Abstract
An approach for the preparation of polysubstituted indole-2-carbonitriles through a cross-coupling reaction of compounds 1-(but-2-ynyl)-1H-indole-2-carbonitriles and 1-benzyl-3-iodo-1H-indole-2-carbonitriles is described. The reactivity of indole derivatives with iodine at position 3 was studied using cross-coupling reactions. The Sonogashira, Suzuki–Miyaura, Stille and Heck cross-couplings afforded a variety of di-, tri- and tetra-substituted indole-2-carbonitriles.
1. Introduction
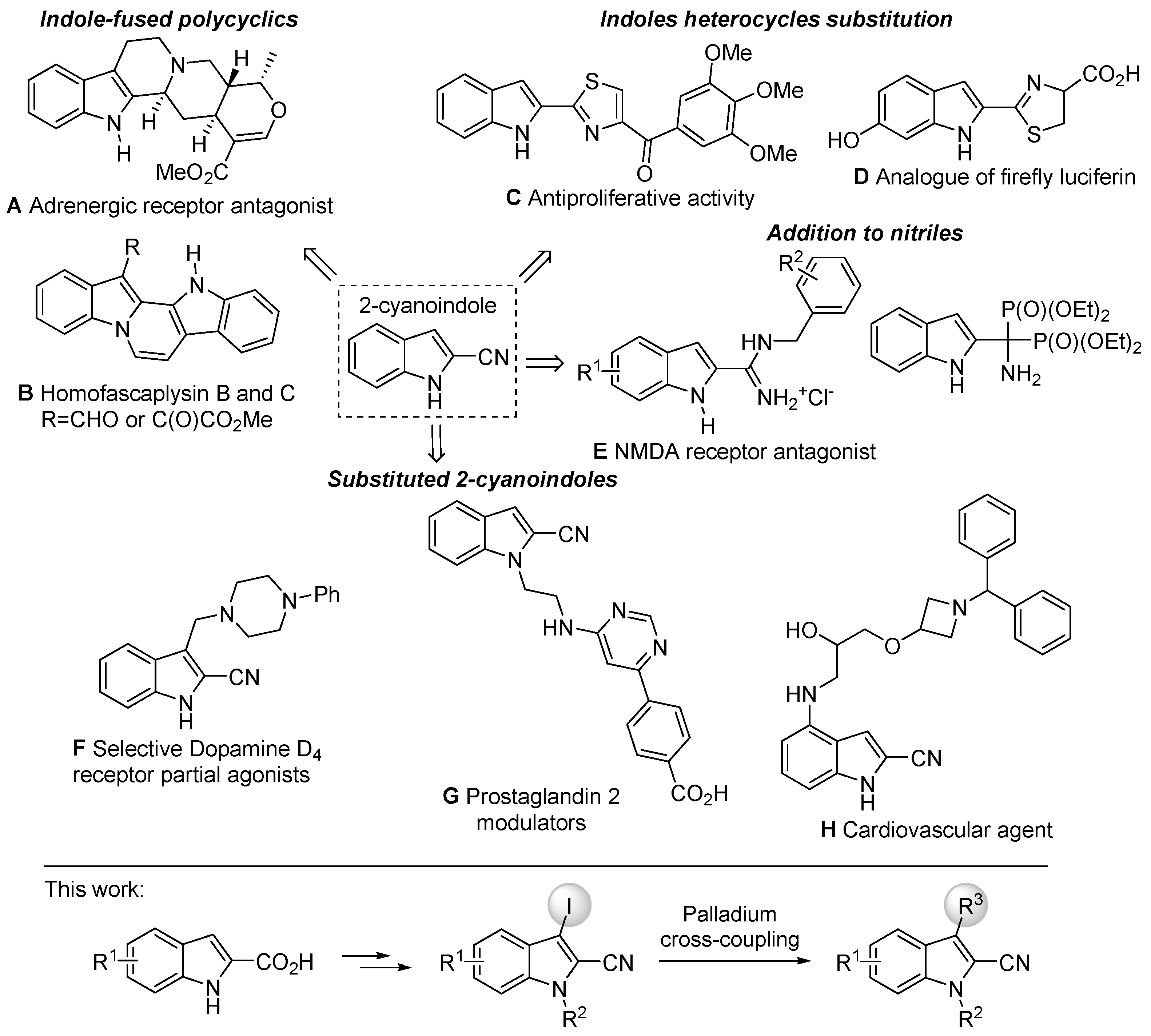
Indole skeletons exist as key building blocks in drugs, natural products, pharmaceuticals, alkaloids and agrochemicals and exhibit potent and wide-ranging biological activities [1,2,3,4,5]. The indole scaffold represents probably one of the most important structural subunits for the discovery of new drug candidates [6,7,8,9]. In particular, the derivatives of 2-cyanoindoles gained considerable attention in recent years because of their great importance in biological sciences, and they are also of interest thanks to this nitrile function [10,11,12]. The 2-cyanoindole unit is an example of structural motif building blocks and effective precursors for the synthesis of various indole-fused polycycles [13,14,15,16,17,18,19], substituted 2-cyanoindoles [20,21,22,23,24], addition to nitriles [25,26] and indole heterocycle substitution [27,28]. These compounds exhibit a wide range of biological activities (Figure 1). They are widely used in medicinal chemistry and pharmacological research as antagonist molecules. For example, adrenergic antagonist A [14] is a drug that inhibits the function of adrenergic receptors. There are also α-adrenoreceptors that are located on vascular smooth muscle. Antagonists reduce or block the signals of agonists. They can be drugs, which are added to the body for therapeutic reasons, or endogenous ligands. Analog D [27] of firefly luciferin is a compound of the class of luciferins, light-emitting chemical compounds. It is found in many species of fireflies (Lampyridae). It is the substrate of luciferase, an enzyme that catalyzes its oxidation into oxyluciferin with concomitant hydrolysis of a molecule of ATP into AMP and PPi accompanied by the emission of a photon of yellow light characteristic of these insects. NMDA receptor antagonists E [25] are a class of drugs that work to antagonize or inhibit the action of the N-Methyl-D-aspartate receptor (NMDAR). They are commonly used as anesthetics for animals and humans; the state of anesthesia they induce is referred to as dissociative anesthesia. The dopamine D4 receptor (D4R) F [20] plays important roles in cognition, attention and decision making. Novel D4R-selective ligands have promise in medication development for neuropsychiatric conditions, including Alzheimer’s disease and substance use disorders. Prostaglandin E2 (PGE2) modulator G [21], subtype (EP2), which is a metabolite of arachidonic acid that binds with and regulates cellular responses to PGE2, is associated with numerous physiological and pathological events in a wide range of tissues. As a stimulatory G protein-coupled receptor, PGE2-induced EP2 activation can activate adenylate cyclase, leading to increased cytoplasmic cAMP levels and activation of protein kinase A. Finally, compound H [22] is considered as an antiarrhythmic agent. Additionally, the cyano group is a valuable and readily available functional group for the preparation of various functional groups such as amines, amides, esters, ketones and their carboxyl derivatives [29].

Figure 1.
Biologically relevant compounds, featuring 2-cyanoindole cores.
Due to their importance, the development of efficient methodologies for the preparation and functionalization of various cyanoindoles has been the subject of intense research efforts. Direct incorporation of the nitrile function to substituted indoles has been accomplished through a variety of methods. These methods involved various sources of a cyano group including: acetonitrile [30], tert-butylisocyanide [31], nitromethane [32], benzyl cyanide [33], Beller’s NCTS (N-cyano-N-phenyl-p-toluenesulfonamide) [34] or Zn(CN)2 [35,36].
Palladium-catalyzed cross-coupling reactions are among the most successful transformations in organic synthesis. Thanks to all research work carried out over the years, a large variety of C–C and C–X bond formations and numerous highly active catalytic combinations are currently available [37,38,39,40,41]. The broad interest of this cross-coupling methodology is thus found in many fields of application [42,43]. Driven by our interest in the preparation of substituted 2-cyanoindoles and in conjunction with our successful previous research on palladium cross-coupling reactions, we explored the reactivity of 3-iodo-indole-2-carbonitrile of the residual iodine. This approach allowed the preparation of novel substituted 2-cyanoindoles in position 3 (Figure 1).
The aim of this work was to synthesize new 1H-indole-2-carbonitrile derivatives, which could also be useful for drug design. Moreover, the substitution in this type of product in position 3 is important for the development of new molecules with biological interests. The reactivity of iodine in this position was studied using some cross-coupling reactions such as Sonogashira, Suzuki–Miyaura, Stille and Heck reactions, which provided a wide variety of molecules. We report the preparation of 3-iodo-2-carbonitrile derivatives as precursors through the use of the C–I bond in coupling reactions to access a molecular diversity (Figure 1).
2. Results and Discussion
2.1. Synthesis of 1H-Indole-2-carbonitriles
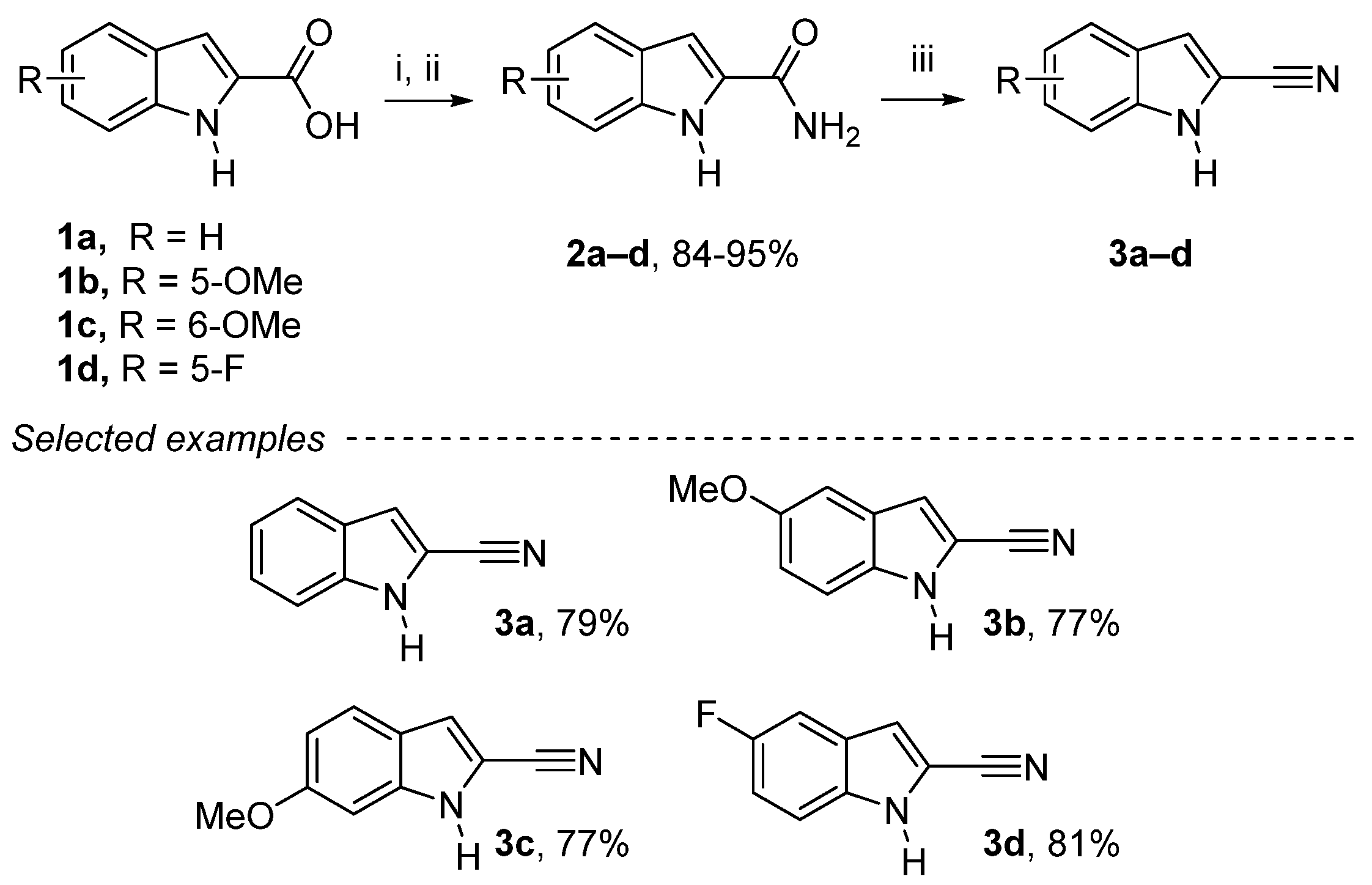
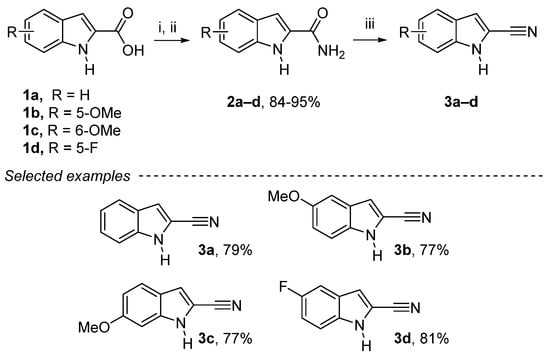
We began our studies with the reaction of thionyl chloride of 1a–d with a catalytic amount of DMF, which gives access to acyl chloride that reacts with a solution of 25% ammonia to give 1H-indole-2-carboxamide derivatives 2a–d in good yields (Scheme 1). The reaction with phosphorus(V) oxychloride on the carboxamide afforded the corresponding 1H-indole-2-carbonitriles derivatives 3a–d in good yields (65–85%), following a modified procedure (see Experimental Section) [28].
Scheme 1.
Reagents and conditions: (i) SOCl2 (1.2 equiv.), DMF cat., dry CHCl3, reflux, 24 h; (ii) NH3 25%, room temperature (rt), 2 h; (iii) POCl3 (4 equiv.), dry CHCl3, reflux, 3 h.
2.2. Synthesis of 1-(3-Phenylprop-2-yn-1-yl)-1H-indole-2-carbonitrile Derivatives
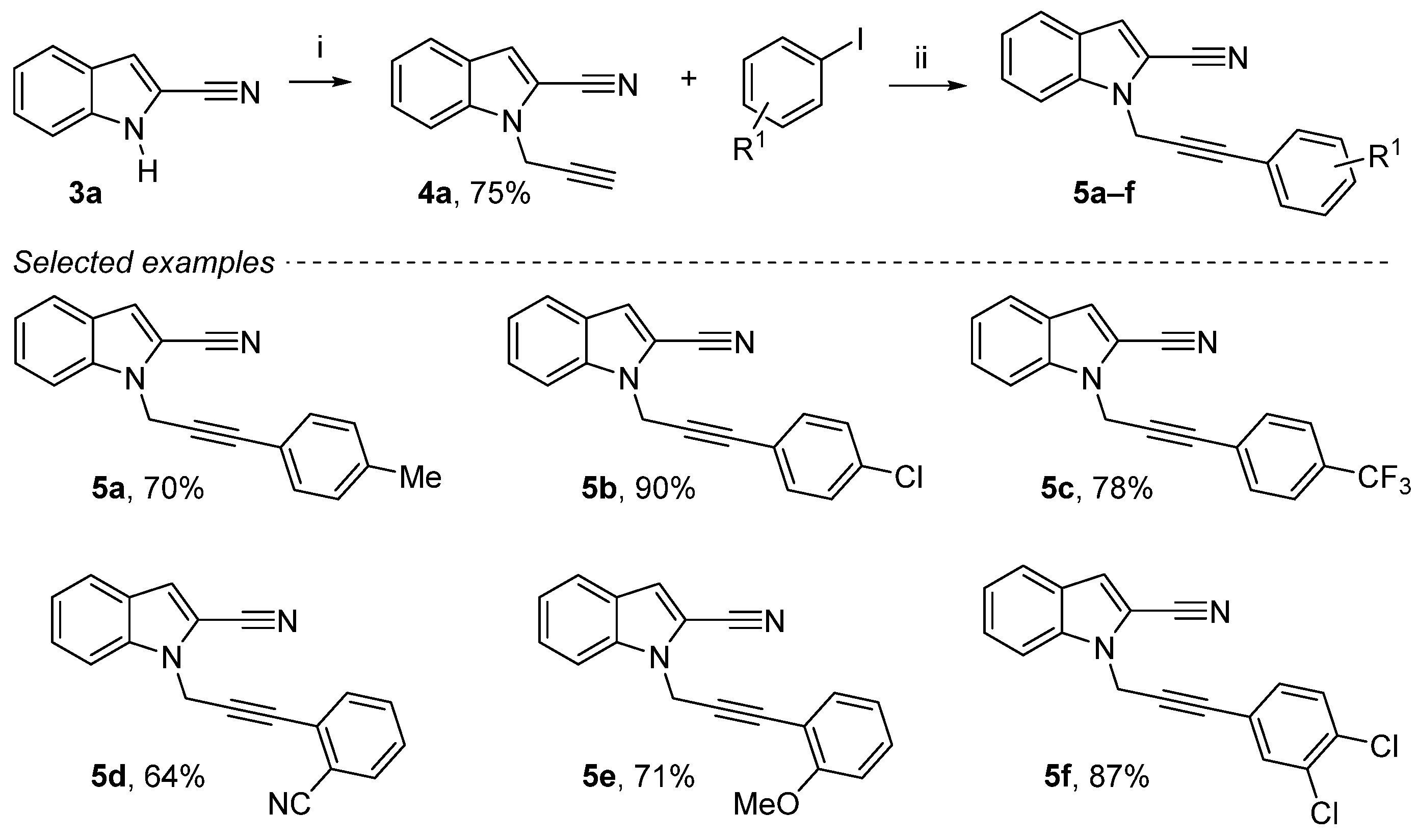
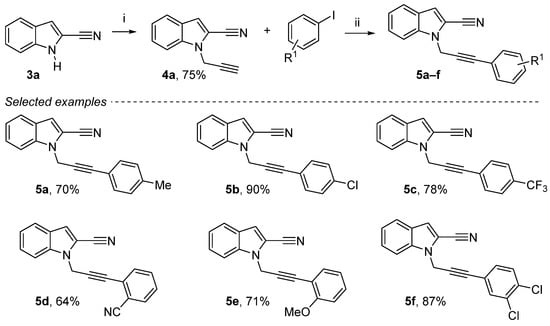
Propargylation reaction (NaH then propargyl bromide in DMF) provides the desired 1-(prop-2-yn-1-yl)-1H-indole-2-carbonitrile 4a in a yield of 75% [10,44]. An efficient method for palladium-catalyzed homocoupling reaction of terminal alkyne in the synthesis of 1H-indole-2-carbonitrile derivatives was carried out (Scheme 2). In the presence of 10 mol % of PdCl2(PPh3)2, 10 mol % of CuI and 3 equiv. of Et3N, homocoupling of various aromatic iodines gave the corresponding dissymmetric alkynes 1-(3-phenylprop-2-yn-1-yl)-1H-indole-2-carbonitrile derivatives 5a–f in moderate to good yields (64–90%) [16]. This Sonogashira cross-coupling reaction was run with various substitutions using electron donor groups and electron-withdrawing groups on the aromatic ring, either in ortho, meta or para.
