil. Front. Microbiol. 2018, 9, 3129. [Google Scholar] [CrossRef] [PubMed]
- Wychodnik, K.; Gałęzowska, G.; Rogowska, J.; Potrykus, M.; Plenis, A.; Wolska, L. Poultry farms as a potential source of environmental pollution by pharmaceuticals. Molecules 2020, 25, 1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zekker, I.; Kivirüüt, A.; Rikmann, E.; Mandel, A.; Jaagura, M.; Tenno, T.; Artemchuk, O.; Rubin, S.d.; Tenno, T. Enhanced efficiency of nitritating-anammox sequencing batch reactor achieved at low decrease rates of oxidation–reduction potential. Environ. Eng. Sci. 2019, 36, 350–360. [Google Scholar] [CrossRef]
- Zekker, I.; Raudkivi, M.; Artemchuk, O.; Rikmann, E.; Priks, H.; Jaagura, M.; Tenno, T. Mainstream-sidestream wastewater switching promotes anammox nitrogen removal rate in organic-rich, low-temperature streams. Environ. Technol. 2021, 42, 3073–3082. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, L.; Cai, Q.; Xu, Y.; Xie, Z.; Liu, J.; Ning, X. Simultaneous reduction of antibiotics and antibiotic resistance genes in pig manure using a composting process with a novel microbial agent. Ecotox. Environ. Safe 2021, 208, 111724. [Google Scholar] [CrossRef]
- Ho, Y.B.; Zakaria, M.P.; Latif, P.A.; Saari, N. Degradation of veterinary antibiotics and hormone during broiler manure composting. Bioresour. Technol. 2013, 131, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Selvam, A.; Xu, D.L.; Zhao, Z.Y.; Wong, J.W.C. Fate of tetracycline, sulfonamide and fluoroquinolone resistance genes and the changes in bacterial diversity during composting of swine manure. Bioresour. Technol. 2012, 126, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.Y.; Yang, X.P.; Li, Q.; Wu, L.H.; Shen, Q.R.; Zhao, F.J. Changes in antibiotic concentrations and antibiotic resistome during commercial composting of animal manures. Environ. Pollut. 2016, 219, 182–190. [Google Scholar] [CrossRef]
- Kong, Z.J.; Wang, X.Q.; Liu, Q.M.; Li, T.; Chen, X.; Chai, L.F.; Liu, D.Y.; Shen, Q.R. Evolution of various fractions during the windrow composting of chicken manure with rice chaff. J. Environ. Manag. 2018, 207, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Shen, Z.Q.; Yang, C.P.; Zhou, Y.X.; Li, X.; Zeng, G.M.; Ai, S.J.; He, H.J. Effects of C/N ratio and bulking agent on speciation of Zn and Cu and enzymatic activity during pig manure composting. Int. Biodeterior. Biodegrad. 2017, 119, 429–436. [Google Scholar] [CrossRef]
- Xiao, R.; Guo, D.; Ali, A.; Mi, S.S.; Liu, T.; Ren, C.Y.; Li, R.H.; Zhang, Z.Q. Accumulation, ecological-health risks assessment, and source apportionment of heavy metals in paddy soils: A case study in Hanzhong, Shaanxi, China. Environ. Pollut. 2019, 248, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.W.; Wang, B.; Huang, J.; Deng, S.B.; Yu, G. Pharmaceuticals and personal care products in the aquatic environment in China: A review. J. Hazard. Mater. 2013, 262, 189–211. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Li, S.; Xu, Y. Novel-integrated process for production of bio-organic fertilizer via swine manure composting. Environ. Eng. Res. 2021, 26, 190522. [Google Scholar] [CrossRef]
