Structure, Bioactivity and Analytical Methods for the Determination of Yucca Saponins
Abstract
:1. Introduction
2. Structure of Yucca Saponins
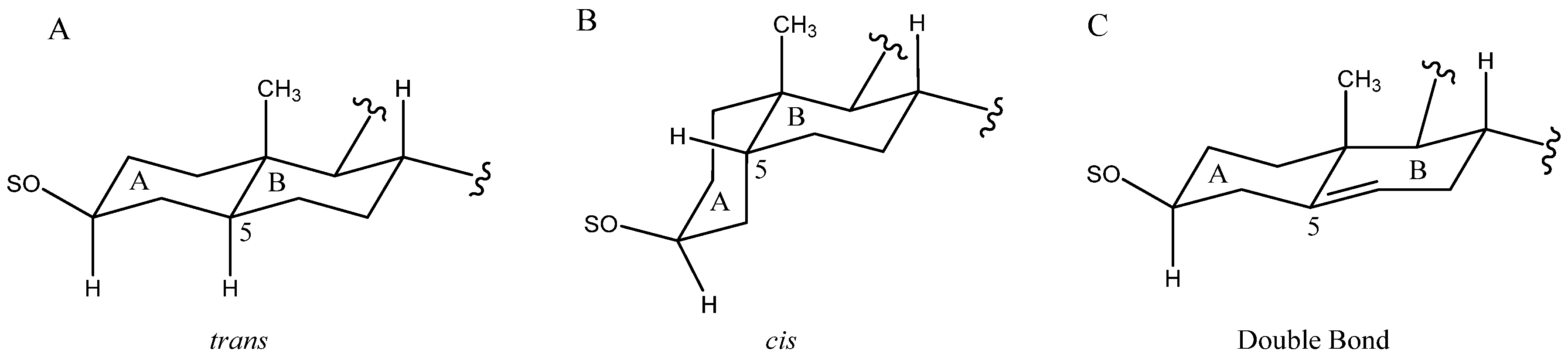
2.1. Aglycone
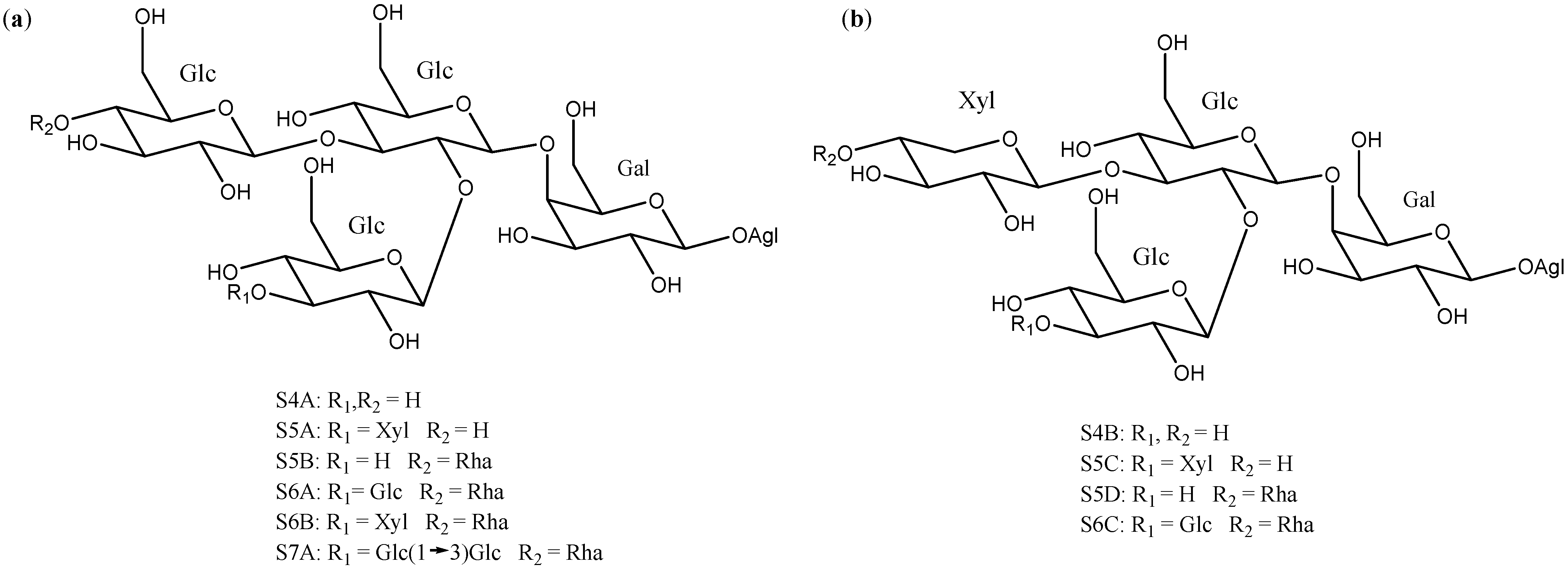
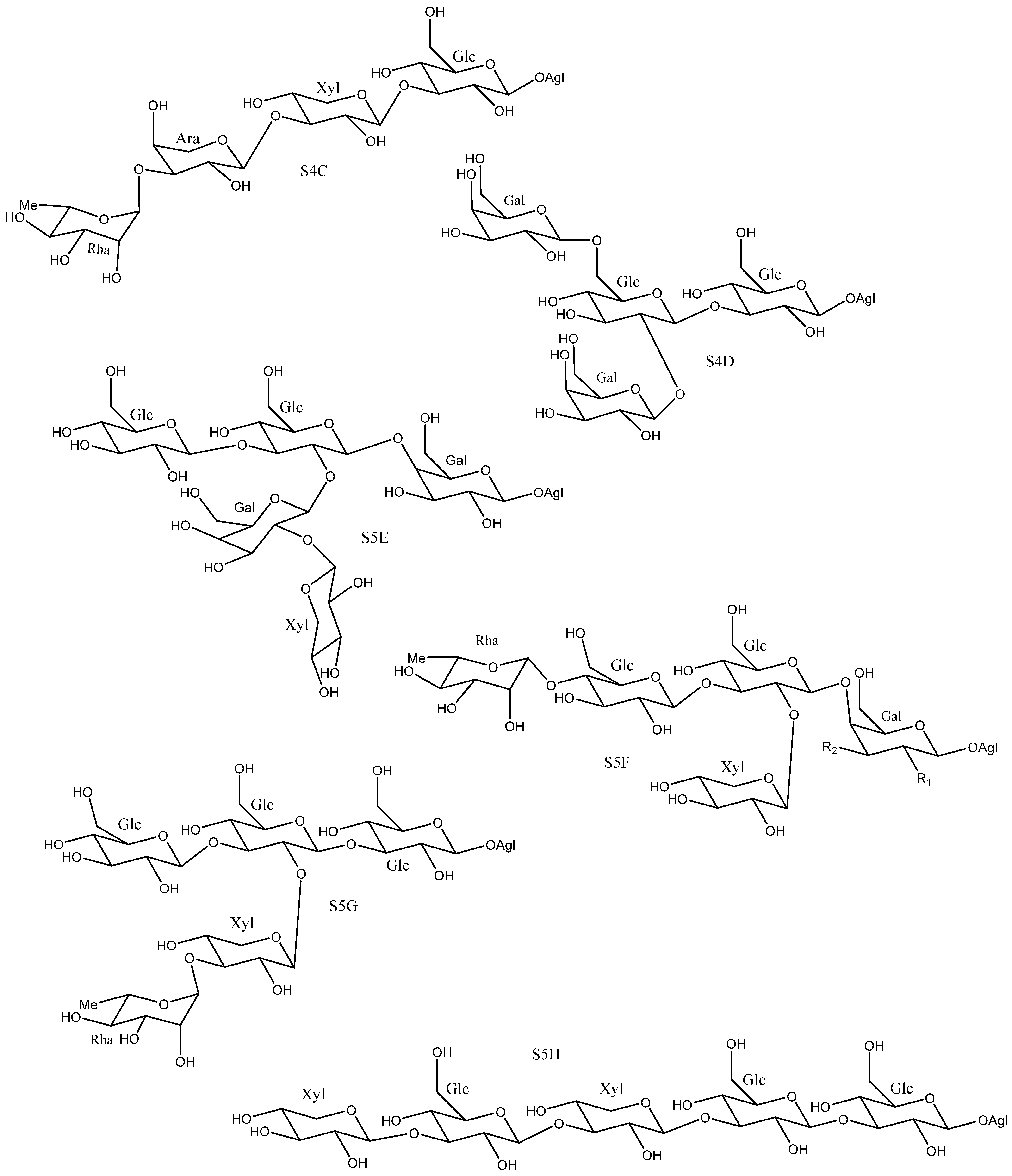
2.2. Sugar Chains
2.3. Saponins in the Yucca Genus
3. Bioactivities by Yucca Saponins
3.1. Cytotoxicity: Study of the Structure-Activity Relationships (SAR)
3.2. Antifungal: Study of the Structure-Activity Relationships (SAR)
3.3. Selection of Active Saponins
3.3.1. Degalactotigonin (2)
3.3.2. Timosaponin AIII (20)
3.3.3. Timosaponin BII (50)
4. Analytical Methods for the Steroidal Saponins in Yucca
5. Materials and Methods
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Patel, S. Yucca: A medicinally significant genus with manifold therapeutic attributes. Nat. Prod. Bioprospect. 2012, 2, 231–234. [Google Scholar] [CrossRef] [Green Version]
- Clary, K.H.; Simpson, B.B. Sistemática y evolución del género Yucca (Agavaceae): Evidencias de análisis nunfológicos y moleculares. Bot. Sci. 1995, 56, 77–88. [Google Scholar] [CrossRef] [Green Version]
- Simmons-Boyce, J.L.; Tinto, W.F. Steroidal Saponins and Sapogenins from the Agavaceae Family. Nat. Prod. Commun. 2007, 2, 99–114. [Google Scholar] [CrossRef]
- Sparg, S.G.; Light, M.E.; Van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef]
- Vincken, J.P.; Heng, L.; de Groot, A.; Gruppen, H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry 2007, 68, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Skhirtladze, A.; Perrone, A.; Montoro, P.; Benidze, M.; Kemertelidze, E.; Pizza, C.; Piacente, S. Steroidal saponins from Yucca gloriosa L. rhizomes: LC-MS profiling, isolation and quantitative determination. Phytochemistry 2011, 72, 126–135. [Google Scholar] [CrossRef]
- Montoro, P.; Skhirtladze, A.; Perrone, A.; Benidze, M.; Kemertelidze, E.; Piacente, S. Determination of steroidal glycosides in Yucca gloriosa flowers by LC/MS/MS. J. Pharm. Biomed. Anal. 2010, 52, 791–795. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Pecio, Ł.; Stochmal, A.; Oleszek, W. Qualitative and quantitative analysis of steroidal saponins in crude extract and bark powder of Yucca schidigera Roezl. J. Agric. Food Chem. 2011, 59, 8058–8064. [Google Scholar] [CrossRef]
- Piacente, S.; Pizza, C.; Oleszek, W. Saponins and phenolics of Yucca schidigera Roezl: Chemistry and bioactivity. Phytochem. Rev. 2005, 4, 177–190. [Google Scholar] [CrossRef]
- Report Insights. Yucca Schidigera Plant Extract Market 2020 by Manufacturers, Regions Analysis(Europe, Asia Pacific, North America and South America), Type and Application, Forecast to 2025; Report Insights: Maharashtra, India, 2020. [Google Scholar]
- Yokosuka, A.; Suzuki, T.; Tatsuno, S.; Mimaki, Y. Steroidal glycosides from the underground parts of Yucca glauca and their cytotoxic activities. Phytochemistry 2014, 101, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Diab, Y.; Ioannou, E.; Emam, A.; Vagias, C.; Roussis, V. Desmettianosides A and B, bisdesmosidic furostanol saponins with molluscicidal activity from Yucca desmettiana. Steroids 2012, 77, 686–690. [Google Scholar] [CrossRef]
- Pérez, A.J.; Calle, J.M.; Simonet, A.M.; Guerra, J.O.; Stochmal, A.; Macías, F.A. Bioactive steroidal saponins from Agave offoyana flowers. Phytochemistry 2013, 95, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Miyakoshi, M.; Tamura, Y.; Masuda, H.; Mizutani, K.; Tanaka, O.; Ikeda, T.; Ohtani, K.; Kasai, R.; Yamasaki, K. Antiyeast steroidal saponins from Yucca schidigera (Mohave Yucca), a new anti-food-deteriorating agent. J. Nat. Prod. 2000, 63, 332–338. [Google Scholar] [CrossRef]
- Xie, L.; Lee, D.Y.-W.; Shang, Y.; Cao, X.; Wang, S.; Liao, J.; Zhang, T.; Dai, R. Characterization of spirostanol glycosides and furostanol glycosides from anemarrhenae rhizoma as dual targeted inhibitors of 5-lipoxygenase and Cyclooxygenase-2 by employing a combination of affinity ultrafiltration and HPLC/MS. Phytomedicine 2020, 77, 153284. [Google Scholar] [CrossRef]
- Oleszek, W.; Sitek, M.; Stochmal, A.; Piacente, S.; Pizza, C.; Cheeke, P. Steroidal saponins of Yucca schidigera Roezl. J. Agric. Food Chem. 2001, 49, 4392–4396. [Google Scholar] [CrossRef]
- Han, F.Y.; Song, X.Y.; Chen, J.J.; Yao, G.D.; Song, S.J. Timosaponin AIII: A novel potential anti-tumor compound from Anemarrhena asphodeloides. Steroids 2018, 140, 125–130. [Google Scholar] [CrossRef]
- Dragalin, I.P.; Gulya, A.P.; Krokhmalyuk, V.V.; Kintya, P.K. The structure of yuccoside E from Yucca filamentosa. Chem. Nat. Compd. 1975, 11, 772–774. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, C.R.; Zhang, Y.J. New Steroidal Saponins from the Leaves of Yucca elephantipes. Helv. Chim. Acta 2013, 96, 1807–1813. [Google Scholar] [CrossRef]
- Qu, L.; Wang, J.; Ruan, J.; Yao, X.; Huang, P.; Wang, Y.; Yu, H.; Han, L.; Zhang, Y.; Wang, T. Spirostane-type saponins obtained from Yucca schidigera. Molecules 2018, 23, 167. [Google Scholar] [CrossRef] [Green Version]
- Skhirtladze, A.; Plaza, A.; Montoro, P.; Benidze, M.; Kemertelidze, E.; Pizza, C.; Piacente, S. Furostanol saponins from Yucca gloriosa L. rhizomes. Biochem. Syst. Ecol. 2006, 34, 809–814. [Google Scholar] [CrossRef]
- Nakano, K.; Hara, Y.; Murakami, K.; Takaishi, Y.; Tomimatsu, T. 12-Hydroxy steroidal glycosides from the caudex of Yucca gloriosa. Phytochemistry 1991, 30, 1993–1995. [Google Scholar] [CrossRef]
- Nakano, K.; Hara, Y.; Murakami, K.; Takaishi, Y.; Tomimatsu, T.; Midzuta, Y.; Hara, Y.; Murakami, K.; Takaishi, Y.; Tomimatsu, T. 12-Keto steroidal glycosides from the caudex of Yucca gloriosa. Phytochemistry 1991, 30, 633–636. [Google Scholar] [CrossRef]