Scheme 2.
Reagents and conditions: (i) NaH (1.3 equiv.), DMF, 0 °C, 15 min, propargyl bromide 80 wt. % in toluene (1.3 equiv.), 0 °C to rt, 4 h; (ii) 4a (1 equiv.), aryl iodide (1.3 equiv.), Et3N (3 equiv.), CuI (10 mol%), PdCl2(PPh3)2 (10 mol%), DMF, rt, overnight.
2.3. Synthesis of 1-Benzyl-3-iodo-1H-indole-2-carbonitrile Derivatives
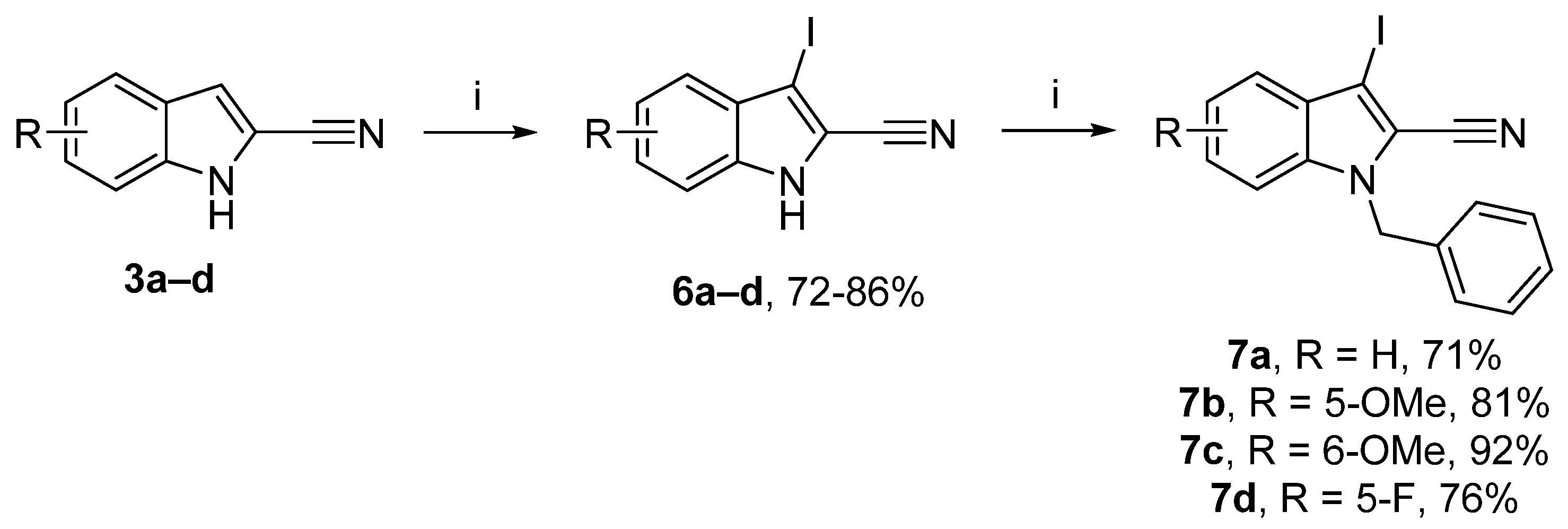
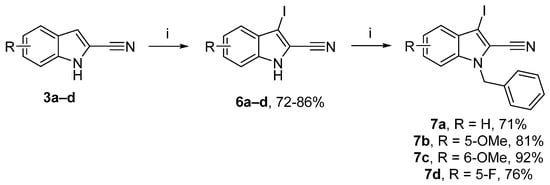
A new series of 2-cyanoindole was also synthesized with an alkynyl substituent in position 3. First, aromatic electrophilic substitution in position 3 by iodine with potassium hydroxide was undertaken (Scheme 3) [45]. The resulting iodine derivatives 6a–d were obtained in yields above 80%. Finally, a protection reaction was carried out with benzyl bromide to give the corresponding 1-benzyl-3-iodo-1H-indole-2-carbonitrile derivatives 7a–d in good yields (71–92%) [46].
Scheme 3.
Reagents and conditions: (i) 3a–d (1 equiv.), KOH (3.6 equiv.), DMF, rt, 30 min, then I2 (1 equiv.), 0 °C to rt, 4 h; (ii) NaH (1.2 equiv.), DMF, 0 °C, 30 min, benzyl bromide (1.3 equiv.), 0 °C to rt.
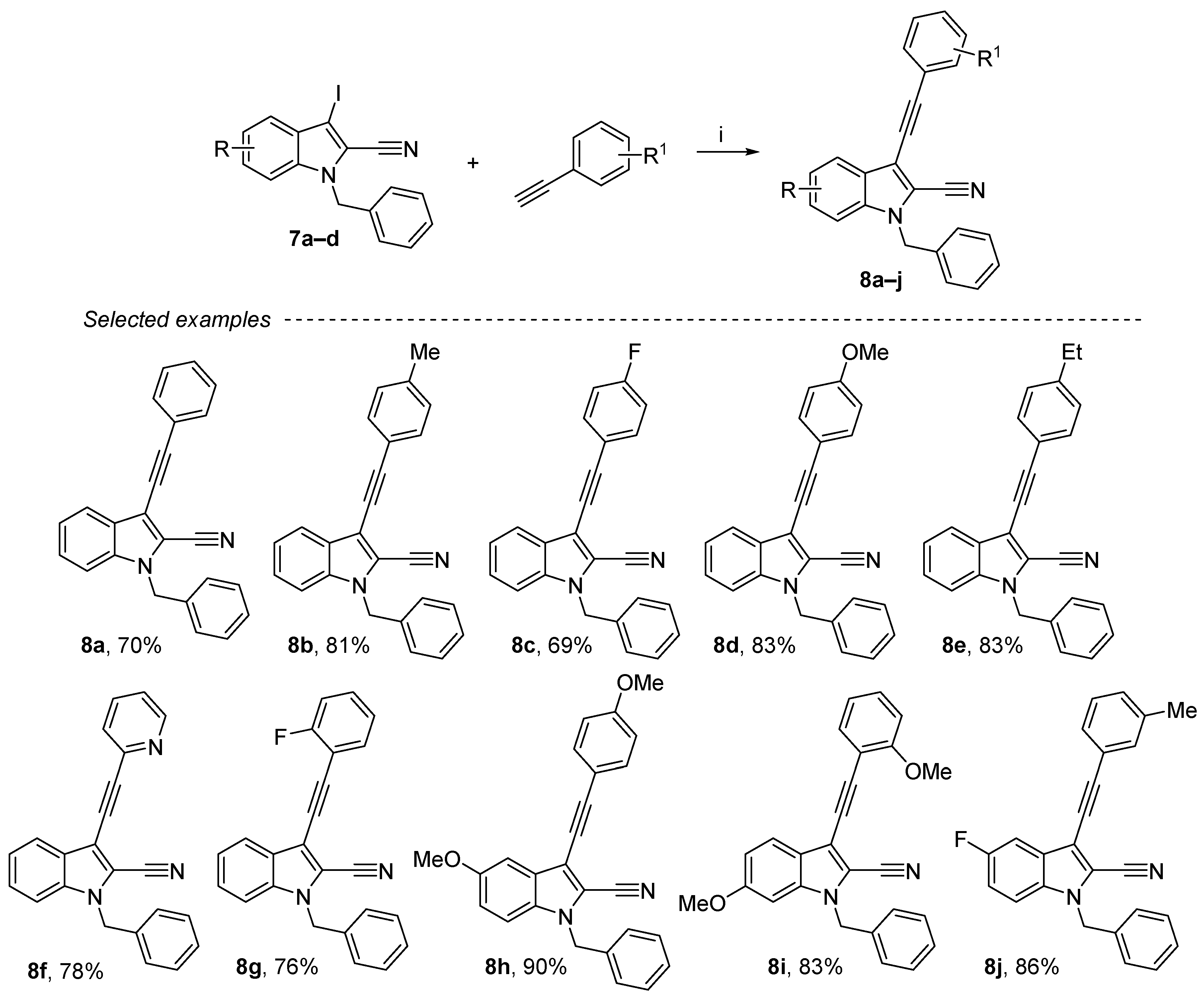
2.4. Sonogashira Reaction on the 1-Benzyl-3-iodo-1H-indole-2-carbonitrile Derivatives
Sonogashira cross-coupling was performed using 7a–d as substrates (Scheme 4) [47,48]. The alkynyl substituent in position 3 has been formed with phenylacetylene derivatives, 10 mol% of palladium (II) and 10 mol% of copper iodide. The reaction of 1-benzyl-3-iodo-1H-indole-2-carbonitrile with a variety of aromatic alkynes containing both electron-donating and electron-withdrawing substituents was also examined. Thus, compounds 8a–j were obtained in moderate to good yields (69–90%), and this reaction was carried out with various substituents, either in ortho (F, OMe), meta (Me) or para (Et, F, OMe) and also with pyridine derivatives. These products were purified either by crystallization or by chromatography on silica gel.
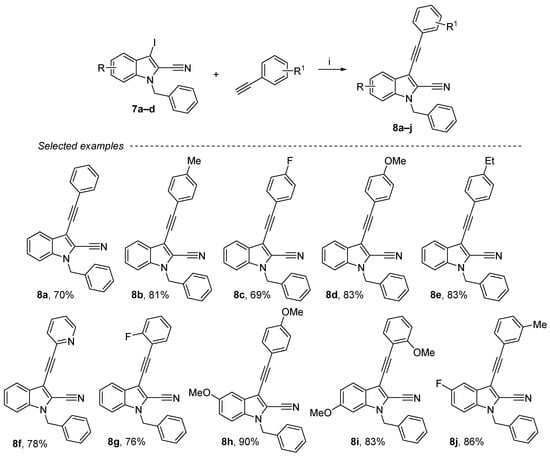
Scheme 4.
Reagents and conditions: (i) 4a (1 equiv.), aryl iodide derivative (1.3 equiv.), Et3N (3 equiv.), CuI (10 mol%), PdCl2(PPh3)2 (10 mol%), DMF, rt, overnight.
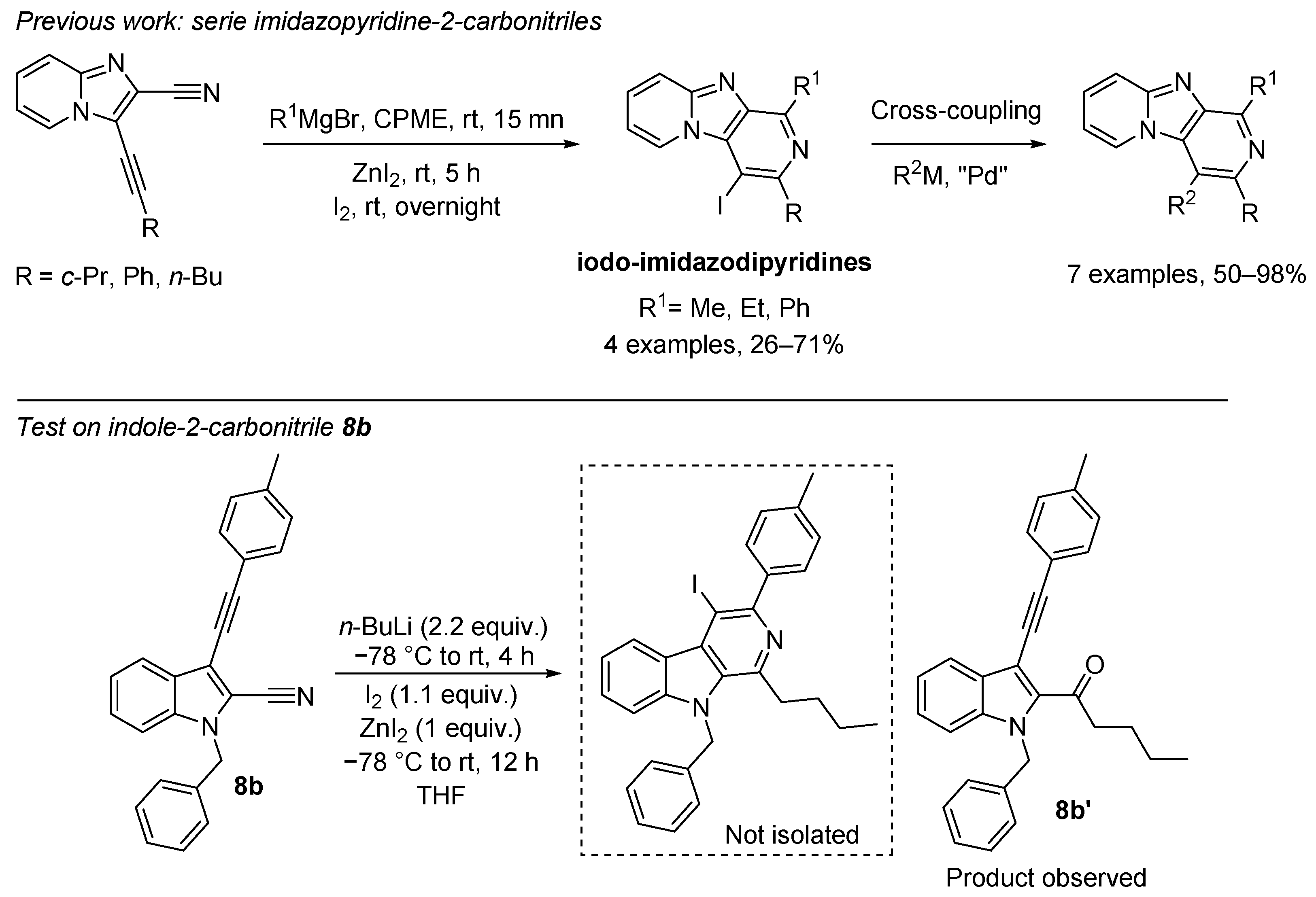
Previously, our group reported on the access to iodo-imidazodipyridines from imidazopyridine-2-carbonitriles promoted by Grignard reagent in the presence of iodine and ZnI2 through a 6-endo-dig cyclization [49]. We wished to extend these results to the indole core. Some additional tests of magnesium ethyl bromide on compound 8b either in diethyl ether or in cyclopentylmethylether (CPME) were made. Changing reaction times and temperatures were carried out. The 6-endo-dig cyclization product was not isolated, and the addition of Grignard reagent on the cyano group provided the corresponding ketone in a very low yield (4%). However, a test with n-BuLi as the organometallic agent yielded the corresponding ketone 8b’ with a full conversion rate, and no cyclization product was observed (Scheme 5). Further efforts to studies on the mechanism and synthetic applications for this type of cyclization are underway in our laboratory.
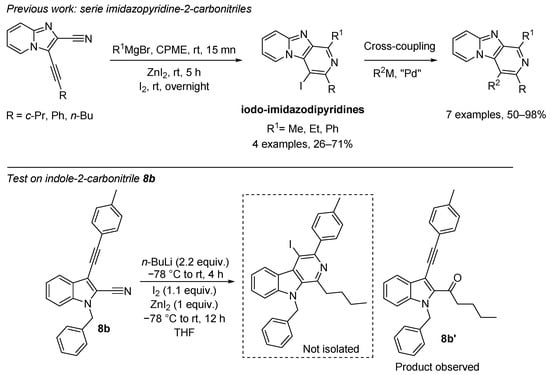
Scheme 5.
Addition of n-BuLi on compound 8b: access to ketone 8b’.
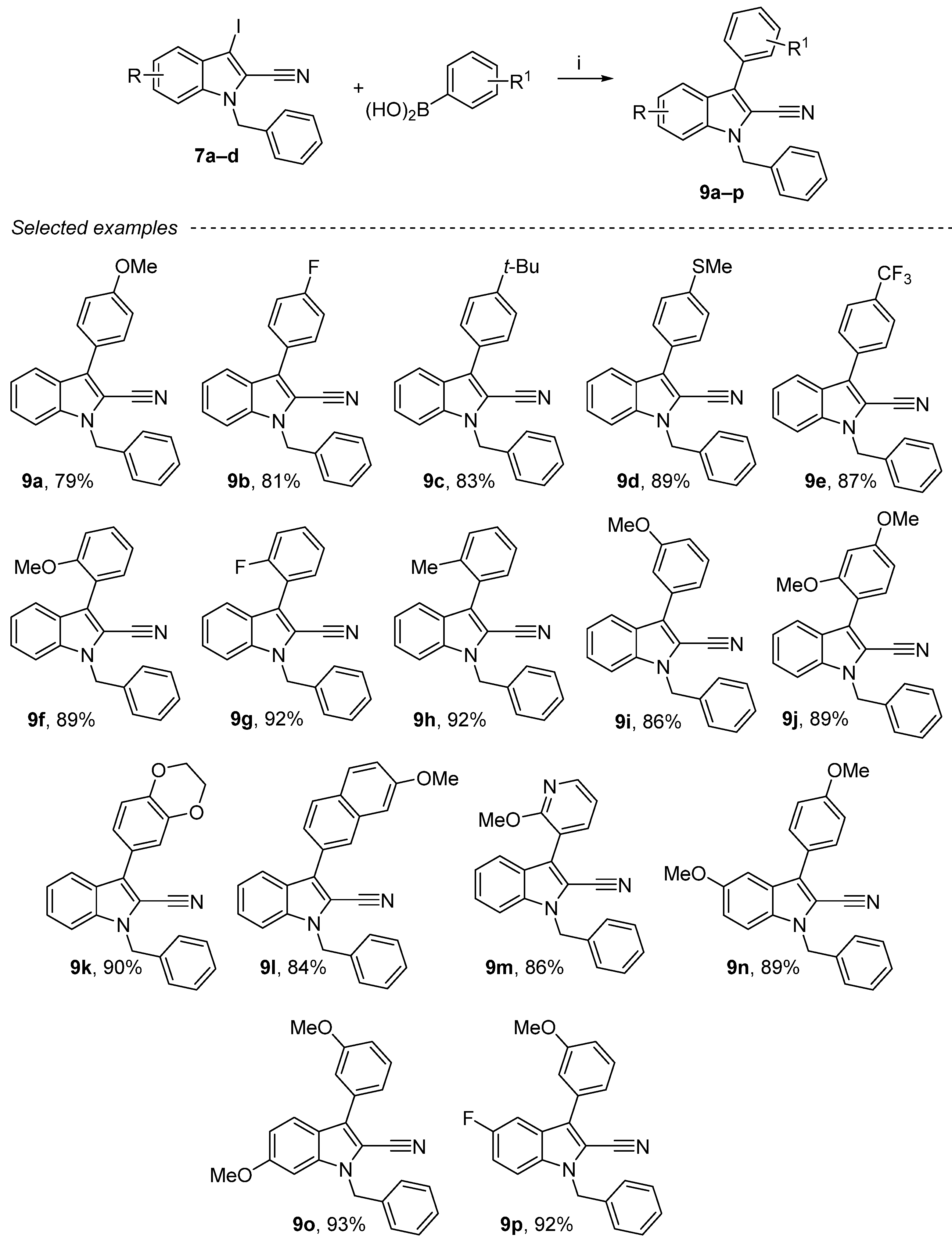
2.5. Suzuki Reaction on the 1-Benzyl-3-iodo-1H-indole-2-carbonitrile Derivatives
Suzuki cross-coupling is one of the most efficient methods for the construction of C–C bonds. Although several other methods are available for this purpose, the Suzuki cross-coupling reaction, which produces biaryls, has proven to be the most popular in recent times. The key advantages of this coupling are the commercial availability of diverse boronic acids that are environmentally safer than other organometallic reagents. Another cross-coupling on nitrile compounds was also performed. The Suzuki reaction was carried out herein with a slight excess of boronic acid using NaHCO3 as base and tetrakistriphenylphosphine (10 mol%) as a catalyst in toluene/water mixture as a solvent (Scheme 6) [50,51,52]. Several compounds were synthesized with different ortho-, meta- or para-aryls substituted by electron donor groups (Me, Et, tBu, OMe) or electron-drawing groups (F, Cl) and with naphthalene derivative. Good yields (79–93%) were also obtained when substituted phenyl groups were used in this cross-coupling reaction (9a–p). These products were purified either by crystallization or by chromatography on silica gel.