- Li, K.; Cao, R.; Mo, S.; Yao, R.; Ren, Z.; Wu, J. Swine manure composting with compound microbial inoculants: Removal of antibiotic resistance genes and their associations with microbial community. Front. Microbiol. 2020, 11, 592592. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.Z.; Zhang, X.Y.; Liao, X.D.; Wu, Y.B.; Mi, J.D.; Wang, Y. Effect of different proportions of three microbial agents on ammonia mitigation during the composting of layer manure. Molecules 2019, 24, 2513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Chu, L.; Daryanto, S.; Lü, L.; Ala, M.; Wang, L. Sand dune stabilization changes the vegetation characteristics and soil seed bank and their correlations with environmental factors. Sci. Total Environ. 2019, 648, 500–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolliver, H.; Gupta, S.; Noll, S. Antibiotic degradation during manure composting. J. Environ. Qual. 2008, 37, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Chai, R.S.; Huang, L.D.; Li, L.L.; Gielen, G.; Wang, H.L.; Zhang, Y.S. Degradation of tetracyclines in pig manure by composting with rice straw. Int. J. Environ. Res. Public Health 2016, 13, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Ben, W.W.; Zhang, Y.; Yang, M.; Qiang, Z.M. Effects of thermophilic composting on oxytetracycline, sulfamethazine, and their corresponding resistance genes in swine manure. Environ. Sci.-Process. Impacts 2015, 17, 1654–1660. [Google Scholar] [CrossRef]
- Charuaud, L.; Jarde, E.; Jaffrezic, A.; Thomas, M.F.; Le Bot, B. Veterinary pharmaceutical residues from natural water to tap water: Sales, occurrence and fate. J. Hazard. Mater. 2019, 361, 169–186. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, F.; Zhu, D.; Sun, J. Environmental fate of tetracycline antibiotics: Degradation pathway mechanisms, challenges, and perspectives. Environ. Sci. Eur. 2021, 33, 64. [Google Scholar] [CrossRef]
- Andriamanohiarisoamanana, F.J.; Ihara, I.; Yoshida, G.; Umetsu, K. Kinetic study of oxytetracycline and chlortetracycline inhibition in the anaerobic digestion of dairy manure. Bioresour. Technol. 2020, 315, 123810. [Google Scholar] [CrossRef]
- Yu, Y.S.; Chen, L.J.; Fang, Y.; Jia, X.B.; Chen, J.C. High temperatures can effectively degrade residual tetracyclines in chicken manure through composting. J. Hazard. Mater. 2019, 380, 120862. [Google Scholar] [CrossRef]
- Varel, V.H.; Wells, J.E.; Shelver, W.L.; Rice, C.P.; Armstrong, D.L.; Parker, D.B. Effect of anaerobic digestion temperature on odour, coliforms and chlortetracycline in swine manure or monensin in cattle manure. J. Appl. Microbiol. 2012, 112, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Zekker, I.; Artemchuk, O.; Rikmann, E.; Ohimai, K.; Dhar Bhowmick, G.; Madhao Ghangrekar, M.; Burlakovs, J.; Tenno, T. Start-up of anammox sbr from non-specific inoculum and process acceleration methods by hydrazine. Water 2021, 13, 350. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [Green Version]
