Research Progress on Fumonisin B1 Contamination and Toxicity: A Review
Abstract
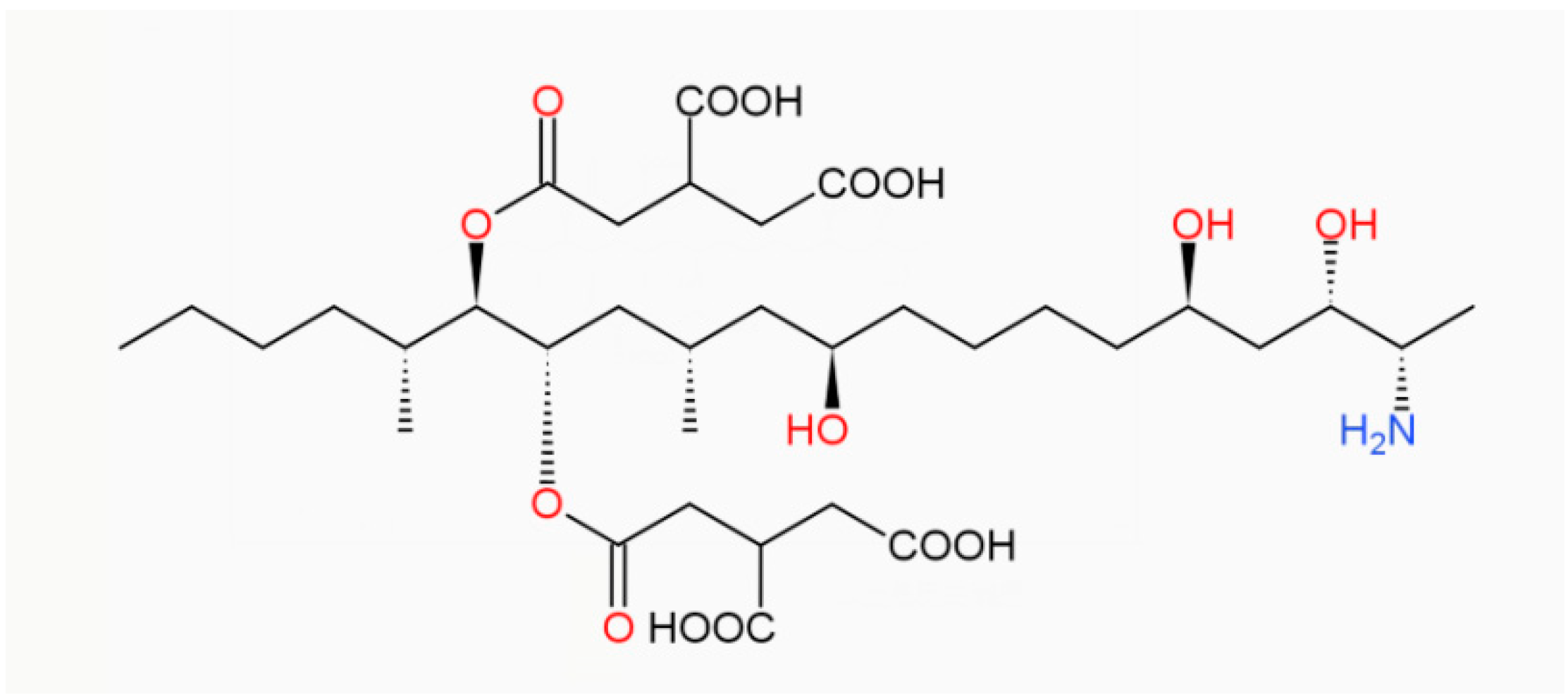
:1. Introduction
2. Contamination Caused by FB1
| Commodity | Country | Positives/Total | Content | Reference |
|---|---|---|---|---|
| Agricultural products | ||||
| Maize | South Africa (Limpopo Province) | 49/54 | 101–53,863 μg/kg | [30] |
| Maize | Algeria | 29/30 | 289–42,143 μg/kg | [31] |
| Rise | Ecuador (Guayas) | 3/20 | 22.6–54.3 μg/kg | [32] |
| Rise | Ecuador (Los Ríos) | 7/23 | 17.9–1146.4 μg/kg | [32] |
| Maize grains | Iran | 56/82 | 15/155 μg/kg | [33] |
| Cereal mixtures | Brazil | 99/105 | 137.8 ± 257.4 μg/g (Mean) | [34] |
| Corn samples (moldy in 1993–1995) | Hungary | 51/69 | 0.05–75.1 mg/kg | [35] |
| Corn samples (mold-free in 1994) | Hungary | 7/23 | 0.06–5.1 mg/kg | [35] |
| Maize | Spain | 48/55 | 0.2–19.2 μg/g | [36] |
| Barley | Spain | 21/29 | 0.2–11.6 μg/g | [36] |
| Wheat | Spain | 8/17 | 0.2–8.8 μg/g | [36] |
| Soybeans | Spain | 1/1 | 8.7 μg/g | [36] |
| Oats | Spain | 29/100 | 62.3–217.4 μg/kg | [37] |
| Maize | China | 166/249 | 530–10,315 μg/kg | [38] |
| Corn | China (Huantai) | 28/30 | nd–12.5 mg/kg | [39] |
| Rice | China (Huantai) | 8/9 | nd–0.4 mg/kg | [39] |
| Wheat flour | China (Huantai) | 8/9 | nd–0.4 mg/kg | [39] |
| Corn | China (Huaian) | 43/43 | 0.2–5.9 mg/kg | [39] |
| Rice | China (Huaian) | 9/10 | nd–0.3 mg/kg | [39] |
| Wheat flour | China (Huaian) | 5/7 | nd–0.4 mg/kg | [39] |
| Corn | China (Fusui) | 29/34 | nd–37.0 mg/kg | [39] |
| Rice | China (Fusui) | 9/10 | nd–0.5 mg/kg | [39] |
| Human food products | ||||
| Corn grits | Brazil | 2/2 | 0.17–1.23 μg/g | [40] |
| Corn meal | Brazil | 9/9 | 0.56–4.93 μg/g | [40] |
| Degerminated corn | Brazil | 8/11 | nd–4.52 μg/g | [40] |
| Popcorn | Brazil | 4/9 | nd–1.72 μg/g | [40] |
| Precooked corn flour | Brazil | 4/6 | nd–1.79 μg/g | [40] |
| Industrial beers | Brazil | 56/114 | 201–1568 μg/L | [41] |
| Cornmeal | Brazil | 25/32 | 33–1208 μg/kg | [42] |
| Corn-flour | Brazil | 19/25 | 114.4–558.6 μg/kg | [42] |
| Popcorn | Brazil | 32/39 | 102.0–1127.3 μg/kg | [42] |
| Polenta | Brazil | 2/2 | 149.0–214.2 μg/kg | [42] |
| Breakfast cereals (corn-based) | Canada | 30/34 | nd–1980 μg/kg | [43] |
| Breakfast cereals (oat-based | Canada | 5/19 | nd–57 μg/kg | [43] |
| Breakfast cereals (rice-based) | Canada | 2/29 | nd–5 μg/kg | [43] |
| Breakfast cereals (wheat-based) | Canada | 5/29 | nd–51 μg/kg | [43] |
| Broa (typical Portuguese maize bread) | Portugal | 24/80 | nd–448 μg/kg | [44] |
| Cornmeal | Portugal | 41/41 | 50–1300 μg/kg | [45] |
| Sweet corn | Portugal | 36/41 | 50–400 μg/kg | [45] |
| Popcorn grain | Japan | 49/57 | 67.5 μg/kg (Mean) 354 μg/kg (Maximum) | [46] |
| Corn grits | Japan | 46/46 | 104 μg/kg (Mean) 1380 μg/kg (Maximum) | [46] |
| Corn snacks | Japan | 41/50 | 113 μg/kg (Mean) 1670 μg/kg (Maximum) | [46] |
| Animal feeds | ||||
| Feed samples (2007) | South African | 20/24 | 5289 ± 1034 μg/kg (Mean) | [4] |
| Feed samples (2008) | South African | 19/24 | 5021 ± 844 μg/kg (Mean) | [4] |
| Feed samples (2006) | Bulgarian | 24/25 | 5564.1 ± 584.4 μg/kg (Mean) | [3] |
| Feed samples (2007) | Bulgarian | 23/25 | 3254.5 ± 480.6 μg/kg (Mean) | [3] |
| Compound feedstuff | China | 284/300 | 20–6568 μg/kg | [24] |
| Concentrated feedstuff | China | 60/60 | 23–6239 μg/kg | [24] |
| Premixing feedstuff | China | 60/60 | 341–6004 μg/kg | [24] |
| Cattle feeds (breeding) | Korea | 10/10 | 365 ± 6.23–13,900 ± 179 ng/g | [23] |
| Cattle feeds (lactation) | Korea | 8/8 | 411 ± 149–2160 ± 471 ng/g | [23] |
| Cattle feeds (fattening) | Korea | 32/32 | 430 ± 173–13,300 ± 2070 ng/g | [23] |
| Swine feeds (breeding) | Korea | 30/42 | 363 ± 142–14,900 ± 72.6 ng/g | [23] |
| Swine feeds (fattening) | Korea | 8/8 | 1510 ± 174–14,600 ± 120 ng/g | [23] |
| Poultry feeds (layer) | Korea | 22/24 | 73.2 ± 15.4–12,800 ± 1460 ng/g | [23] |
| Poultry feeds (broiler) | Korea | 17/22 | 1380 ± 169–14,600 ± 187 ng/g | [23] |
| Cat food samples (maize) | Poland | - | 10.0–15.6 | [25] |
| Cat food samples (maize and wheat) | Poland | - | 15.0–20.8 | [25] |
| Dog food samples (maize) | Poland | - | 29.5–55.5 | [25] |
| Dog food samples (maize and wheat) | Poland | - | 26.5–57.0 | [25] |
| Dog food samples (wheat) | Poland | - | 29.6–37.1 | [25] |
| Human Foods and Animal Feeds | Maximum Levels (mg/kg) | |
|---|---|---|
| 1 | Human foods | |
| 2 | Unprocessed maize, with the exception of unprocessed maize intended to be processed by wet milling | 4 |
| 3 | Maize intended for direct human consumption, maize- based foods for direct human consumption, with the exception of foodstuffs listed in 3 and 4 | 1 |
| 4 | Maize-based breakfast cereals and maize-based snacks | 0.8 |
| 1 | Processed maize-based foods and baby foods for infants and young children | 0.2 |
| Animal feeds | ||
| Maize by-products | 60 | |
| Complete and complimentary feedstuffs for pigs, Equidae, rabbits | 5 | |
| Complete and complimentary feedstuffs for poultry, calves, lambs, kids | 20 | |
| Complete and complimentary feedstuffs for adult ruminants and mink | 50 | |
| Complete and complimentary feedstuffs for fish | 10 |
| Country | Ingestion Population | Exposure Levels | Reference |
|---|---|---|---|
| South Korea | [50.2 ng/g × 0.1 g per person per day]/57.6 kg | 0.087 ng/kg of body weight per day | [47] |
| China (Huantai) | - | 92.4 μg per day | [39] |
| China (Huaian) | - | 460.0 μg per day | [39] |
| China (Fusui) | 138.6 μg per day | [39] | |
| China (Shandong Province) | - | 0.02 µg/kg bw/day | [48] |
| Algerian | - | 10.86 µg/kg bw/day | [31] |
| Brazil (rural areas with higher corn intake) | A 70 kg adult | 1276 ng/kg bw/day | [40] |
| Brazil (urban and some rural areas) | A 70 kg adult | 392 ng/kg bw/day | [40] |
| Tanzania | Children aged 6–12 months | 2 μg/kg body weight/day | [27] |
| Iran (Isfahan 1998) | 3.3 g maize/person (60 kg)/day | 0.009 μg/kg b.w./day | [49] |
| Iran (Isfahan 1998) | 3.3 g maize/person (60 kg)/day | 0.012 μg/kg b.w./day | [49] |
| Iran (Mazandaran 1998) | 3.3 g maize/person (60 kg)/day | 0.125 μg/kg b.w./day | [49] |
| Iran (Mazandaran 1998) | 3.3 g maize/person (60 kg)/day | 0.175 μg/kg b.w./day | [49] |
| Iran (Mazandaran 1998) | 3.3 g maize/person (60 kg)/day | 0.338 μg/kg b.w./day | [49] |
| Mexico | For men of 73.3 kg bw | 4.12 µg/kg bw/day | [50] |
| Mexico | For women of 65.8 kg bw | 3.00 µg/kg bw/day | [50] |