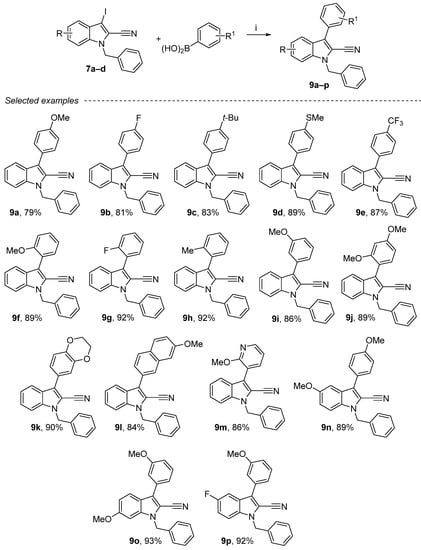
Scheme 6.
Reagents and conditions: (i) 7a–d (1 equiv.), boronic acids (1.2 equiv.), sat. aq. NaHCO3, EtOH/toluene = 3/2, Pd(PPh3)4 10 mol%, 130 °C, 4 h.
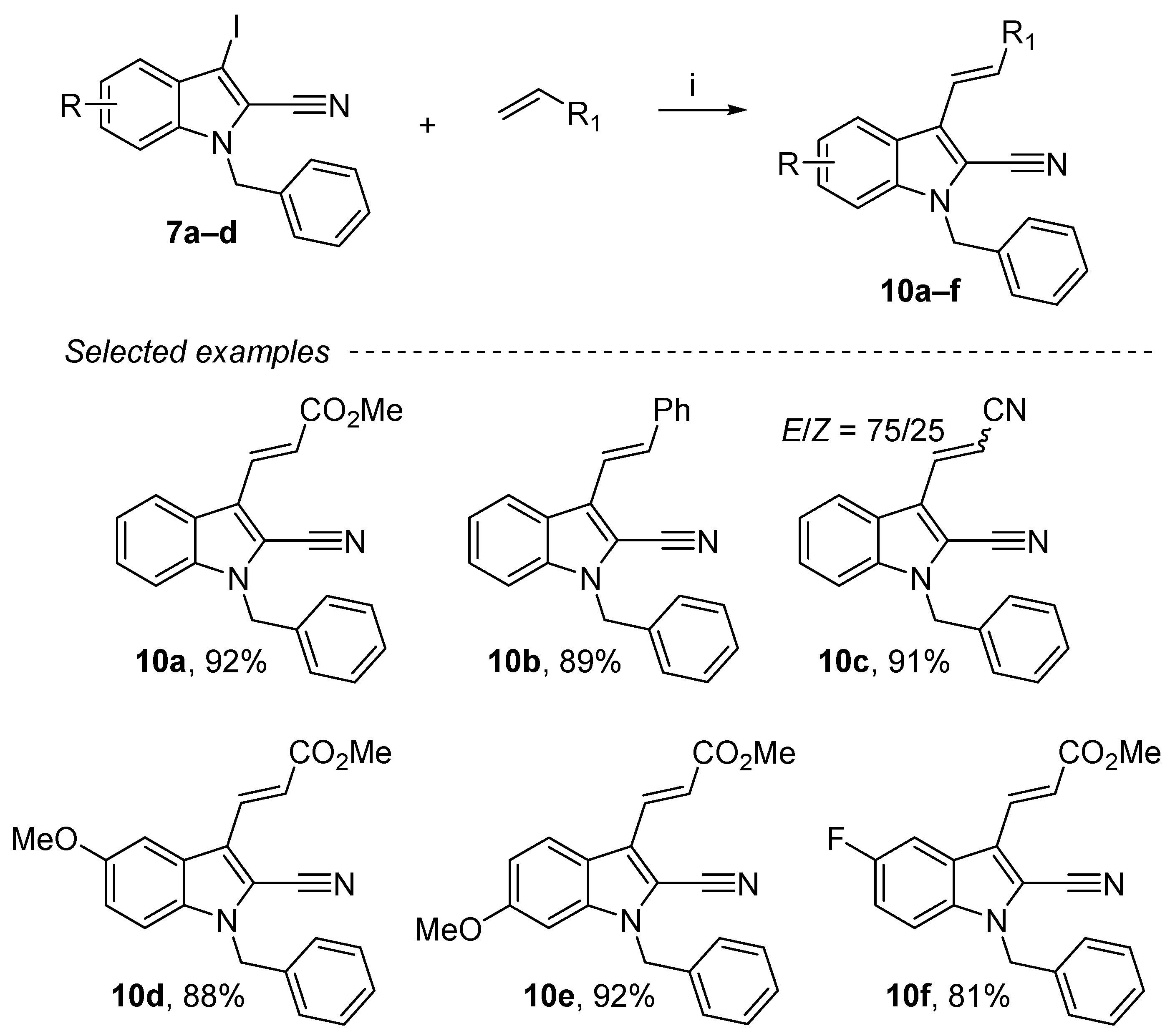
2.6. Heck Reaction on the 1-Benzyl-3-iodo-1H-indole-2-carbonitrile Derivatives
Among all palladium-catalyzed cross-couplings, the Mizoroki–Heck reaction allows a direct coupling between heteroatomic compounds and alkenes [53]. This reaction allowed the creation of a sigma bond between two sp2-hybridized carbon atoms through a C–H activation. Compared to other cross-coupling processes, this one presents practical and economic advantages such as using simple and readily available materials. Under conditions (DMF, 80 °C, 24 h), coupling of 1-benzyl-3-iodo-1H-indole-2-carbonitrile derivatives in the presence of KOAc (6 equiv.), n-Bu4NCl (2 equiv.), Pd(OAc)2 (4 mol%) with diverse olefins afforded a series of cyanoindoles substituted in position 3 (10a–f) in excellent yields (81–92%) (Scheme 7). The E-configuration products were mainly obtained except for the acrylonitrile, for which a mixture of two isomers (E/Z= 75/25) was observed.
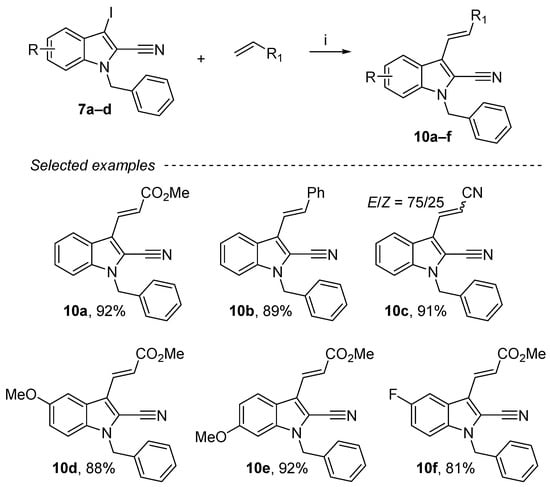
Scheme 7.
Reagents and conditions: (i) 7a–d (1 equiv.), alkenes (1.2 equiv.), KOAc (6 equiv.), n-Bu4NCl (2 equiv.), Pd(OAc)2 4 mol%, DMF, 80 °C, 24 h.
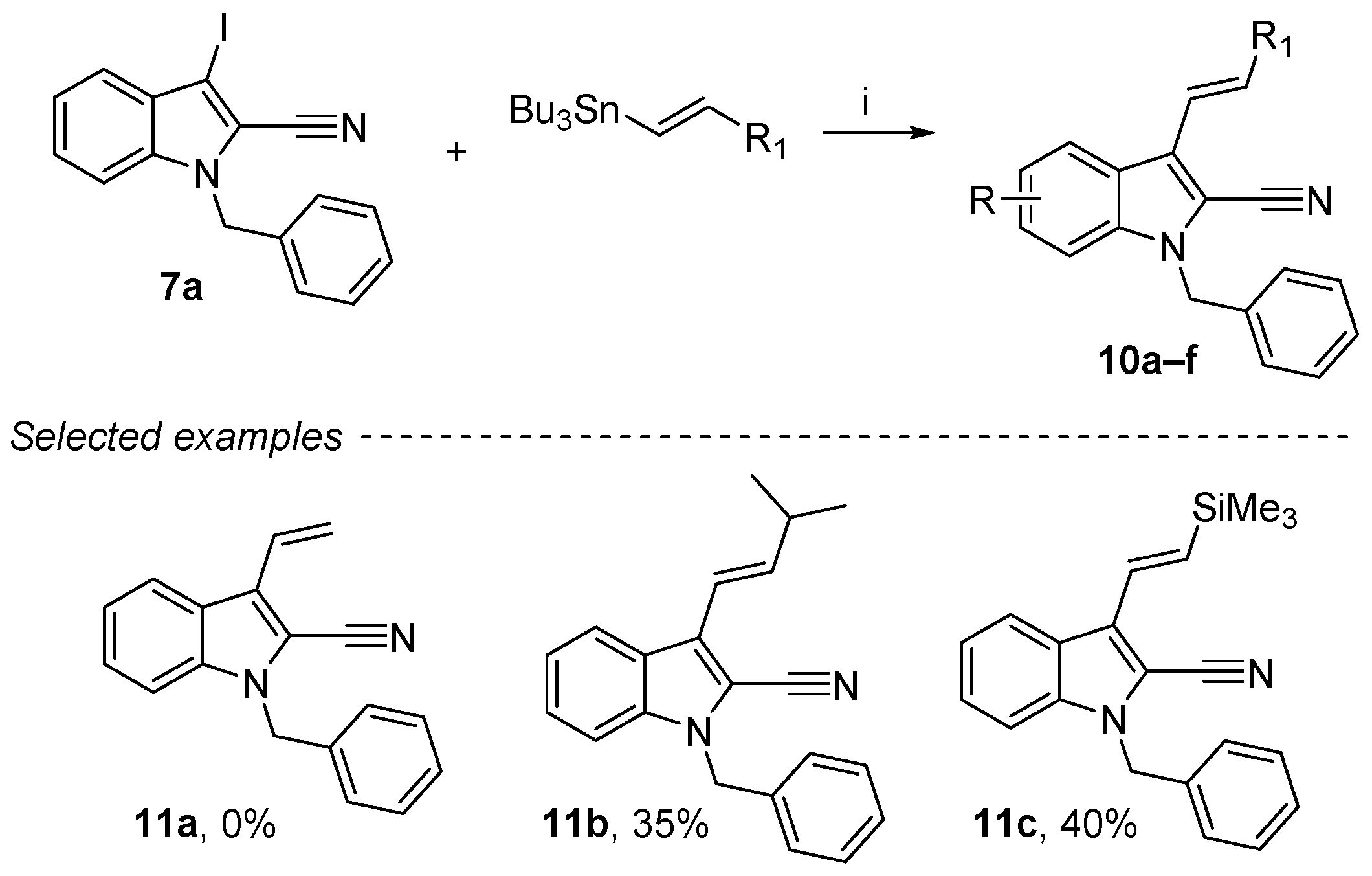
2.7. Stille Reaction on the 1-Benzyl-3-iodo-1H-indole-2-carbonitrile Derivatives
Stille coupling was performed using an organotin compound and a catalytic amount of dichlorobis(acetonitrile)palladium(II) in DMF at 40°C (Scheme 8) [54,55]. Under these conditions, the coupling with compound 7a was successfully achieved, and two examples of coupling products (11b and 11c) were obtained in low to moderate yields (35–40%). No coupling product with tributyl(vinyl)stannane could be obtained (11a, 0%). Finally, these compounds were purified by column chromatography with a stationary phase composed of 10% powdered anhydrous K2CO3 and silica to remove all traces of organotin impurities [56].
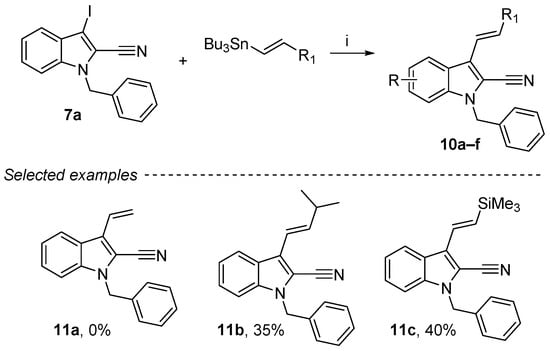
Scheme 8.
Reagents and conditions: (i) 7a (1 equiv.), vinyltin derivatives (1.2 equiv.), PdCl2MeCN2 (10 mol%), DMF, 40 °C, 48 h.
3. Materials and Methods
3.1. General Information
The reagents were purchased from commercial suppliers and used without further purification. Melting points were determined on Büchi B-540 apparatus and are uncorrected. All solvents were dried following the procedure described by Armarego et Chai [57]. 1H NMR and 13C NMR spectra (from supplementary) were recorded on a Bruker Avance 300 MHz at 300 and 75 MHz, respectively. 1H NMR spectra were recorded in CDCl3 or referenced the residual CHCl3 at 7.26 ppm (2.50 ppm for DMSO-d6); 13C NMR and J-mod spectra were referenced to the central peak of CDCl3 at 77.0 ppm (39.52 ppm for DMSO-d6). 19F NMR was recorded at 282 MHz on the same instrument, using the CFCl3 as internal reference (δ 0.0). Chemical shifts were reported in parts per million (ppm, δ), and coupling constants (J) were given in Hertz (Hz). Abbreviations for signal coupling are as follows: s, singlet; d, doublet; t, triplet; q, quartet; quin, quintet; sextuplet; sext, dd, doublet of doublets; dq, doublet of quartets; m, multiplet. High-resolution mass spectra (HRMS) were obtained by the electrospray ionization time-of-flight (ESI) mass spectrometry. Thin-layer chromatography (TLC) was performed on TLC silica gel 60 F254. Compounds were visualized under UV light (λ = 254 nm) and/or by immersion in a KMnO4 solution followed by heating. Products were purified by flash column chromatography on silica gel (0.04–0.063 mm) using various mixtures of EtOAc and petroleum ether (35–60 °C fraction) as eluent. Heating was performed using a magnetic stirrer hotplate and an appropriate-sized heating block. Tributyl(vinyl)stannane was prepared from vinylmagnesium bromide and bis(tributyltin) oxide [58,59]. (E)-1-(Tributylstannyl)-2-(trimethylsilyl)ethene was prepared by hydrostannation of (trimethylsilyl)acetylene [60]. (E)-Tributyl(3-methylbut-1-en-1-yl)stannane was prepared by the method described by Chong [61]. The compound’s name follows the IUPAC recommendations.
3.2. Experimental Section
3.2.1. General Procedure for the Synthesis of Nitriles (Series 3)
To a solution of amide (4.0 g, 25.0 mmol, 1.0 equiv.) in chloroform (75 mL) was added dropwise phosphorus oxychloride (9.24 mL, 99.1 mmol, 4.0 equiv.). The mixture was stirred under reflux for 3 h and then cooled to room temperature. The reaction mixture was quenched by a 25% NH4OH aq. Solution (20 mL) and the resulting aqueous layer were extracted with Et2O (3 × 30 mL). The organic layers were combined, dried over MgSO4 and concentrated under reduced pressure. The crude product was purified by column chromatography using petroleum ether/ethyl acetate (80:20) as eluent.
1H-Indole-2-carbonitrile (3a): Yellow solid, 79%, 2.83 g. 1H NMR (300 MHz, CDCl3): δ = 8.66 (bs, 1H, NH), 7.68 (dq, J = 8.1 Hz, 0.9 Hz, 1H, Ar-H), 7.45–7.36 (m, 2H, Ar-H), 7.25–7.19 (m, 2H, Ar-H) ppm. 13C NMR (75 MHz, CDCl3): δ = 137.1 (Cquat), 126.3 (Ar-CH), 126.2 (Cquat), 122.1 (Ar-CH), 121.7 (Ar-CH), 114.6 (Cquat), 114.5 (Ar-CH), 112.0 (Ar-CH), 106.0 (Cquat) ppm. These spectroscopic data correspond to the reported data in reference [28].
5-Methoxy-1H-indole-2-carbonitrile (3b): Yellow solid, 77%, 2.85 g. 1H NMR (300 MHz, CDCl3): δ = 8.57 (bs, 1H, NH), 7.31 (dd, J = 9.7 Hz, J = 0.9 Hz, 1H, Ar-H), 7.12 (dd, J = 2.1 Hz, J = 0.9 Hz, 1H, Ar-H), 7.07 (d, J = 2.4 Hz, 1H, Ar-H), 7.06–7.03 (m, 1H, Ar-H), 6.83 (d, J = 1.3 Hz, 1H, Ar-H), 3.85 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 155.5 (Cquat), 132.3 (Cquat), 126.9 (Cquat), 118.1 (Ar-CH), 114.4 (Cquat), 114.0 (Ar-CH), 112.7 (Ar-CH), 106.6 (Cquat), 102.1 (Ar-CH), 55.8 (OCH3) ppm. These spectroscopic data correspond to the reported data in reference [45].