- Kishor, N.; Sati, P.; Sakakibarat, J.; Kaiya, T. A spirostanol glycoside from Yucca aloifolia. Phytochemistry 1992, 31, 706–707. [Google Scholar] [CrossRef]
- Kishor, N. A new molluscicidal spirostanol glycoside of Yucca aloifolia. J. Nat. Prod. 1990, 53, 1557–1559. [Google Scholar] [CrossRef]
- Bahuguna, S.; Kishor, N.; Sati, O.P.; Sati, S.P.; Sakakibara, J.; Kaiya, T. A new spirostanol glycoside from Yucca aloifolia. J. Nat. Prod. 1991, 54, 863–865. [Google Scholar] [CrossRef]
- Plock, A.; Beyer, G.; Hiller, K.; Gründemann, E.; Krause, E.; Nimtz, M.; Wray, V. Application of MS and NMR to the structure elucidation of complex sugar moieties of natural products: Exemplified by the steroidal saponin from Yucca filamentosa L. Phytochemistry 2001, 57, 489–496. [Google Scholar] [CrossRef]
- Qu, L.; Ruan, J.; Wu, S.; Huang, P.; Yan, J.; Yu, H.; Zhang, Y.; Wang, T. Separation and bioactive assay of 25R/S-spirostanol saponin diastereomers from Yucca schidigera roezl (Mojave) stems. Molecules 2018, 23, 2562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemertelidze, E.; Benidze, M.; Skhirtladze, A. Steroidal glycosides from the leaves of Yucca gloriosa L. Bull. Georg. Natl. Acad. Sci. 2011, 5, 158–163. [Google Scholar]
- Nakano, K.; Matsuda, E.; Tsurumi, K.; Yamasaki, T.; Murakami, K.; Takaishi, Y.; Tomimatsu, T. The steroidal glycosides of the flowers of Yucca gloriosa. Phytochemistry 1988, 27, 3235–3239. [Google Scholar] [CrossRef]
- Favel, A.; Kemertelidze, E.; Benidze, M.; Fallague, K.; Regli, P. Antifungal activity of steroidal glycosides from Yucca gloriosa L. Phyther. Res. 2005, 19, 158–161. [Google Scholar] [CrossRef]
- Kemertelidze, É.P.; Benidze, M.M.; Skhirtladze, A.V. Steroid compounds from Yucca gloriosa L. introduced into Georgia and their applications. Pharm. Chem. J. 2009, 43, 45–47. [Google Scholar] [CrossRef]
- Jin, Y.-L.; Kuk, J.-H.; Oh, K.-T.; Kim, Y.-J.; Piao, X.-L.; Park, R.-D. A new steroidal saponin, yuccalan, from the leaves of Yucca smalliana. Arch. Pharm. Res. 2007, 30, 543–546. [Google Scholar] [CrossRef]
- Eskander, J.; Sakka, O.K.; Harakat, D.; Lavaud, C. Steroidal saponins from the leaves of Yucca de-smetiana and their in vitro antitumor activity: Structure activity relationships through a molecular modeling approach. Med. Chem. Res. 2013, 22, 4877–4885. [Google Scholar] [CrossRef]
- Nakano, K.; Yamasaki, T.; Imamura, Y.; Murakami, K.; Takaishi, Y.; Tomimatsu, T. The steroidal glycosides from the caudex of Yucca gloriosa. Phytochemistry 1989, 28, 1215–1217. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.J.; Jacob, M.R.; Li, X.C.; Yang, C.R. Steroidal saponins from the stem of Yucca elephantipes. Phytochemistry 2008, 69, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Grishkovets, V.L.; Karpov, A.; Iksanovr, S.V.; Chirva, V.Y. A steroid glycoside from the seeds of Yucca macrocarpa. Chem. Nat. Compd. 1998, 34, 738–740. [Google Scholar] [CrossRef]
- Fang, M.; Gu, L.; Gu, G.; Fang, J. Facile synthesis and antitumor activities of timosaponin AIII and its analogs. J. Carbohydr. Chem. 2012, 31, 187–202. [Google Scholar] [CrossRef]
- Yang, C.R.; Zhang, Y.; Jacob, M.R.; Khan, S.I.; Zhang, Y.J.; Li, X.C. Antifungal activity of C-27 steroidal saponins. Antimicrob. Agents Chemother. 2006, 50, 1710–1714. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.C.; Fan, M.C.; Wang, S.Y.; Huang, J.W. Identification of the Solanum nigrum extract component involved in controlling cabbage black leaf spot disease. J. Agric. Food Chem. 2011, 59, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Jiang, J.; Zhang, T.; Chen, F.; Zheng, Y.; Han, M.; Hu, Y. Application of Degalactotigonin in Preparing Pharmaceutical for Preventing and Treating Influenza Virus Infection 2018. Patent CN 107595868 A, 19 January 2018. [Google Scholar]
- Kang, L.-P.; Wu, K.-L.; Yu, H.-S.; Pang, X.; Liu, J.; Han, L.-F.; Zhang, J.; Zhao, Y.; Xiong, C.-Q.; Song, X.-B.; et al. Steroidal saponins from Tribulus terrestris. Phytochemistry 2014, 107, 182–189. [Google Scholar] [CrossRef]
- Chakraborty, D.; Maity, A.; Jha, T.; Mondal, N.B. Spermicidal and contraceptive potential of Desgalactotigonin: A prospective alternative of Nonoxynol-9. PLoS ONE 2014, 9, e107164. [Google Scholar] [CrossRef]
- Chakraborty, D.; Jain, C.K.; Maity, A.; Ghosh, S.; Choudhury, S.R.; Jha, T.; Majumder, H.K.; Mondal, N.B. Chenopodium album metabolites act as dual topoisomerase inhibitors and induce apoptosis in the MCF7 cell line. Medchemcomm 2016, 7, 837–844. [Google Scholar] [CrossRef]
- Le Tuan Anh, H.; Tran, P.T.; Thao, D.T.; Trang, D.T.; Dang, N.H.; Van Cuong, P.; Van Kiem, P.; Van Minh, C.; Lee, J.-H. Degalactotigonin, a steroidal glycoside from Solanum nigrum, induces apoptosis and cell cycle arrest via inhibiting the EGFR signaling pathways in pancreatic cancer cells. Biomed. Res. Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Tsumagari, H.; Honbu, T.; Nohara, T. Cytotoxic activity of steroidal glycosides from solanum plants. Biol. Pharm. Bull. 2003, 26, 1198–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mimaki, Y.; Yokosuka, A.; Kuroda, M.; Sashida, Y. Cytotoxic activities and structure-cytotoxic relationships of steroidal saponins. Biol. Pharm. Bull. 2001, 24, 1286–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Jia, Q.; Wu, M.S.; Xie, X.; Wang, Y.; Song, G.; Zou, C.Y.; Tang, Q.; Lu, J.; Huang, G.; et al. Degalactotigonin, a natural compound from Solanum nigrum L., inhibits growth and metastasis of osteosarcoma through GSK3β inactivation-mediated repression of the Hedgehog/gli1 pathway. Clin. Cancer Res. 2018, 24, 130–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuroda, M.; Mimaki, Y.; Hasegawa, F.; Yokosuka, A.; Sashida, Y.; Sakagami, H. Steroidal glycosides from the bulbs of Camassia leichtlinii and their cytotoxic activities. Chem. Pharm. Bull. 2001, 49, 726–731. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.M.; Zhang, Y.J.; Yang, C.R. Spirostanol and furostanol glycosides from the fresh tubers of Polianthes tuberosa. J. Nat. Prod. 2004, 67, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, T.; Koyano, T.; Kowithayakorn, T.; Sakai, S.; Kawahara, N.; Goda, Y.; Yamaguchi, N.; Ishibashi, M. New chlorogenin hexasaccharide isolated from Agave fourcroydes with cytotoxic and cell cycle inhibitory activities. Bioorganic Med. Chem. 2004, 12, 3841–3845. [Google Scholar] [CrossRef]