| Hungary | All maize-product consumers | 0.045–0.120 µg/kg bw/day | [51] |
3. Toxic Mechanism of FB1
3.1. Effects on Sphingolipids
3.2. Oxidative Stress
3.3. Endoplasmic Reticulum Stress
3.4. TNF Signaling Pathway
4. Toxic Effects of FB1
4.1. Immunotoxicity
4.2. Organ Toxicity
4.2.1. Toxic Effects of FB1 on the Liver
4.2.2. Toxic Effects of FB1 on the Kidney
4.2.3. Toxic Effects of FB1 on the Intestinal Tract
4.2.4. Toxic Effects of FB1 on the Heart and Lungs
| Animal Species | Method of Administration and Dosage | Duration | Effects | References |
|---|---|---|---|---|
| Mammals | ||||
| Holstein calf | Intravenous. 1mg/kg b.w. | 4 days | Elevated sphingol and sphingosine concentrations in the liver, severe liver and bile duct damage, impaired liver function, apoptosis of liver cells. | [122] |
| Holstein calves | Mixed into the feed and fed 2.36 mg/kg bw, increasing to 3.54 mg/kg bw after 23 weeks | 239~253 days | There was karyomegaly of hepatocellular nuclei, with occasional dense, shrunken hepatocyte nuclei and mitotic figures of hepatocytes. Billiary epithelial cells exhibited mild anisokaryosis and piling on of the epithelium. | [123] |
| Pigs | Mixed into the feed and fed. 20 mg/kg b.w | 10 days | Relative increase in liver weight and vacuolar or fatty degeneration in hepatocytes. | [124] |
| Pigs | Mixed into the feed and fed. 10 mg/kg b.w | 3 months | Degenerative changes in proximal tubules, hyperaemia of vessels and peritubular capillaries, activation of capillary endothelium, mononuclear proliferation in the kidney interstitium, perivascular or pericapillary edema in kidneys, etc. | [85] |
| Pigs | Intravenous. 1 mg/kg b.w | 4 days | Mild pulmonary edema was present. In the liver, there was scattered hepatocyte apoptotic cell death and mitosis. | [125] |
| Piglets | Mixed into the feed and fed. 92 mg/kg b.w. | 4~7 days | Fatal pulmonary edema. | [126] |
| Rats | Feed. 30 mg/kg b.w. | 7 days | Pulmonary congestion, alveolar edema. | [127] |
| Rats | Feed. 50 mg/kg or 150 mg/kg | 2 years | There was evidence of sustained nephrotoxicity manifested as basophilia, apoptosis, cell regeneration, and simple tubule hyperplasia, affecting proximal convoluted tubules in the deep cortex, extending into the outer region of the outer stripe of outer medulla. | [128] |
| Rats | Mixed into the feed and fed. 5 mg/kg | 42 days | FB1 caused histological alterations in duodenum, cecum, and intestine, including partial shedding of villous epithelial cells and inflammatory cell infiltration. | [116] |
| F344/N/Nctr Br rats | Mixed into the feed and fed. 484 mg/kg | 28 days | Induction of apoptosis and mitosis of hepatocytes in female rats. Induced apoptosis and regeneration of tubular epithelial cells in male rats. | [129] |
| Horses | Intravenous.0.2 mg/kg b.w. | 7~28 days | Symptoms such as cyanosis, dyspnea and oedema of the mucous membranes and mild pulmonary oedema. | [130] |
| Male New Zealand rabbit | Feed, 1.5 mg/kg b.w. | 21 days | Liver and kidney congestion with moderate vacuolar degeneration of the liver. | [131] |
| Poultry | ||||
| Japanese quail | 200 mg/kg FB1and Fusarium fujikuroi culture material (MCM), supplying 100 mg/kg M | 28 days | Cardiomyocytes thin and form many irregularly sized fluid vesicles between the myoplasm and myogenic fibers. | [132] |
4.2.5. Toxic Effects of FB1 on the Brain
4.2.6. Toxic Effects of FB1 on Human Organs
| Cell Type | Dosage | Duration | Effects | References |
|---|---|---|---|---|
| Gastric epithelial cell line (AGS) and human colon adenocarcinoma cell line (SW742). | 4.5~72 mg/L | 72 h | Increased levels of pro-inflammatory cytokines such as IL-1β and TNF-α and decreased IL-8 levels in gastric and colonic cell lines in a concentration-dependent manner. This effect may underlie the development or progression of inflammation and subsequent atrophy of the stomach and intestine. | [79] |
| Human esophageal epithelial cells (HET-IA) | 1 μM or 100 μM | 5 days | 1 μM fumonisin B1 had no effect on the clonal growth of HET-1A, but 100 μM fumonisin B1 inhibited the clonal growth of HET-1A by 75%. Morphological observations showed that fumonisin B1 induced apoptosis of HET-1A cells. | [148] |
| Human oesophageal carcinoma (SNO)cells | 1.25 and 10 μM or 20 μM | - | FB1 induced apoptosis in SNO cells, as evidenced by decreased survival, phosphatidylserine externalization, increased Bax protein expression, and DNA fragmentation. Caspase-dependent apoptosis started at 1.25 and 10 μM FB1, but execution at 20 μM FB1 may be mediated by a caspase-independent pathway. | [72] |
| Human embryonic kidney (HEK-293) cells | 25 μM | 48 h | HEK-293 cells are resistant to the apoptotic effects of FB1, which enhances cell survival by forming sphingosine-1-phosphate. This finding is only applicable to HEK-293 cells, and resistance to other tissues needs further study. | [149] |
| Human proximal tubule-derived cells (IHKE cells) | 10 μM | 24 h | Both caspase 3 activity and DNA fragmentation were significantly increased. | [150] |
| Normal human keratino- cytes (NHKc) | 1 μM or 10 μM | 5 days | When the concentration reached 1 μM, fumonisin B1 had no effect on the growth of keratin-forming cells, while 10 μM fumonisin B1 inhibited the clonal growth of keratin-forming cells by 42%. | [148] |
| NHKc | 10 μM or 100 μM | 4–8 days | Increased intracellular lipids in NHKc, growth inhibition at FB1 of 10 μM, and DNA fragmentation at 100 μM, all due to accumulation of sphinganine (SA). | [151] |
| NHKc | 100 μM | 2 days | The clone-forming ability of NHKc decreased to 44.5% of the control level and almost disappeared after 4 days. | [152] |
4.3. Reproductive Toxicity
5. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dutton, M.F.; Kinsey, A. Occurrence of mycotoxins in cereals and animal feedstuffs in Natal, South Africa 1994. Mycopathologia 1995, 131, 31–36. [Google Scholar] [CrossRef]
