Absolute Antioxidant Activity of Five Phenol-Rich Essential Oils
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phenolic Compositions of the Essential Oils
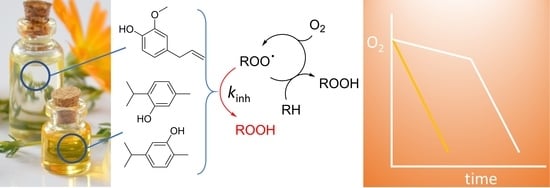
2.2. Autoxidation of Reference Substrates Inhibited by Essential Oils
2.3. Study Limitations and Future Directions
3. Materials and Methods
3.1. Materials
3.2. GC-MS and GC-FID Analysis of the EOs
3.3. Inhibited Autoxidation Studies
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Saleh, M.A.; Clark, S.; Woodard, B.; Deolu-Sobogun, S.A. Antioxidant and free radical scavenging activities of essential oils. Ethn. Dis. 2010, 20 (Suppl. S1), 78–82. [Google Scholar]
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Malandrino, G.; Cafiso, V.; Stefani, S.; Geraci, C. Oregano and Thyme Essential Oils Encapsulated in Chitosan Nanoparticles as Effective Antimicrobial Agents against Foodborne Pathogens. Molecules 2021, 26, 4055. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, L. (Ed.) Essential Oils as Natural Food Additives: Composition, Applications, Antioxidant and Antimicrobial Properties; Nova Science Publishing: New York, NY, USA, 2012; ISBN 978-1-62100-241-3. [Google Scholar]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Otoni, C.G.; Pontes, S.F.O.; Medeiros, E.A.A.; Soares, N.D.F. Edible films from methylcellulose and nanoemulsions of clove bud (Syzygium aromaticum) and oregano (Origanum vulgare) essential oils as shelf life extenders for sliced bread. J. Agric. Food Chem. 2014, 62, 5214–5219. [Google Scholar] [CrossRef]
- Stea, S.; Beraudi, A.; De Pasquale, D. Essential Oils for Complementary Treatment of Surgical Patients: State of the Art. Evid.-Based Compl. Altern. Med. 2014. [Google Scholar] [CrossRef] [Green Version]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, A. Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef] [Green Version]
- Sadgrove, N.; Jones, G. A Contemporary Introduction to Essential Oils: Chemistry, Bioactivity and Prospects for Australian Agriculture. Agriculture 2015, 5, 48–102. [Google Scholar] [CrossRef] [Green Version]
- Hüsnü, K.C.B.; Franz, C. Essential Oils Used in Veterinary Medicine. In Handbook of Essential Oils: Science, Technology, and Applications, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2020; p. 14. [Google Scholar]
- Spada, M.; Cuzman, O.A.; Tosini, I.; Galeotti, M.; Sorella, F. Essential oils mixtures as an eco-friendly biocidal solution for a marble statue restoration. Int. Biodeter. Biodegrad. 2021, 163, 105280. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant and Anti-Inflammatory Activities of Essential Oils: A Short Review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef] [Green Version]
- do Nascimento, L.D.; Barbosa de Moraes, A.A.; Santana da Costa, K.; Pereira Galúcio, J.M.; Taube, P.S.; Costa, C.M.L.; Cruz, J.N.; de Aguiar Andrade, E.H.; de Faria, L.J.G. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Industrial Crops and Products 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Methods to measure the antioxidant activity of phytochemicals and plant extracts. J. Agric. Food Chem. 2018, 66, 3324–3329. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, L.; Pratt, D.A. Antioxidants in chemistry and biology. In Encyclopedia of radicals in chemistry, biology and materials; Chatgilialoglu, C., Studer, A., Eds.; Wiley Publishing: Chirchester, UK, 2012; Volume 3, pp. 1623–1677. [Google Scholar]