- Candra, E.; Matsunaga, K.; Fujiwara, H.; Mimaki, Y.; Sashida, Y.; Yamakuni, T.; Ohizumi, Y. Two steroidal saponins from Camassia cusickii induce L1210 cell death through the apoptotic mechanism. Can. J. Physiol. Pharmacol. 2001, 79, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.F.; Lin, Y.Y.; Kong, L.Y. Steroids from the roots of Asparagus officinalis and their cytotoxic activity. J. Integr. Plant Biol. 2008, 50, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Sautour, M.; Miyamoto, T.; Lacaille-Dubois, M.A. Bioactive steroidal saponins from Smilax medica. Planta Med. 2006, 72, 667–670. [Google Scholar] [CrossRef]
- Sy, L.K.; Lok, C.N.; Wang, J.Y.; Liu, Y.; Cheng, L.; Wan, P.K.; Leung, C.T.; Cao, B.; Kwong, W.L.; Chang, R.C.C.; et al. Identification of “sarsasapogenin-aglyconed” timosaponins as novel Aβ-lowering modulators of amyloid precursor protein processing. Chem. Sci. 2016, 7, 3206–3214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Xu, J.; Wang, M.; Xia, X.; Dai, R.; Zhao, Y. Steroidal saponins and sapogenins from fenugreek and their inhibitory activity against α-glucosidase. Steroids 2020, 161, 108690. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.H.; Lee, E.J.; Kim, J.S.; Kang, S.S.; Ha, H.; Lee, H.Y.; Kim, C.; Lee, J.H.; Son, K.H. Rat growth-hormone release stimulators from fenugreek seeds. Chem. Biodivers. 2008, 5, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, K.-H.; Lee, I.-S.; Park, J.Y.; Na, Y.-C.; Chung, W.-S.; Jang, H.-J. Apoptosis and G2/M cell cycle arrest induced by a timosaponin A3 from Anemarrhena asphodeloides Bunge on AsPC-1 pancreatic cancer cells. Phytomedicine 2019, 56, 48–56. [Google Scholar] [CrossRef]
- Park, B.K.; So, K.S.; Ko, H.J.; Kim, H.J.; Kwon, K.S.; Kwon, Y.S.; Son, K.H.; Kwon, S.Y.; Kim, H.P. Therapeutic Potential of the Rhizomes of Anemarrhena asphodeloides and Timosaponin A-III in an Animal Model of Lipopolysaccharide-Induced Lung Inflammation. Biomol. Ther. 2018, 26, 553–559. [Google Scholar] [CrossRef]
- Cong, Y.; Wang, L.; Peng, R.; Zhao, Y.; Bai, F.; Yang, C.; Liu, X.; Wang, D.; Ma, B.; Cong, Y. Timosaponin AIII induces antiplatelet and antithrombotic activity via Gq-mediated signaling by the thromboxane A2 receptor. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kang, L.-P.; Zhang, J.; Cong, Y.; Li, B.; Xiong, C.-Q.; Zhao, Y.; Tan, D.-W.; Yu, H.; Yu, Z.-Y.; Cong, Y.-W.; et al. Steroidal glycosides from the rhizomes of Anemarrhena asphodeloides and their antiplatelet aggregation activity. Planta Med. 2012, 78, 611–616. [Google Scholar] [CrossRef]
- Xu, Y.; Xue, J.; Huang, R. Timosaponin Composition, and Preparation Method and Application Thereof in Treating Viral Miocarditis 2020. Patent CN 110882266 A, 17 March 2020. [Google Scholar]
- Wang, H.Q.; Liu, M.; Wang, L.; Lan, F.; Zhang, Y.H.; Xia, J.E.; Xu, Z.D.; Zhang, H. Identification of a novel BACE1 inhibitor, timosaponin A-III, for treatment of Alzheimer’s disease by a cell extraction and chemogenomics target knowledgebase-guided method. Phytomedicine 2020, 75, 153244. [Google Scholar] [CrossRef]
- Wang, N.; Xu, P.; Wang, X.; Yao, W.; Wang, B.; Wu, Y.; Shou, D. Timosaponin AIII attenuates inflammatory injury in AGEs-induced osteoblast and alloxan-induced diabetic osteoporosis zebrafish by modulating the RAGE/MAPK signaling pathways. Phytomedicine 2020, 75, 153247. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, J.; Sun, X.; Huang, X.; Li, L.; Liu, Q.; Song, S. Steroids from the rhizome of Anemarrhena asphodeloides and their cytotoxic activities. Bioorg. Med. Chem. Lett. 2016, 26, 3081–3085. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-F.; Zhou, J.; Zhang, M.-J.; Zhang, M.; Huang, X.-F. Cytotoxic steroidal saponins from the rhizome of Anemarrhena asphodeloides. Steroids 2020, 155, 108557–108561. [Google Scholar] [CrossRef]
- Carpinteyro Díaz, A.E.; Herfindal, L.; Rathe, B.A.; Sletta, K.Y.; Vedeler, A.; Haavik, S.; Fossen, T. Cytotoxic saponins and other natural products from flowering tops of Narthecium ossifragum L. Phytochemistry 2019, 164, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Y.; Han, F.Y.; Chen, J.J.; Wang, W.; Zhang, Y.; Yao, G.D.; Song, S.J. Timosaponin AIII, a steroidal saponin, exhibits anti-tumor effect on taxol-resistant cells in vitro and in vivo. Steroids 2019, 146, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Y.; Zhao, W.R.; Xiao, Y.; Zhou, X.M.; Huang, C.; Shi, W.T.; Zhang, J.; Ye, Q.; Chen, X.L.; Tang, J.Y. Antiangiogenesis effect of timosaponin AIII on HUVECs in vitro and zebrafish embryos in vivo. Acta Pharmacol. Sin. 2020, 41, 260–269. [Google Scholar] [CrossRef]
- Nho, K.J.; Chun, J.M.; Kim, H.K. Induction of mitochondria-dependent apoptosis in HepG2 human hepatocellular carcinoma cells by timosaponin A-III. Environ. Toxicol. Pharmacol. 2016, 45, 295–301. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, X.; Xiang, L.; Huang, Y.; Wang, Z.; He, X. Furostanol saponins from Chinese onion induce G2/M cell-cycle arrest and apoptosis through mitochondria-mediate pathway in HepG2 cells. Steroids 2019, 148, 11–18. [Google Scholar] [CrossRef]