6-Methoxy-1H-indole-2-carbonitrile (3c): Yellow solid, 77%, 2.85 g. 1H NMR (300 MHz, CDCl3): δ = 8.71 (bs, 1H, NH), 7.52 (d, J = 8.8 Hz, 1H, Ar-H), 7.13 (d, J = 1.3 Hz, 1H, Ar-H), 6.88 (dd, J = 8,8 Hz, 2.1 Hz, 1H, Ar-H), 6.83 (d, J = 1.3 Hz, 1H, Ar-H), 3.86 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 159.7 (Cquat), 138.2 (Cquat), 122.9 (Ar-CH), 120.6 (Cquat), 114.9 (Ar-CH), 114.8 (Cquat), 113.4 (Ar-CH), 104.8 (Cquat), 93.8 (CH), 55.7 (OCH3) ppm. These spectroscopic data correspond to the reported data in reference [27].
5-Fluoro-1H-indole-2-carbonitrile (3d): Yellow solid, 81%, 2.90 g. 1H NMR (300 MHz, CDCl3): δ = 8.64 (bs, 1H, NH), 7.37 (dd, J = 9.0 Hz, 4.3 Hz, 1H, Ar-H), 7.32 (dd, J = 8.9 Hz, 2.5 Hz, 1H, Ar-H), 7.20–7.12 (m, 2H, Ar-CH) ppm. 13C NMR (75 MHz, CDCl3): δ = 158.7 (d, J = 238.6 Hz, Cquat), 133.6 (Cquat), 126.6 (d, J = 10.6 Hz, Cquat), 115.7 (d, J = 27.1 Hz, Ar-CH), 114.3 (d, J = 5.4 Hz, Ar-CH), 114.1 (Cquat), 113.0 (d, J = 9.5 Hz, Ar-CH), 107.7 (Cquat), 106.5 (d, J = 23.8 Hz, Ar-CH) ppm. 19F NMR (282 MHz, CDCl3): δ = −119.9 ppm. These spectroscopic data correspond to the reported data in reference [45].
3.2.2. Typical Procedure for the Synthesis of Propargyl Compound 4
In a two-neck round bottom flask, NaH 60% (0.60 g,15.0 mmol, 1.3 equiv.) was dissolved in DMF (15 mL), and indole (7.03 mmol, 1 equiv.) in DMF (10 mL) was added dropwise at 0 °C under argon. The mixture was stirred for 30 min at room temperature. Propargyl bromide 80% (11.4 mmol, 1.3 equiv.) was diluted in DMF and added dropwise into the flask at 0 °C under argon. After 3–4 h of stirring at room temperature, the mixture was hydrolyzed with sat. aq. NH4Cl (10 mL), extracted with Et2O (7 × 20 mL), and the combined organic layers were washed with brine (6 × 10 mL), dried over MgSO4 and evaporated under reduced pressure.
1-(Prop-2-yn-1-yl)-1H-indole-2-carbonitrile (4a): Yellow solid, 75%, 0.750 g. 1H NMR (300 MHz, CDCl3): δ = 7.69 (dt, J = 8.1 Hz, 1H, Ar-H), 7.51 (dd, J = 8.4 Hz, J = 0.9 Hz, 1H, Ar-H), 7.48–7.41 (m, 1H, Ar-H), 7.29–7.19 (m, 2H, Ar-H), 5.04 (s, 2H, CH2), 2.40 (t, J = 2.5 Hz, 1H, CH) ppm. 13C NMR (75 MHz, CDCl3): δ = 137.3 (Cquat), 126.6 (Cquat), 126.4 (Ar-CH), 122.7 (Ar-CH), 122.0 (Ar-CH), 114.2 (Ar-CH), 113.2 (Cquat), 110.7 (Ar-CH), 109.6 (Cquat), 76.5 (Cquat), 74.2 (CH), 34.4 (CH2) ppm. These spectroscopic data correspond to the reported data in reference [10].
3.2.3. General Procedure for the Synthesis of Nitriles (Series 5)
A dried Schlenk tube was charged with compound 4a (1.6 mmol, 1 equiv.), aryl iodide derivative (2.1 mmol, 1.3 equiv.) and DMF (5 mL). The solution was cooled to 0 °C for 15 min under argon ant Et3N (4.8 mmol, 3 equiv.) was added into the Schlenk. CuI (0.16 mmol, 10 mol%) and PdCl2(PPh3)2 (0.16 mmol, 10 mol%) were added, and the mixture was stirred overnight at room temperature. The mixture was hydrolyzed with sat. aq. NH4Cl (10 mL) and extracted with Et2O (7 × 20 mL). The organic layers were washed with brine (2 × 10 mL), dried over MgSO4 and evaporated under reduced pressure.
1-(3-(p-Tolyl)prop-2-yn-1-yl)-1H-indole-2-carbonitrile (5a): Yellow solid, 70%, 252 mg. mp 96–98 °C. 1H NMR (300 MHz, CDCl3): δ = 7.69 (d, J = 8.1 Hz, 1H, Ar-H), 7.59 (dd, J = 8.5 Hz, 8.0 Hz, 1H, Ar-H), 7.45 (ddd, J = 8.4 Hz, J = 7.0 Hz, J = 1.1 Hz, 1H, Ar-H), 7.29 (d, J = 8.1 Hz, 2H), 7.27–7.21 (m, 2H), 7.09 (d, J = 7.9 Hz, 2H), 5.25 (s, 2H, CH2), 2.32 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 139.1 (Cquat), 137.3 (Cquat), 131.8 (2Ar-CH), 129.1 (2Ar-CH), 126.5 (Cquat), 126.2 (Ar-CH), 122.5 (Ar-CH), 121.8 (Ar-CH), 118.8 (Cquat), 113.9 (Ar-CH), 113.4 (Cquat), 110.8 (Ar-CH), 109.5 (Cquat), 85.9 (Cquat), 81.2 (Cquat), 35.7 (CH2), 21.5 (CH3) ppm. HRMS (ESI): calcd. for C19H15N2 (M + H)+ 271.12352; found 271.12347.
1-(3-(4-Chlorophenyl)prop-2-yn-1-yl)-1H-indole-2-carbonitrile (5b): Yellow solid, 90%, 353 mg). mp 98–100 °C. 1H NMR (300 MHz, CDCl3): δ = 7.69 (d, J = 8.1 Hz, 1H, Ar-H), 7.56 (d, J = 8.4 Hz, 1H, Ar-H), 7.46 (ddd, J = 8.1 Hz, J = 7.0 Hz, J = 1.0 Hz, 1H, Ar-H), 7.33 (d, J = 8.6 Hz, 2H, Ar-H), 7.30–7.22 (m, 4H, Ar-H), 5.25 (s, 2H, CH2) ppm. 13C NMR (75 MHz, CDCl3): δ = 137.3 (Cquat), 135.1 (Cquat), 133.2 (2Ar-CH), 128.8 (2Ar-CH), 126.6 (Cquat), 126.4 (Ar-CH), 122.7 (Ar-CH), 122.0 (Ar-CH), 120.4 (Cquat), 114.2 (Ar-CH), 113.3 (Cquat), 110.7 (Ar-CH), 109.6 (Cquat), 84.7 (Cquat), 82.9 (Cquat), 35.6 (CH2) ppm. HRMS (ESI): calcd. for C18H12ClN2 (M + H)+ 291.06890; found 291.06901.
1-(3-(4-(Trifluoromethyl)phenyl)prop-2-yn-1-yl)-1H-indole-2-carbonitrile (5c): Yellow solid, 78%, 341 mg. mp 110–112 °C. 1H NMR (300 MHz, CDCl3): δ = 7.71 (d, J = 8.1 Hz, 1H, Ar-H), 7.60–7.53 (m, 3H, Ar-H), 7.53–7.42 (m, 3H, Ar-H), 7.29 (m, 2H), 7.29 (dd, J = 7.1 Hz, J = 0.9 Hz, 1H, Ar-H), 7.24 (s, 1H, Ar-H), 5.28 (s, 2H, CH2) ppm. 13C NMR (75 MHz, CDCl3): δ = 137.4 (Cquat), 132.3 (2Ar-CH), 130.7 (q, J = 32.5 Hz, Cquat), 126.6 (Cquat), 125.7 (q, J = 1.3 Hz, Cquat), 125.4 (q, J = 3.7 Hz, 2Ar-CH), 123.9 (q, J = 272.8 Hz, Cquat), 122.7 (Ar-CH), 122.1 (Ar-CH), 114.3 (Ar-CH), 113.3 (Cquat), 110.6 (Ar-CH), 109.6 (Cquat), 84.4 (Cquat), 84.3 (Cquat), 35.6 (CH2) ppm. HRMS (ESI): calcd. for C19H12F3N2 (M + H)+ 325.09471; found 325.09395.
1-(3-(2-Cyanophenyl)prop-2-yn-1-yl)-1H-indole-2-carbonitrile (5d): Yellow solid, 64%, 379 mg. mp 137–139 °C. 1H NMR (300 MHz, CDCl3): δ = 7.71–7.62 (m, 2H, Ar-H), 7.54–7.47 (m, 3H, Ar-H), 7.46–7.39 (m, 2H, Ar-H), 7.29–7.22 (m, 2H, Ar-H), 5.34 (s, 2H, CH2) ppm. 13C NMR (75 MHz, CDCl3): δ = 137.4 (Cquat), 132.9 (Ar-CH), 132.7 (Ar-CH), 132.5 (Ar-CH), 129.2 (Ar-CH), 126.63 (Ar-CH), 126.6 (Cquat), 125.8 (Cquat), 122.6 (Ar-CH), 122.1 (Ar-CH), 117.3 (Cquat), 115.7 (Cquat), 114.4 (Ar-CH), 113.3 (Cquat), 111.0 (Ar-CH), 109.5 (Cquat), 88.3 (Cquat), 81.9 (Cquat), 35.6 (CH2) ppm. HRMS (ESI): calcd. for C19H11N3 (M + H)+ 282.10257; found 282.10179.
1-(3-(2-Methoxyphenyl)prop-2-yn-1-yl)-1H-indole-2-carbonitrile (5e): Yellow solid, 71%, 269 mg. mp 96–98 °C. 1H NMR (300 MHz, CDCl3): δ = 7.70–7.64 (m, 2H, Ar-H), 7.45 (ddd, J = 8.5 Hz, J = 7.1 Hz, J = 1.1 Hz, 1H, Ar-H), 7.35 (dd, J = 7.5 Hz, J = 1.6 Hz, 1H, Ar-H), 7.32–7.19 (m, 3H, 3Ar-H), 6.87 (td, J = 7.5 Hz, J = 0.9 Hz, 1H, Ar-H), 6.84 (d, J = 8.4 Hz, Ar-H), 5.29 (s, 2H, CH2), 3.84 (s, OCH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 160.4 (Cquat), 137.4 (Cquat), 133.9 (Ar-CH), 130.4 (Ar-CH), 126.6 (Cquat), 126.1 (Ar-CH), 122.5 (Ar-CH), 121.8 (Ar-CH), 120.5 (Ar-CH), 113.9 (Ar-CH), 113.4 (Cquat), 111.2 (2Ar-CH + Cquat), 110.8 (Ar-CH), 109.6 (Cquat), 85.8 (Cquat), 82.4 (Cquat), 55.8 (OCH3), 36.1 (CH2) ppm. HRMS (ESI): calcd. for C19H15N2O (M + H)+ 287.11844; found 287.11835.
1-(3-(3,4-Dichlorophenyl)prop-2-yn-1-yl)-1H-indole-2-carbonitrile (5f): Yellow solid, 87%, 381 mg. mp 115–117 °C. 1H NMR (300 MHz, CDCl3): δ = 7.70 (d, J = 8.1 Hz, 1H, Ar-H), 7.54 (d, J = 8.4 Hz, 1H, Ar-H), 7.50–7.44 (m, 2H, Ar-H), 7.36 (d, J = 8.3 Hz, 1H, Ar-H), 7.27 (dd, J = 7.0 Hz, J = 1.0 Hz, 1H, Ar-H), 7.23 (s, 1H, Ar-H), 7.20 (dd, J = 8.4 Hz, J = 1.9 Hz, 1H, Ar-H), 5.25 (s, 2H, CH2) ppm. 13C NMR (75 MHz, CDCl3): δ = 137.3 (Cquat), 133.5 (Ar-CH), 132.7 (Cquat), 131.1 (Ar-CH), 130.5 (Ar-CH), 126.6 (Cquat), 126.5 (Ar-CH), 122.7 (Ar-CH), 122.1 (Ar-CH), 121.8 (Cquat), 114.3 (Ar-CH), 113.3 (Cquat), 111.8 (Cquat), 110.5 (Ar-CH), 109.6 (Cquat), 83.9 (Cquat), 83.5 (Cquat), 35.5 (CH2) ppm. HRMS (ESI): calcd. for C18H1035Cl2N2 (M + H)+ 325.02938; found 325.02841.
3.2.4. General Procedure for the Synthesis of Nitriles (Series 6)
To a solution of 1H-indole-2-carbonitrile (2.0 g, 14.1 mmol, 1.0 equiv.) in DMF (10 mL), KOH (0.79 g, 50.3 mmol, 3.6 equiv.) was added in small portions. The mixture was stirred for 30 min at room temperature. Then, a solution of iodine (3.57 g, 14.1 mmol, 1.0 equiv.) in DMF (3 mL) was added dropwise at 0 °C, and the mixture was stirred for 4 h at room temperature. The mixture was then poured into a mixture of water (600 mL) and sat. aq. NH4Cl (40 mL) and stirred for 30 min. The precipitate was filtered on a Büchner funnel and dried under vacuum for 2 h.
3-Iodo-1H-indole-2-carbonitrile (6a): White solid, 77%, 2.90 g. 1H NMR (300 MHz, CDCl3): δ = 8.98 (bs, 1H, NH), 7.49 (dq, J = 8.1 Hz, J = 0.9 Hz, 1H, Ar-H), 7.47–7.39 (m, 2H, Ar-H), 7.30 (ddd, J = 8.1 Hz, J = 6.2 Hz, J = 1.8 Hz, 1H, Ar-H) ppm. 13C NMR (75 MHz, DMSO-d6): δ = 137.0 (Cquat), 128.9 (Cquat), 126.6 (Ar-CH), 122.0 (Ar-CH), 121.8 (Ar-CH), 114.3 (Cquat), 112.8 (Ar-CH), 111.4 (Cquat), 72.0 (Cquat) ppm. These spectroscopic data correspond to the reported data in reference [46].
3-Iodo-5-methoxy-1H-indole-2-carbonitrile (6b): White solid, 78%, 2.70 g. 1H NMR (300 MHz, DMSO-d6): δ = 12.74 (bs, 1H, NH), 7.40 (dd, J = 9.0 Hz, J = 0.4 Hz, 1H, Ar-H), 7.05 (dd, J = 9.0 Hz, 2.5 Hz, 1H, Ar-H), 6.77 (d, J = 2.3 Hz, 1H, Ar-H), 3.82 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, DMSO-d6): δ = 155.4 (Cquat), 132.1 (Cquat), 129.3 (Cquat), 118.3 (Ar-CH), 114.4 (Cquat), 114.0 (Ar-CH), 111.2 (Cquat), 101.3 (Ar-CH), 70.8 (Cquat), 55.4 (OCH3) ppm. HRMS (ESI): calcd. for C10H8IN2O (M + H)+ 298.96813; found 298.96711.
3-Iodo-6-methoxy-1H-indole-2-carbonitrile (6c): White solid, 81%, 2.80 g. 1H NMR (300 MHz, CDCl3): δ = 9.09 (bs, 1H, NH), 7.31 (d, J = 9.2 Hz, 1H, Ar-H), 6.92 (dd, J = 9.2 Hz, 2.1 Hz, 1H, Ar-H), 6.80 (d, J = 2.1 Hz, 1H, Ar-H), 3.87 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 160.6 (Cquat), 137.8 (Cquat), 124.0 (Cquat), 123.6 (Ar-CH), 114.4 (Ar-CH), 114.4 (Cquat), 110.4 (Cquat), 93.8 (Ar-CH), 72.5 (Cquat), 55.8 (OCH3) ppm. HRMS (ESI): calcd. for C10H8IN2O (M + H)+ 298.96813; found 298.96741.
5-Fluoro-3-iodo-1H-indole-2-carbonitrile (6d): White solid, 73%, 2.60 g. 1H NMR (300 MHz, DMSO-d6): δ = 12.05 (bs, 1H, NH), 8.03 (ddd, J = 9.0 Hz, 4.3 Hz, 0.4 Hz, 1H, Ar-H), 7.69 (td, J = 9.2 Hz, J = 2.5 Hz, 1H, Ar-H), 7.58 (ddd, J = 9.0 Hz, 2.5 Hz, 0.4 Hz, 1H, Ar-H) ppm. 13C NMR (75 MHz, Acetone-d6): δ = 159.9 (d, J =238.1 Hz, Cquat), 134.6 (Cquat), 130.6 (d, J = 10.7 Hz, Cquat), 116.6 (d, J = 27.2 Hz, Ar–CH), 115.3 (d, J = 9.5 Hz, Ar–CH), 114.7 (Cquat), 114.0 (Cquat), 107.1 (d, J = 24.9 Hz, Ar–CH), 70.1 (d, J = 5.6 Hz, Cquat) ppm. 19F NMR (282 MHz, CDCl3): δ = −119.6 Hz. HRMS (ESI): calcd. for C9H5FIN2 (M + H) + 286.94814; found 286.20350.
3.2.5. General Procedure for the Synthesis of Nitriles (Series 7)
To compound 6a (2.0 g, 7.4 mmol, 1 equiv.) in DMF (10 mL), NaH (0.17 g, 44 mmol, 1.2 equiv.) was added portion-wise at 0 °C under argon. The mixture was stirred for 30 min at room temperature. Then, benzyl bromide (0.57 mL, 0.0048 mol, 1.3 equiv.) was added dropwise at 0 °C. The mixture was stirred at room temperature for 3–4 h and hydrolyzed with sat. aq. NH4Cl (10 mL). The aqueous phase was extracted with DCM (3 × 20 mL), and the combined organic layers were dried over MgSO4 and concentrated under reduced pressure. The product was purified by column chromatography using petroleum ether/ethyl acetate (80:20) as eluent.