- Widyasari-Mehta, A.; Kartika, H.R.; Suwito, A.; Kreuzig, R. Laboratory testing on the removal of the veterinary antibiotic doxycycline during long-term liquid pig manure and digestate storage. Chemosphere 2016, 149, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Spielmeyer, A. Occurrence and fate of antibiotics in manure during manure treatments: A short review. Sustain. Chem. Pharm. 2018, 9, 76–86. [Google Scholar] [CrossRef]
- Zhang, M.; He, L.Y.; Liu, Y.S.; Zhao, J.L.; Liu, W.R.; Zhang, J.N.; Chen, J.; He, L.K.; Zhang, Q.Q.; Ying, G.G. Fate of veterinary antibiotics during animal manure composting. Sci. Total Environ. 2019, 650, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gu, J.; Wang, X.; Song, Z.; Dai, X.; Guo, H.; Yu, J.; Zhao, W.; Lei, L. Enhanced removal of antibiotic resistance genes and mobile genetic elements during swine manure composting inoculated with mature compost. J. Hazard. Mater. 2021, 411, 125135. [Google Scholar] [CrossRef] [PubMed]
- Jia, A.; Wan, Y.; Xiao, Y.; Hu, J.Y. Occurrence and fate of quinolone and fluoroquinolone antibiotics in a municipal sewage treatment plant. Water Res. 2012, 46, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, X.; Yu, X.L.; Pu, C.J.; Sun, Y.; Luo, W.H. Dissipation and persistence of sulfonamides, quinolones and tetracyclines in anaerobically digested biosolids and compost during short-term storage under natural conditions. Sci. Total Environ. 2019, 684, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Sciscenko, I.; Arques, A.; Varga, Z.; Bouchonnet, S.; Monfort, O.; Brigante, M.; Mailhot, G. Significant role of iron on the fate and photodegradation of enrofloxacin. Chemosphere 2021, 270, 129791. [Google Scholar] [CrossRef]
- Yang, B.; Meng, L.; Xue, N.D. Removal of five fluoroquinolone antibiotics during broiler manure composting. Environ. Technol. 2018, 39, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, B.; Nguyen, D.D.; Chaudhary, D.K.; Chang, S.W.; Kim, J.; Lee, S.R.; Shin, J.; Jeon, B.H.; Chung, S.; Lee, J. Influence of biochar on physico-chemical and microbial community during swine manure composting process. J. Environ. Manag. 2019, 232, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Z.; Awasthi, M.K.; Jiang, Y.H.; Li, R.H.; Ren, X.N.; Zhao, J.C.; Shen, F.; Wang, M.J.; Zhang, Z.Q. Evaluation of medical stone amendment for the reduction of nitrogen loss and bioavailability of heavy metals during pig manure composting. Bioresour. Technol. 2016, 220, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Field Guide to Compost Use; U.S. Composting Council: Amherst, MA, USA, 2001.

| Characteristics | Broiler Manure | Rice Hull | ||||
|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | ||