- Wang, Y.; Xiang, L.; Yi, X.; He, X. Potential anti-inflammatory steroidal saponins from the berries of Solanum nigrum L. (European Black Nightshade). J. Agric. Food Chem. 2017, 65, 4262–4272. [Google Scholar] [CrossRef]
- Nath, L.R.; Gorantla, J.N.; Thulasidasan, A.K.T.; Vijayakurup, V.; Shah, S.; Anwer, S.; Joseph, S.M.; Antony, J.; Veena, K.S.; Sundaram, S.; et al. Evaluation of uttroside B, a saponin from Solanum nigrum Linn, as a promising chemotherapeutic agent against hepatocellular carcinoma. Sci. Rep. 2016, 6, 36318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.F.; Li, X.C.; Yang, H.Y.; Wang, J.J.; Yang, C.R. Inhibitory effects of some steroidal saponins on human spermatozoa in vitro. Planta Med. 1996, 62, 130–132. [Google Scholar] [CrossRef]
- Chen, W.; Li, R.; Zhu, S.; Ma, J.; Pang, L.; Ma, B.; Du, L.; Jin, Y. Nasal timosaponin BII dually sensitive in situ hydrogels for the prevention of Alzheimer’s disease induced by lipopolysaccharides. Int. J. Pharm. 2020, 578, 119115. [Google Scholar] [CrossRef]
- Huo, Z.; Qiu, Y.; Chu, Z.; Yin, P.; Lu, W.; Yan, Y.; Wan, N.; Chen, Z. Electrospinning preparation of Timosaponin B-II-loaded PLLA nanofibers and their antitumor recurrence activities in vivo. J. Nanomater. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Ji, K.-Y.; Kim, K.M.; Kim, Y.H.; Im, A.-R.; Lee, J.Y.; Park, B.; Na, M.; Chae, S. The enhancing immune response and anti-inflammatory effects of Anemarrhena asphodeloides extract in RAW 264.7 cells. Phytomedicine 2019, 59, 152789–152797. [Google Scholar] [CrossRef]
- Yuan, Y.L.; Lin, B.Q.; Zhang, C.F.; Cui, L.L.; Ruan, S.X.; Yang, Z.L.; Li, F.; Ji, D. Timosaponin B-II ameliorates palmitate-induced insulin resistance and inflammation via IRS-1/PI3K/Akt and IKK/NF- κ B pathways. Am. J. Chin. Med. 2016, 44, 755–769. [Google Scholar] [CrossRef]
- Ma, T.; Sun, J.; Li, X.; Ma, Y.; Liu, L.; Guo, L.; Liu, Q.; Sun, Y. Optimization of extraction for Anemarrhena asphodeloides Bge. using silica gel-based vortex-homogenized matrix solid-phase dispersion and rapid identification of antioxidant substances. J. Sep. Sci. 2020, 43, 2180–2192. [Google Scholar] [CrossRef] [PubMed]
- Du, M.J.; Chen, J.P.; Lian, B.W.; Mei, W.J.; Yu, J.X.; Chen, L.S.; Deng, S.B.; Zhong, Y.M.; Yu, C.Q. Cardioprotective effects of timosaponin B-II isolated from Anemarrhena rhizome in a zebrafish model. Pharmazie 2020, 75, 201–204. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Yu, Y.; Yan, L.L.; Li, C.; Han, J.Y.; Qin, Z.F.; Dai, Y.; Yao, Z.H.; Zhou, H.; Yao, X.S. Discovery of cardio-protective constituents of Gualou Xiebai Decoction, a classical traditional Chinese medicinal formula. Phytomedicine 2019, 54, 318–327. [Google Scholar] [CrossRef]
- Deng, X.Y.; Chen, J.J.; Li, H.Y.; Ma, Z.Q.; Ma, S.P.; Fu, Q. Cardioprotective effects of timosaponin B II from Anemarrhenae asphodeloides Bge on isoproterenol-induced myocardial infarction in rats. Chem. Biol. Interact. 2015, 240, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xue, J.; Huang, R. Timosaponin Composition and Application Thereof in Treating Viral Myocarditis 2019. Patent CN 110623965 A, 31 December 2019. [Google Scholar]
- Kim, Y.S.; Suh, W.S.; Park, K.J.; Choi, S.U.; Lee, K.R. Allimacrosides A–E, new steroidal glycosides from Allium macrostemon Bunge. Steroids 2017, 118, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, G.; Wang, N.; Yang, M.; Wang, Z.; Wang, X.; Yao, X. New furostanol saponins from the bulbs of Allium macrostemon Bunge and their cytotoxic activity. Pharmazie 2007, 62, 544–548. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, J.; Fu, Q.; Cheng, L.; Wu, L.; Zhang, W.; Zhang, Y.; Jin, Y.; Zhang, C. Anti-inflammatory activities of compounds isolated from the rhizome of Anemarrhena asphodeloides. Molecules 2018, 23, 2631. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Peng, J. Identification of New Constituents in Allium macrostemon Bunge and A. chinense G. Don Bulbs and Their Anti-Blood Platelet Agglutination Activities 1995. Patent CN 1102186A, 3 May 1995. [Google Scholar]
- Mimaki, Y.; Kameyama, A.; Kuroda, M.; Sashida, Y.; Hirano, T.; Oka, K.; Koike, K.; Nikaido, T. Steroidal glycosides from the underground parts of Hosta plantaginea var. japonica and their cytostatic activity on leukaemia HL-60 cells. Phytochemistry 1997, 44, 305–310. [Google Scholar] [CrossRef]
- Mimaki, Y.; Kuroda, M.; Kameyama, A.; Yokosuka, A.; Sashida, Y. Steroidal saponins from the rhizomes of Hosta sieboldii and their cytostatic activity on HL-60 cells. Phytochemistry 1998, 48, 1361–1369. [Google Scholar] [CrossRef]
- Kim, C.S.; Kim, S.Y.; Moon, E.; Lee, M.K.; Lee, K.R. Steroidal constituents from the leaves of Hosta longipes and their inhibitory effects on nitric oxide production. Bioorganic Med. Chem. Lett. 2013, 23, 1771–1775. [Google Scholar] [CrossRef]
- Sidana, J.; Singh, B.; Sharma, O.P. Saponins of Agave: Chemistry and bioactivity. Phytochemistry 2016, 130, 22–46. [Google Scholar] [CrossRef]
- Uematsu, Y.; Hirata, K.; Saito, K.; Kudo, I. Spectrophotometric determination of saponin in Yucca extract used as food additive. J. AOAC Int. 2000, 83, 1451–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paray, B.A.; El-Basuini, M.F.; Alagawany, M.; Albeshr, M.F.; Farah, M.A.; Dawood, M.A.O. Yucca schidigera usage for healthy aquatic animals: Potential roles for sustainability. Animals 2021, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Fayed, W.M.A.; Khalil, R.H.; Sallam, G.R.; Mansour, A.T.; Elkhayat, B.K.; Omar, E.A. Estimating the effective level of Yucca schidigera extract for improvement of the survival, haematological parameters, immunological responses and Water quality of European seabass juveniles (dicentrarchus labrax). Aquac. Rep. 2019, 15, 100208–100214. [Google Scholar] [CrossRef]