- Shephard, G.S.; Thiel, P.G.; Stockenström, S.; Sydenham, E.W. Worldwide Survey of Fumonisin Contamination of Corn and Corn-Based Products. J. AOAC Int. 1996, 79, 671–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoev, S.; Dutton, M.; Njobeh, P.; Mosonik, J.; Steenkamp, P. Mycotoxic nephropathy in Bulgarian pigs and chickens: Complex aetiology and similarity to Balkan Endemic Nephropathy. Food Addit. Contam. Part A 2010, 27, 72–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoev, S.D.; Denev, S.; Dutton, M.F.; Njobeh, P.B.; Mosonik, J.S.; Steenkamp, P.A.; Petkov, I. Complex etiology and pathology of mycotoxic nephropathy in South African pigs. Mycotoxin Res. 2009, 26, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, W.C.; Jaskiewicz, K.; Marasas, W.F.; Thiel, P.G.; Horak, R.M.; Vleggaar, R.; Kriek, N.P. Fumonisins-novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl. Environ. Microbiol. 1988, 54, 1806–1811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezuidenhout, S.C.; Gelderblom, W.C.A.; Gorst-Allman, C.P.; Horak, R.M.; Marasas, W.F.O.; Spiteller, G.; Vleggaar, R. Structure elucidation of the fumonisins, mycotoxins from Fusarium moniliforme. J. Chem. Soc. Chem. Commun. 1988, 11, 743–745. [Google Scholar] [CrossRef]
- FAO. Evaluation of Certain Contaminants in Food; FAO: Rome, Italy, 2017; pp. 1–166. [Google Scholar]
- Ahangarkani, F.; Rouhi, S.; Azizi, I.G. A review on incidence and toxicity of fumonisins. Toxin Rev. 2014, 33, 95–100. [Google Scholar] [CrossRef]
- Waes, J.G.-V.; Starr, L.; Maddox, J.; Aleman, F.; Voss, K.A.; Wilberding, J.; Riley, R.T. Maternal fumonisin exposure and risk for neural tube defects: Mechanisms in an In Vivo mouse model. Birth Defects Res. Part A Clin. Mol. Teratol. 2005, 73, 487–497. [Google Scholar] [CrossRef]
- Missmer, S.A.; Suarez, L.; Felkner, M.; Wang, E.; Merrill, A.; Rothman, K.; Hendricks, K.A. Exposure to Fumonisins and the Occurrence of Neural Tube Defects along the Texas–Mexico Border. Environ. Health Perspect. 2006, 114, 237–241. [Google Scholar] [CrossRef]
- Stoev, S.D. Foodborne mycotoxicoses, risk assessment and underestimated hazard of masked mycotoxins and joint mycotoxin effects or interaction. Environ. Toxicol. Pharmacol. 2015, 39, 794–809. [Google Scholar] [CrossRef] [PubMed]
- Stoev, S.D. Food Safety and Increasing Hazard of Mycotoxin Occurrence in Foods and Feeds. Crit. Rev. Food Sci. Nutr. 2013, 53, 887–901. [Google Scholar] [CrossRef]
- Hanvi, D.M.; Lawson-Evi, P.; De Boevre, M.; Goto, C.E.; De Saeger, S.; Eklu-Gadegbeku, K. Natural occurrence of mycotoxins in maize and sorghum in Togo. Mycotoxin Res. 2019, 35, 321–327. [Google Scholar] [CrossRef]
- Ponce-García, N.; Serna-Saldivar, S.O.; García-Lara, S. Fumonisins and their analogues in contaminated corn and its processed foods—A review. Food Addit. Contam. Part A 2018, 35, 2183–2203. [Google Scholar] [CrossRef]
- Alizadeh, A.M.; Roshandel, G.; Roudbarmohammadi, S.; Roudbary, M.; Sohanaki, H.; Ghiasian, S.A.; Taherkhani, A.; Semnani, S.; Aghasi, M. Fumonisin B1 Contamination of Cereals and Risk of Esophageal Cancer in a High Risk Area in Northeastern Iran. Asian Pac. J. Cancer Prev. 2012, 13, 2625–2628. [Google Scholar] [CrossRef] [Green Version]
- Cendoya, E.; Monge, M.D.P.; Chiacchiera, S.M.; Farnochi, M.C.; Ramirez, M.L. Influence of water activity and temperature on growth and fumonisin production by Fusarium proliferatum strains on irradiated wheat grains. Int. J. Food Microbiol. 2018, 266, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Rheeder, J.; van der Westhuizen, L.; Imrie, G.; Shephard, G.S. Fusariumspecies and fumonisins in subsistence maize in the former Transkei region, South Africa: A multi-year study in rural villages. Food Addit. Contam. Part B 2016, 9, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Liverpool-Tasie, L.S.O.; Turna, N.S.; Ademola, O.; Obadina, A.; Wu, F. The occurrence and co-occurrence of aflatoxin and fumonisin along the maize value chain in southwest Nigeria. Food Chem. Toxicol. 2019, 129, 458–465. [Google Scholar] [CrossRef] [PubMed]
- van Rensburg, B.J.; McLaren, N.; Flett, B. Grain colonization by fumonisin-producing Fusarium spp. and fumonisin synthesis in South African commercial maize in relation to prevailing weather conditions. Crop. Prot. 2017, 102, 129–136. [Google Scholar] [CrossRef]
- Bryła, M.; Waśkiewicz, A.; Szymczyk, K.; Jędrzejczak, R. Effects of pH and Temperature on the Stability of Fumonisins in Maize Products. Toxins 2017, 9, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arroyo-Manzanares, N.; Rodríguez-Estévez, V.; Arenas-Fernández, P.; García-Campaña, A.M.; Gámiz-Gracia, L. Occurrence of Mycotoxins in Swine Feeding from Spain. Toxins 2019, 11, 342. [Google Scholar] [CrossRef] [Green Version]
- Pietsch, C. Risk assessment for mycotoxin contamination in fish feeds in Europe. Mycotoxin Res. 2020, 36, 41–62. [Google Scholar] [CrossRef] [Green Version]
- Seo, D.-G.; Phat, C.; Kim, D.-H.; Lee, C. Occurrence of Fusarium Mycotoxin Fumonisin B1 and B2 in Animal Feeds in Korea. Mycotoxin Res. 2013, 29, 159–167. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Zheng, H.; He, C.; Zhang, H. T-2 toxin, zearalenone and fumonisin B1in feedstuffs from China. Food Addit. Contam. Part B 2013, 6, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Witaszak, N.; Waśkiewicz, A.; Bocianowski, J.; Stępień, Ł. Contamination of Pet Food with Mycobiota and Fusarium Mycotoxins—Focus on Dogs and Cats. Toxins 2020, 12, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirima, C.P.; Kimanya, M.E.; Kinabo, J.L.; Routledge, M.; Srey, C.; Wild, C.P.; Gong, Y.Y. Dietary exposure to aflatoxin and fumonisin among Tanzanian children as determined using biomarkers of exposure. Mol. Nutr. Food Res. 2013, 57, 1874–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamala, A.; Kimanya, M.; Lachat, C.; Jacxsens, L.; Haesaert, G.; Kolsteren, P.; Ortiz, J.; Tiisekwa, B.; De Meulenaer, B. Risk of Exposure to Multiple Mycotoxins from Maize-Based Complementary Foods in Tanzania. J. Agric. Food Chem. 2017, 65, 7106–7114. [Google Scholar] [CrossRef] [PubMed]
- De Nijs, M.; Van Egmond, H.P.; Nauta, M.; Rombouts, F.M.; Notermans, S.H.W. Assessment of Human Exposure to Fumonisin B1. J. Food Prot. 1998, 61, 879–884. [Google Scholar] [CrossRef]
- Peters, J.; Van Dam, R.; Van Doorn, R.; Katerere, D.; Berthiller, F.; Haasnoot, W.; Nielen, M.W.F. Mycotoxin profiling of 1000 beer samples with a special focus on craft beer. PLoS ONE 2017, 12, e0185887. [Google Scholar] [CrossRef] [Green Version]
- Phoku, J.; Dutton, M.; Njobeh, P.; Mwanza, M.; Egbuta, M.; Chilaka, C. Fusarium infection of maize and maize-based products and exposure of a rural population to fumonisin B1in Limpopo Province, South Africa. Food Addit. Contam. Part A 2012, 29, 1743–1751. [Google Scholar] [CrossRef]