1-Benzyl-3-iodo-1H-indole-2-carbonitrile (7a): White solid, 71%, 1.90 g. mp 144–146 °C. 1H NMR (300 MHz, CDCl3): δ = 7.50 (dt, J = 8.1 Hz, 1.0 Hz, 1H, Ar-H), 7.41–7.38 (m, 1H, Ar-H), 7.37–7.27 (m, 5H, Ar-H), 7.18 (dd, J = 7.5 Hz, J = 2.0 Hz, 2H, Ar-H), 5.51 (s, 2H, CH2) ppm. 13C NMR (75 MHz, CDCl3): δ = 137.4 (Cquat), 135.6 (Cquat), 129.8 (Cquat), 129.2 (2Ar-CH), 128.5 (Ar-CH), 127.2 (Ar-CH), 127.0 (2Ar-CH), 123.2 (Ar-CH), 122.6 (Ar-CH), 115.4 (Cquat), 113.4 (Cquat), 111.1 (Ar-CH), 70.6 (Cquat), 50.1 (CH2) ppm. HRMS (ESI): calcd. for C16H12IN2 (M + H)+ 359.00397; found 359.00293.
1-Benzyl-3-iodo-5-methoxy-1H-indole-2-carbonitrile (7b): White solid, 81%, 2.10 g. 1H NMR (300 MHz, CDCl3): δ = 7.35–7.27 (m, 3H, Ar-H), 7.20 (d, J = 9.1 Hz, 1H, Ar-H), 7.15 (dd, J = 7.5 Hz, J = 2.4 Hz, 2H, Ar-H), 7.04 (dd, J = 9.1 Hz, J = 2.4 Hz, 1H, Ar-H), 6.82 (d, J = 2.4 Hz, 1H, Ar-H), 5.47 (s, 2H, CH2), 3.88 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 156.3 (Cquat), 135.7 (Cquat), 132.7 (Cquat), 130.2 (Cquat), 129.2 (2Ar-CH), 128.4 (Ar-CH), 126.9 (2Ar-CH), 119.1 (Ar-CH), 115.3 (Cquat), 113.5 (Cquat), 112.2 (Ar-CH), 102.7 (Ar-CH), 69.4 (Cquat), 55.9 (OCH3), 50.2 (CH2) ppm. HRMS (ESI): calcd. for C17H14IN2O (M + H)+ 389.01453; found 389.01495.
1-Benzyl-3-iodo-6-methoxy-1H-indole-2-carbonitrile (7c): White solid, 92%, 2.40 g. 1H NMR (300 MHz, CDCl3): δ = 7.38–7.28 (m, 4H, Ar-H), 7.17 (dd, J = 7.6 Hz, J = 2.1 Hz, 2H, Ar-H), 6.92 (dd, J = 8.9 Hz, J = 2.1 Hz, 1H, Ar-H), 6.66 (d, J = 2.1 Hz, 1H, Ar-H), 5.43 (s, 2H, CH2), 3.81 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 160.4 (Cquat), 138.5 (Cquat), 135.6 (Cquat), 129.2 (Ar-CH), 128.4 (Ar-CH), 127.0 (Ar-CH), 124.2 (Cquat), 124.0 (Ar-CH), 114.2 (Cquat), 113.9 (Ar-CH), 113.8 (Cquat), 92.9 (Ar-CH), 70.9 (Cquat), 55.8 (OCH3), 50.0 (CH2) ppm. HRMS (ESI): calcd. for C17H14IN2O (M + H)+ 389.01508; found 389.01492.
1-Benzyl-5-fluoro-3-iodo-1H-indole-2-carbonitrile (7d): White solid, 76%, 2.0 g. 1H NMR (300 MHz, CDCl3): δ = 7.37–7.27 (m, 4H, Ar-H), 7.26–7.23 (m, 1H, Ar-H), 7.19–7.11 (m, 3H, Ar-H), 5.49 (s, 2H, CH2) ppm. 13C NMR (75 MHz, CDCl3): δ = 159.3 (d, J = 241.0 Hz, Cquat), 135.3 (Cquat), 134.0 (Cquat), 130.3 (d, J = 10.6 Hz, Cquat), 129.3 (2Ar–CH), 128.6 (Ar–CH), 126.9 (2Ar–CH), 116.9 (Cquat), 116.5 (d, J = 27.1 Hz, Ar–CH), 113.0 (Cquat), 112.4 (d, J = 9.4 Hz, Ar–CH), 107.8 (d, J = 24.7 Hz, Ar–CH), 69.4 (d, J = 5.6 Hz, Cquat), 50.4 (CH2) ppm. 19F (282 MHz, CDCl3): δ = −119.9 ppm. HRMS (ESI): calcd. for C16H11FIN2 (M + H)+ 376.99509; found 376.99497.
3.2.6. General Procedure for Sonogashira Coupling (Series 8)
A Schlenk tube was charged with phenylacetylene (1.08 mmol, 1.3 equiv.), Et3N (0.34 mL, 2.52 mmol, 3.0 equiv.), DMF (3 mL), PdCl2(PPh3)2 (58 mg, 0.083 mmol, 10 mol%), compound 7a (300 mg, 0.837 mmol, 1 equiv.) and CuI (160 mg, 0.084 mmol, 10 mol%). The tube was evacuated and backfilled with argon, and the mixture was stirred at room temperature overnight. Then, the mixture was hydrolyzed with sat. aq. NH4Cl (10 mL), extracted by Et2O (3 × 20 mL), and the combined organic layers were washed with brine (6 × 10 mL), dried over MgSO4 and concentrated under reduced pressure. The product was purified by column chromatography using petroleum ether/ethyl acetate (80:20) as eluent.
1-Benzyl-3-(phenylethynyl)-1H-indole-2-carbonitrile (8a): Yellow solid, 70%, 195 mg. mp 125–127 °C. 1H NMR (300 MHz, CDCl3): δ = 7.88 (dt, J = 8.0 Hz, J = 1.0 Hz, 1H, Ar-H), 7.67–7.62 (m, 2H, Ar-H), 7.44–7.39 (m, 4H, Ar-H), 7.36–7.28 (m, 5H, Ar-H), 7.20 (dd, J = 7.6 Hz, 1.9 Hz, 2H, Ar-H), 5.48 (s, 2H, CH2) ppm. 13C NMR (75 MHz, CDCl3): δ = 137.1 (Cquat), 135.6 (Cquat), 131.9 (2Ar-CH), 129.2 (2Ar-CH), 128.8 (Ar-CH), 128.6 (2Ar-CH), 128.4 (Ar-CH), 127.5 (Cquat), 127.0 (Ar-CH), 127.0 (2Ar-CH), 123.0 (Cquat), 122.4 (Ar-CH), 121.8 (Ar-CH), 112.8 (2Cquat), 111.1 (Ar-CH), 109.8 (Cquat), 97.2 (Cquat), 79.6 (Cquat), 49.6 (CH2) ppm. HRMS (ESI): calcd. for C24H17N2 (M + H)+ 333.13863; found 333.13760.
1-Benzyl-3-(p-tolylethynyl)-1H-indole-2-carbonitrile (8b): Yellow solid, 81%, 235 mg. mp 163–165°C. 1H NMR (300 MHz, CDCl3): δ = 7.86 (d, J = 8.2 Hz, 1H, Ar-H), 7.51 (d, J = 8.1 Hz, 2H, Ar-H), 7.43–7.26 (m, 6H, Ar-H), 7.22–7.16 (m, 3H, Ar-H), 5.48 (s, 2H, CH2), 2.40 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 139.0 (Cquat), 137.1 (Cquat), 135.6 (Cquat), 131.7 (Ar-CH), 129.3 (Ar-CH), 129.1 (Ar-CH), 128.4 (Ar-CH), 127.5 (Cquat), 127.0 (Ar-CH), 122.3 (Ar-CH), 121.8 (Ar-CH), 119.9 (Cquat), 112.9 (Cquat), 112.6 (Cquat), 111.0 (Ar-CH), 110.1 (Cquat), 97.4 (Cquat), 79.0 (Cquat), 49.5 (CH2), 21.7 (CH3) ppm. HRMS (ESI): calcd. for C25H19N2 (M + H)+ 347.15482; found 346.15489.
1-Benzyl-3-((4-fluorophenyl)ethynyl)-1H-indole-2-carbonitrile (8c): Brown solid, 69%, 202 mg. mp 180–182 °C. 1H NMR (300 MHz, CDCl3): δ = 7.85 (dt, J = 8.0 Hz, J = 1.0 Hz, 1H, Ar-H), 7.60 (dd, J = 8.9 Hz, J = 5.4 Hz, 2H, Ar-H), 7.40 (ddd, J = 8.4 Hz, J = 6.6 Hz, J = 1.2 Hz, 1H, Ar-H), 7.35–7.27 (m, 5H, Ar-H), 7.21–7.18 (m, 2H, Ar-H), 7.08 (t, J = 8.7 Hz, 2H, Ar-H), 5.48 (s, 2H, CH2) ppm. 13C NMR (75 MHz, CDCl3): δ = 162.9 (d, J = 250.3 Hz, Cquat), 137.1 (Cquat), 135.6 (Cquat), 133.8 (d, J = 8.4 Hz, Ar-CH), 129.2 (2Ar–CH), 128.5 (Ar-CH), 127.5 (Cquat), 127.1 (Ar-CH), 127.0 (2Ar-CH), 122.4 (Ar-CH), 121.7 (Ar-CH), 119.1 (d, J = 3.5 Hz, Cquat), 115.9 (d, J = 22.1 Hz, Ar-CH), 112.9 (Cquat), 112.8 (Cquat), 111.1 (Ar-CH), 109.7 (Cquat), 96.0 (Cquat), 79.4 (Cquat), 49.6 (CH2) ppm. 19F NMR (282 MHz, CDCl3): δ = −110.2 ppm. HRMS (ESI): calcd. for C24H16FN2 (M-H)+ 351.12920; found 351.12816.
1-Benzyl-3-((4-methoxyphenyl)ethynyl)-1H-indole-2-carbonitrile (8d): Brown solid, 83%, 252 mg. mp 137–139 °C. 1H NMR (300 MHz, CDCl3): δ = 7.85 (dt, J = 8.0 Hz, J = 1.0 Hz, 1H, Ar-H), 7.56 (d, J = 8.9 Hz, 2H, Ar-H), 7.40 (ddd, J = 8.4 Hz, J = 6.7 Hz, J = 1.2 Hz, 1H, Ar-H), 7.36–7.24 (m, 6H, Ar-H), 7.19 (dd, J = 7.8 Hz, J = 2.0 Hz, 1H, Ar-H), 6.91 (d, J = 8.9 Hz, 2H, Ar-H), 5.47 (s, 2H, CH2), 3.85 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 160.0 (Cquat), 137.0 (Cquat), 135.6 (Cquat), 133.3 (2Ar-CH), 129.1 (2Ar-CH), 128.3 (Ar-CH), 127.4 (Cquat), 126.9 (2Ar-CH), 122.2 (Ar-CH), 121.7 (Ar-CH), 115.0 (Cquat), 114.2 (Ar-CH), 112.9 (Cquat), 112.4 (Cquat), 111.0 (Ar-CH), 110.2 (Cquat), 97.2 (Cquat), 78.3 (Cquat), 55.4 (OCH3), 49.4 (CH2) ppm. HRMS (ESI): calcd. for C25H19N2O (M + H)+ 363.14919; found 363.14962.
1-Benzyl-3-((4-ethylphenyl)ethynyl)-1H-indole-2-carbonitrile (8e): Brown solid, 83%, 251 mg. mp 94–96 °C.1H NMR (300 MHz, CDCl3): δ = 7.86 (dt, J = 8.0 Hz, J = 1.0 Hz, 1H, Ar-H), 7.54 (d, J = 8.3 Hz, 2H, Ar-H), 7.40 (ddd, J = 8.4 Hz, J = 6.6 Hz, J = 1.2 Hz, 1H, Ar-H), 7.36–7.28 (m, 5H, Ar-H), 7.24–7.18 (m, 4H, Ar-H), 5.48 (s, 2H, CH2), 2.69 (q, J = 7.6 Hz, 2H, CH2), 1.26 (t, J = 7.6 Hz, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 145.3 (Cquat), 137.0 (Cquat), 135.6 (Cquat), 131.8 (2Ar-CH), 129.1 (2Ar-CH), 128.4 (Ar-CH), 128.1 (2Ar-CH), 127.5 (Cquat), 127.0 (3Ar-CH), 122.3 (Ar-CH), 121.8 (Ar-CH), 120.1 (Cquat), 112.9 (Cquat), 112.6 (Cquat),111.0 (Ar-CH), 110.1 (Cquat), 97.4 (Cquat), 79.0 (Cquat), 49.5 (CH2), 29.0 (CH2), 15.5 (CH3) ppm. HRMS (ESI): calcd. for C26H21N2 (M + H)+ 361.16993; found 361.16903.
1-Benzyl-3-(pyridin-3-yl-ethynyl)-1H-indole-2-carbonitrile (8f): Brown solid, 78%, 216 mg. mp 138–140 °C. 1H NMR (300 MHz, CDCl3): δ = 8.67 (ddd, J = 4.9 Hz, J = 1.8 Hz, J = 1.0 Hz, 1H, Ar-H), 7.93 (dt, J = 8.1 Hz, J = 1.0 Hz, 1H, Ar-H), 7.72 (dt, J = 7.8 Hz, J = 1.8 Hz, 1H, Ar-H), 7.63 (dt, J = 7.8 Hz, J = 1.2 Hz, 1H, Ar-H), 7.41 (ddd, J = 8.4 Hz, J = 6.6 Hz, J = 1.2 Hz, 1H, Ar-H), 7.37–7.26 (m, 6H, Ar-H), 7.19 (dd, J = 7.7 Hz, J = 2.1 Hz, 2H, Ar-H), 5.50 (s, 2H, CH2) ppm. 13C NMR (75 MHz, CDCl3): δ = 150.1 (Ar-CH), 143.0 (Cquat), 136.9 (Cquat), 136.2 (Ar-CH), 135.3 (Cquat), 129.1 (2Ar-CH), 128.3 (Ar-CH), 127.6 (Ar-CH), 127.4 (Cquat), 127.0 (Ar-CH), 126.8 (2Ar-CH), 123.0 (Ar-CH), 122.5 (Ar-CH), 121.8 (Ar-CH), 113.5 (Cquat), 112.4 (Cquat), 111.0 (Ar-CH), 108.5 (Cquat), 95.9 (Cquat), 79.5 (Cquat), 49.5 (CH2) ppm. HRMS (ESI): calcd. for C23H16N3 (M + H)+ 334.13387; found 334.13301.
1-Benzyl-3-((2-fluorophenyl)ethynyl)-1H-indole-2-carbonitrile (8g): Brown solid, 76%, 222 mg. mp 178–180 °C. 1H NMR (300 MHz, CDCl3): δ = 7.88 (dt, J = 8.0 Hz, J = 0.9 Hz, 1H, Ar-H), 7.60 (td, J = 7.2 Hz, J = 1.9 Hz, 1H, Ar-H), 7.41 (ddd, J = 8.4 Hz, J = 6.7 Hz, J = 1.2 Hz, 1H, Ar-H), 7.38–7.27 (m, 6H, Ar-H), 7.21–7.11 (m, 4H, Ar-H), 5.50 (s, 2H, CH2) ppm. 13C NMR (75 MHz, CDCl3): δ = 162.7 (d, J = 252.3 Hz, Cquat), 137.0 (Cquat), 135.5 (Cquat), 133.5 (Ar-CH), 130.5 (d, J = 7.9 Hz, Ar-CH), 129.1 (2Ar-CH), 128.4 (Ar-CH), 127.5 (Cquat), 127.1 (Ar-CH), 127.0 (2Ar-CH), 124.1 (d, J = 3.7 Hz, Ar-CH), 122.6 (Ar-CH), 121.8 (Ar-CH), 115.7 (d, J = 20.7 Hz, Ar-CH), 112.8 (Cquat), 112.7 (Cquat), 111.6 (d, J = 15.7 Hz, Cquat), 111.1 (Ar-CH), 109.3 (Cquat), 90.4 (Cquat), 84.7 (Cquat), 49.5 (CH2) ppm. 19F NMR (282 MHz, CDCl3): δ = −112.1 ppm. HRMS (ESI): calcd. for C24H16FN2 (M + H)+ 351.12920; found 351.12956.
1-Benzyl-5-methoxy-3-((4-methoxyphenyl)ethynyl)-1H-indole-2-carbonitrile (8h): Brown solid, 90%, 272 mg. 1H NMR (300 MHz, CDCl3): δ = 7.57 (d, J = 8.9 Hz, 2H, Ar-H), 7.35–7.28 (m, 3H, Ar-H), 7.20 (d, J = 9.1 Hz, 1H, Ar-H), 7.16 (dd, J = 7.4 Hz, J = 2.4 Hz, 2H, Ar-H), 7.04 (dd, J = 9.1 Hz, J 2.5 Hz, 1H, Ar-H), 6.91 (d, J = 8.9 Hz, 2H, Ar-H), 5.44 (s, 2H, CH2), 3.89 (s, 3H, OCH3), 3.85 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 160.0 (Cquat), 156.1 (Cquat), 135.7 (Cquat), 133.4 (2Ar-CH), 132.4 (Cquat), 129.1 (2Ar-CH), 128.4 (Ar-CH), 128.1 (Cquat), 126.9 (2Ar-CH), 118.6 (Ar-CH), 115.1 (Cquat), 114.2 (2Ar-CH), 113.0 (Cquat), 112.6 (Cquat), 112.1 (Ar-CH), 109.3 (Cquat), 101.6 (Ar-CH), 97.0 (Cquat), 78.4 (Cquat), 55.9 (OCH3), 55.5 (OCH3), 49.7 (CH2) ppm. HRMS (ESI): calcd. for C26H21N2O2 (M + H)+ 393.15975; found 393.16029.
1-Benzyl-6-methoxy-3-((2-methoxyphenyl)ethynyl)-1H-indole-2-carbonitrile (8i): Brown solid, 83%, 252 mg. 1H NMR (300 MHz, CDCl3): δ = 7.74 (d, J = 8.8 Hz, 1H, Ar-H), 7.56 (dd, J = 7.6 Hz, J = 1.6 Hz, 1H, Ar-H), 7.39–7.28 (m, 4H, Ar-H), 7.17 (dd, J = 7.6 Hz, J = 1.6 Hz, 2H, Ar-H), 6.98 (dd, J = 7.6 Hz, J = 1.0 Hz, 1H, Ar-H), 6.96–6.90 (m, 2H, Ar-H), 6.67 (d, J = 2.1 Hz, 1H, Ar-H), 5.41 (s, 2H, CH2), 3.96 (s, 3H, OCH3), 3.80 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 160.3 (Cquat), 160.1 (Cquat), 138.2 (Cquat), 135.7 (Cquat), 133.5 (Ar-CH), 130.2 (Ar-CH), 129.1 (2Ar-CH), 128.3 (Ar-CH), 126.9 (2Ar-CH), 122.7 (Ar-CH), 121.8 (Cquat), 120.6 (Ar-CH), 113.4 (Ar-CH), 113.2 (Cquat), 112.4 (Cquat), 111.4 (Cquat), 111.0 (Ar-CH), 110.5 (Cquat), 93.5 (Cquat), 93.2 (Ar-CH), 83.8 (Cquat), 56.0 (OCH3), 55.7 (OCH3), 49.4 (CH2) ppm. HRMS (ESI): calcd. for C26H21N2O2 (M + H)+ 393.15975; found 393.15951.