- Sun, D.; Shi, B.; Tong, M.; Yan, S. Improved performance and immunological responses as a result of dietary Yucca schidigera extract supplementation in broilers. Ital. J. Anim. Sci. 2018, 17, 511–517. [Google Scholar] [CrossRef] [Green Version]
- Cheeke, P.R. Actual and Potential Applications of Yucca Schidigera and Quillaja Saponaria Saponins in Human and Animal Nutrition. In Saponins in Food, Feedstuffs and Medicinal Plants; Springer: Dordrecht, The Netherlands, 2000; pp. 241–254. [Google Scholar]
- Cheeke, P.R.; Piacente, S.; Oleszek, W. Anti-inflammatory and anti-arthritic effects of Yucca schidigera: A review. J. Inflamm. 2006, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Franco Ospina, L.A.; Castro Guerrero, J.P.; Ocampo Buendía, Y.C.; Pájaro Bolívar, I.B.; Díaz Castillo, F. Actividad antiinflamatoria, antioxidante y antibacteriana de dos especies del género Tabebuia. Rev. Cuba Plantas Med. 2013, 18, 34–46. [Google Scholar]
- Guo, C.; Li, L.; Yang, X.; Meng, Z.; Li, F.; Zhang, C.; Yang, Z. Protective effects of timosaponin B-II on high glucose-induced apoptosis in human umbilical vein endothelial cells. Environ. Toxicol. Pharmacol. 2014, 37, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Arencibia, D.F.; Rosario, L.A.; Curveco, D.L. Principal assays that to determine the cytotoxicity of a substance, some considerations and their utility. Retell Rev. Toxicol. Linea 2009, 42–52. [Google Scholar]
- Chae, S.U.; Lee, M.Y. Cosmetics Containing Anemarrhena asphodeloides Extract 2014. Patent WO 2014092325 A1, 19 June 2014. [Google Scholar]
- Liu, M.; Bi, X.; Hong, Z.; Fan, C.; Wang, Z.; Xu, X.; Gao, S. Application of Timosaponin BI, Timosaponin IA, and Timosaponin AIII in the Preparation of Anti Cognitive Disorder Drugs 2019. Patent CN 110585224 A, 20 December 2019. [Google Scholar]
- Olas, B.; Urbańska, K.; Bryś, M. Saponins as modulators of the blood coagulation system and perspectives regarding their use in the prevention of venous thromboembolic incidents. Molecules 2020, 25, 5171. [Google Scholar] [CrossRef]
- Nian, S.-H.; Li, H.-J.; Liu, E.-H.; Li, P. Comparison of α-glucosidase inhibitory effect and bioactive constituents of Anemarrhenae Rhizoma and Fibrous Roots. J. Pharm. Biomed. Anal. 2017, 145, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Lian, B.; Liu, Y. Gynecological Compound Composition Based on Clotrimazole and Timosaponin B2 2019. Patent CN 109431968 A, 8 March 2019. [Google Scholar]
- Tenon, M.; Feuillère, N.; Roller, M.; Birtić, S. Rapid, cost-effective and accurate quantification of Yucca schidigera Roezl. steroidal saponins using HPLC-ELSD method. Food Chem. 2017, 221, 1245–1252. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, R.; Ohno, H.; Murakami, T.; Shirataki, Y. Improving quality control of yucca extracts used as food additives by screening antimicrobial activity using NMR metabolomics. J. Nat. Med. 2020, 74, 306–310. [Google Scholar] [CrossRef]
- Do Canto, G.S.; Treter, J.; Yang, S.; Borré, G.L.; Peixoto, M.P.G.; Ortega, G.G. Evaluation of foam properties of saponin from Ilex paraguariensis A. St. Hil. (Aquifoliaceae) fruits. Braz. J. Pharm. Sci. 2010, 46, 237–243. [Google Scholar] [CrossRef] [Green Version]
- San Martín, R.; Briones, R. Quality control of commercial quillaja (Quillaja saponaria Molina) extracts by reverse phase HPLC. J. Sci. Food Agric. 2000, 80, 2063–2068. [Google Scholar] [CrossRef]
- Böttcher, S.; Drusch, S. Interfacial properties of saponin extracts and their impact on foam characteristics. Food Biophys. 2016, 11, 91–100. [Google Scholar] [CrossRef]
- Ross, J.; Miles, G.D. An apparatus for comparison of foaming properties of soaps and detergents. Oil Soap 1941, 18, 99–102. [Google Scholar] [CrossRef]
- Baccou, J.C.; Lambert, F.; Sauvaire, Y. Spectrophotometric method for the determination of total steroidal sapogenin. Analyst 1977, 102, 458–465. [Google Scholar] [CrossRef]
- Wang, Y.; McAllister, T.A. A modified spectrophotometric assay to estimate deglycosylation of steroidal saponin to sapogenin by mixed ruminal microbes. J. Sci. Food Agric. 2010, 90, 1811–1818. [Google Scholar] [CrossRef]
- Sastre, F.; Ferreira, F.; Pedreschi, F. TLC fingerprint of phenolics and saponins in commercial extracts of Yucca schidigera Roezl. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 698–701. [Google Scholar] [CrossRef]
- Zhao, Y.; McCauley, J.; Pang, X.; Kang, L.; Yu, H.; Zhang, J.; Xiong, C.; Chen, R.; Ma, B. Analytical and semipreparative separation of 25 (R/S)-spirostanol saponin diastereomers using supercritical fluid chromatography. J. Sep. Sci. 2013, 36, 3270–3276. [Google Scholar] [CrossRef]
- El Sayed, A.M.; Basam, S.M.; El-Naggar, E.-M.B.A.; Marzouk, H.S.; El-Hawary, S. LC–MS/MS and GC–MS profiling as well as the antimicrobial effect of leaves of selected Yucca species introduced to Egypt. Sci. Rep. 2020, 10, 17778–17792. [Google Scholar] [CrossRef]
- Sastre, F.; Ferreira, F.; Pedreschi, F. MALDI-TOF mass spectrometry and reversed-phase HPLC-ELSD chromatography for structural and quantitative studies of major steroid saponins in commercial extracts of Yucca schidigera Roezl. J. Pharm. Biomed. Anal. 2016, 120, 270–282. [Google Scholar] [CrossRef]
- Ruan, J.; Qu, L.; Zhao, W.; Gao, C.; Huang, P.; Zheng, D.; Han, L.; Yu, H.; Zhang, Z.; Zhang, Y.; et al. Identification and Structural Analysis of Spirostanol Saponin from Yucca schidigera by Integrating Silica Gel Column Chromatography and Liquid Chromatography/Mass Spectrometry Analysis. Molecules 2020, 25, 3848. [Google Scholar] [CrossRef] [PubMed]
- Sastre, F.; Ferreira, F.; Pedreschi, F. A systematic approach for the chromatographic fractionation and purification of major steroid saponins in commercial extracts of Yucca schidigera Roezl. J. Chromatogr. B 2017, 1046, 235–242. [Google Scholar] [CrossRef] [PubMed]