- Li, B.; Pratt, D.A. Methods for determining the efficacy of radical-trapping antioxidants. Free Radic. Biol. Med. 2015, 82, 187–202. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radic. Res. 2015, 49, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kykkidou, S.; Giatrakou, V.; Papavergou, A.; Kontominas, M.G.; Savvaidis, I.N. Effect of thyme essential oil and packaging treatments on fresh Mediterranean swordfish fillets during storage at 4 degrees C. Food Chem. 2009, 115, 169–175. [Google Scholar] [CrossRef]
- Fasseas, M.K.; Mountzouris, K.C.; Tarantilis, P.A.; Polissiou, M.; Zervas, G. Antioxidant activity in meat treated with oregano and sage essential oils. Food Chem. 2008, 106, 1188–1194. [Google Scholar] [CrossRef]
- Harpaz, S.; Glatman, L.; Drabkin, V.; Gelman, A. Effects of herbal essential oils used to extend the shelf life of freshwater-reared asian sea bass fish (Lates calcarifer). J. Food Prot. 2003, 66, 410–417. [Google Scholar] [CrossRef]
- Sampaio, B.; Edrada-Ebel, R.; Da Costa, F. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: A model for environmental metabolomics of plants. Sci Rep. 2016, 6, 29265. [Google Scholar] [CrossRef] [Green Version]
- Koşar, M.; Demirci, B.; Demirci, F.; Can Başer, K.H. Effect of maturation on the composition and biological activity of the essential oil of a commercially important satureja species from turkey: Satureja cuneifolia Ten. (Lamiaceae). J. Agric. Food Chem. 2008, 56, 2260–2265. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef]
- McGrath, A.J.; Garrett, G.E.; Valgimigli, L.; Pratt, D.A. The redox chemistry of sulfenic acids. J. Am. Chem. Soc. 2010, 132, 16759–16761. [Google Scholar] [CrossRef]
- Amorati, R.; Pedulli, G.F.; Pratt, D.A.; Valgimigli, L. TEMPO reacts with oxygen-centered radicals under acidic conditions. Chem. Commun. 2010, 46, 5139–5141. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Valgimigli, L.; Dinér, P.; Bakhtiari, K.; Saeedi, M.; Engman, L. Multi-faceted reactivity of alkyltellurophenols towards peroxyl radicals: Catalytic antioxidant versus thiol-depletion effect. Chem. Eur. J. 2013, 19, 7510–7522. [Google Scholar] [CrossRef] [PubMed]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef] [Green Version]
- Costa, R.; De Fina, M.R.; Valentino, M.R.; Dugo, P.; Mondello, L. Reliable Identification of Terpenoids and Related Compounds by using Linear Retention Indices Interactively with Mass Spectrometry Search. Nat. Prod. Commun. 2007, 2, 413–418. [Google Scholar] [CrossRef]
- Cicchetti, E.; Merle, P.; Chaintreau, A. Quantitation in gas chromatography: Usual practices and performances of a response factor database. Flavour Fragr. J. 2008, 23, 450–459. [Google Scholar] [CrossRef]
- Guo, Y.; Baschieri, A.; Amorati, R.; Valgimigli, L. Synergic antioxidant activity of γ-terpinene with phenols and polyphenols enabled by hydroperoxyl radicals. Food Chem. 2021, 345, 128468. [Google Scholar] [CrossRef]
- Lucarini, M.; Pedulli, G.F.; Valgimigli, L. Do Peroxyl Radicals Obey the Principle That Kinetic Solvent Effects on H-Atom Abstraction Are Independent of the Nature of the Abstracting Radical? J. Org. Chem. 1998, 63, 4497–4499. [Google Scholar] [CrossRef]
- Foti, M.C.; Ingold, K.U. Mechanism of inhibition of lipid peroxidation by gamma-terpinene, an unusual and potentially useful hydrocarbon antioxidant. J. Agric. Food Chem. 2003, 51, 2758–2765. [Google Scholar] [CrossRef] [Green Version]
- Baschieri, A.; Pulvirenti, L.; Muccilli, V.; Amorati, R.; Tringali, C. Chain-breaking antioxidant activity of hydroxylated and methoxylated magnolol derivatives: The role of H-bonds. Org. Biomol. Chem. 2017, 15, 6177–6184. [Google Scholar] [CrossRef]