- Mahdjoubi, C.K.; Arroyo-Manzanares, N.; Hamini-Kadar, N.; García-Campaña, A.M.; Mebrouk, K.; Gámiz-Gracia, L. Multi-Mycotoxin Occurrence and Exposure Assessment Approach in Foodstuffs from Algeria. Toxins 2020, 12, 194. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, J.; Van Camp, J.; Mestdagh, F.; Donoso, S.; De Meulenaer, B. Mycotoxin co-occurrence in rice, oat flakes and wheat noodles used as staple foods in Ecuador. Food Addit. Contam. Part A 2013, 30, 2165–2176. [Google Scholar] [CrossRef]
- Chehri, K.; Jahromi, S.T.; Reddy, K.R.N.; Abbasi, S.; Salleh, B. Occurrence of Fusarium spp. and Fumonisins in Stored Wheat Grains Marketed in Iran. Toxins 2010, 2, 2816–2823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peluque, E.; Neres, N.; Michelin, E.; Reis, T.; Rosim, R.; Oliveira, C.; Sousa, R.; Corrêa, B.; Fernandes, A. Fumonisin B1in cereal mixtures marketed in Brazil. Food Addit. Contam. Part B 2014, 7, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, B.; Bajmócy, E.; Glávits, R.; Fenyvesi, A.; Tanyi, J. Fumonisin B1 Contamination of Maize and Experimental Acute Fumonisin Toxicosis in Pigs. J. Vet. Med. Ser. B 1998, 45, 171–181. [Google Scholar] [CrossRef]
- Castellá, G.; Bragulat, M.R.; Cabañes, F.J. Surveillance of fumonisins in maize-based feeds and cereals from Spain. J. Agric. Food Chem. 1999, 47, 4707–4710. [Google Scholar] [CrossRef] [PubMed]
- Tarazona, A.; Gómez, J.; Mateo, F.; Jiménez, M.; Mateo, E. Potential Health Risk Associated with Mycotoxins in Oat Grains Consumed in Spain. Toxins 2021, 13, 421. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Y.; Li, R.; Pang, M.; Liu, Y.; Dong, J. Natural occurrence of fumonisins B1 and B2 in maize from eight provinces of China in 2014. Food Addit. Contam. Part B 2017, 10, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Wang, S.; Hu, X.; Su, J.; Zhang, Y.; Xie, Y.; Zhang, H.; Tang, L.; Wang, J.-S. Co-contamination of aflatoxin B1and fumonisin B1in food and human dietary exposure in three areas of China. Food Addit. Contam. Part A 2011, 28, 461–470. [Google Scholar] [CrossRef]
- Mac, M., Jr.; Soares, L.M.V. Fumonisins B1and B2in Brazilian corn-based food products. Food Addit. Contam. 2000, 17, 875–879. [Google Scholar] [CrossRef]
- Piacentini, K.C.; Rocha, L.; Fontes, L.C.; Carnielli, L.; Reis, T.; Corrêa, B. Mycotoxin analysis of industrial beers from Brazil: The influence of fumonisin B1 and deoxynivalenol in beer quality. Food Chem. 2017, 218, 64–69. [Google Scholar] [CrossRef]
- Bordin, K.; Rosim, R.; Neeff, D.; Rottinghaus, G.; Oliveira, C. Assessment of dietary intake of fumonisin B1 in São Paulo, Brazil. Food Chem. 2014, 155, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Roscoe, V.; Lombaert, G.A.; Huzel, V.; Neumann, G.; Melietio, J.; Kitchen, D.; Kotello, S.; Krakalovich, T.; Trelka, R.; Scott, P.M. Mycotoxins in breakfast cereals from the Canadian retail market: A 3-year survey. Food Addit. Contam. Part A 2008, 25, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Lino, C.; Silva, L.; Pena, A.; Fernández, M.; Mañes, J. Occurrence of fumonisins B1 and B2 in broa, typical Portuguese maize bread. Int. J. Food Microbiol. 2007, 118, 79–82. [Google Scholar] [CrossRef] [Green Version]
- Martins, H.M.; Almeida, I.; Marques, M.C.; Guerra, M. Fumonisins and deoxynivalenol in corn-based food products in Portugal. Food Chem. Toxicol. 2008, 46, 2585–2587. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, K.; Nakajima, M.; Tabata, S.; Ishikuro, E.; Tanaka, T.; Norizuki, H.; Itoh, Y.; Fujita, K.; Kai, S.; Tsutsumi, T.; et al. Four-Year Surveillance for Ochratoxin A and Fumonisins in Retail Foods in Japan. J. Food Prot. 2010, 73, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.; Yoon, Y.; Kim, K.; Shim, W.-B.; Kuzmina, N.; Oh, K.-S.; Lee, J.-O.; Kim, D.-S.; Suh, J.; Lee, S.-H.; et al. Fumonisins B1 and B2 in Agricultural Products Consumed in South Korea: An Exposure Assessment. J. Food Prot. 2009, 72, 436–440. [Google Scholar] [CrossRef]
- Jiang, D.; Li, F.; Zheng, F.; Zhou, J.; Li, L.; Shen, F.; Chen, J.; Li, W. Occurrence and dietary exposure assessment of multiple mycotoxins in corn-based food products from Shandong, China. Food Addit. Contam. Part B 2019, 12, 10–17. [Google Scholar] [CrossRef]
- Yazdanpanah, H.; Shephard, G.S.; Marasas, W.F.O.; van der Westhuizen, L.; Rahimian, H.; Safavi, S.N.; Eskandari, P.; Ghiasian, S.A. Human Dietary Exposure to Fumonisin B1 from Iranian Maize Harvested During 1998–2000. Mycopathologia 2006, 161, 395–401. [Google Scholar] [CrossRef]
- Gilbert-Sandoval, I.; Wesseling, S.; Rietjens, I.M.C.M. Occurrence and Probabilistic Risk Assessment of Fumonisin B1, Fumonisin B2 and Deoxynivalenol in Nixtamalized Maize in Mexico City. Toxins 2020, 12, 644. [Google Scholar] [CrossRef]
- Zentai, A.; Szeitzné-Szabó, M.; Mihucz, G.; Szeli, N.; Szabó, S.; Kovács, M. Occurrence and Risk Assessment of Fumonisin B1 and B2 Mycotoxins in Maize-Based Food Products in Hungary. Toxins 2019, 11, 709. [Google Scholar] [CrossRef] [Green Version]
- Schertz, H.; Dänicke, S.; Frahm, J.; Schatzmayr, D.; Dohnal, I.; Bichl, G.; Schwartz-Zimmermann, H.E.; Colicchia, S.; Breves, G.; Teifke, J.P.; et al. Biomarker Evaluation and Toxic Effects of an Acute Oral and Systemic Fumonisin Exposure of Pigs with a Special Focus on Dietary Fumonisin Esterase Supplementation. Toxins 2018, 10, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Chen, J.; Wei, Z.; Wang, Y.; Long, M.; Wu, W.; Kuca, K. Fumonisin B1: Mechanisms of toxicity and biological detoxification progress in animals. Food Chem. Toxicol. 2021, 149, 111977. [Google Scholar] [CrossRef]
- Wang, E.; Ross, P.F.; Wilson, T.M.; Riley, R.T.; Merrill, J.A.H. Increases in Serum Sphingosine and Sphinganine and Decreases in Complex Sphingolipids in Ponies Given Feed Containing Fumonisins, Mycotoxins Produced by Fusarium moniliforme. J. Nutr. 1992, 122, 1706–1716. [Google Scholar] [CrossRef] [PubMed]
- Wangia, R.N.; Githanga, D.P.; Xue, K.S.; Tang, L.; Anzala, O.A.; Wang, J.-S. Validation of urinary sphingolipid metabolites as biomarker of effect for fumonisins exposure in Kenyan children. Biomarkers 2019, 24, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.T.; Merrill, A.H., Jr. Ceramide synthase inhibition by fumonisins: A perfect storm of perturbed sphingolipid metabolism, signaling, and disease. J. Lipid Res. 2019, 60, 1183–1189. [Google Scholar] [CrossRef] [Green Version]
- Cabello-Verrugio, C.; Simon, F.; Trollet, C.; Santibañez, J.F. Oxidative Stress in Disease and Aging: Mechanisms and Therapies. Oxidative Med. Cell. Longev. 2017, 2017, 4310469. [Google Scholar] [CrossRef]