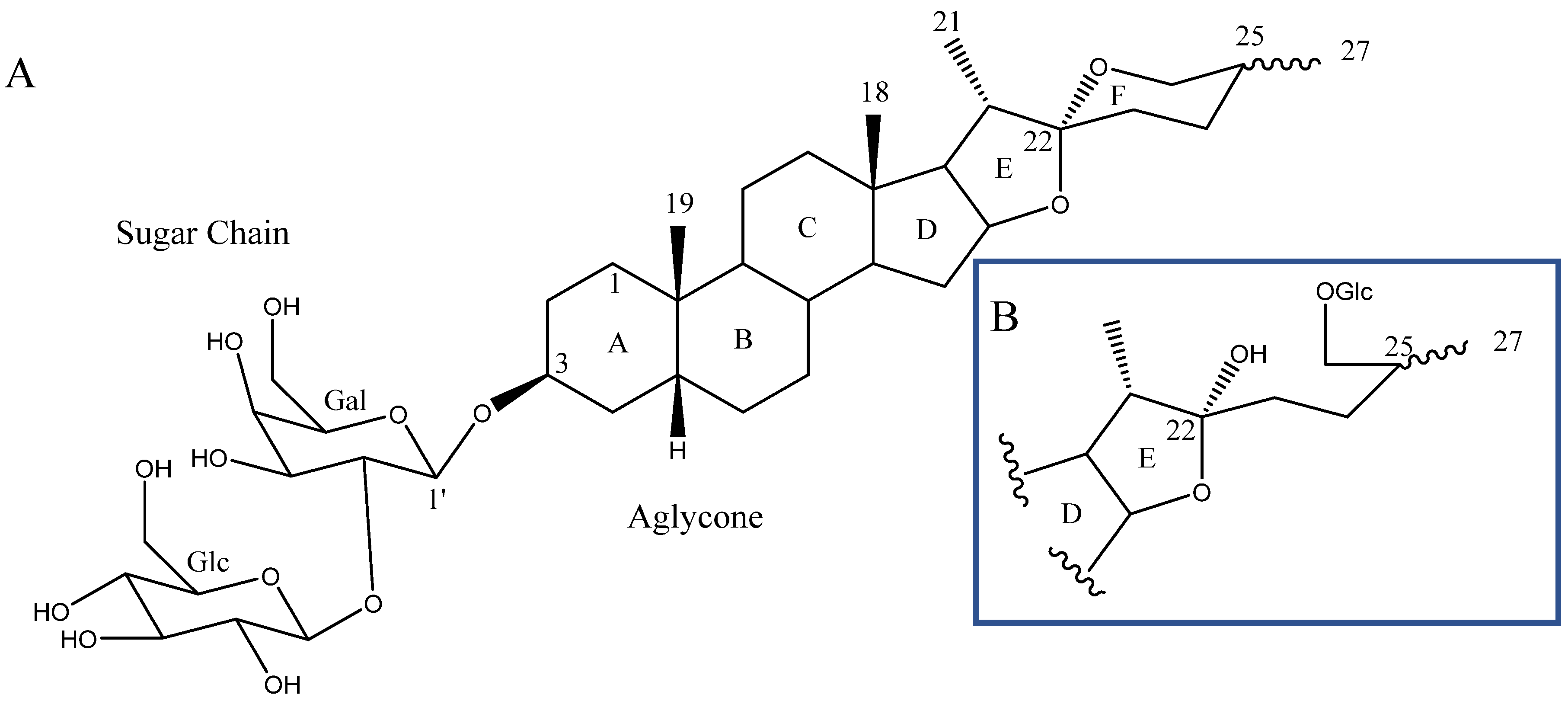

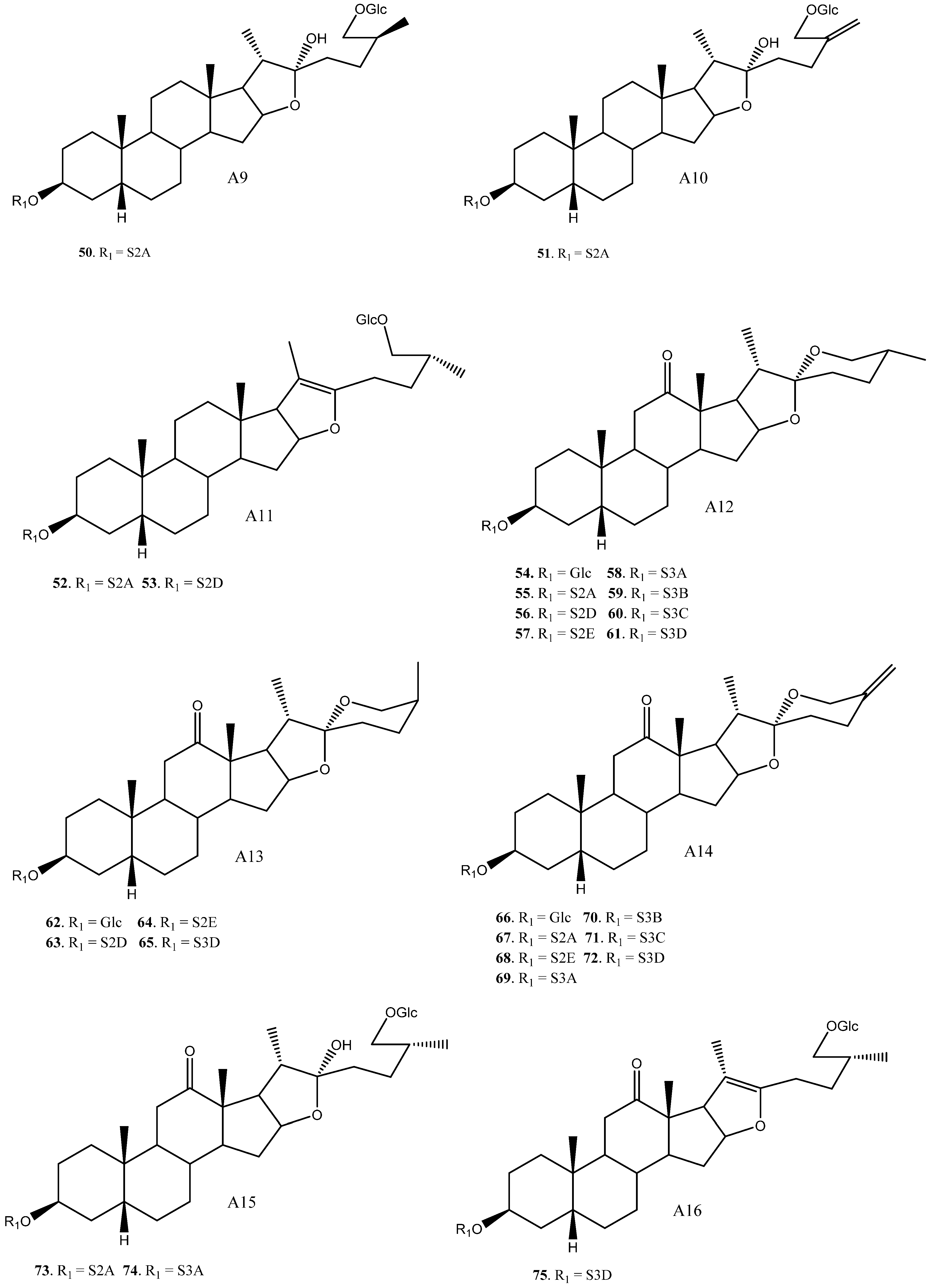
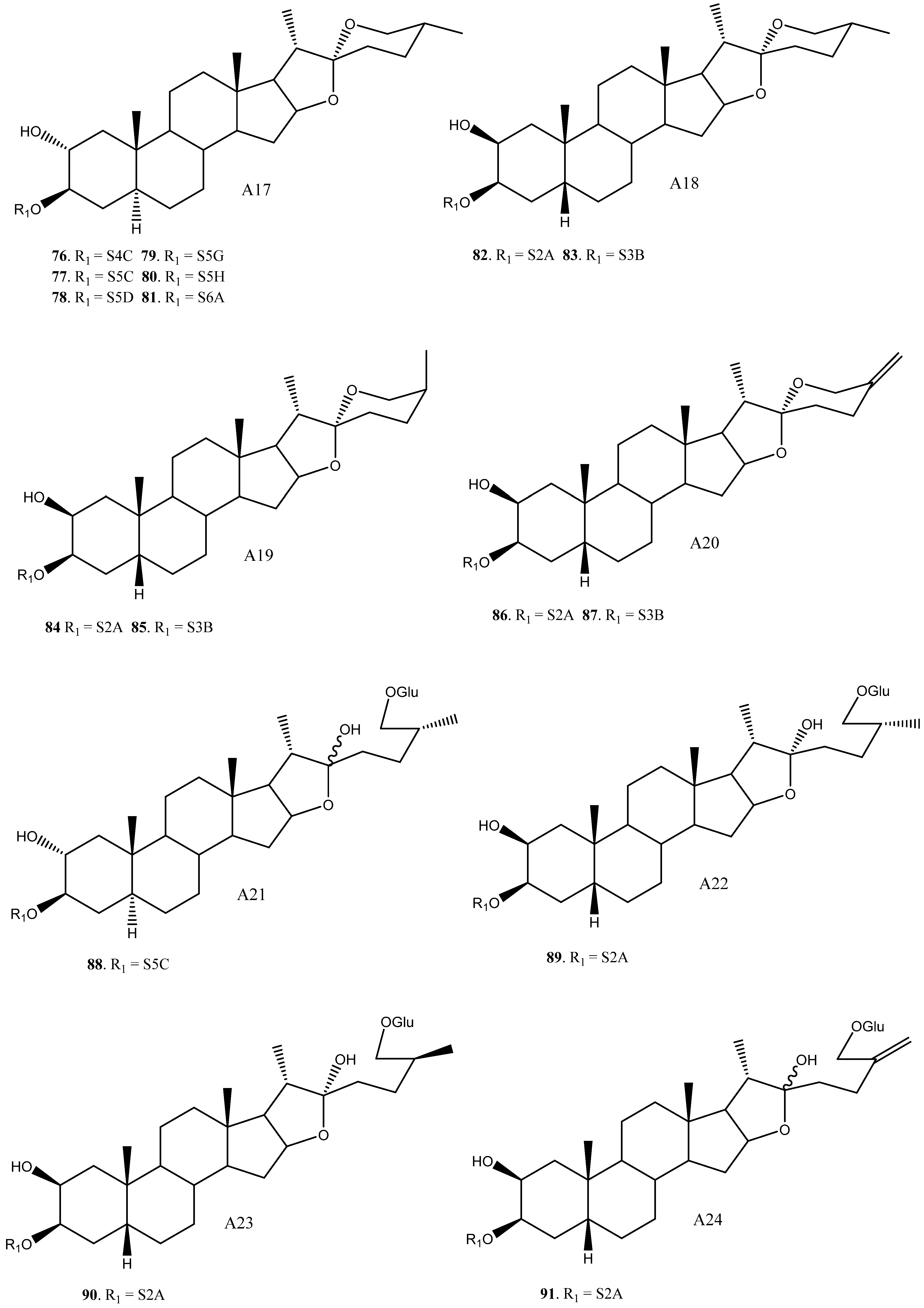
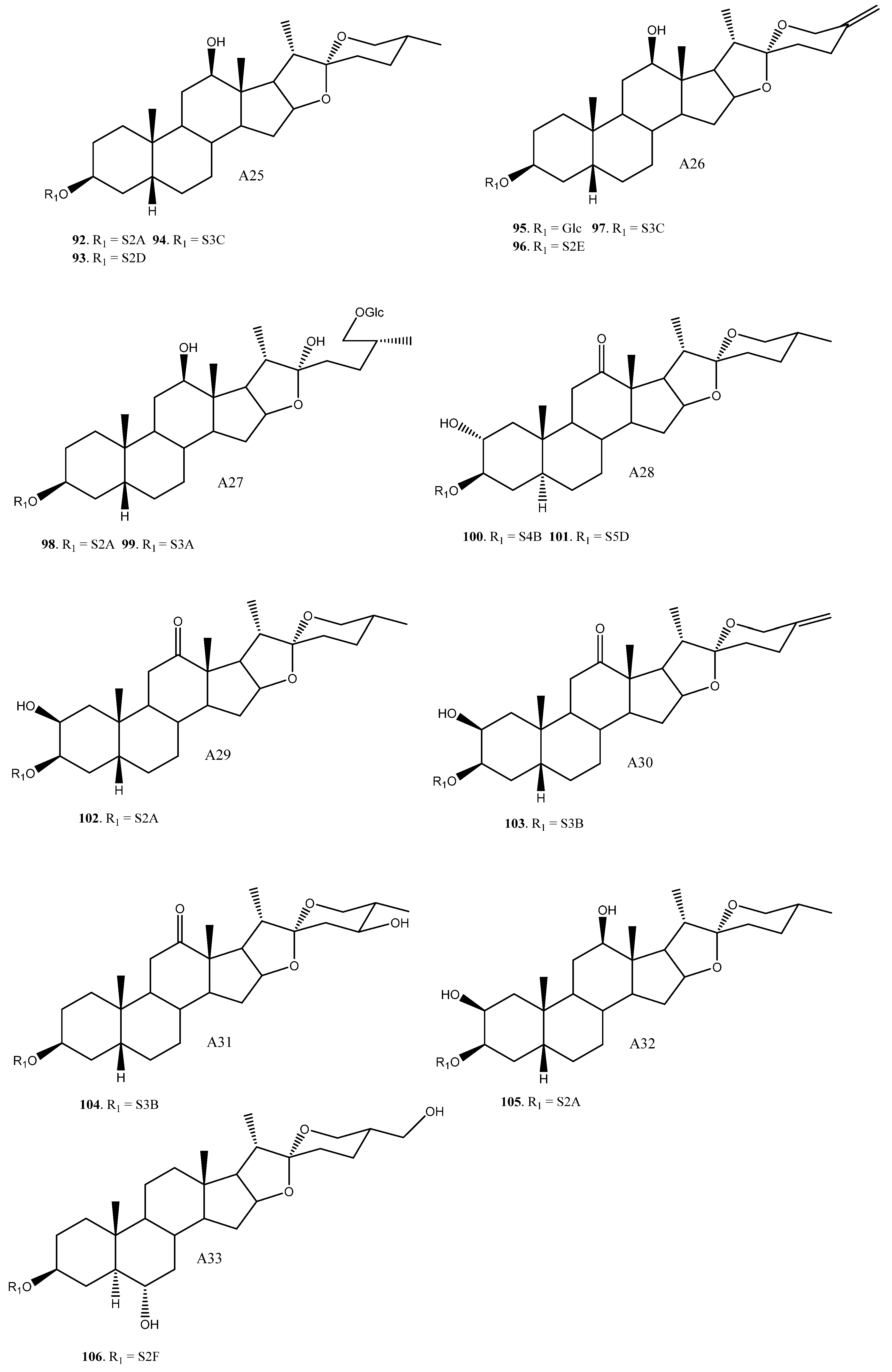


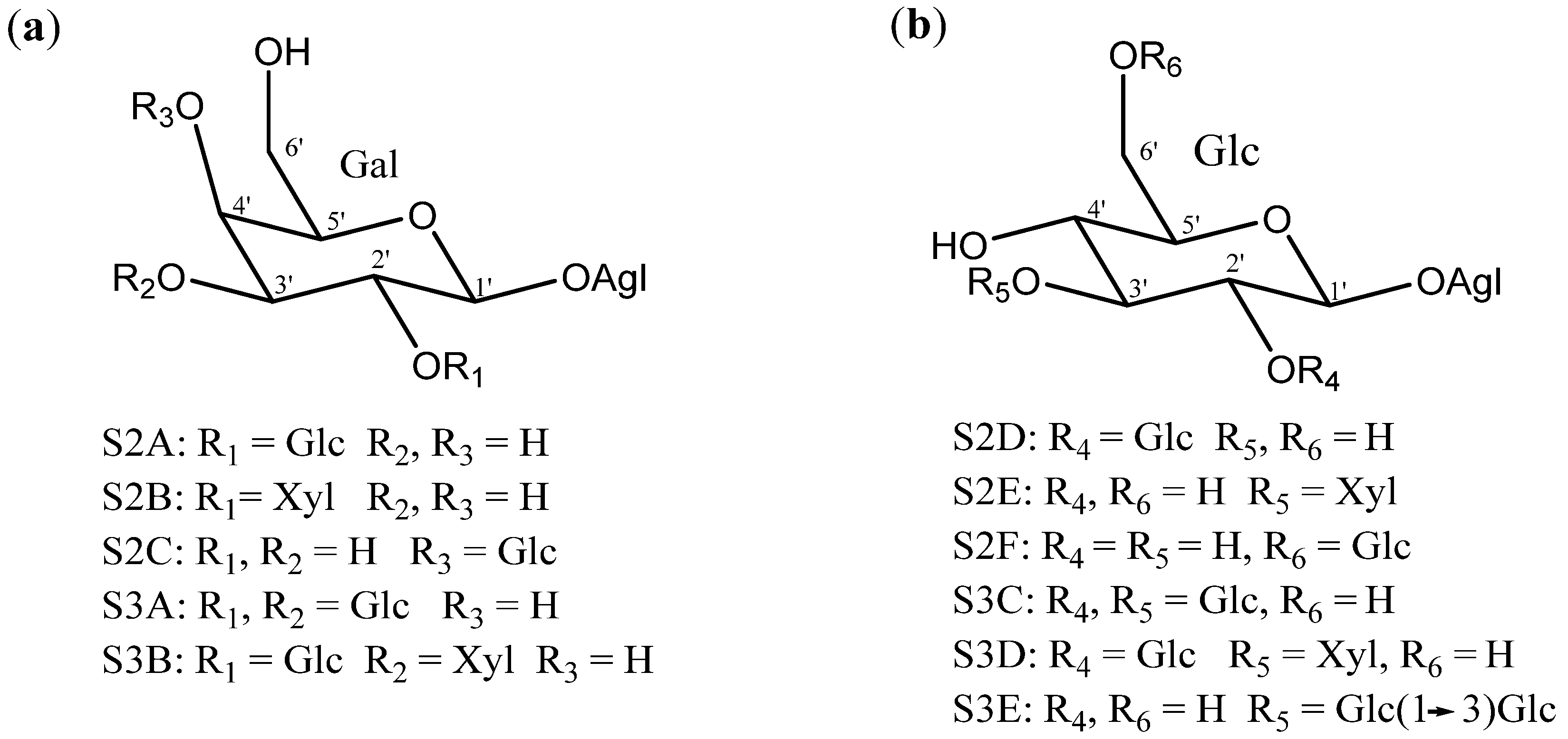
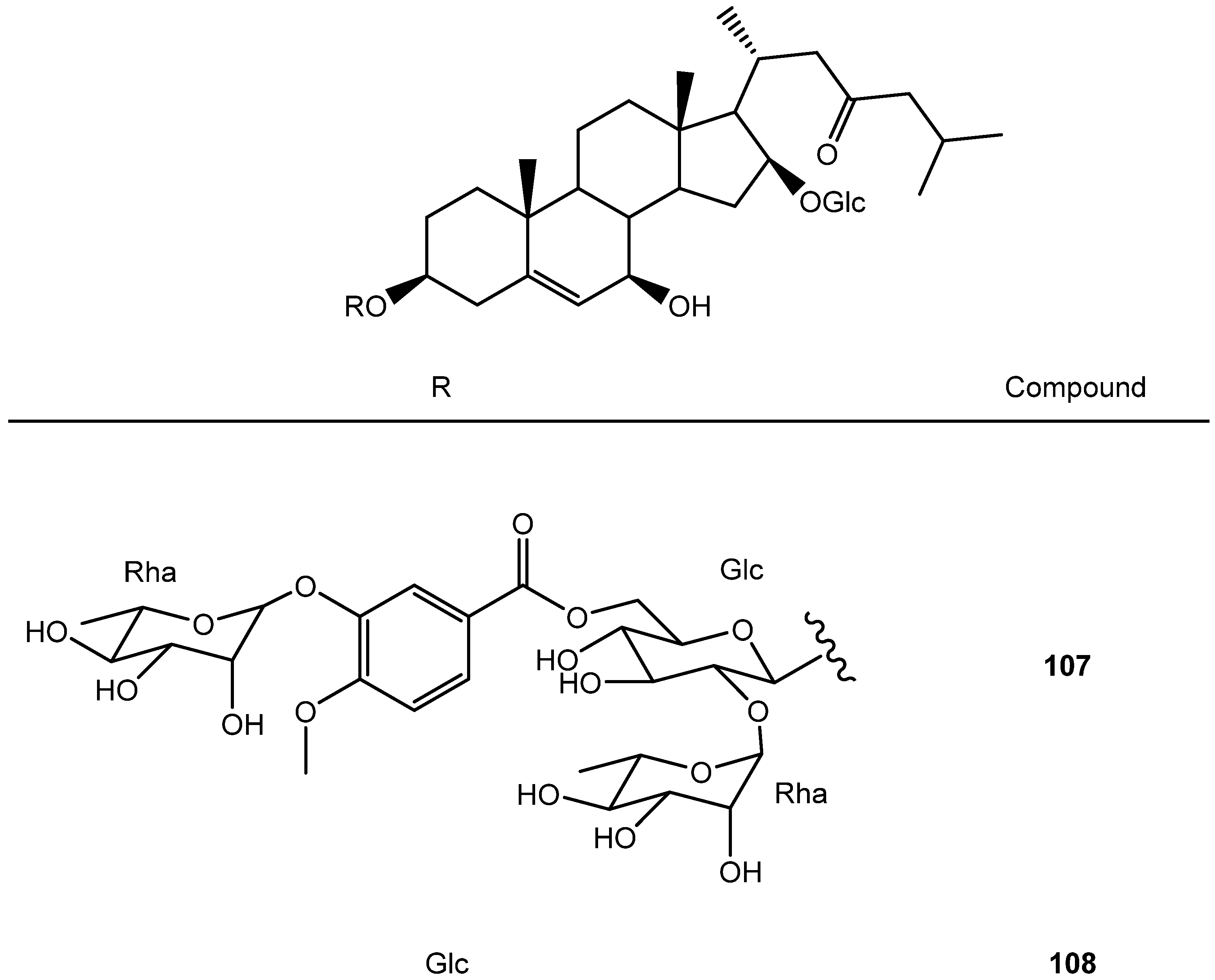
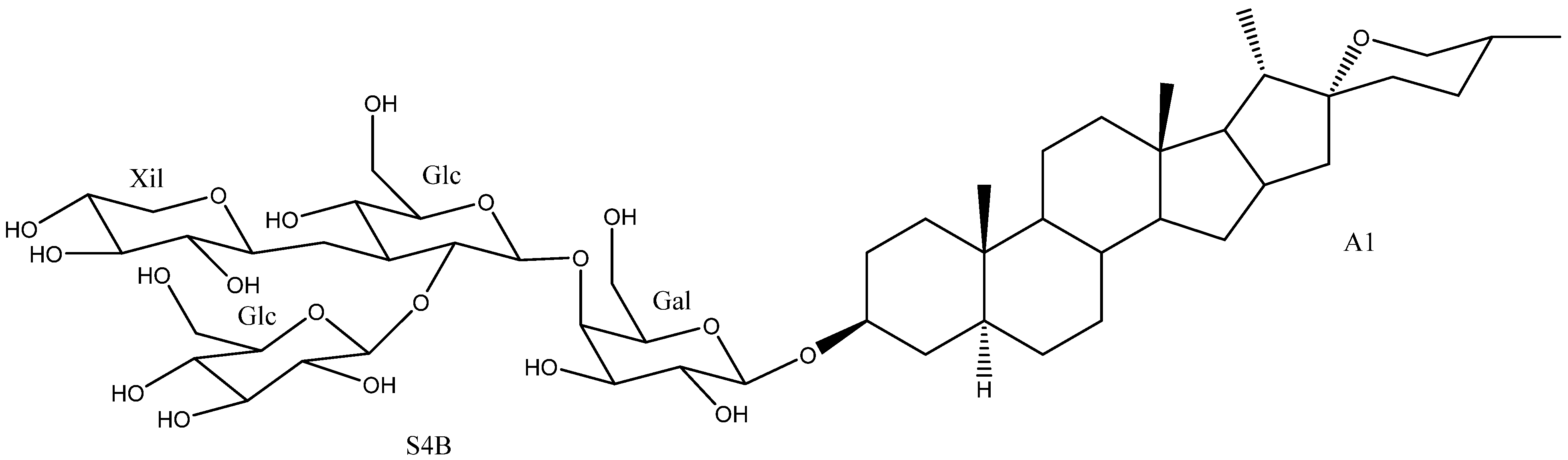

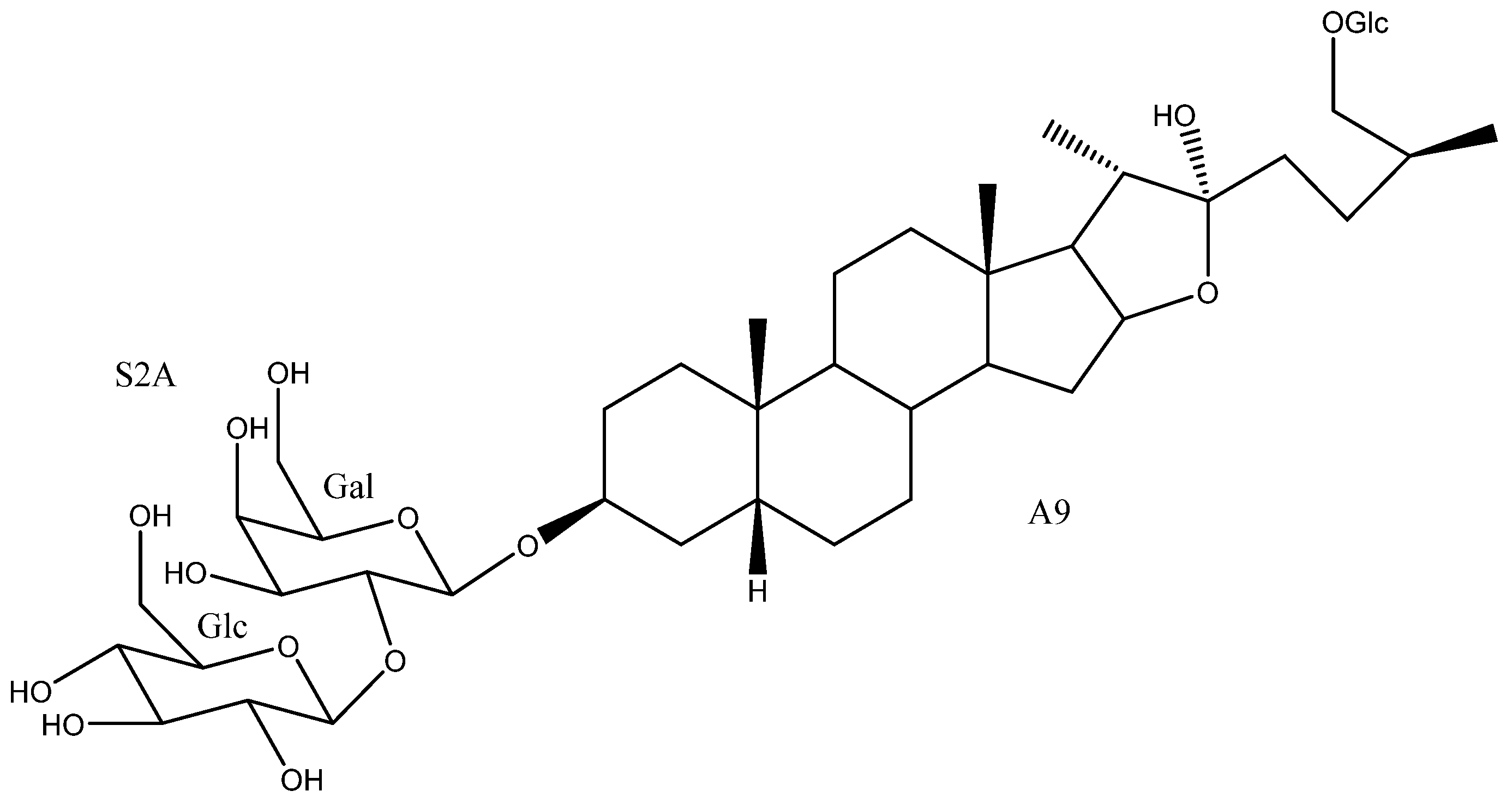
| N° | Name | Aglycone | Sugar Chain | Yucca Species | Bioactivities | Refs. |
|---|---|---|---|---|---|---|
| 1 | Yuccaloeside A | A1 | S2A | Y. gloriosa | Cytotoxic (HeLa IC50 = 12 μM) | [7,29,32,38] |
| 2 | Degalactotigonin | A1 | S4B | Y. gloriosa | See Section 3.3.1 | [7,29,39,40,41,42,43,44,45,46,47,48] |
| 3 | Yuccaloeside B | A1 | S5B | Y. gloriosa | Cytotoxic (MSC-2 y HGF LD50 = 1.9 y 20 μM) Antifungal (Various strings, MIC 0.78→100 μM) | [7,31,32,49] |
| 4 | A1 | S5C | Y. gloriosa | Cytotoxic (Vero IC50 = 7.5 μM y HeLa IC50 = 7.2 μM) Antifungal (Various strings IC50 0.4–15 μM) | [30,39,50] | |
| 5 | A1 | S5D | Y. gloriosa | [32] | ||
| 6 | Desmettianoside C | A1 | S5E | Y. desmetiana | Cytotoxic (HCT116, MCF7, A549, HepG2 IC50 = 2.4, 2.6, 10.2, 1.1 μM) | [34] |
| 7 | A1 | S5F | Y. gloriosa | [29] | ||
| 8 | Yuccaloeside C | A1 | S6A | Y. gloriosa | Cytotoxic (HeLa IC50 = 4.8 μM, HSC-2 y HGF LD50 = 1.0, 3.1 μM y L1210 EC50 = 0.1 μM) Antifungal (Various strings, MIC 0.39→100 μM) | [7,31,32,49,51,52] |
| 9 | A1 | S6B | Y. gloriosa | [29] | ||
| 10 | Yuccaloeside E | A1 | S6C | Y. gloriosa | [7,29] | |
| 11 | A2 | Glc | Y. schidigera | Cytotoxic (HO-8910, L1210, SW620 IC50 = 24.83, 12.33, >100 μM) Antifungal (Inactive) | [28,53,54] | |
| 12 | YS-II/(25R) Timosaponin AIII | A2 | S2A | Y. gloriosa, glauca, elephantipes, desmetiana | Cytotoxic (HCT116, MCF7, A549, HL60 IC50 = 4.9, 4.0, 8.4–16.5, 3.1 μM) Antifungal (C. albicans y C. neoformans IC50 = 5.0, 6.0 μM) | [6,11,34,35,36] |
| 13 | Elephanoside A | A2 | S2B | Y. elephantipes | Antifungal (inactive) | [36] |
| 14 | YS-I/Schidigera-saponin D5 | A2 | S2D | Y. gloriosa | Cytotoxic (SW620 IC50 = 63.37 μM) Antifungal * (Various Strings MIC 3.13→100 μM) | [6,14,28,35] |
| 15 | YS-IV/Schidigera-saponin D4 | A2 | S3A | Y. gloriosa, schidigera, elephantipes | Antifungal * (Various Strings MIC 3.13–50 μM y C. neoformans y C. albicans IC50 = 15 μM) | [14,35,36] |
| 16 | Saponin YSM2/Schidigera-saponin D2 | A2 | S3B | Y. schidigera, glauca | Antifungal * (Various Strings MIC 1.56→100 μM) Cytotoxic (HL60, A549 IC50 = 4.2, 5.9 µM) | [11,14] |
| 17 | YS-III | A2 | S3C | Y. gloriosa, schidigera | Antifungal (Various strings MIC 3.13–6.25 μM) | [14,35] |
| 18 | Saponin YSM4/Schidigera-saponin D1 | A2 | S3D | Y. schidigera | Cytotoxic (SW620 IC50 = 69.17 μM) Antifungal * (Various strings MIC 3.13→100 μM) | [14,28] |
| 19 | Asparagoside A | A3 | Glc | Y. schidigera | Cytotoxic (SW620 IC50 = 60.26 μM) Cytotoxic (SW620 IC50 = 60.26 μM) Growth Hormone releasing effect (10% release of GH) Anti-alzheimer (IC50 = 6.0 μM) | [28,55,56,57] |
| 20 | Timosaponin AIII | A3 | S2A | Y. macrocarpa, gloriosa | See Section 3.3.2 | [15,35,37,55,58,59,60,61,62,63,64,65,66,67,68,69,70] |
| 21 | Schidigera-saponin D5 | A3 | S2D | Y. schidigera | Cytotoxic (SW620 IC50 = 33.91 μM) Antifungal * (Various strings, MIC 3.13→100 μM) | [14,16,28] |
| 22 | Saponin YSM3/Schidigera-saponin D4 | A3 | S3A | Y.schidigera | Antifungal * (Various strings, MIC 3.13–50 μM) | [14] |
| 23 | Saponin YSM2/Schidigera-saponin D2 | A3 | S3B | Y. schidigera | Antifungal * (Various strings, MIC 3.13→100 μM) | [14] |
| 24 | A3 | S3C | Y. schidigera | Antifungal * (Various strings, MIC 3.13→100 μM) | [14] | |
| 25 | Saponin YSM4/Schidigera-saponin D1 | A3 | S3D | Y. schidigera | Cytotoxic (SW620 IC50 = 12.02 μM) Antifungal * (Various strings, MIC 3.13→100 μM) | [14,16,28] |
| 26 | Yuccoside E | A3 | S4D | Y. filamentosa | [18] | |
| 27 | Elephanoside G | A4 | S3E | Y. elephantipes | [19] | |
| 28 | Yucca spirostanoside A1 | A5 | Glc | Y. schidigera | [20] | |
| 29 | 25(27)-ene-Timosaponin AIII | A5 | S2A | Y. glauca | Cytotoxic (Various cell lines IC50 = 2.5–13.14 μM) | [11,65] |
| 30 | Yucca spirostanoside A2 | A5 | S2E | Y. schidigera | [20] | |
| 31 | Saponin YSM1/Schidigera-saponin A2 | A5 | S3B | Y. schidigera, glauca | Cytotoxic (HL60, A549 IC50 = 4.9, 6 μM) Antifungal (Various strings, MIC 3.13→100 μM) | [11,14] |
| 32 | Schidigera-saponin A3 | A5 | S3C | Y. schidigera | Antifungal (Various strings, MIC 3.13→100 μM) | [14,20] |
| 33 | Schidigera-saponin A1 | A5 | S3D | Y. schidigera | Antifungal (Various strings MIC 3.13–12.5 μM) | [14,20] |
| 34 | A6 | S2A | Y. gloriosa | [29] | ||
| 35 | Desmettianoside B | A6 | S4A | Y. desmetiana | Cytotoxic (Various cell lines IC50 1.76–25.07 μM) Anti-inflammatory (Inactive) Molluscicide (LC100 = 11 mg/L) | [12,71,72] |
| 36 | Uttroside B | A6 | S4B | Y. gloriosa | Cytotoxic (Various strings, IC50 0.5–18.83 μM) Anti-inflammatory (inactive) Platelet aggregation (Inhibition 15.6%) | [29,42,46,50,72,73] |
| 37 | Desmettianoside A | A6 | S5A | Y. desmetiana | Molluscicide (LC100 = 6 mg/L) | [12] |
| 38 | A6 | S5B | Y. gloriosa | [29] | ||
| 39 | A6 | S5D | Y. gloriosa | [29] | ||
| 40 | Furcreafurostatin | A6 | S6A | Y. gloriosa | [29] | |
| 41 | A6 | S7A | Y. gloriosa | [29] | ||
| 42 | (25R) Timosaponin BII | A7 | S2A | Y. gloriosa, glauca, desmetiana | Cytotoxic (HL60, A549, HCT116, MCF7, HepG2 IC50 = 3.7, 7.0, >100, >100, >100 μM). | [11,21,34] |
| 43 | A7 | S2A | Y. gloriosa | Cytotoxic (A549, HCT116, MCF7, HepG2 IC50 = >100, >100, >100, >100 μM) | [11,21] | |
| 44 | A7 | S2C | Y. glauca | Cytotoxic (HL60, A549 IC50 = 14.3, 20 μM) | [11] | |
| 45 | Disporoside C | A7 | S2D | Y. gloriosa, schidigera | [16,21] | |
| 46 | Elephanoside B | A7 | S3A | Y. elephantipes | Antifungal (Inactive) | [36] |