| Moisture content (%) | 78.44 ± 0.69 | 77.34 ± 0.89 | 77.46 ± 1.61 | 78.3 ± 0.39 | 79.97 ± 1.35 | 12.59 ± 0.23 |
| pH | 5.66 ± 0.18 | 5.58 ± 0.21 | 5.73 ± 0.24 | 5.84 ± 0.07 | 5.62 ± 0.09 | 6.86 ± 0.33 |
| EC (us/cm) | 590 ± 24.15 | 626 ± 32.93 | 608 ± 27.58 | 617 ± 19.60 | 604 ± 9.43 | ND |
| TN (%) | 5.05 ± 0.02 | 4.55 ± 0.01 | 4.54 ± 0.17 | 4.52 ± 0.07 | 4.62 ± 0.04 | 0.42 ± 0.01 |
| C/N | 9.47 ± 0.05 | 10.57 ± 0.02 | 10.61 ± 0.41 | 10.63 ± 0.34 | 10.38 ± 0.21 | 111.39 ± 3.26 |
| TP (%) | 1.16 ± 0.04 | 1.05 ± 0.01 | 1.11 ± 0.07 | 1.12 ± 0.01 | 1.05 ± 0.02 | 0.09 ± 0.01 |
| OM (%) | 86.21 ± 0.11 | 86.56 ± 0.08 | 86.67 ± 0.19 | 86.52 ± 0.22 | 86.33 ± 0.23 | 84.21 ± 0.15 |
| Cu (mg/kg) | 60.29 ± 1.19 | 77.4 ± 0.3 | 78.09 ± 0.75 | 30.59 ± 4.03 | ||
| Zn (mg/kg) | 500.26 ± 12.16 | 644.65 ± 10.25 | 655.81 ± 12.02 | 63.2 ± 1.56 | ||
| Pb (mg/kg) | 0.62 ± 0.03 | 0.78 ± 0.04 | 0.83 ± 0.27 | 0 | ||
| As(mg/kg) | 0.22 ± 0.01 | 0.27 ± 0.02 | 0.32 ± 0.02 | 0.66 ± 0.03 | ||
| Hg (mg/kg) | 0.005 ± 0.001 | 0.005 ± 0.002 | 0.004 ± 0.003 | 0 | ||
| Ni (mg/kg) | 3.02 ± 0.01 | 3.46 ± 0.37 | 4.02 ± 0.33 | 1.33 ± 0.16 | ||
| Cr (mg/kg) | 218.13 ± 19.53 | 398.01 ± 6.36 | 418.94 ± 1.88 | 26.51 ± 2.60 | ||
| Concentration of DOX (mg/kg) | 250.4 ± 3.2 | 143.2 ± 7.2 | ||||
| Concentration of GAT (mg/kg) | 185.9 ± 13.97 | 146.8 ± 10.1 | ||||
| Antibiotics | Treatments | Fitting Equation | Slope (k) | R2 | Half-Life (t1/2) (Days) |
|---|---|---|---|---|---|
| GAT | T4 | ln C/C0 = −0.5011X + 0.812 | −0.5011 | 0.9703 | 1.38 |
| T5 | ln C/C0 = −0.5251X + 0.7539 | −0.5251 | 0.9655 | 1.32 | |
| DOX | T4 | ln C/C0 = −0.0437X − 0.5954 | −0.0437 | 0.7452 | 15.86 |
| T5 | ln C/C0 = −0.0504X − 0.5085 | −0.0504 | 0.7812 | 13.75 |
| Time (Day) | Cu mg/kg | Zn mg/kg | Cr mg/kg | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | |
| 1 | 25.18 ± 1.62 | 34.43 ± 0.81 | 34.49 ± 1.39 | 204.92 ± 5.23 | 249.77 ± 0.42 | 262.74 ± 7.28 | 119.53 ± 10.01 | 172.29 ± 15.08 | 195.29 ± 21.59 |
| 3 | 28.37 ± 1.15 | 33.48 ± 0.48 | 36.49 ± 0.88 | 220.21 ± 4.10 | 271.96 ± 9.05 | 283.25 ± 10.96 | 150.54 ± 6.30 | 208.20 ± 17.40 | 224.33 ± 9.82 |
| 5 | 30.04 ± 0.07 | 35.25 ± 3.04 | 37.11 ± 1.11 | 196.79 ± 14.14 | 256.44 ± 11.74 | 274.04 ± 7.14 | 129.04 ± 1.43 | 212.07 ± 7.23 | 216.96 ± 1.65 |
| 7 | 27.32 ± 3.72 | 33.17 ± 2.36 | 35.39 ± 0.16 | 199.25 ± 6.58 | 257.47 ± 8.77 | 272.58 ± 2.33 | 137.38 ± 3.22 | 203.11 ± 4.11 | 206.76 ± 15.46 |
| 10 | 28.66 ± 0.48 | 58.15 ± 3.61 | 50.19 ± 0.81 | 210.45 ± 7.92 | 261.67 ± 10.11 | 270.84 ± 3.96 | 143.2 ± 7.14 | 212.4 ± 1.77 | 207.42 ± 1.03 |
| 15 | 32.59 ± 3.79 | 47.5 ± 4.95 | 55.83 ± 1.34 | 245.34 ± 13.19 | 273.21 ± 20.77 | 289.98 ± 17.06 | 134.52 ± 0.80 | 219.44 ± 8.66 | 237.66 ± 16.92 |
| 20 | 45.67 ± 2.71 | 38.84 ± 0.72 | 44.48 ± 1.03 | 232.09 ± 5.29 | 298.09 ± 13.62 | 302.29 ± 18.37 | 152.33 ± 5.93 | 238.55 ± 10.17 | 246.87 ± 4.05 |
| 25 | 37.45 ± 3.43 | 45.34 ± 3.24 | 47.34 ± 4.04 | 257.56 ± 15.30 | 277.55 ± 7.94 | 288.79 ± 23.32 | 147.87 ± 6.69 | 221.76 ± 6.02 | 243.99 ± 5.52 |