- Arumugam, T.; Pillay, Y.; Ghazi, T.; Nagiah, S.; Abdul, N.S.; Chuturgoon, A.A. Fumonisin B1-induced oxidative stress triggers Nrf2-mediated antioxidant response in human hepatocellular carcinoma (HepG2) cells. Mycotoxin Res. 2019, 35, 99–109. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Q.; Wan, D.; Liu, Q.; Chen, D.; Liu, Z.; Martínez-Larrañaga, M.R.; Martínez, M.-A.; Anadón, A.; Yuan, Z. Fumonisins: Oxidative stress-mediated toxicity and metabolism In Vivo and In Vitro. Arch. Toxicol. 2016, 90, 81–101. [Google Scholar] [CrossRef]
- Stockmann-Juvala, H.; Mikkola, J.; Naarala, J.; Loikkanen, J.; Elovaara, E.; Savolainen, K. Oxidative Stress Induced by Fumonisin B1in Continuous Human and Rodent Neural Cell Cultures. Free Radic. Res. 2004, 38, 933–942. [Google Scholar] [CrossRef]
- Yuan, Q.; Jiang, Y.; Fan, Y.; Ma, Y.; Lei, H.; Su, J. Fumonisin B1 Induces Oxidative Stress and Breaks Barrier Functions in Pig Iliac Endothelium Cells. Toxins 2019, 11, 387. [Google Scholar] [CrossRef] [Green Version]
- Mobio, T.A.; Tavan, E.; Baudrimont, I.; Anane, R.; Carratù, M.; Sanni, A.; Gbeassor, M.F.; Shier, T.W.; Narbonne, J.-F.; Creppy, E.E. Comparative study of the toxic effects of fumonisin B1 in rat C6 glioma cells and p53-null mouse embryo fibroblasts. Toxicology 2003, 183, 65–75. [Google Scholar] [CrossRef]
- Cheng, Y.; Ren, X.; Gowda, A.S.; Shan, Y.; Zhang, L.; Yuan, Y.-S.; Patel, R.; Wu, H.; Huber-Keener, K.; Yang, J.W.; et al. Interaction of Sirt3 with OGG1 contributes to repair of mitochondrial DNA and protects from apoptotic cell death under oxidative stress. Cell Death Dis. 2013, 4, e731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demirel, G.; Alpertunga, B.; Ozden, S. Role of fumonisin B1 on DNA methylation changes in rat kidney and liver cells. Pharm. Biol. 2015, 53, 1302–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arumugam, T.; Ghazi, T.; Chuturgoon, A. Fumonisin B1 Epigenetically Regulates PTEN Expression and Modulates DNA Damage Checkpoint Regulation in HepG2 Liver Cells. Toxins 2020, 12, 625. [Google Scholar] [CrossRef]
- Yu, S.; Jia, B.; Liu, N.; Yu, D.; Zhang, S.; Wu, A. Fumonisin B1 triggers carcinogenesis via HDAC/PI3K/Akt signalling pathway in human esophageal epithelial cells. Sci. Total Environ. 2021, 787, 147405. [Google Scholar] [CrossRef]
- Arumugam, T.; Ghazi, T.; Chuturgoon, A.A. Fumonisin B1 alters global m6A RNA methylation and epigenetically regulates Keap1-Nrf2 signaling in human hepatoma (HepG2) cells. Arch. Toxicol. 2021, 95, 1367–1378. [Google Scholar] [CrossRef]
- Matsuzawa, A.; Ichijo, H. Stress-Responsive Protein Kinases in Redox-Regulated Apoptosis Signaling. Antioxid. Redox Signal. 2005, 7, 472–481. [Google Scholar] [CrossRef]
- Kim, S.H.; Singh, M.P.; Sharma, C.; Kang, S.C. Fumonisin B1 actuates oxidative stress-associated colonic damage via apoptosis and autophagy activation in murine model. J. Biochem. Mol. Toxicol. 2018, 32, e22161. [Google Scholar] [CrossRef]
- Li, H.; Wang, M.; Kang, W.; Lin, Z.; Gan, F.; Huang, K. Non-cytotoxic dosage of fumonisin B1 aggravates ochratoxin A-induced nephrocytotoxicity and apoptosis via ROS-dependent JNK/MAPK signaling pathway. Toxicology 2021, 457, 152802. [Google Scholar] [CrossRef]
- Khan, R.; Phulukdaree, A.; Chuturgoon, A.A. Concentration-dependent effect of fumonisin B1 on apoptosis in oesophageal cancer cells. Hum. Exp. Toxicol. 2017, 37, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Oakes, S.A.; Papa, F.R. The Role of Endoplasmic Reticulum Stress in Human Pathology. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 173–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, L.; Yuan, X.; Le, G.; Lin, Z.; Gan, F.; Li, H.; Huang, K. Fumonisin B1 induces nephrotoxicity via autophagy mediated by mTORC1 instead of mTORC2 in human renal tubule epithelial cells. Food Chem. Toxicol. 2021, 149, 112037. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Jia, B.; Liu, N.; Yu, D.; Wu, A. Evaluation of the Individual and Combined Toxicity of Fumonisin Mycotoxins in Human Gastric Epithelial Cells. Int. J. Mol. Sci. 2020, 21, 5917. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Jia, B.; Yang, Y.; Liu, N.; Wu, A. Involvement of PERK-CHOP pathway in fumonisin B1- induced cytotoxicity in human gastric epithelial cells. Food Chem. Toxicol. 2019, 136, 111080. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, E.; Yin, S.; Zhao, C.; Fan, L.; Hu, H. Activation of the IRE1α Arm, but not the PERK Arm, of the Unfolded Protein Response Contributes to Fumonisin B1-Induced Hepatotoxicity. Toxins 2020, 12, 55. [Google Scholar] [CrossRef] [Green Version]
- Dugyala, R.R.; Sharma, R.P.; Tsunoda, M.; Riley, R.T. Tumor necrosis factor-alpha as a contributor in fumonisin B1 toxicity. J. Pharmacol. Exp. Ther. 1998, 285, 317–324. [Google Scholar]
- Mahmoodi, M.; Alizadeh, A.M.; Sohanaki, H.; Rezaei, N.; Amini-Najafi, F.; Khosravi, A.R.; Hosseini, S.-K.; Safari, Z.; Hydarnasab, D.; Khori, V. Impact of fumonisin B1 on the production of inflammatory cytokines by gastric and colon cell lines. Iran. J. Allergy Asthma Immunol. 2012, 11, 165–173. [Google Scholar]
- Chen, J.; Yang, S.; Huang, S.; Yan, R.; Wang, M.; Chen, S.; Cai, J.; Long, M.; Li, P. Transcriptome study reveals apoptosis of porcine kidney cells induced by fumonisin B1 via TNF signalling pathway. Food Chem. Toxicol. 2020, 139, 111274. [Google Scholar] [CrossRef]
- Régnier, M.; Gourbeyre, P.; Pinton, P.; Napper, S.; Laffite, J.; Cossalter, A.; Bailly, J.; Lippi, Y.; Bertrand-Michel, J.; Bracarense, A.P.F.; et al. Identification of Signaling Pathways Targeted by the Food Contaminant FB1: Transcriptome and Kinome Analysis of Samples from Pig Liver and Intestine. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [Green Version]
- Abbès, S.; Ben Salah-Abbès, J.; Jebali, R.; Ben Younes, R.; Oueslati, R. Interaction of aflatoxin B1and fumonisin B1 in mice causes immunotoxicity and oxidative stress: Possible protective role using lactic acid bacteria. J. Immunotoxicol. 2016, 13, 46–54. [Google Scholar] [CrossRef]