- Kallel, I.; Hadrich, B.; Gargouri, B.; Chaabane, A.; Lassoued, S.; Gdoura, R.; Bayoudh, A.; Messaoud, E.B. Optimization of Cinnamon (Cinnamomum zeylanicum Blume) Essential Oil Extraction: Evaluation of Antioxidant and Antiproliferative Effects. Evid.-Based Compl. Altern. Med. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, K.; Kaushal, S.; Rani, R. Chemical Composition, Antioxidant and Antifungal Potential of Clove (Syzygium aromaticum) Essential Oil, its Major Compound and its Derivatives. J. Essent. Oil Bear. Plants 2019, 22, 1195–1217. [Google Scholar] [CrossRef]
- Wołosik, K.; Knaś, M.; Zalewska, A.; Niczyporuk, M.; Przystupa, A.W. The importance and perspective of plant-based squalene in cosmetology. J. Cosmet. Sci. 2013, 64, 59–66. [Google Scholar]
- Chen, H.; Liu, R.H. Potential Mechanisms of Action of Dietary Phytochemicals for Cancer Prevention by Targeting Cellular Signaling Transduction Pathways. J. Agric. Food Chem. 2018, 66, 3260–3276. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.H.; Couvreur, P. Squalene: A natural triterpene for use in disease management and therapy. Adv. Drug Deliv. Rev. 2009, 61, 1412–1426. [Google Scholar] [CrossRef]
- Baschieri, A.; Pizzol, R.; Guo, Y.; Amorati, R.; Valgimigli, L. Calibration of squalene, p-cymene, and sunflower oil as standard oxidizable substrates for quantitative antioxidant testing. J. Agric. Food Chem. 2019, 67, 6902–6910. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Zotova, J.; Baschieri, A.; Valgimigli, L. Antioxidant Activity of Magnolol and Honokiol: Kinetic and Mechanistic Investigations of Their Reaction with Peroxyl Radicals. J. Org. Chem. 2015, 80, 10651–10659. [Google Scholar] [CrossRef]
- de Saint Laumer, J.-Y.; Cicchetti, E.; Merle, P.; Egger, J.; Chaintreau, A. Quantification in Gas Chromatography: Prediction of Flame Ionization Detector Response Factors from Combustion Enthalpies and Molecular Structures. Anal. Chem. 2010, 82, 6457–6462. [Google Scholar] [CrossRef]
- Baschieri, A.; Daci Ajvazi, M.; Folifack Tonfack, J.L.; Valgimigli, L.; Amorati, R. Explaining the antioxidant activity of some common non-phenolic components of essential oils. Food Chem. 2017, 232, 656–663. [Google Scholar] [CrossRef]
- Kulisic, T.; Radonic, A.; Mladen Milos, M. Inhibition of lard oxidation by fractions of different essential oils. Grasas y Aceites 2005, 56, 284–291. [Google Scholar] [CrossRef] [Green Version]
- Lucarini, M.; Pedulli, G.F.; Valgimigli, L.; Amorati, R.; Minisci, F. Thermochemical and Kinetic Studies of a Bisphenol Antioxidant. J. Org. Chem. 2001, 66, 5456–5462. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, L.; Lucarini, M.; Pedulli, G.F.; Ingold, K.U. Does beta-carotene really protect vitamin E from oxidation? J. Am. Chem. Soc. 1997, 119, 8095–8096. [Google Scholar] [CrossRef]
- Haidasz, E.A.; Meng, D.; Amorati, R.; Baschieri, A.; Ingold, K.U.; Valgimigli, L.; Pratt, D.A. Acid Is Key to the Radical-Trapping Antioxidant Activity of Nitroxides. J. Am. Chem. Soc. 2016, 138, 5290–5298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, H.; Shanks, D.; Engman, L.; Amorati, R.; Pedulli, G.F.; Valgimigli, L. Long-lasting antioxidant protection: A regenerable BHA analogue. J. Org. Chem. 2010, 75, 7535–7541. [Google Scholar] [CrossRef] [PubMed]
- Denisov, E.T.; Azatyan, V.V. Inhibition of Chain Reactions; Gordon and Breach Science Publishing: London, UK, 2000; ISBN 90-6994-002-7. [Google Scholar]
- Denisov, E.T.; Afanas’ev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biology; CRC Press: Boca Raton, FL, USA, 2005; ISBN 0-8247-5356-9. [Google Scholar]