| 47 | A7 | S3D | Y. schidigera | [16] | ||
| 48 | A8 | Glc | Y. elephantipes | [19] | ||
| 49 | A8 | S2C | Y. elephantipes | Spermicidal (Inactive, >80% motility) | [19,74] | |
| 50 | Timosaponin BII | A9 | S2A | Y. glauca | See Section 3.3.3 | [11,15,75,76,77,78,79,80,81,82,83] |
| 51 | Macrostemonoside O/Timosaponin L | A10 | S2A | Y. glauca | Cytotoxic (Various cell lanes, IC50 = 4.4–16.34 μM) Platelet aggregation (Inactive) | [11,61,84,85] |
| 52 | Macrostemonoside F | A11 | S2A | Y. gloriosa | Platelet aggregation (inhibition, IC50 = 0.02 mM) Anti-inflammatory (NO reduced 62.89%) | [6,86,87] |
| 53 | A11 | S2D | Y. gloriosa | [6] | ||
| 54 | (25R)-Yucca spirostanoiside E1 | A12 | Glc | Y. schidigera | Cytotoxic (SW620, Inactive) | [28] |
| 55 | Elephanoside H | A12 | S2A | Y. gloriosa, glauca, elephantipes | Cytotoxic (HL60, A549, Inactive) | [6,11,19] |
| 56 | (25R)-Yucca spirostanoside E3 | A12 | S2D | Y. schidigera | Cytotoxic (SW620 IC50 = 29.81 μM) | [28] |
| 57 | A12 | S2E | Y. schidigera | Cytotoxic (SW620, Inactive) | [28] | |
| 58 | YS-VIII | A12 | S3A | Y. gloriosa, elephantipes | Antifungal (inactive) | [6,23,36] |
| 59 | A12 | S3B | Y. glauca | Cytotoxic (HL60, A549, Inactive) | [11] | |
| 60 | YS-VII | A12 | S3C | Y. gloriosa, schidigera | [6,16,23] | |
| 61 | Schidigera-saponin E1 | A12 | S3D | Y. schidigera | Antifungal * (Various strings, inactive or low activity) | [14,16] |
| 62 | (25S)-Yucca spirostanoside E1 | A13 | Glc | Y. schidigera | Cytotoxic (SW620, Inactive) | [28] |
| 63 | (25S)-Yucca spirostanoside E3 | A13 | S2D | Y. schidigera | Cytotoxic (SW620 IC50 = 55.90 μM) | [28] |
| 64 | (25S)-Yucca spirostanoside E2 | A13 | S2E | Y. schidigera | Cytotoxic (SW620, Inactive) | [28] |
| 65 | Schidigera-saponin E1 | A13 | S3D | Y. schidigera | Antifungal* (Various strings, inactive or low activity) | [14] |
| 66 | Yucca spirostanoside C1 | A14 | Glc | Y. schidigera | [20] | |
| 67 | 25(27)-Ene-elephanoside H | A14 | S2A | Y. glauca | Cytotoxic (HL60, A549, Inactive) | [11] |
| 68 | Yucca spirostanoside C2 | A14 | S2E | Y. schidigera | [20] | |
| 69 | Yucca spirostanoside C3 | A14 | S3A | Y. schidigera | [20] | |
| 70 | A14 | S3B | Y. glauca | Cytotoxic (HL60, A549, Inactive) | [11] | |
| 71 | A14 | S3C | Y. gloriosa, schidigera | [6,20] | ||
| 72 | Schidigera-saponin B1 | A14 | S3D | Y. schidigera | Antifungal (Various strings, inactive or low activity) | [14] |
| 73 | Elephanoside D | A15 | S2A | Y. glauca, elephantipes | Cytotoxic (HL60, A549, Inactive) Antifungal (inactive) | [11,36] |
| 74 | Elephanoside C | A15 | S3A | Y. elephantipes | Antifungal (inactive) | [36] |
| 75 | A16 | S3D | Y. schidigera | [16] | ||
| 76 | A17 | S4C | Y. aloifolia | Molluscicide (LC100 = 10 ppm) | [25] | |
| 77 | A17 | S5C | Y. gloriosa | [30] | ||
| 78 | A17 | S5D | Y. gloriosa | Cytotoxic (HL60 IC50 1.7–6.5 μM) Anti-alzheimer (NO production IC50 = 13.16) | [7,30,88,89,90] | |
| 79 | A17 | S5G | Y. aloifolia | [24] | ||
| 80 | A17 | S5H | Y. aloifolia | [26] | ||
| 81 | A17 | S6A | Y. filamentosa | [27] | ||
| 82 | (25R)-2β-Hydroxytimosaponin AIII | A18 | S2A | Y. gloriosa, schidigera, glauca | Cytotoxic (HL60, A549 IC50 = 11.3, >20 μM) Antifungal * (Various strings, inactive or low activity) | [11,14,35] |
| 83 | Schidigera-saponin F1 | A18 | S3B | Y. schidigera | Antifungal * (Various strings, inactive or low activity) | [14] |
| 84 | Timosaponin AII/Schidigera-saponin F2 | A19 | S2A | Y. schidigera | Antifungal * (Various strings, inactive or low activity) | [14,16] |
| 85 | Schidigera-saponin F1 | A19 | S3B | Y. schidigera | Antifungal * (Various strings, inactive or low activity) | [14] |
| 86 | 25(27)-Ene-2β-hydroxytimosaponin AIII/Schidigera-saponin C2 | A20 | S2A | Y. schidigera, glauca | Cytotoxic (HL60, A549 IC50 = 5.0, >20 μM) Antifungal (Various strings, inactive or low activity) | [11,14,20] |
| 87 | Schidigera-saponin C1 | A20 | S3B | Y. schidigera | Antifungal (Various strings, inactive or low activity) | [14,20] |
| 88 | YG4 | A21 | S5C | Y. gloriosa | [30] | |
| 89 | A22 | S2A | Y. glauca | Cytotoxic (HL60, A549 IC50 = 17.8, >20 μM) | [11] | |
| 90 | Timosaponin N | A23 | S2A | Y. glauca | Cytotoxic (HL60, A549 IC50 9.2, >20 μM) | [11] |
| 91 | 25(27)-Ene-Macrostemonoside J | A24 | S2A | Y. glauca | Cytotoxic (HL60, A549 IC50 = 13.3, >20 μM) | [11] |
| 92 | (25R)-12β-Hydroxytimosaponin AIII/YS-XIII | A25 | S2A | Y. gloriosa, glauca | Cytotoxic (HL60, A549, Inactive) | [11,22] |
| 93 | A25 | S2D | Y. gloriosa | [6] | ||
| 94 | A25 | S3C | Y. gloriosa | [22] | ||
| 95 | Yucca spirostanoside B1 | A26 | Glc | Y. schidigera | [20] | |
| 96 | Yucca spirostanoside B2 | A26 | S2E | Y. schidigera | [20] | |
| 97 | Yucca spirostanoside B3c | A26 | S3C | Y. schidigera | [20] | |
| 98 | Elephanoside E | A27 | S2A | Y. elephantipes | Antifungal (inactive) | [36] |
| 99 | Elephanoside F | A27 | S3A | Y. elephantipes | Antifungal (inactive) | [36] |
| 100 | YS-IX | A28 | S4B | Y. gloriosa | Cytotoxic (HL60 IC50 = 7.2 μM) | [23,89] |
| 101 | YS-X | A28 | S5D | Y. gloriosa | Cytotoxic (HL60, 40% growth inhibition at 10 μM) | [23,89] |
| 102 | YS-VI | A29 | S2A | Y. gloriosa | [23] | |
| 103 | A30 | S3B | Y. schidigera | [20] | ||
| 104 | A31 | S3B | Y. glauca | Cytotoxic (HL60, A549, inactive) | [11] | |
| 105 | A32 | S2A | Y. gloriosa | [22] | ||
| 106 | Yuccalan | A33 | S2F | Y. smalliana | Antifungal (Fusarium oxysporum and Rhizoctonia solani, low activity) | [33] |

| Compound | R1 | R2 | R3 | R4 | C-25 | IC50 HL-60 (µM) | IC50 A549 (µM) |
|---|---|---|---|---|---|---|---|
| 29 | H | S2A | H, H | H | Δ25(27) | 2.5 ± 0.47 | 7.3 ± 0.63 |
| 67 | H | S2A | O | H | Δ25(27) | >20 | |
| 70 | H | S3B | O | H | Δ25(27) | >20 | |