- Keck, B.B.; Bodine, A.B. The Effects of Fumonisin B1 on viability and mitogenic response of avian immune cells. Poult. Sci. 2006, 85, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Taranu, I.; Marin, D.E.; Bouhet, S.; Pascale, F.; Bailly, J.-D.; Miller, J.D.; Pinton, P.; Oswald, I.P. Mycotoxin Fumonisin B1 Alters the Cytokine Profile and Decreases the Vaccinal Antibody Titer in Pigs. Toxicol. Sci. 2005, 84, 301–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoev, S.D.; Gundasheva, D.; Zarkov, I.; Mircheva, T.; Zapryanova, D.; Denev, S.; Mitev, Y.; Daskalov, H.; Dutton, M.; Mwanza, M.; et al. Experimental mycotoxic nephropathy in pigs provoked by a mouldy diet containing ochratoxin A and fumonisin B1. Exp. Toxicol. Pathol. 2012, 64, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, Y.; Xia, B.; Xiao, Q.; Wang, Q.; Sun, W.; Zhang, H.; He, C. The immunosuppressive characteristics of FB1 by inhibition of maturation and function of BMDCs. Int. Immunopharmacol. 2017, 47, 206–211. [Google Scholar] [CrossRef]
- Yao, Z.G.; Zhang, X.H.; Hua, F.; Wang, J.L.; Xing, X.; Yan, X.; Xing, L.X. Effects of fumonisin B1 on HLA class I antigen presentation and processing pathway in GES-1 cells In Vitro. Hum. Exp. Toxicol. 2011, 30, 379–390. [Google Scholar] [CrossRef]
- Li, Z.; Cui, J.; Zhang, X.; Kang, W. Aflatoxin G1 reduces the molecular expression of HLA-I, TAP-1 and LMP-2 of adult esophageal epithelial cells In Vitro. Toxicol. Lett. 2010, 195, 169–173. [Google Scholar] [CrossRef]
- Mary, V.S.; Theumer, M.G.; Arias, S.L.; Rubinstein, H.R. Reactive oxygen species sources and biomolecular oxidative damage induced by aflatoxin B1 and fumonisin B1 in rat spleen mononuclear cells. Toxicology 2012, 302, 299–307. [Google Scholar] [CrossRef]
- Kachlek, M.; Szabó-Fodor, J.; Bodnár, Z.B.; Horvatovich, K.; Kovács, M. Preliminary results on the interactive effects of deoxynivalenol, zearalenone and fumonisin B1 on porcine lymphocytes. Acta Vet. Hung. 2017, 65, 340–353. [Google Scholar] [CrossRef] [Green Version]
- Mwanza, M.; Kametler, L.; Bonai, A.; Rajli, V.; Kovacs, M.; Dutton, M.F. The cytotoxic effect of fumonisin B1 and ochratoxin A on human and pig lymphocytes using the Methyl Thiazol Tetrazolium (MTT) assay. Mycotoxin Res. 2009, 25, 233–238. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Ding, S.-T.; Chang, M.-H. Effect of fumonisins on macrophage immune functions and gene expression of cytokines in broilers. Arch. Anim. Nutr. 2006, 60, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Grenier, B.; Dohnal, I.; Shanmugasundaram, R.; Eicher, S.D.; Selvaraj, R.K.; Schatzmayr, G.; Applegate, T.J. Susceptibility of Broiler Chickens to Coccidiosis When Fed Subclinical Doses of Deoxynivalenol and Fumonisins—Special Emphasis on the Immunological Response and the Mycotoxin Interaction. Toxins 2016, 8, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pósa, R.; Magyar, T.; Stoev, S.D.; Glávits, R.; Donko, T.; Repa, I.; Kovács, M. Use of Computed Tomography and Histopathologic Review for Lung Lesions Produced by the Interaction between Mycoplasma hyopneumoniaeand Fumonisin Mycotoxins in Pigs. Vet. Pathol. 2013, 50, 971–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oswald, I.; Desautels, C.; Laffitte, J.; Fournout, S.; Peres, S.Y.; Odin, M.; Le Bars, P.; Le Bars, J.; Fairbrother, J.M. Mycotoxin Fumonisin B 1 Increases Intestinal Colonization by Pathogenic Escherichia coli in Pigs. Appl. Environ. Microbiol. 2003, 69, 5870–5874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burel, C.; Tanguy, M.; Guerre, P.; Boilletot, E.; Cariolet, R.; Queguiner, M.; Postollec, G.; Pinton, P.; Salvat, G.; Oswald, I.; et al. Effect of Low Dose of Fumonisins on Pig Health: Immune Status, Intestinal Microbiota and Sensitivity to Salmonella. Toxins 2013, 5, 841–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halloy, D.J.; Gustin, P.G.; Bouhet, S.; Oswald, I. Oral exposure to culture material extract containing fumonisins predisposes swine to the development of pneumonitis caused by Pasteurellamultocida. Toxicology 2005, 213, 34–44. [Google Scholar] [CrossRef]
- Kovács, M.; Pósa, R.; Tuboly, T.; Donko, T.; Repa, I.; Tossenberger, J.; Szabó-Fodor, J.; Stoev, S.; Magyar, T. Feed exposure to FB1 can aggravate pneumonic damages in pigs provoked by P. multocid a. Res. Vet. Sci. 2016, 108, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Meyer, K.; Mohr, K.; Bauer, J.; Horn, P.; Kovacs, M. Residue formation of fumonisin B1 in porcine tissues. Food Addit. Contam. 2003, 20, 639–647. [Google Scholar] [CrossRef]
- Enongene, E.; Sharma, R.; Bhandari, N.; Voss, K.; Riley, R. Disruption of sphingolipid metabolism in small intestines, liver and kidney of mice dosed subcutaneously with fumonisin B1. Food Chem. Toxicol. 2000, 38, 793–799. [Google Scholar] [CrossRef]
- Dassi, M.; Souto, N.S.; Braga, A.C.M.; Freitas, M.L.; Vasconcelos, C.; Oliveira, M.; Furian, A.F. Effects of repeated fumonisin B1 exposure on markers of oxidative stress in liver, kidneys, and lungs of C57BL/6 mice. J. Environ. Sci. Health Part B 2018, 53, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, D.; Auby, A.; Bluteau, C.; Bailly, J.-D.; Guerre, P. Determination of Fumonisin B1 in animal tissues with immunoaffinity purification. J. Chromatogr. B 2008, 870, 140–144. [Google Scholar] [CrossRef]
- Loiseau, N.; Polizzi, A.; Dupuy, A.; Therville, N.; Rakotonirainy, M.; Loy, J.; Viadere, J.-L.; Cossalter, A.-M.; Bailly, J.-D.; Puel, O.; et al. New insights into the organ-specific adverse effects of fumonisin B1: Comparison between lung and liver. Arch. Toxicol. 2015, 89, 1619–1629. [Google Scholar] [CrossRef]
- Gelderblom, W.; Cawood, M.; Snyman, S.; Marasas, W. Fumonisin B1 dosimetry in relation to cancer initiation in rat liver. Carcinogenesis 1994, 15, 209–214. [Google Scholar] [CrossRef]
- Ewuola, E.O. Organ traits and histopathology of rabbits fed varied levels of dietary fumonisin B1. J. Anim. Physiol. Anim. Nutr. 2009, 93, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; He, Q.; Sharma, R.P. Amelioration of fumonisin B1 hepatotoxicity in mice by depletion of T cells with anti-Thy-1.2. Toxicology 2006, 223, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Howard, P.C.; Eppley, R.M.; Stack, M.E.; Warbritton, A.; Voss, K.A.; Lorentzen, R.J.; Kovach, R.M.; Bucci, T.J. Fumonisin b1 carcinogenicity in a two-year feeding study using F344 rats and B6C3F1 mice. Environ. Health Perspect. 2001, 109 (Suppl. 2), 277–282. [Google Scholar] [CrossRef] [Green Version]
- Burger, H.-M.; Abel, S.; Gelderblom, W. Modulation of key lipid raft constituents in primary rat hepatocytes by fumonisin B1—Implications for cancer promotion in the liver. Food Chem. Toxicol. 2018, 115, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.T.; Hinton, D.M.; Chamberlain, W.J.; Bacon, C.W.; Wang, E.; Merrill, J.A.H.; Voss, K.A. Dietary Fumonisin B1 Induces Disruption of Sphingolipid Metabolism in Sprague-Dawley Rats: A New Mechanism of Nephrotoxicity. J. Nutr. 1994, 124, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Szabó, A.; Fébel, H.; Ali, O.; Kovács, M. Fumonisin B1 induced compositional modifications of the renal and hepatic membrane lipids in rats—Dose and exposure time dependence. Food Addit. Contam. Part A 2019, 36, 1722–1739. [Google Scholar] [CrossRef] [PubMed]