1-Benzyl-5-fluoro-3-(m-tolylethynyl)-1H-indole-2-carbonitrile (8j): Brown solid, 86%, 250 mg. 1H NMR (300 MHz, CDCl3): δ = 7.55–7.46 (m, 3H), 7.38–7.27 (m, 4H), 7.25–7.08 (m, 5H), 5.46 (s, 2H), 2.40 (s, 3H) ppm. 13C NMR (75 MHz, CDCl3): δ = 159.2 (d, J = 241.2 Hz, Cquat), 139.2 (Cquat), 135.4 (Cquat), 133.7 (Cquat), 131.8 (2Ar-CH), 129.4 (2Ar-CH), 129.3 (2Ar-CH), 128.6 (Ar-CH), 128.1 (d, J = 10.4 Hz, Cquat), 126.9 (2Ar-CH), 119.7 (Cquat), 116.2 (d, J = 27.0 Hz, Ar-CH), 114.0 (Cquat), 112.5 (Cquat), 112.3 (d, J = 9.1 Hz, Ar-CH), 109.8 (d, J = 5.4 Hz, Cquat), 106.5 (d, J = 24.0 Hz), 97.8 (Cquat), 78.4 (Cquat), 49.9 (CH2), 21.7 (CH3) ppm. 19F NMR (282 MHz, CDCl3): δ = −120.3 ppm. HRMS (ESI): calcd. for C25H18FN2 (M + H)+ 365.14540; found 365.14514.
3.2.7. General Procedure for Suzuki Coupling (Series 9)
A Schlenk tube was charged with toluene (3 mL), ethanol (2 mL), sat. aq. NaHCO3 (1.5 mL), compound 7a (300 mg, 0.83 mmol, 1 equiv.) and boronic acid (1.2 mmol, 1.5 equiv.). The tube was vigorously stirred for few minutes and Pd(PPh3)4 (90 mg, 0.083 mmol, 10 mol%) was added to the solution. The mixture was heated at 130 °C for 4 h and then cooled to room temperature. The mixture was extracted with EtOAc (3 × 10 mL), and the combined organic layers were washed with water (2 × 10 mL) and dried over MgSO4. Solvents were removed under reduced pressure, and the residue was purified by column chromatography using petroleum ether/EtOAc as eluent (80:20).
1-Benzyl-3-(4-methoxyphenyl)-1H-indole-2-carbonitrile (9a): Brown solid, 79%, 221 mg. mp 153–155 °C. 1H NMR (300 MHz, CDCl3): δ = 7.85 (dt, J = 8.2 Hz, J = 0.9 Hz, 1H, Ar-H), 7.67 (d, J = 8.9 Hz, 2H, Ar-H), 7.39–7.35 (m, 2H, Ar-H), 7.33–7.28 (m, 3H, Ar-H), 7.26–7.20 (m, 3H, Ar-H), 7.07 (d, J = 8.9 Hz, 2H, Ar-H), 5.52 (s, 2H, CH2), 3.89 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 159.7 (Cquat), 138.0 (Cquat), 136.2 (Cquat), 130.1 (2Ar-CH), 129.1 (2Ar-CH), 128.5 (Cquat), 128.2 (2Ar-CH), 127.1 (2Ar-CH), 126.5 (Ar-CH), 125.3 (Cquat), 124.4 (Cquat), 121.8 (2Ar-CH), 114.7 (2Ar-CH), 114.5 (Cquat), 110.9 (Ar-CH), 107.2 (Cquat), 55.5 (OCH3), 49.2 (CH2) ppm. HRMS (ESI): calcd. for C23H19N2O (M + H)+ 339.14919; found 339.14965.
1-Benzyl-3-(4-fluorophenyl)-1H-indole-2-carbonitrile (9b): Brown solid, 81%, 220 mg. mp 120–122 °C. 1H NMR (300 MHz, CDCl3): δ = 7.82 (dt, J = 8.2 Hz, J = 0.9 Hz, 1H, Ar-H), 7.70 (dd, J = 8.8 Hz, J = 5.3 Hz, 2H, Ar-H), 7.42–7.39 (m, 2H, Ar-H), 7.37–7.27 (m, 3H, Ar-H), 7.25–7.19 (m, 3H, Ar-H), 5.53 (s, 2H, CH2) ppm. 13C NMR (75 MHz, CDCl3): δ = 162.6 (d, J = 248.0 Hz, Cquat), 137.9 (Cquat), 136.0 (Cquat), 130.6 (d, J = 8.1 Hz, Ar-CH), 129.1 (2Ar-CH), 128.3 (Ar-CH), 127.9 (d, J = 3.3 Hz, Cquat), 127.5 (Cquat), 127.0 (2Ar-CH), 126.6 (Ar-CH), 125.1 (Cquat), 122.1 (Ar-CH), 121.4 (Ar-CH), 116.2 (d, J = 21.6 Hz, Ar-CH), 114.1 (Cquat), 111.0 (Ar-CH), 107.5 (Cquat), 49.2 (CH2) ppm. 19F NMR (282 MHz, CDCl3): δ = −113.2 ppm. HRMS (ESI): calcd. for C22H16FN2 (M + H)+ 327.12975; found 327.12988.
1-Benzyl-3-(4-(tert-butyl)phenyl)-1H-indole-2-carbonitrile (9c): Brown solid, 83%, 252 mg. mp 149–151 °C. 1H NMR (300 MHz, CDCl3): δ = 7.92 (dt, J = 8.2 Hz, J = 0.8 Hz, 1H, Ar-H), 7.72 (d, J = 8.5 Hz, 2H, Ar-H), 7.57 (d, J = 8.5 Hz, 2H, Ar-H), 7.40–7.37 (m, 2H, Ar-H), 7.35–7.207 (m, 6H, Ar-H), 5.51 (s, 2H, CH2), 1.41 (s, 9H, C(CH3)3) ppm. 13C NMR (75 MHz, CDCl3): δ = 151.2 (Cquat), 138.0 (Cquat), 136.2 (Cquat), 129.1 (2Ar-CH), 129.0 (Cquat), 128.6 (Cquat), 128.5 (2Ar-CH), 128.2 (Ar-CH), 127.0 (2Ar-CH), 126.5 (Ar-CH), 126.1 (2Ar-CH), 125.0 (Cquat), 121.9 (Ar-CH), 121.8 (Ar-CH), 114.5 (Cquat), 111.0 (Ar-CH), 107.4 (Cquat), 49.1 (CH2), 34.8 (Cquat), 31.4 (C(CH3)3) ppm. HRMS (ESI): calcd. for C26H25N2 (M + H)+ 365.20177; found 365.20152.
1-Benzyl-3-(4-(methylthio)phenyl)-1H-indole-2-carbonitrile (9d): Yellow viscous liquid, 89%, 263 mg. 1H NMR (300 MHz, CDCl3): δ = 7.85 (dd, J = 8.2 Hz, J = 0.9 Hz, 1H, Ar-H), 7.67 (d, J = 8.6 Hz, 2H, Ar-H), 7.42–7.38 (m, 4H, Ar-H), 7.36–7.29 (m, 3H, Ar-H), 7.26–7.22 (m, 3H, Ar-H), 5.53 (s, 2H, CH2), 2.55 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 138.8 (Cquat), 138.0 (Cquat), 136.1 (Cquat), 129.2 (2Ar-CH), 129.1 (2Ar-CH), 128.6 (Cquat), 128.3 (Ar-CH), 128.1 (Cquat), 127.1 (4Ar-CH), 126.6 (Ar-CH), 126.6 (Ar-CH), 125.1 (Cquat), 122.0 (Ar-CH), 121.7 (Ar-CH), 114.3 (Cquat), 111.0 (Ar-CH), 107.4 (Cquat), 49.2 (CH2), 15.8 (CH3) ppm. HRMS (ESI): calcd. for C23H19N2S (M + H)+ 355.12635; found 355.12680.
1-Benzyl-3-(4-(trifluoromethyl)phenyl)-1H-indole-2-carbonitrile (9e): Brown liquid, 87%, 272 g. 1H NMR (300 MHz, CDCl3): δ = 7.86 (d, J = 8.5 Hz, 2H, Ar-H), 7.85 (dd, J = 8.2 Hz, J = 0.9 Hz, 1H, Ar-H), 7.79 (d, J = 8.5 Hz, 2H, Ar-H), 7.45–7.42 (m, 2H, Ar-H), 7.38–7.28 (m, 4H, Ar-H), 7.27–7.22 (m, 2H, Ar-H), 5.55 (s, 2H, CH2) ppm. 13C NMR (75 MHz, CDCl3): δ = 137.9 (Cquat), 135.8 (Cquat), 135.7 (q, J = 1.2 Hz, Cquat), 130.0 (q, J = 32.7 Hz, Cquat), 129.2 (2Ar-CH), 129.1 (2Ar-CH), 128.4 (Ar-CH), 127.1 (2Ar-CH), 126.9 (Ar-CH), 126.7 (Cquat), 126.1 (q, J = 3.8 Hz, 2Ar-CH), 124.9 (Cquat), 124.2 (q, J = 272.2 Hz, Cquat), 122.5 (Ar-CH), 121.3 (Ar-CH), 113.8 (Cquat), 111.2 (Ar-CH), 108.1 (Cquat), 49.3 (CH2) ppm. 19F NMR (282 MHz, CDCl3): δ = −62.5 ppm. HRMS (ESI): calcd. for C23H16F3N2 (M + H)+ 377.12656; found 377.12516.
1-Benzyl-3-(2-methoxyphenyl)-1H-indole-2-carbonitrile (9f): Brown solid, 89%, 250 mg. mp 132–134 °C. 1H NMR (300 MHz, CDCl3): δ = 7.91 (dd, J = 8.2 Hz, J = 0.9 Hz, 1H, Ar-H), 7.52–7.40 (m, 4H, Ar-H), 7.37–7.27 (m, 5H, Ar-H), 7.25–7.23 (m, 2H, Ar-H), 6.99 (ddd, J = 8.2 Hz, J = 2.6 Hz, J = 1.0 Hz, 1H, Ar-H), 5.54 (s, 2H, CH2), 3.90 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 160.2 (Cquat), 138.0 (Cquat), 136.1 (Cquat), 133.2 (Cquat), 130.2 (Ar-CH), 129.1 (2Ar-CH), 128.4 (Cquat), 128.3 (Ar-CH), 127.0 (2Ar-CH), 126.6 (Ar-CH), 125.2 (Cquat), 122.0 (Ar-CH), 121.8 (Ar-CH), 121.3 (Ar-CH), 114.2 (Ar-CH), 114.1 (Ar-CH), 111.0 (Ar-CH), 109.1 (Cquat), 107.7 (Cquat), 55.5 (CH2), 49.2 (OCH3) ppm. HRMS (ESI): calcd. for C23H19N2O (M + H)+ 339.14919; found 339.14959.
1-Benzyl-3-(2-fluorophenyl)-1H-indole-2-carbonitrile (9g): White solid, 92%, 250 mg. mp 151–153 °C. 1H NMR (300 MHz, CDCl3): δ = 7.69 (ddt, J = 8.2 Hz, J = 2.2 Hz, J = 1.0 Hz, 1H, Ar-H), 7.62 (m, 1H, Ar-H), 7.48–7.39 (m, 4H, Ar-H), 7.37–7.29 (m, 4H, Ar-H), 7.28–7.21 (m, 3H, Ar-H), 5.55 (s, 2H, CH2) ppm. 13C NMR (75 MHz, CDCl3): δ = 159.9 (d, J = 249.1 Hz, Cquat), 137.6 (Cquat), 136.0 (Cquat), 131.7 (d, J = 3.1 Hz, Ar-CH), 130.2 (d, J = 8.2 Hz, Ar-CH), 129.1 (2Ar-CH), 128.3 (Ar-CH), 127.1 (2Ar-CH), 126.5 (Ar-CH), 125.7 (Cquat), 124.7 (d, J = 3.6 Hz, Ar-CH), 122.0 (d, J = 3.2 Hz, Ar-CH), 122.0 (Ar-CH), 119.6 (d, J = 15.4 Hz, Cquat), 116.5 (d, J = 22.1 Hz, Ar-CH), 113.6 (Cquat), 111.9 (Ar-CH), 109.1 (Cquat), 104.5 (Cquat), 49.4 (CH2) ppm. 19F NMR (282 MHz, CDCl3): δ = −112.1 ppm. HRMS (ESI): calcd. for C22H16FN2 (M + H)+ 327.12975; found 327.13012.
1-Benzyl-3-(o-tolyl)-1H-indole-2-carbonitrile (9h): Brown solid, 92%, 246 mg. mp 130–132 °C. 1H NMR (300 MHz, CDCl3): δ = 7.49 (dt, J = 8.1 Hz, J = 1.0 Hz, 1H, Ar-H), 7.42–7.37 (m, 5H, Ar-H), 7.36–7.32 (m, 4H, Ar-H), 7.27–7.18 (m, 3H, Ar-H), 5.55 (s, 2H, CH2), 2.31 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 137.5 (Cquat), 137.3 (Cquat), 136.2 (Cquat), 131.0 (Ar-CH), 130.9 (Cquat), 130.7 (Ar-CH), 129.1 (2Ar-CH), 128.7 (Cquat), 128.6 (Ar-CH), 128.2 (Ar-CH), 127.0 (2Ar-CH), 126.4 (Ar-CH), 126.3 (Cquat), 126.0 (Ar-CH), 122.0 (Ar-CH), 121.6 (Ar-CH), 113.8 (Cquat), 110.9 (Ar-CH), 108.9 (Cquat), 49.2 (CH2), 20.3 (CH3) ppm. HRMS (ESI): calcd. for C23H19N2 (M + H)+ 323.15428; found 323.15463.
1-Benzyl-3-(3-methoxyphenyl)-1H-indole-2-carbonitrile (9i): Solid orange, 86%, 241 mg. mp 131–133 °C. 1H NMR (300 MHz, CDCl3): δ = 7.91 (d, J = 8.2 Hz, 1H, Ar-H), 7.46 (t, J = 7.9 Hz, 1H, Ar-H), 7.41–7.39 (m, 2H, Ar-H), 7.37–7.27 (m, 5H, Ar-H), 7.25–7.23 (m, 2H, Ar-H), 6.99 (ddd, J = 8.2 Hz, J = 2.5 Hz, J = 0.8 Hz, 1H, Ar-H), 5.54 (s, 2H, CH2), 3.90 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 160.2 (Cquat), 138.0 (Cquat), 136.1 (Cquat), 133.2 (Cquat), 130.2 (Ar-CH), 129.1 (2Ar-CH), 128.4 (Cquat), 128.3 (Ar-CH), 127.1 (2Ar-CH), 126.6 (Ar-CH), 125.2 (Cquat), 122.0 (Ar-CH), 121.8 (Ar-CH), 121.3 (Ar-CH), 114.2 (Cquat), 114.2 (Ar-CH), 114.1 (Ar-CH), 111.0 (Ar-CH), 107.7 (Cquat), 55.5 (CH3), 49.2 (CH2) ppm. HRMS (ESI): calcd. for C23H19N2O (M + H)+ 339.14919; found 339.14965.
1-Benzyl-3-(2,4-dimethoxyphenyl)-1H-indole-2-carbonitrile (9j): Brown liquid, 89%, 248 mg. 1H NMR (300 MHz, CDCl3): δ = 7.64 (dt, J = 8.1 Hz, J = 0.9 Hz, 2H, Ar-H), 7.42 (d, J = 8.8 Hz, 1H, Ar-H), 7.34–7.27 (m, 5H, Ar-H), 7.24–7.14 (m, 3H, Ar-H), 6.63 (s, 1H, Ar-H), 6.62 (dd, J = 7.6 Hz, J = 2.4 Hz, 1H, Ar-H), 5.49 (s, 2H, CH2), 3.87 (s, 3H, OCH3), 3.84 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 161.3 (Cquat), 158.1 (Cquat), 137.6 (Cquat), 136.4 (Cquat), 132.2 (Ar-CH), 129.0 (2Ar-CH), 128.1 (Ar-CH), 127.1 (2Ar-CH), 126.2 (Cquat), 126.0 (Ar-CH), 124.7 (Cquat), 122.4 (Ar-CH), 121.3 (Ar-CH), 114.3 (Cquat), 113.3 (Cquat), 110.7 (Ar-CH), 109.1 (Cquat), 104.9 (Ar-CH), 99.2 (Ar-CH), 55.6 (OCH3), 55.4 (OCH3), 49.2 (CH2) ppm. HRMS (ESI): calcd. for C24H21N2O2 (M + H)+ 369.16030; found 369.16135.
1-Benzyl-3-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-1H-indole-2-carbonitrile (9k): Brown solid, 90%, 273 mg. mp 140–142 °C. 1H NMR (300 MHz, CDCl3): δ = 7.87 (d, J = 8.2 Hz, 2H, Ar-H), 7.38–7.36 (m, 2H, Ar-H), 7.35–7.27 (m, 4H, Ar-H), 7.25–7.21 (m, 4H, Ar-H), 7.02 (d, J = 8.3 Hz, 2H, Ar-H), 5.51 (s, 2H, CH2), 4.32 (s, 4H, 2 x CH2) ppm. 13C NMR (75 MHz, CDCl3): δ = 144.0 (Cquat), 143.7 (Cquat), 137.8 (Cquat), 136.1 (Cquat), 129.0 (2Ar-CH), 128.1 (Ar-CH), 128.1 (Cquat), 127.0 (2Ar-CH), 126.4 (Ar-CH), 125.1 (2Cquat), 122.0 (Ar-CH), 121.8 (Ar-CH), 121.7 (Ar-CH), 118.0 (Ar-CH), 117.6 (Ar-CH), 114.3 (Cquat), 110.8 (Ar-CH), 107.2 (Cquat), 64.5 (CH2), 64.4 (CH2), 49.0 (CH2) ppm. HRMS (ESI): calcd. for C24H19N2O2 (M + H)+ 367.14465; found 367.14425.