| 30 | 43.66 ± 0.83 | 46.97 ± 1.28 | 53.66 ± 0.36 | 244.53 ± 6.32 | 301.39 ± 7.40 | 319.62 ± 5.61 | 158.52 ± 2.35 | 228.39 ± 11.10 | 257.88 ± 8.84 |
| 35 | 42.81 ± 2.18 | 47.86 ± 0.78 | 52.78 ± 2.68 | 258.20 ± 3.57 | 312.21 ± 12.10 | 333.68 ± 9.62 | 160.16 ± 3.24 | 223.98 ± 5.68 | 248.02 ± 7.05 |
| p | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Time (Day) | As mg/kg | Pb mg/kg | Hg mg/kg | ||||||
| T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | |
| 1 | 0.608 ± 0.02 | 0.558 ± 0.01 | 0.611 ± 0.07 | 0.215 ± 0.03 | 0.247 ± 0.06 | 0.29 ± 0.13 | 0.008 ± 0.003 | 0.011 ± 0.001 | 0.012 ± 0.003 |
| 3 | 0.568 ± 0.01 | 0.513 ± 0.13 | 0.643 ± 0.02 | 0.276 ± 0.18 | 0.221 ± 0.17 | 0.345 ± 0.06 | 0.011 ± 0 | 0.018 ± 0.001 | 0.012 ± 0.001 |
| 5 | 0.621 ± 0.03 | 0.574 ± 0.04 | 0.587 ± 0.01 | 0.356 ± 0.19 | 0.429 ± 0.08 | 0.478 ± 0.05 | 0.046 ± 0.001 | 0.029 ± 0.008 | 0.028 ± 0.005 |
| 7 | 0.849 ± 0.21 | 0.632 ± 0.06 | 0.628 ± 0.05 | 0.317 ± 0.12 | 0.505 ± 0.13 | 0.390 ± 0.13 | 0.026 ± 0.001 | 0.014 ± 0.002 | 0.034 ± 0.01 |
| 10 | 0.569 ± 0.04 | 0.545 ± 0.02 | 0.665 ± 0.03 | 0.315 ± 0.02 | 0.344 ± 0.18 | 0.376 ± 0.01 | 0.01 ± 0.002 | 0.017 ± 0.004 | 0.011 ± 0.002 |
| 15 | 0.634 ± 0.11 | 0.667 ± 0.01 | 0.81 ± 0.19 | 0.565 ± 0.07 | 0.515 ± 0.24 | 0.436 ± 0.04 | 0.016 ± 0.003 | 0.03 ± 0.01 | 0.015 ± 0 |
| 20 | 0.597 ± 0.06 | 0.768 ± 0.22 | 0.673 ± 0.01 | 0.448 ± 0.15 | 0.537 ± 0.04 | 0.599 ± 0.03 | 0.018 ± 0.01 | 0.021 ± 0.01 | 0.024 ± 0.008 |
| 25 | 0.692 ± 0.03 | 0.643 ± 0.01 | 0.742 ± 0.12 | 0.421 ± 0.02 | 0.479 ± 0.05 | 0.766 ± 0.02 | 0.013 ± 0.001 | 0.012 ± 0.002 | 0.013 ± 0.001 |
| 30 | 0.702 ± 0.15 | 0.681 ± 0.01 | 0.725 ± 0.04 | 0.517 ± 0.06 | 0.567 ± 0.10 | 0.521 ± 0.17 | 0.008 ± 0.003 | 0.011 ± 0.001 | 0.026 ± 0.004 |
| 35 | 0.699 ± 0.09 | 0.698 ± 0.12 | 0.758 ± 0.01 | 0.495 ± 0.11 | 0.575 ± 0.02 | 0.650 ± 0.09 | 0.021 ± 0.012 | 0.010 ± 0.001 | 0.013 ± 0.001 |
| p | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, L.; Wang, Y.; Huang, B.; Ma, J.; Chen, X. Dissipation Dynamics of Doxycycline and Gatifloxacin and Accumulation of Heavy Metals during Broiler Manure Aerobic Composting. Molecules 2021, 26, 5225. https://doi.org/10.3390/molecules26175225
Chu L, Wang Y, Huang B, Ma J, Chen X. Dissipation Dynamics of Doxycycline and Gatifloxacin and Accumulation of Heavy Metals during Broiler Manure Aerobic Composting. Molecules. 2021; 26(17):5225. https://doi.org/10.3390/molecules26175225
Chicago/Turabian StyleChu, Lei, Yongcui Wang, Bin Huang, Jian Ma, and Xin Chen. 2021. "Dissipation Dynamics of Doxycycline and Gatifloxacin and Accumulation of Heavy Metals during Broiler Manure Aerobic Composting" Molecules 26, no. 17: 5225. https://doi.org/10.3390/molecules26175225
APA StyleChu, L., Wang, Y., Huang, B., Ma, J., & Chen, X. (2021). Dissipation Dynamics of Doxycycline and Gatifloxacin and Accumulation of Heavy Metals during Broiler Manure Aerobic Composting. Molecules, 26(17), 5225. https://doi.org/10.3390/molecules26175225