- Bondy, G.; Barker, M.; Lombaert, G.; Armstrong, C.; Fernie, S.; Gurofsky, S.; Huzel, V.; Savard, M.; Curran, I. A comparison of clinical, histopathological and cell-cycle markers in rats receiving the fungal toxins fumonisin B1 or fumonisin B2 by intraperitoneal injection. Food Chem. Toxicol. 2000, 38, 873–886. [Google Scholar] [CrossRef]
- Szabó, A.; Szabó-Fodor, J.; Kachlek, M.; Mézes, M.; Balogh, K.; Glávits, R.; Ali, O.; Zeebone, Y.Y.; Kovács, M. Dose and Exposure Time-Dependent Renal and Hepatic Effects of Intraperitoneally Administered Fumonisin B1 in Rats. Toxins 2018, 10, 465. [Google Scholar] [CrossRef] [Green Version]
- Hannun, Y.A.; Obeid, L. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Sancak, D.; Ozden, S. Global histone modifications in Fumonisin B1 exposure in rat kidney epithelial cells. Toxicol. Vitr. 2015, 29, 1809–1815. [Google Scholar] [CrossRef] [PubMed]
- Lallès, J.-P.; Lessard, M.; Oswald, I.P.; David, J.-C. Consumption of fumonisin B1 for 9 days induces stress proteins along the gastrointestinal tract of pigs. Toxicon 2010, 55, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Zhu, X.; Li, X.; Ouyang, H.; Wang, K.; Li, X. Assessment of ionic homeostasis imbalance and cytochrome P450 system disturbance in mice during fumonisin B1 (FB1) exposure. Chemosphere 2020, 251, 126393. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, H.; Li, X.; Yuan, Q.; Su, J.; Yang, L.; Ning, L.; Lei, H. Fumonisin B1 damages the barrier functions of porcine intestinal epithelial cells In Vitro. J. Biochem. Mol. Toxicol. 2019, 33, e22397. [Google Scholar] [CrossRef]
- Constable, P.D.; Smith, G.; Rottinghaus, G.E.; Haschek, W.M. Ingestion of Fumonisin B1-Containing Culture Material Decreases Cardiac Contractility and Mechanical Efficiency in Swine. Toxicol. Appl. Pharmacol. 2000, 162, 151–160. [Google Scholar] [CrossRef]
- Come, J.; Cambaza, E.; Ferreira, R.; Da Costa, J.M.C.; Carrilho, C. Esophageal cancer in Mozambique: Should mycotoxins be a concern? Pan Afr. Med. J. 2019, 33, 187. [Google Scholar] [CrossRef]
- Constable, P.D.; Smith, G.; Rottinghaus, G.E.; Tumbleson, M.E.; Haschek, W.M. Fumonisin-induced blockade of ceramide synthase in sphingolipid biosynthetic pathway alters aortic input impedance spectrum of pigs. Am. J. Physiol. Circ. Physiol. 2003, 284, H2034–H2044. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, S.-H.; Constable, P.D.; Smith, G.; Haschek, W.M. Effects of Exogenous Sphinganine, Sphingosine, and Sphingosine-1-Phosphate on Relaxation and Contraction of Porcine Thoracic Aortic and Pulmonary Arterial Rings. Toxicol. Sci. 2005, 86, 194–199. [Google Scholar] [CrossRef] [Green Version]
- Mathur, S.; Constable, P.D.; Eppley, R.M.; Waggoner, A.L.; Tumbleson, M.E.; Haschek, W.M. Fumonisin B1 Is Hepatotoxic and Nephrotoxic in Milk-Fed Calves. Toxicol. Sci. 2001, 60, 385–396. [Google Scholar] [CrossRef] [Green Version]
- Baker, D.C.; Rottinghaus, G.E. Chronic Experimental Fumonisin Intoxication of Calves. J. Vet. Diagn. Investig. 1999, 11, 289–292. [Google Scholar] [CrossRef] [Green Version]
- Ali, O.; Szabó-Fodor, J.; Fébel, H.; Mézes, M.; Balogh, K.; Glávits, R.; Kovács, M.; Zantomasi, A.; Szabó, A. Porcine Hepatic Response to Fumonisin B1 in a Short Exposure Period: Fatty Acid Profile and Clinical Investigations. Toxins 2019, 11, 655. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.; Constable, P.D.; Eppley, R.M.; Tumbleson, M.E.; Gumprecht, L.A.; Haschek-Hock, W.M. Purified fumonisin B(1) decreases cardiovascular function but does not alter pulmonary capillary permeability in swine. Toxicol. Sci. 2000, 56, 240–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haschek, W.M.; Gumprecht, L.A.; Smith, G.; Tumbleson, M.E.; Constable, P.D. Fumonisin toxicosis in swine: An overview of porcine pulmonary edema and current perspectives. Environ. Health Perspect. 2001, 109 (Suppl. 2), 251–257. [Google Scholar] [CrossRef] [PubMed]
- Salam, G.A.; Mehlab, E.; El-Shishtawy, M. Fumonisin Lung Toxicity: Gross and Microscopic Changes are Dose and Time Dependent. J. Am. Sci. 2012, 8, 729–736. [Google Scholar]
- Hard, G.C.; Howard, P.C.; Kovatch, R.M.; Bucci, T.J. Rat Kidney Pathology Induced by Chronic Exposure to Fumonisin B1 Includes Rare Variants of Renal Tubule Tumor. Toxicol. Pathol. 2001, 29, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Howard, P.C.; Warbritton, A.; Voss, K.A.; Lorentzen, R.J.; Thurman, J.D.; Kovach, R.M.; Bucci, T.J. Compensatory regeneration as a mechanism for renal tubule carcinogenesis of fumonisin B1 in the F344/N/Nctr BR rat. Environ. Health Perspect. 2001, 109 (Suppl. 2), 309–314. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.; Constable, P.D.; Foreman, J.H.; Eppley, R.M.; Waggoner, A.L.; Tumbleson, M.E.; Haschek, W.M. Cardiovascular changes associated with intravenous administration of fumonisin B1 in horses. Am. J. Vet. Res. 2002, 63, 538–545. [Google Scholar] [CrossRef]
- Orsi, R.; Oliveira, C.; Dilkin, P.; Xavier, J.; Direito, G.; Corrêa, B. Effects of oral administration of aflatoxin B1 and fumonisin B1 in rabbits (Oryctolagus cuniculus). Chem. Interact. 2007, 170, 201–208. [Google Scholar] [CrossRef]
- Sharma, D.; Asrani, R.K.; LeDoux, D.R.; Rottinghaus, G.E.; Gupta, V.K. Toxic Interaction between Fumonisin B1 and Moniliformin for Cardiac Lesions in Japanese Quail. Avian Dis. 2012, 56, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Foreman, J.H.; Constable, P.D.; Waggoner, A.L.; Lévy, M.; Eppley, R.; Smith, G.W.; Tumbleson, M.E.; Haschek, W.M. Neurologic Abnormalities and Cerebrospinal Fluid Changes in Horses Administered Fumonisin B1 Intravenously. J. Vet. Intern. Med. 2004, 18, 223–230. [Google Scholar] [CrossRef]
- Banczerowski-Pelyhe, I.; Détári, L.; Világi, I.; Al, E. Nerve conduction velocity and spinal reflexes may change in rats after fumonisin B1 exposure. Acta Biol. Hung. 2002, 53, 413–422. [Google Scholar] [CrossRef]
- Sandmeyer, L.S.; Vujanovic, V.; Petrie, L.; Campbell, J.R.; Bauer, B.S.; Allen, A.L.; Grahn, B.H. Optic neuropathy in a herd of beef cattle in Alberta associated with consumption of moldy corn. Can. Vet. J. 2015, 56, 249–256. [Google Scholar] [PubMed]