- Loshadkin, D.; Roginsky, V.; Pliss, E. Substituted p-hydroquinones as a chain-breaking antioxidant during the oxidation of styrene. Int. J. Chem. Kinet. 2002, 34, 162–171. [Google Scholar] [CrossRef]



| EO | d (g/mL, 20 °C) | Component | % (p/p) |
|---|---|---|---|
| T. vulgaris, L. (thyme) | 0.912 | α-Thujene | 1.6 ± 0.1 |
| α-Pinene | 3.8 ± 0.1 | ||
| Camphene | 8.6 ± 0.2 | ||
| β-Pinene | 2.6 ± 0.1 | ||
| p-Cymene | 45.3 ± 1.2 | ||
| Thymol | 4.0 ± 0.1 | ||
| Carvacrol | 33.9 ± 0.7 | ||
| O. vulgare, L. (oregano) | 0.948 | α-Phellandrene | 0.4 ± 0.02 |
| α-Pinene | 0.7 ± 0.1 | ||
| Myrcene | 1.2 ± 0.1 | ||
| α-Terpinene | 1.1 ± 0.1 | ||
| p-Cymene | 14.1 ± 0.3 | ||
| γ-Terpinene | 4.9 ± 0.1 | ||
| Linalool | 2.1 ± 0.1 | ||
| Thymol | 0.4 ± 0.03 | ||
| Carvacrol | 69.2 ± 1.4 | ||
| β-Caryophyllene | 1.6 ± 0.2 | ||
| S. hortensis, L. (savory) | 0.937 | α-Pinene | 1.8 ± 0.1 |
| Camphene | 0.9 ± 0.1 | ||
| β-Pinene | 0.2 ± 0.02 | ||
| Myrcene | 1.3 ± 0.1 | ||
| α-Terpinene | 2.3 ± 0.1 | ||
| p-Cymene | 20.0 ± 0.8 | ||
| Limonene | 0.9 ± 0.04 | ||
| Eucalyptol | 0.6 ± 0.02 | ||
| γ-Terpinene | 17.0 ± 0.6 | ||
| Thymol | 1.7 ± 0.1 | ||
| Carvacrol | 46.6 ± 1.7 | ||
| Thymol Acetate | 0.6 ± 0.02 | ||
| β-Caryophyllene | 1.6 ± 0.1 | ||
| Aromadendrene | 0.9 ± 0.03 | ||
| δ-Cadinene | 0.3 ± 0.02 | ||
| Caryophyllene Oxide | 0.4 ± 0.02 | ||
| E. caryophyllus, Spreng (clove bud) | 1.041 | Eugenol | 80.8 ± 1.7 |
| β-Caryophyllene | 8.9 ± 0.5 | ||
| Humulene | 1.1 ± 0.2 | ||
| Eugenyl Acetate | 9.1 ± 0.6 | ||
| C. zeylanicum, Blume (cinnamon) | 1.043 | α-Pinene | 2.7 ± 0.1 |
| Camphene | 0.8 ± 0.03 | ||
| β-Pinene | 0.4 ± 0.02 | ||
| α-Phellandrene | 1.7 ± 0.1 | ||
| p-Cymene | 1.9 ± 0.1 | ||
| Linalool | 2.1 ± 0.2 | ||
| Eugenol | 81.4 ± 1.6 | ||
| β-Caryophyllene | 4.8 ± 0.3 | ||
| Eugenyl Acetate | 3.7 ± 0.1 | ||
| Caryophyllene Oxide | 0.2 ± 0.04 | ||
| Benzyl Benzoate | 0.3 ± 0.04 |
| EO | Phenol | [Phenol]/M 1 | Σ[Phenol]/M 2 | [AH]app/M 3 | kinh/M−1s−1(cumene) 4 | kinh/M−1s−1(squalene) 5 |
|---|---|---|---|---|---|---|
| T. vulgaris, L. | carvacrol | 2.3 × 10−6 | 2.6 × 10−6 | (2.5 ± 0.1) × 10−6 | (1.5 ± 0.1) × 104 | (1.0 ± 0.3) × 104 |
| thymol | 2.7 × 10−7 | |||||
| S. hortensis, L. | carvacrol | 3.1 × 10−6 | 3.2 × 10−6 | (2.9 ± 0.3) × 10−6 | (1.3 ± 0.1) × 104 | (9.8 ± 1.5) × 103 |
| thymol | 1.1 × 10−7 | |||||
| O. vulgare, L. | carvacrol | 4.6 × 10−6 | 4.6 × 10−6 | (4.8 ± 0.2) × 10−6 | (1.3 ± 0.2) × 104 | (9.5 ± 0.9) × 103 |
| thymol | 0.3 × 10−7 | |||||
| E. caryophyllus, Spreng | eugenol | 4.9 × 10−6 | 4.9 × 10−6 | (4.6 ± 0.3) × 10−6 | (5.5 ± 0.5) × 103 | (5.7 ± 0.6) × 103 |
| C. zeylanicum, Blume | eugenol | 5.0 × 10−6 | 5.0 × 10−6 | (4.4 ± 0.4) × 10−6 | (4.9 ± 0.3) × 103 | (4.8 ± 0.4) × 103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Pizzol, R.; Gabbanini, S.; Baschieri, A.; Amorati, R.; Valgimigli, L. Absolute Antioxidant Activity of Five Phenol-Rich Essential Oils. Molecules 2021, 26, 5237. https://doi.org/10.3390/molecules26175237
Guo Y, Pizzol R, Gabbanini S, Baschieri A, Amorati R, Valgimigli L. Absolute Antioxidant Activity of Five Phenol-Rich Essential Oils. Molecules. 2021; 26(17):5237. https://doi.org/10.3390/molecules26175237
Chicago/Turabian StyleGuo, Yafang, Romeo Pizzol, Simone Gabbanini, Andrea Baschieri, Riccardo Amorati, and Luca Valgimigli. 2021. "Absolute Antioxidant Activity of Five Phenol-Rich Essential Oils" Molecules 26, no. 17: 5237. https://doi.org/10.3390/molecules26175237
APA StyleGuo, Y., Pizzol, R., Gabbanini, S., Baschieri, A., Amorati, R., & Valgimigli, L. (2021). Absolute Antioxidant Activity of Five Phenol-Rich Essential Oils. Molecules, 26(17), 5237. https://doi.org/10.3390/molecules26175237