1-Benzyl-3-(6-methoxynaphthalen-2-yl)-1H-indole-2-carbonitrile (9l): Brown solid, 84%, 272 mg. mp 147–149 °C. 1H NMR (300 MHz, CDCl3): δ = 8.13 (s, 1H, Ar-H), 7.96–7.81 (m, 4H, Ar-H), 7.41–7.19 (m, 10H, Ar-H), 5.52 (s, 2H, CH2), 3.94 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 158.3 (Cquat), 138.0 (Cquat), 136.2 (Cquat), 134.3 (Cquat), 129.8 (Ar-CH), 129.2 (Cquat), 129.1 (2Ar-CH), 128.7 (Cquat), 128.2 (Ar-CH), 127.8 (Ar-CH), 127.7 (Ar-CH), 127.2 (Ar-CH), 127.1 (Cquat), 127.0 (2Ar-CH), 126.6 (Ar-CH), 125.4 (Cquat), 121.9 (Ar-CH), 121.8 (Ar-CH), 119.5 (Ar-CH), 114.4 (Cquat), 111.0 (Ar-CH), 107.6 (Cquat), 105.8 (Ar-CH), 55.5 (OCH3), 49.2 (CH2) ppm. HRMS (ESI): calcd. for C27H21N2O (M + H)+ 389.16484; found 389.16527.
1-Benzyl-3-(2-methoxypyridin-3-yl)-1H-indole-2-carbonitrile (9m): Brown liquid, 86%, 241 mg. 1H NMR (300 MHz, CDCl3): δ = 8.27 (dd, J = 5.0 Hz, J = 1.9 Hz, 1H, Ar-H), 7.83 (dd, J = 7.3 Hz, J = 1.9 Hz, 1H, Ar-H), 7.63 (d, J = 8.2 Hz, 2H, Ar-H), 7.39–7.37 (m, 3H, Ar-H), 7.34–7.29 (m, 3H, Ar-H), 7.27–7.19 (m, 2H, Ar-H), 7.05 (dd, J = 7.3 Hz, J = 5.1 Hz, 1H, Ar-H), 5.54 (s, 2H, CH2), 4.03 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 161.3 (Cquat), 147.0 (Ar-CH), 139.9 (Ar-CH), 137.6 (Cquat), 136.1 (Cquat), 129.1 (2Ar-CH), 128.3 (Ar-CH), 127.1 (2Ar-CH), 126.4 (Ar-CH), 125.8 (Cquat), 122.8 (Cquat), 122.1 (Ar-CH), 121.8 (Ar-CH), 117.0 (Ar-CH), 115.3 (Cquat), 113.7 (Cquat), 111.0 (Ar-CH), 109.4 (Cquat), 53.6 (CH2), 49.4 (OCH3) ppm. HRMS (ESI): calcd. for C22H18N3O (M + H)+ 340.14499; found 340.14471.
1-Benzyl-5-methoxy-3-(4-methoxyphenyl)-1H-indole-2-carbonitrile (9n): Brown solid, 89%, 254 mg. mp 152–154 °C. 1H NMR (300 MHz, CDCl3): δ = 7.65 (d, J = 8.8 Hz, 2H, Ar-H), 7.37–7.27 (m, 3H, Ar-H), 7.26–7.18 (m, 4H, Ar-H), 7.10–7.02 (m, 3H, Ar-H), 5.48 (s, 2H, CH2), 3.89 (s, 3H, OCH3), 3.82 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 159.5 (Cquat), 155.7 (Cquat), 136.3 (Cquat), 133.3 (Cquat), 129.9 (2Ar-CH), 129.1 (2Ar-CH), 128.2 (Ar-CH), 127.6 (Cquat), 127.0 (2Ar-CH), 125.6 (Cquat), 124.5 (Cquat), 118.0 (Ar-CH), 114.7 (2Ar-CH), 114.5 (Cquat), 111.9 (Ar-CH), 107.5 (Cquat), 101.6 (Ar-CH), 55.9 (OCH3), 55.5 (OCH3), 49.3 (CH2) ppm. HRMS (ESI): calcd. for C24H21N2O2 (M + H)+ 369.15975; found 369.16033.
1-Benzyl-6-methoxy-3-(3-methoxyphenyl)-1H-indole-2-carbonitrile (9o): Brown solid, 93%, 264 mg. mp 150–152 °C. 1H NMR (300 MHz, CDCl3): δ = 7.78 (d, J = 8.9 Hz, 1H, Ar-H), 7.46 (t, J = 7.9 Hz, 1H, Ar-H), 7.41–7.22 (m, 7H, Ar-H), 6.99 (dd, J = 7.9 Hz, J = 2.1 Hz, 1H, Ar-H), 6.93 (dd, J = 8.9 Hz, J = 2.1 Hz, 1H, Ar-H), 6.75 (d, J = 2.1 Hz, 1H, Ar-H), 5.49 (s, 2H, CH2), 3.91 (s, 3H, OCH3), 3.84 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 160.2 (Cquat), 159.8 (Cquat), 139.1 (Cquat), 136.1 (Cquat), 133.3 (Cquat), 130.2 (Ar-CH), 129.1 (2Ar-CH), 128.8 (Cquat), 128.2 (Ar-CH), 127.0 (2Ar-CH), 122.6 (Ar-CH), 121.2 (Ar-CH), 119.5 (Cquat), 114.6 (Cquat), 114.2 (Ar-CH), 114.0 (Ar-CH), 113.1 (Ar-CH), 106.4 (Cquat), 93.1 (Ar-CH), 55.7 (OCH3), 55.5 (OCH3), 49.1 (CH2) ppm. HRMS (ESI): calcd. for C24H21N2O2 (M + H)+ 369.15975; found 369.16017.
1-Benzyl-5-fluoro-3-(3-methoxyphenyl)-1H-indole-2-carbonitrile (9p): Yellow viscous liquid, 98%, 279 mg. 1H NMR (300 MHz, CDCl3): δ = 7.51 (dd, J = 7.4 Hz, J = 1.6 Hz, 1H), 7.44 (td, J = 8.1 Hz, J = 1.7 Hz, 1H), 7.36–7.23 (m, 1H), 7.16–7.08 (m, 3H), 5.52 (s, 2H), 3.90 (s, 3H) ppm. 13C NMR (75 MHz, CDCl3): δ = 158.7 (d, J = 238.7 Hz, Cquat), 156.9 (Cquat), 136.0 (Cquat), 134.1 (Cquat), 131.4 (Ar-CH), 130.0 (Ar-CH), 129.1 (2Ar-CH), 128.3 (Ar-CH), 127.0 (2Ar-CH), 126.4 (d, J = 10.5 Hz, Cquat), 124.2 (d, J = 5.5 Hz, Cquat), 121.0 (Ar-CH), 120.1 (Cquat), 115.2 (d, J = 26.8 Hz, Ar-CH), 113.7 (Cquat), 111.8, (d, J = 9.4 Hz, Ar-CH), 111.5 (Ar-CH), 110.8 (Cquat), 106.8 (d, J = 24.1 Hz, Ar-CH), 55.4 (OCH3), 49.5 (CH2) ppm. 19F NMR (282 MHz, CDCl3): δ = −121.7 ppm. HRMS (ESI): calcd. for C23H18FN2O (M + H)+ 357.14032; found 357.14081.
3.2.8. General Procedure for Heck Coupling (Series 10)
Compound 7a (300 mg, 0.083 mmol, 1 equiv.), alkene (1.2 mmol, 1.5 equiv), KOAc (50 mg, 0.5 mmol, 6 equiv.), Pd(OAc)2 (0.74 mg, 0.003 mmol, 0.04 equiv.), tBuNH4Cl(46 mg, 0.166 mmol, 2 equiv.) and DMF (5 mL) were introduced in a sealed tube. The tube was vacuumed and backfilled with argon. The reaction mixture was stirred at 80 °C for 24 h and then cooled to room temperature. The reaction mixture was quenched with water, filtered through a Celite© pad and washed with EtOAc (100 mL). Then, the resulting organic layer was washed with sat. aq. NH4Cl (2 × 40 mL) and brine (3 × 40 mL). The organic layers were dried over MgSO4 and concentrated under reduced pressure. The crude product was purified by column chromatography using petroleum ether/EtOAc as eluent (70:30).
(E)-Methyl-3-(1-benzyl-2-cyano-1H-indol-3-yl) acrylate (10a): Brown solid, 92%, 244 mg. 1H NMR (300 MHz, CDCl3): δ = 7.99 (d, J = 16.2 Hz, 1H, CH=CH), 7.95 (dt, J = 8.2 Hz, J = 0.9 Hz, 1H, Ar-H), 7.46–7.30 (m, 6H, Ar-H), 7.18 (m, 2H, Ar-H), 6.76 (d, J = 16.2 Hz, 1H, CH=CH), 5.50 (s, 2H, CH2), 3.85 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 167.5 (Cquat), 138.1 (Cquat), 135.3 (Cquat), 134.0 (CH=CH), 129.2 (2Ar-CH), 128.5 (Ar-CH), 127.1 (Ar-CH), 127.0 (2Ar-CH), 124.9 (Cquat), 123.2 (Ar-CH), 121.8 (Cquat), 121.6 (Ar-CH), 119.1 (CH=CH), 112.8 (Cquat), 111.7 (Cquat), 111.5 (Ar-CH), 52.0 (OCH3), 49.6 (CH2) ppm. HRMS (ESI): calcd. for C20H17N2O2 (M + H)+ 317.12900; found 317.12946.
(E)-1-Benzyl-3-styryl-1H-indole-2-carbonitrile (10b): Brown solid, 89%, 257 mg. 1H NMR (300 MHz, CDCl3): δ = 8.06 (dt, J = 8.1 Hz, J = 0.7 Hz, 1H), 7.66–7.58 (m, 2H, Ar-H), 7.51 (d, J = 16.5 Hz, CH=CH), 7.45–7.29 (m, 10H, 9Ar-H + CH=CH), 7.23–7.20 (m, 2H, Ar-H), 5.45 (s, 2H, CH2) ppm. 13C NMR (75 MHz, CDCl3): δ = 138.1 (Cquat), 137.3 (Cquat), 135.9 (Cquat), 131.2 (CH=CH), 129.1 (2Ar-CH), 128.9 (2ArCH), 128.2 (Ar-CH), 128.1 (Ar-CH), 126.9 (2Ar-CH), 126.6 (Ar-CH), 126.5 (2Ar-CH), 125.2 (Cquat), 124.6 (Cquat), 122.0 (CH=CH), 121.7 (Ar-CH), 118.4 (Ar-CH), 113.9 (Cquat), 111.0 (Ar-CH), 108.6 (Cquat), 49.1 (CH2) ppm. HRMS (ESI): calcd. for C24H19N2 (M + H)+ 335.15482; found 335.15532.
1-Benzyl-3-(3-cyanoprop-1-en-1-yl)-1H-indole-2-carbonitrile (10c): Brown solid, 81%, 192 mg. 1H NMR (300 MHz, CDCl3): δ = 8.09 (d, J = 8.1 Hz, 1H, Z-isomer), 7.79 (d, J = 8.2 Hz, 1H, E-isomer), 7.61 (d, J = 16.7 Hz, 1H, E-isomer), 7.52–7.29 (m, Ar-CH), 7.23–7.18 (m, Ar-CH), 6.14 (d, J = 16.7 Hz, 1H, E-isomer), 5.64 (d, J = 12.1 Hz, 1H, Z-isomer), 5.48 (s, 2H) ppm. 13C NMR E-isomer (75 MHz, CDCl3): δ = 139.5 (CH=CH), 137.9 (Cquat), 134.9 (Cquat), 129.2 (2Ar-CH), 128.6 (Ar-CH), 127.4 (Ar-CH), 126.9 (2Ar-CH), 124.1 (Cquat), 123.7 (Ar-CH), 120.8 (Ar-CH), 120.7 (Cquat), 118.3 (Cquat), 111.2 (Cquat), 111.7 (Ar-CH), 111.3 (Cquat), 96.9 (CH=CH), 49.6 (CH2) ppm. HRMS (ESI): calcd. for C19H14N3 (M + H)+ 284.11877; found 284.11842.
(E)-Methyl-3-(1-benzyl-2-cyano-5-methoxy-1H-indol-3-yl) acrylate (10d): Brown solid, 88%, 236 mg. 1H NMR (300 MHz, CDCl3): δ = 7.97 (d, J = 16.2 Hz, 1H, CH=CH), 7.36–7.28 (m, 3H, Ar-CH), 7.27–7.23 (m, 2H, Ar-CH), 7.16 (dd, J = 7.5 Hz, J = 2.0 Hz, 2H, Ar-CH), 7.06 (dd, J = 9.2 Hz, J = 2.3 Hz, 1H, Ar-CH), 6.69 (d, J = 16.2 Hz, 1H, CH=CH) 5.46 (s, 2H, CH2), 3.88 (s, 3H, OCH3), 3.85 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 167.6 (Cquat), 156.6 (Cquat), 135.3 (Cquat), 134.1 (CH=CH), 133.2 (Cquat), 129.2 (2Ar-CH), 128.5 (Ar-CH), 126.9 (2Ar-CH), 125.5 (Cquat), 120.8 (Cquat), 118.4 (Ar-CH), 118.1 (Ar-CH), 112.9 (Cquat), 112.4 (Ar-CH), 111.3 (Cquat), 101.6 (Ar-CH), 55.9 (OCH3), 51.9 (OCH3), 49.7 (CH2) ppm. HRMS (ESI): calcd. for C21H19N2O3 (M + H)+ 347.13957; found 347.14053.
(E)-Methyl-3-(1-benzyl-2-cyano-6-methoxy-1H-indol-3-yl) acrylate (10e): Brown solid, 92%, 247 mg. 1H NMR (300 MHz, CDCl3): δ = 7.90 (d, J = 16.1 Hz, 1H, CH=CH), 7.76 (d, J = 9.0 Hz, 1H, Ar-CH), 7.35–7.28 (m, 3H, Ar-CH), 7.21–7.13 (m, 2H, Ar-CH),6.94 (dd, J = 8.9 Hz, J = 2.0 Hz, 1H, Ar-CH), 6.71 (s, 1H, Ar-CH), 6.68 (d, J = 16.1 Hz, 1H, CH=CH), 5.40 (s, 2H, CH2), 3.83 (s, 3H, OCH3), 3.80 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 167.4 (Cquat), 159.9 (Cquat), 139.3 (Cquat), 135.2 (Cquat), 134.6 (Cquat), 133.9 (CH=CH), 129.2 (2Ar-CH), 128.4 (Ar-CH), 126.9 (2Ar-CH), 122.2 (Ar-CH), 122.0 (Cquat), 118.8 (Ar-CH), 113.9 (Ar-CH), 113.0 (Cquat), 110.4 (Cquat), 93.6 (CH=CH), 55.6 (OCH3), 51.8 (OCH3), 49.3 (CH2) ppm. HRMS (ESI): calcd. for C21H19N2O3 (M + H)+ 347.13957; found 347.14046.
(E)-Methyl-3-(1-benzyl-2-cyano-5-fluoro-1H-indol-3-yl)acrylate (10f): Brown solid, 81%, 215 mg. 1H NMR (300 MHz, CDCl3): δ = 7.92 (d, J = 16.2 Hz, 1H, CH=CH), 7.57 (dd, J = 9.1 Hz, J = 2.4 Hz, 1H, Ar-CH), 7.40–7.27 (m, 4H, Ar-CH), 7.22–7.12 (m, 3H, Ar-CH), 6.67 (d, J = 16.2 Hz, 1H, CH=CH), 5.48 (s, 2H, CH2), 3.85 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 167.3 (Cquat), 159.6 (d, J = 241.4 Hz, Cquat), 134.6 (Cquat), 133.5 (CH=CH), 129.3 (2Ar-CH), 128.7 (Ar-CH), 126.9 (2Ar-CH), 125.2 (d, J = 10.1 Hz, Cquat), 121.4 (d, J = 5.3 Hz, Cquat), 119.2 (CH=CH), 116.2 (d, J = 26.8 Hz, Ar-CH), 112.9 (Cquat), 112.7 (d, J = 9.6 Hz, Ar-CH), 112.4 (Cquat), 106.5 (d, J = 24.5 Hz, Ar-CH), 52.0 (OCH3), 49.9 (CH2) ppm. 19F NMR (282 MHz, CDCl3): δ = −118.71 ppm. HRMS (ESI): calcd. for C20H16FN2O2 (M + H)+ 335.11958; found 335.12021.
3.2.9. General Procedure for Stille Coupling (Series 11)
A Schlenk flask was charged with compound 7a (300 mg, 0.84 mmol, 1 equiv.), DMF (5 mL), vinyl tin derivatives (1.008 mmol, 1.2 equiv.) and PdCl2(MeCN)2 (21.8 mg, 0.084 mmol, 10 mol%) under argon. The mixture was stirred at 40 °C for 1 week. Then, the reaction mixture was added to a mixture of KF (2 M) and ethyl acetate (50:50), stirred for 30 min at room temperature and filtered through a Celite© pad. Solvents were removed under reduced pressure, and the crude product was purified by column chromatography with a stationary phase composed of 10% powdered anhydrous K2CO3 and silica, using petroleum ether/ethyl acetate as eluent (99.9:0.1).
(E)-1-Benzyl-3-(3-methylbut-1-en-1-yl)-1H-indole-2-carbonitrile (11b): Brown oil, 35%, 88 mg. 1H NMR (300 MHz, CDCl3): δ = 7.90 (dt, J = 8.2 Hz, J = 0.9 Hz, Hz, 1H), 7.39–7.26 (m, 5H, Ar-CH), 7.25–7.13 (m, 3H, Ar-CH), 6.63 (d, J = 16.2 Hz, 1H, CH=CH), 6.54 (dd, J = 16.2 Hz, J = 6.2 Hz, 1H, CH=CH), 5.44 (s, 2H, CH2), 2.56 (oct, J = 6.6 Hz, 1H, CH(CH3)2), 1.16 (d, J = 6.7 Hz, 6H, 2xCH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 141.8 (CH=CH), 138.1 (Cquat), 136.2 (Cquat), 129.1 (2Ar-CH), 128.2 (Ar-CH), 127.0 (Cquat), 126.9 (2Ar-CH), 126.5 (Ar-CH), 125.8 (Cquat), 124.8 (Cquat), 121.8 (Ar-CH), 121.6 (CH=CH), 116.9 (Ar-CH), 114.1 (Cquat), 110.9 (Ar-CH), 49.0 (CH2), 32.5 (CH(CH3)2), 22.6 (2xCH3) ppm. HRMS (ESI): calcd. for C21H21N2 (M + H)+ 301.17047; found 301.17038.
(E)-1-Benzyl-3-(2-(trimethylsilyl)vinyl)-1H-indole-2-carbonitrile (11c): Orange oil, 40%, 111 mg. 1H NMR (300 MHz, CDCl3): δ = 8.01 (dt, J = 8.2 Hz,J = 0.8 Hz, 1H, Ar-CH), 7.41–7.23 (m, 6H, Ar-CH), 7.17 (d, J = 19.6 Hz, 1H, CH=CH), 7.16 (dd, J = 7.9 Hz, J = 2.2 Hz, 2H, Ar-CH), 6.82 (d, J = 19.5 Hz, 1H, CH=CH), 5.45 (s, 2H), 0.22 (s, 9H, 3xCH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 138.0 (Cquat), 135.9 (Cquat), 133.6 (CH=CH), 133.0 (Ar-CH), 129.0 (2Ar-CH), 128.2 (Ar-CH), 126.8 (2Ar-CH), 126.5 (Ar-CH), 124.7 (Cquat), 117.0 (Cquat), 113.8 (Cquat), 110.9 (Ar-CH), 108.5 (Cquat), 49.0 (CH2), −1.16 (3xCH3) ppm. HRMS (ESI): calcd. for C21H23N2Si (M + H)+ 331.16305; found 335.16298.