- Kovacic, S.; Pepeljnjak, S.; Petrinec, Z.; Klarić, M. Fumonisin B1 Neurotoxicity in Young Carp (Cyprinus Carpio L.). Arch. Ind. Hyg. Toxicol. 2009, 60, 419–426. [Google Scholar] [CrossRef]
- Marasas, W.F.O.; Riley, R.T.; Hendricks, K.A.; Stevens, V.L.; Sadler, T.W.; Waes, J.G.-V.; Missmer, S.A.; Cabrera, J.; Torres, O.; Gelderblom, W.C.A.; et al. Fumonisins Disrupt Sphingolipid Metabolism, Folate Transport, and Neural Tube Development in Embryo Culture and In Vivo: A Potential Risk Factor for Human Neural Tube Defects among Populations Consuming Fumonisin-Contaminated Maize. J. Nutr. 2004, 134, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Voss, K.A.; Riley, R.T.; Waes, J.G.-V. Fumonisin B1 induced neural tube defects were not increased in LM/Bc mice fed folate-deficient diet. Mol. Nutr. Food Res. 2014, 58, 1190–1198. [Google Scholar] [CrossRef]
- Kigen, G.; Busakhala, N.; Kamuren, Z.; Rono, H.; Kimalat, W.; Njiru, E. Factors associated with the high prevalence of oesophageal cancer in Western Kenya: A review. Infect. Agents Cancer 2017, 12, 59. [Google Scholar] [CrossRef] [Green Version]
- Xue, K.S.; Tang, L.; Sun, G.; Wang, S.; Hu, X.; Wang, J.-S. Mycotoxin exposure is associated with increased risk of esophageal squamous cell carcinoma in Huaian area, China. BMC Cancer 2019, 2019, 1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.-K.; Wang, T.-T.; Huang, G.-L.; Shi, R.-F.; Yang, L.-G.; Sun, G.-J. Stimulation of the proliferation of human normal esophageal epithelial cells by fumonisin B1 and its mechanism. Exp. Ther. Med. 2014, 7, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.-C.; Shi, Z.-Z.; Xue, L.-Y.; Chen, W.; Xu, X.; Han, Y.-L.; Lv, N.; Wang, M.-R. Expression of cell cycle related proteins cyclin D1, p53 and p21WAF1/Cip1 in esophageal squamous cell carcinoma. Hereditas 2010, 32, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Myburg, R.B.; Dutton, M.F.; Chuturgoon, A.A. Cytotoxicity of fumonisin B1, diethylnitrosamine, and catechol on the SNO esophageal cancer cell line. Environ. Health Perspect. 2002, 110, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; Garbetta, A.; D’Antuono, I.; Cardinali, A.; Martino, N.A.; Debellis, L.; Visconti, A. Toxic Mechanisms Induced by Fumonisin B1 Mycotoxin on Human Intestinal Cell Line. Arch. Environ. Contam. Toxicol. 2014, 67, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-K.; Liu, S.; Yang, L.-G.; Shi, R.-F.; Sun, G.-J. Effect of fumonisin B1 on the cell cycle of normal human liver cells. Mol. Med. Rep. 2013, 7, 1970–1976. [Google Scholar] [CrossRef]
- Chuturgoon, A.A.; Phulukdaree, A.; Moodley, D. Fumonisin B1 modulates expression of human cytochrome P450 1b1 in human hepatoma (Hepg2) cells by repressing Mir-27b. Toxicol. Lett. 2014, 227, 50–55. [Google Scholar] [CrossRef]
- Persson, E.C.; Sewram, V.; Evans, A.A.; London, W.T.; Volkwyn, Y.; Shen, Y.-J.; Van Zyl, J.A.; Chen, G.; Lin, W.; Shephard, G.S.; et al. Fumonisin B1 and risk of hepatocellular carcinoma in two Chinese cohorts. Food Chem. Toxicol. 2012, 50, 679–683. [Google Scholar] [CrossRef] [Green Version]
- Tolleson, W.H.; Dooley, K.L.; Sheldon, W.G.; Thurman, J.D.; Bucci, T.J.; Howard, P.C. The Mycotoxin Fumonisin Induces Apoptosis in Cultured Human Cells and in Livers and Kidneys of Rats. In Fumonisins in Food; Springer: Boston, MA, USA, 1996; Volume 392, pp. 237–250. [Google Scholar] [CrossRef]
- Sharma, N.; He, Q.; Sharma, R.P. Sphingosine kinase activity confers resistance to apoptosis by fumonisin B1 in human embryonic kidney (HEK-293) cells. Chem. Interact. 2004, 151, 33–42. [Google Scholar] [CrossRef]
- Seefelder, W.; Humpf, H.-U.; Schwerdt, G.; Freudinger, R.; Gekle, M. Induction of apoptosis in cultured human proximal tubule cells by fumonisins and fumonisin metabolites. Toxicol. Appl. Pharmacol. 2003, 192, 146–153. [Google Scholar] [CrossRef]
- Tolleson, W.H.; Couch, L.H.; Melchior, W.B.; Jenkins, G.R.; Muskhelishvili, M.; Muskhelishvili, L.; McGarrity, L.J.; Domon, O.; Morris, S.M.; Howard, P.C. Fumonisin B1 induces apoptosis in cultured human keratinocytes through sphinganine accumulation and ceramide depletion. Int. J. Oncol. 1999, 14, 833–843. [Google Scholar] [CrossRef]
- Tolleson, W.H.; Melchior, W.B.; Morris, S.M.; McGarrity, L.J.; Domon, O.E.; Muskhelishvili, L.; James, S.; Howard, P.C. Apoptotic and anti-proliferative effects of fumonisin B1 in human keratinocytes, fibroblasts, esophageal epithelial cells and hepatoma cells. Carcinogenesis 1996, 17, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Wahhab, M.A.; Hassan, A.M.; Amer, H.A.; Naguib, K.M. Prevention of fumonisin-induced maternal and developmental toxicity in rats by certain plant extracts. J. Appl. Toxicol. 2004, 24, 469–474. [Google Scholar] [CrossRef]
- Somoskői, B.; Kovács, M.; Cseh, S. Effects of T-2 and Fumonisin B1 combined treatment on in vitro mouse embryo development and blastocyst quality. Toxicol. Ind. Health 2018, 34, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, H.; Zhuang, R.; Wang, Y.; Cao, W.; He, Y.; Jiang, Y.; Rui, R.; Ju, S. Fumonisin B1 exposure adversely affects porcine oocyte maturation in vitro by inducing mitochondrial dysfunction and oxidative stress. Theriogenology 2021, 164, 1–11. [Google Scholar] [CrossRef]
- Lumsangkul, C.; Chiang, H.-I.; Lo, N.-W.; Fan, Y.-K.; Ju, J.-C. Developmental Toxicity of Mycotoxin Fumonisin B1 in Animal Embryogenesis: An Overview. Toxins 2019, 11, 114. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Wen, J.; Tang, Y.; Shi, J.; Mu, G.; Yan, R.; Cai, J.; Long, M. Research Progress on Fumonisin B1 Contamination and Toxicity: A Review. Molecules 2021, 26, 5238. https://doi.org/10.3390/molecules26175238
Chen J, Wen J, Tang Y, Shi J, Mu G, Yan R, Cai J, Long M. Research Progress on Fumonisin B1 Contamination and Toxicity: A Review. Molecules. 2021; 26(17):5238. https://doi.org/10.3390/molecules26175238
Chicago/Turabian StyleChen, Jia, Jun Wen, Yating Tang, Jichao Shi, Guodong Mu, Rong Yan, Jing Cai, and Miao Long. 2021. "Research Progress on Fumonisin B1 Contamination and Toxicity: A Review" Molecules 26, no. 17: 5238. https://doi.org/10.3390/molecules26175238
APA StyleChen, J., Wen, J., Tang, Y., Shi, J., Mu, G., Yan, R., Cai, J., & Long, M. (2021). Research Progress on Fumonisin B1 Contamination and Toxicity: A Review. Molecules, 26(17), 5238. https://doi.org/10.3390/molecules26175238