| 59 | H | S3B | O | H | 25R | >20 | |
| 104 | H | S3B | O | OH | 25S | >20 | |
| 12 | H | S2A | H, H | H | 25R | 3.1 ± 0.35 | 8.4 ± 0.34 |
| 92 | H | S2A | β-OH | H | 25R | >20 | |
| 55 | H | S2A | O | H | 25R | >20 | |
| 86 | OH | S2A | H, H | H | Δ25(27) | 5.0 ± 0.09 | >20 |
| 82 | OH | S2A | H, H | H | 25R | 11.3 ± 1.42 | >20 |
| 31 | H | S3B | H, H | H | Δ25(27) | 4.9 ± 0.43 | 6.0 ± 0.65 |
| 16 | H | S3B | H, H | H | 25R | 4.2 ± 0.37 | 5.9 ± 0.43 |
| Cisplatin | 1.7 ± 0.06 | 2.1 ± 1.10 | |||||
| Etoposide | 0.39 ± 0.08 |
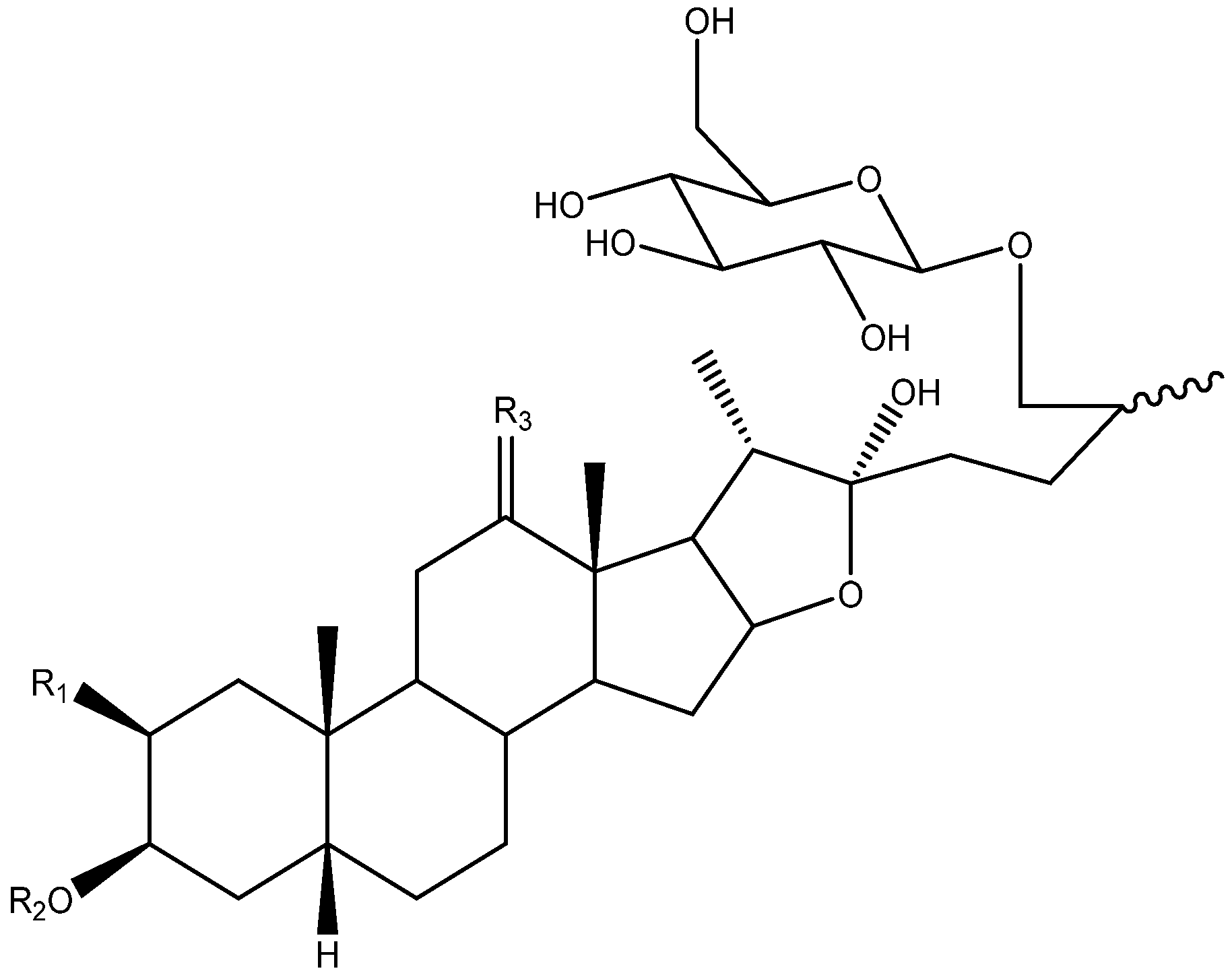
| Compound | R1 | R2 | R3 | C-25 | IC50 HL-60 (µM) | IC50 A549 (µM) |
|---|---|---|---|---|---|---|
| 91 | OH | S2A | H, H | Δ25(27) | 13.3 ± 0.09 | >20 |
| 89 | OH | S2A | H, H | 25R | 17.8 ± 2.47 | >20 |
| 90 | OH | S2A | H, H | 25S | 9.2 ± 1.21 | >20 |
| 51 | H | S2A | H, H | Δ25(27) | 4.4 ± 0.10 | 11.9 ± 0.70 |
| 42 | H | S2A | H, H | 25R | 3.7 ± 0.55 | 7.0 ± 0.29 |
| 50 | H | S2A | H, H | 25S | 3.3 ± 0.15 | 9.3 ± 2.07 |
| 73 | H | S2A | O | 25R | >20 | |
| 44 | H | S2C | H, H | 25R | 14.3 ± 0.07 | >20 |
| Cisplatin | 1.7 ± 0.06 | 2.1 ± 1.10 | ||||
| Etoposide | 0.39 ± 0.08 | - |

| Compound | R1 | R2 | C-25 | IC50 (µM) |
|---|---|---|---|---|
| Control (5-Fluorouracil) | 10.00 ± 0.15 | |||
| 54 | Glc | O | R | >100 |
| 62 | Glc | O | S | >100 |
| 57 | S2E | O | R | >100 |
| 64 | S2E | O | S | >100 |
| 56 | S2D | O | R | 29.81 ± 0.21 |
| 63 | S2D | O | S | 55.90 ± 0.78 |
| 11 | Glc | H, H | R | >100 |
| 19 | Glc | H, H | S | 60.26 ± 4.53 |
| 14 | S2D | H, H | R | 63.37 ± 0.70 |
| 21 | S2D | H, H | S | 33.91 ± 1.27 |
| 18 | S3D | H, H | R | 69.17 ± 1.24 |
| 25 | S3D | H, H | S | 12.02 ± 1.43 |
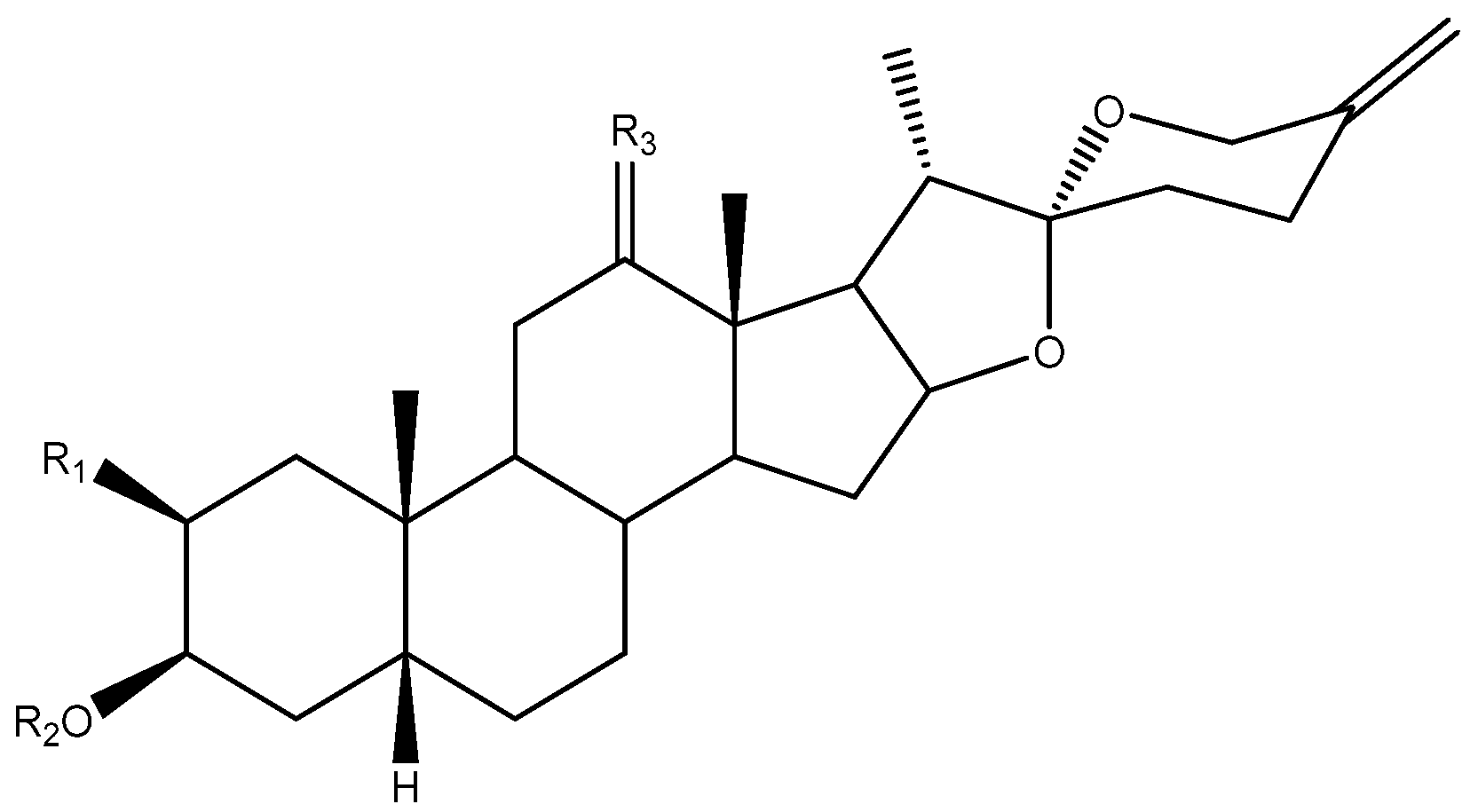
| Compound | R1 | R2 | R3 | Sc | Ca | Ha | Pn | Ka | Dh |
|---|---|---|---|---|---|---|---|---|---|
| 33 | H | S3D | H2 | 3.13 | 6.25 | 3.13 | 3.13 | 12.5 | 6.25 |
| 31 | H | S3B | H2 | 12.5 | 12.5 | 3.13 | 3.13 | >100 | >100 |
| 32 | H | S3C | H2 | 12.5 | 12.5 | 6.13 | 3.13 | >100 | >100 |
| 72 | H | S3D | O | >100 | >100 | >100 | >100 | >100 | >100 |
| 87 | OH | S3B | H2 | 100 | 100 | >100 | 100 | >100 | >100 |
| 86 | OH | S2A | H2 | >100 | >100 | >100 | >100 | >100 | >100 |
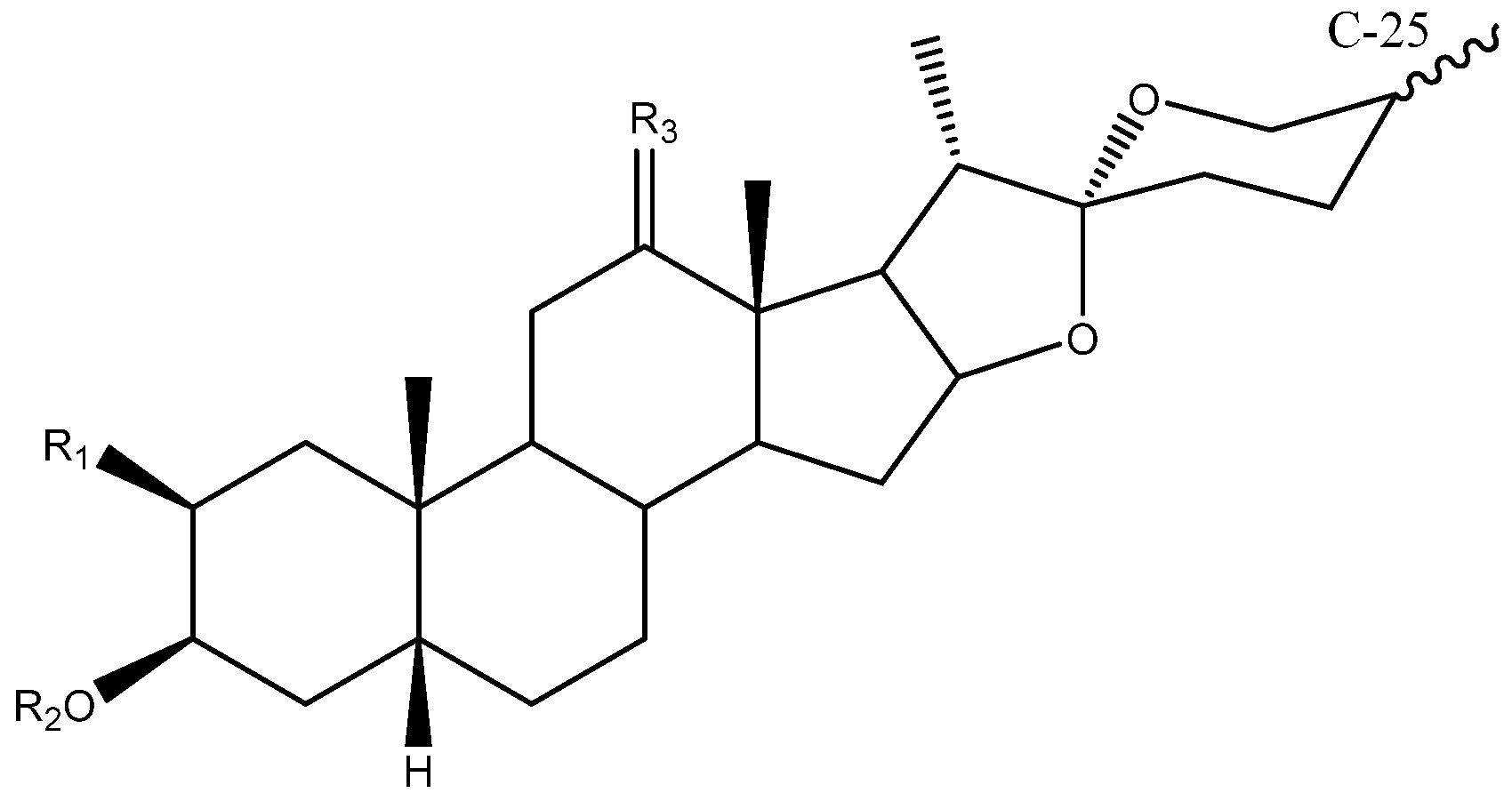
| Compounds | R1 | R2 | R3 | Sc | Ca | Ha | Pn | Ka | Dh |
|---|---|---|---|---|---|---|---|---|---|
| 18 + 25 | H | S3D | H2 | 6.25 | 50 | 3.13 | 3.13 | >100 | 6.25 |
| 16 + 23 | H | S3B | H2 | 25 | >100 | 3.13 | 12.5 | >100 | 50 |
| 24 + 17 | H | S3C | H2 | 6.25 | >100 | 1.56 | 3.13 | >100 | 6.25 |
| 22 + 15 | H | S3A | H2 | 12.5 | 25 | 3.13 | 6.25 | 50 | 6.25 |
| 21 + 14 | H | S2D | H2 | 12.5 | 12.5 | 6.25 | 3.13 | >100 | >100 |
| 61 + 65 | H | S3D | O | 100 | >100 | 100 | >100 | >100 | >100 |
| 83 + 85 | OH | S3B | H2 | 100 | >100 | >100 | >100 | >100 | 100 |
| 84 + 82 | OH | S2A | H2 | >100 | >100 | >100 | 100 | >100 | >100 |
| Activity | Description | Value | Value Range | Refs. |
|---|---|---|---|---|
| Cytotoxic | Reduces the viability of cancer cells. | IC50/EC50 | 0.35–22.1 µM/0.02–5.12 µM | [38,58,65,66,67,68,70,79] |
| Anti-inflammatory | Can moderate the physiological response to inflammation. | IC50/% BALF recruitment | 1.21, 1.82 µM/64% lower (50mg/kg) | [15,59] |
| Effect on platelet aggregation | Inhibition or induction to platelet aggregation. | IC50/% inhibition | 4.36 µM/80–90% (2–50 µM) | [60,61] |
| Anti-Alzheimer | Prevents and improves the condition of Alzheimer’s patients. | IC50/% Aβ42 reduction | 2.3–7.45 µM/42% (5 µM) | [55,63] |
| Anti-diabetic osteoporosis | Prevents and improves the condition of patients with diabetes-related osteoporosis. | Better anti-AGEs and anti-osteoporosis effects | 0.1 µM | [64] |
| Activity | Description | Value | Value Range | Refs. |
|---|---|---|---|---|
| Cytotoxic | Reduces the viability of cancer cells. | IC50/% tumor growth inhibition | 3.3, 9.3 µM/65–84% (10–15% wt) | [11,76] |
| Anti-inflammatory | Can moderate the physiological response to inflammation. | IC50/% damaged cells viability increment. | 0.77, 1.57 µM/61.5–74.8% (5–50 µM) | [15,78] |
| Effect on platelet aggregation | Inhibition or induction to platelet aggregation. | Concentration to inhibit ADP-induced aggregation | 50–100 µM | [79,103] |
| Anti-Alzheimer | Prevents and improves the condition of Alzheimer’s patients. | Inhibition of IL6, IL1β and TNFα expressions. | 0.313–5 mg/mL | [75] |
| Anti-diabetic | Prevents and improves the condition of diabetic patients. | α-glucosidase inhibition | [104] | |
| Cardioprotective | Preventive and reparative capacity against cardiac disorders. | Reversal of ISO-induced disorders/overall improvement of the conditions | 50 mg/kg–100 mg/kg/100–200 µM | [80,82] |
| Antiapoptotic | Protecting HUVECs against high-glucose- induced apoptosis. | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez, G.G.; Durán, A.G.; Macías, F.A.; Simonet, A.M. Structure, Bioactivity and Analytical Methods for the Determination of Yucca Saponins. Molecules 2021, 26, 5251. https://doi.org/10.3390/molecules26175251
Jiménez GG, Durán AG, Macías FA, Simonet AM. Structure, Bioactivity and Analytical Methods for the Determination of Yucca Saponins. Molecules. 2021; 26(17):5251. https://doi.org/10.3390/molecules26175251
Chicago/Turabian StyleJiménez, Gabriel G., Alexandra G. Durán, Francisco A. Macías, and Ana M. Simonet. 2021. "Structure, Bioactivity and Analytical Methods for the Determination of Yucca Saponins" Molecules 26, no. 17: 5251. https://doi.org/10.3390/molecules26175251
APA StyleJiménez, G. G., Durán, A. G., Macías, F. A., & Simonet, A. M. (2021). Structure, Bioactivity and Analytical Methods for the Determination of Yucca Saponins. Molecules, 26(17), 5251. https://doi.org/10.3390/molecules26175251