3.2.10. Typical Procedure for Addition of n-BuLi on Compound 8b
A suspension of compound 8b (300 mg, 0.865 mmol) in THF (15 mL) was introduced into a dry, single-necked, round-bottomed flask under argon. The suspension was vigorously stirred, and n-BuLi (2 mmol, 2.2 equiv., 0.17 mL, 1.6 M in hexane) was added at −78 °C. The mixture was stirred magnetically under argon for 4h at room temperature. Then, ZnI2 (276mg, 0.865 mmol, 1 equiv.) and I2 (241 mg, 0.95 mmol, 1.1 equiv.) were added at −78 °C, and the suspension was stirred overnight under argon at rt. The mixture was basified with 2 N aq NaOH (10 mL), extracted with EtOAc (10 mL) and washed with 5% aq Na2S2O3 (10 mL). The organic layer was dried (MgSO4), filtered and concentrated under reduced pressure. The residue was purified by chromatography on silica gel.
1-(1-Benzyl-3-(p-tolylethynyl)-1H-indol-2-yl)pentan-1-one (8b’): Orange solid, 78.0%, 281 mg. 1H NMR (300 MHz, CDCl3): δ = 7.95 (dt, J = 7.9 Hz,J = 0.9 Hz, 1H, Ar-CH), 7.51–7.40 (m, 2H, Ar-CH), 7.30–7.20 (m, 6H, Ar-CH), 7.05–7.02 (m, 2H, Ar-CH), 5.84 (s, 2H, CH2), 3.39 (t, J = 7.3 Hz, 2H, CH2), 2.41 (s, 3H, CH3), 1.72 (q, J = 7.5 Hz, 2H, CH2), 1.37 (sext, J = 7.6 Hz, 2H, CH2), 0.91 (t, J = 7.3 Hz, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δ = 195.2 (Cquat), 138.8 (Cquat), 138.7 (Cquat), 138.2 (Cquat), 135.8 (Cquat), 131.3 (2Ar-CH), 129.4 (2Ar-CH), 128.7 (2Ar-CH), 128.5 (Cquat), 127.3 (Ar-CH), 126.9 (Ar-CH), 126.5 (2Ar-CH), 122.2 (Ar-CH), 121.8 (Ar-CH), 120.5 (Cquat), 111.1 (Ar-CH), 105.9 (Cquat), 98.5 (Cquat), 85.8 (Cquat), 48.7 (CH2), 42.4 (CH2), 27.0 (CH2), 22.6 (CH2), 21.7 (CH3), 14.1 (CH3) ppm. HRMS (ESI): calcd. for C29H28NO (M + H)+ 406.21709; found 406.21720.
4. Conclusions
In summary, we have developed a palladium-catalyzed homocoupling reaction of heteroatoms through C–I bond functionalization using different alkynes, alkenes and aryl derivatives. This approach offers a simple strategy and alternative route for the preparation of heteroaryl nitriles from easily available precursors in good to excellent yields. The present work allows access to novel diversely polysubstituted cyanoindoles via Sonogashira, Suzuki–Miyaura, Heck and Stille cross-coupling reactions.
Supplementary Materials
The following are available online, File S1: 1H- and 13C-NMR or J-mod spectra for compounds 3a–d, 4a, 5a–f, 6a–d, 7a–d, 8a–j, 9a–p, 10a–f, 11a–c and 8b’.
Author Contributions
Conceptualization, A.H.; data curation, Y.C.; formal analysis, A.H., M.R.-Y. and Y.C.; investigation, J.T.; methodology, A.H. and M.C.; project administration, J.T.; validation, Y.C.; resources, J.T.; writing—original draft preparation, J.T., A.H. and M.C.; writing—review and editing, J.T.; visualization, J.T. and Y.C.; supervision, J.T. All authors discussed the results and commented on the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this sudy ara available in Supplementary Materials.
Acknowledgments
We acknowledge Frédéric Montigny (Analysis Department, Tours University) for HRMS analysis. We acknowledge the French Ministry for Research and Innovation for financial support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
Sample Availability
Samples of the compounds 3a–d, 4a, 5a–f, 6a–d, 7a–d, 8a–j, 9a–p, 10a–f, 11a–c and 8b’ are available from the authors.
References
- Humphrey, G.R.; Kuethe, J.T. Practical methodologies for the synthesis of indoles. Chem. Rev. 2006, 106, 2875–2911. [Google Scholar] [CrossRef]
- Katayama, S.; Ae, N.; Nagata, R. Synthesis of tricyclic indole-2-carboxylic acids as potent NMDA-glycine antagonists. J. Org. Chem. 2001, 66, 3474–3483. [Google Scholar] [CrossRef]
- Leboho, T.C.; Michael, J.P.; van Otterlo, W.A.L.; van Vuuren, S.F.; de Koning, C.B. The synthesis of 2- and 3-aryl indoles and 1,3,4,5-tetrahydropyran[4,3-b]indoles and their antibacterial and antifungal activity. Bioorg. Med. Chem. Lett. 2009, 19, 4948–4951. [Google Scholar] [CrossRef]
- Lounasma, M.; Tolvanen, A. Simple indole alkaloids and those with a nonrearranged monoterpenoid unit. Nat. Prod. Rep. 2000, 17, 175–191. [Google Scholar]
- Chen, Z.; Zeng, X.; Yan, B.; Zhao, Y.; Fu, Y. Au-Catalyzed intramolecular annulations toward fused tricyclic [1,3]oxazino[3,4-a]indol-1-ones under extremely mild conditions. RSC Adv. 2015, 5, 100251–100255. [Google Scholar] [CrossRef]
- de Sa alves, F.R.; Barreiro, E.J.; Fraga, C.A.M. From nature to drug discovery: The indole scaffold as a ‘privileged structure’. Mini Rev. Med. Chem. 2009, 9, 782–793. [Google Scholar] [CrossRef]
- Chada, N.; Silakari, O. Indoles as therapeutics of interest in medicinal chemistry: Bird’s eye view. Eur. J. Med. Chem. 2017, 134, 159–184. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, R.K. Medicinal chemistry of indole derivatives: Current to future therapeutic prospectives. Bioorg. Chem. 2019, 89, 103021. [Google Scholar] [CrossRef]
- Kovacikova, L.; Prnva, M.S.; Majekova, M.; Bohac, A.; Karasu, C.; Stefek, M. Development of Novel indole-based bifunctional aldose reductase inhibitors/antioxydants as promising drugs for the treatment of diabetic complications. Molecules 2021, 26, 2867. [Google Scholar] [CrossRef]
- Festa, A.A.; Zalte, R.R.; Golantsov, N.E.; Varlamov, A.V.; Van der Eycken, E.V.; Voskressensky, L.G. DBU-Catalyzed alkyne-imidate cyclization toward 1-alkoxypyrazino[1,2-a] indolesynthesis. J. Org. Chem. 2018, 83, 9305–9311. [Google Scholar] [CrossRef]
- Jaisankar, P.; Srinivasan, P.A. Facile synthesis of 1-phenylsulfonyl-3-substituted-2-cyanoindoles, 1-phenylsulfonyl-2-methyl-3-cyanoindoles, and bifunctional 1-phenyl sulfonylindoles. Synthesis 2006, 14, 2413–2417. [Google Scholar]
- Golantsov, N.E.; Karchava, A.V.; Nosova, V.M.; Yurovskaya, M.A. Stereoselective synthesis of 4-substituted 1,2,3,4,10,10a-hexahydropyrazino[1,2-a]indoles. Russ. Chem. Bull. 2005, 54, 226–230. [Google Scholar] [CrossRef]
- Liang, L.-N.; An, R.; Huang, T.; Xu, M.; Hao, X.-J.; Pan, W.-D.; Liu, S. A simple approach for the syntheses of rutaecarpine and its analogs. Tetrahedron Lett. 2015, 56, 2466–2468. [Google Scholar] [CrossRef]
- Almagro, L.; Fernández-Pérez, F.; Pedreño, M.A. Indole alkaloids from catharanthus roseus: Bioproduction and their effect on human health. Molecules 2015, 20, 2973–3000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hys, V.Y.; Milokhov, D.S.; Keda, T.Y.; Omelchenko, I.V.; Konovalova, I.S.; Shishkina, S.V.; Volovenko, Y.M. Efficient synthesis of seven-membered aza-sultams: Heterofused amino-1,2,4-thiadiazepine dioxides. Tetrahedron 2021, 88, 132149. [Google Scholar] [CrossRef]
- Iioka, R.; Yorozu, K.; Sakai, Y.; Kawai, R.; Hatae, N.; Takashima, K.; Tanabe, G.; Wasada, H.; Yoshimatsu, M. Synthesis of azepino[1,2-a]indole-10-amines via [6+1] annulation of ynenitriles with reformatsky reagent. Eur. J. Org. Chem. 2021, 10, 1553–1558. [Google Scholar] [CrossRef]
- Zalte, R.R.; Festa, A.A.; Golantsov, N.E.; Subramani, K.; Rybakov, V.B.; Varlamov, A.V.; Luque, R.; Voskressensky, L.G. Aza-henry and aza-knoevenagel reactions of nitriles for the synthesis of pyrido[1,2-a]indoles. Chem. Commun. 2020, 56, 6527–6530. [Google Scholar] [CrossRef]
- Festa, A.; Golantsov, N.; Storozhenko, O.; Shumsky, A.; Varlamov, A.; Voskressensky, L. Alcohol-Initiated dinitrile cyclization in basic media: A route toward pyrazino[1,2-a]indole-3-amines. Synlett 2018, 29, 898–903. [Google Scholar]
- Reddy, G.; Kumar, P.; Anand, R.; Mukkanti, K.; Reddy, P. An expedient entry to fused [1,2,5]-triazepinederivatives: Extension to fused [1,2,5]-oxadiazinederivatives, a new class of seven-membered heterocycles. Synlett 2009, 9, 1463–1465. [Google Scholar] [CrossRef]
- Moll, A.; Hübner, H.; Gmeiner, P.; Troschütz, R. Phenylpiperazinylmethylindolecarboxylates and derivatives as selective D4-ligands. Bioorg. Med. Chem. 2002, 10, 1671–1679. [Google Scholar] [CrossRef]
- Heinz, F.; Lyothier, I.; Pothier, J.; Richard-Bildstein, S.; Thierry, S. N-Substituted Indole Derivatives. Patent WO2018210995, 22 November 2018. [Google Scholar]
- Stenzel, W.; Armah, B. Preparation of N-Diarylmethyl-3-[(2-cyanoindol-4-yl)aminopropoxy]azetidines as Cardiovascular agents. Patent DE4002391, 1 August 1991. [Google Scholar]
- Lopchuk, J.M.; Montgomery, W.L.; Jasinski, J.P.; Gorjifard, S.; Gribble, G.W. Manganese(III)-mediated oxidative radical addition of malonates to 2-cyanoindoles. Tetrahedron Lett. 2013, 54, 6142–6145. [Google Scholar] [CrossRef]
- Huang, L.; Wei, Y.; Shi, M. Asymmetric substitutions of o-boc-protected morita–baylis–hillman adducts with pyrrole and indole derivatives. Org. Biomol. Chem. 2012, 10, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Borza, I.; Kolok, S.; Ignácz-Szendrei, G.; Greiner, I.; Tárkányi, G.; Galgóczy, K.; Horváth, C.; Farkas, S. Domány, G. Indole-2-carboxamidines as novel NR2B selective NMDA receptor antagonists. Bioorg. Med. Chem. Lett. 2005, 15, 5439–5441. [Google Scholar] [CrossRef]
- Kaboudin, B.; Esfandiari, H.; Moradi, A.; Kazemi, F.; Aoyama, H. ZnCl2-Mediated double addition of dialkylphosphite to nitriles for the synthesis of 1-aminobisphosphonates. J. Org. Chem. 2019, 84, 14943–14948. [Google Scholar] [CrossRef] [PubMed]
- Woodroofe, C.C.; Meisenheimer, P.L.; Klaubert, D.H.; Kovic, Y.; Rosenberg, J.C.; Behney, C.E.; Southworth, T.L.; Branchini, B.R. Novel heterocyclic analogues of firefly luciferin. Biochemistry 2012, 51, 9807–9813. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, C.-M.; Wang, Z.; Chen, J.; Mohler, M.L.; Li, W.; Dalton, J.T.; Miller, D.D. Design, synthesis, and SAR studies of 4-substituted methoxylbenzoyl-aryl-thiazoles analogues as potent and orally bioavailable anticancer agents. J. Med. Chem. 2011, 54, 4678–4693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larock, R.C. Comprehensive Organic Transformations: A Guide to Functional Group Preparations; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Zhao, M.; Zhang, W.; Shen, Z. Cu-Catalyzed cyanation of indoles with acetonitrile as a cyano source. J. Org. Chem. 2015, 80, 8868–8873. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Huang, X.; Hong, X.; Xu, B. Palladium-assisted regioselective C-H cyanation of heteroarenes using isonitrile as cyanide source. Org. Lett. 2012, 14, 4614–4617. [Google Scholar] [CrossRef]
- Nagase, Y.; Sugiyama, T.; Nomiyama, S.; Yonekura, K.; Tsuchimoto, T. Zinc-Catalyzed direct cyanation of indoles and pyrroles: Nitromethane as a source of a cyano group. Adv. Synth. Catal. 2014, 356, 347–352. [Google Scholar] [CrossRef]
- Yuen, O.Y.; Choy, P.Y.; Chow, W.K.; Wong, W.T.; Kwong, F.Y. Synthesis of 3-cyanoindole derivatives mediated by copper(I) iodide using benzyl cyanide. J. Org. Chem. 2013, 78, 3374–3378. [Google Scholar] [CrossRef]
- Liu, W.; Richter, S.C.; Mei, R.; Feldt, M.; Ackermann, L. Synergistic heterobimetallic manifold for expedient manganese(I)-catalyzed C-H cyanation. Chem. Eur. J. 2016, 22, 17958–17961. [Google Scholar] [CrossRef]
- Zeidan, N.; Bognar, S.; Lee, S.; Lautens, M. Palladium-Catalyzed synthesis of 2-cyanoindoles from 2-gem-dihalovinylanilines. Org. Lett. 2017, 19, 5058–5061. [Google Scholar] [CrossRef]
- Cohen, D.T.; Buchwald, S.L. Mild palladium-catalyzed cyanation of (hetero)aryl halides and triflates in aqueous media. Org. Lett. 2015, 17, 202–205. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Castillo, P.; Buchwald, S.L. Applications of palladium-catalyzed C-N cross-coupling reactions. Chem. Rev. 2016, 116, 12564–12649. [Google Scholar] [CrossRef]
- Sain, S.; Jain, S.; Srivastava, M.; Vishwakarma, R.; Dwivedi, J. Application of palladium-catalyzed cross-coupling reactions in organic synthesis. Curr. Org. Chem. 2019, 16, 1105–1142. [Google Scholar] [CrossRef]
- McGlinchey, M.J.; Nikitin, K. Palladium-catalysed coupling reactions en route to molecular machines: Sterically hindered indenyl and ferrocenyl anthracenes and triptycenes, and biindenyls. Molecules 2020, 25, 1950. [Google Scholar] [CrossRef]
- Cacchi, S.; Fabrizi, G. Update 1: Synthesis and functionalization of indoles through palladium-catalyzed reactions. Chem. Rev. 2011, 111, PR215–PR283. [Google Scholar] [CrossRef]
- Torborg, C.; Beller, M. Recent applications of palladium-catalyzed coupling reactions in the pharmaceutical, agrochemical, and fine chemical industries. Adv. Synth. Catal. 2009, 351, 3027–3043. [Google Scholar] [CrossRef]
- Khairul, W.M.; Daud, A.I.; Hanifaah, N.A.M.; Arshad, S.; Razak, I.A.; Zuki, H.M.; Erben, M.F. Structural study of a novel acetylide-thiourea derivative and its evaluation as a detector of benzene. J. Mol. Struct. 2017, 1139, 353–361. [Google Scholar] [CrossRef]
- Buchmeiser, M.R.; Schareina, T.; Kempe, R.; Wurst, K. Bis(pyrimidine)-based palladium catalysts: Synthesis, X-ray structure and applications in Heck-, Suzuki-, Sonogashira-Hagihara couplings and amination reactions. J. Organomet. Chem. 2001, 634, 39–46. [Google Scholar] [CrossRef]
- Durust, Y.; Sagirli, A.; Kariuki, B.M.; Knight, D.W. [1,3]-Dipolar cycloaddition of N-aryl sydnones to benzothiophene 1,1-dioxide, 1-cyclopropylprop-2-yn-1-ol and 1-(prop-2-ynyl)-1H-indole. Tetrahedron 2014, 70, 6012–6019. [Google Scholar] [CrossRef]
- Sakamoto, T.; Nagano, T.; Kondo, Y.; Yamanaka, H. Palladium-catalyzed coupling reaction of 3-iodoindoles and 3-iodobenzo[b]thiophene with terminal acetylenes. Chem. Pharm. Bull. 1988, 36, 2248–2252. [Google Scholar] [CrossRef] [Green Version]
- Inack-Ngi, S.; Guilloteau, V.; Abarbri, M.; Thibonnet, J. Regioselective copper-mediated synthesis of thieno[2,3-c]pyrane-7-one, indolo[2,3-c]pyrane-1-one, and indolo[3,2-c]pyrane-1-one. J. Org. Chem. 2011, 76, 8347–8354. [Google Scholar] [CrossRef] [PubMed]
- Evano, G.; Blanchard, N.; Toumi, M. Copper-mediated coupling reactions and their applications in natural products and designed biomolecules synthesis. Chem. Rev. 2008, 108, 3054–3131. [Google Scholar] [CrossRef]
- Liang, B.; Dai, M.; Chen, J.; Yang, Z. Copper-free sonogashira coupling reaction with PdCl2 in water under aerobic conditions. J. Org. Chem. 2005, 70, 391–393. [Google Scholar] [CrossRef]
- Enguehard-Gueiffier, C.; Delaye, P.-O.; Penichon, M.; Denevault-Sabourin, C.; Allouchi, H.; Gueiffier, A. Domino 6-endo-dig cyclization/halogenation reactions: Three-component synthesis of 1,3-disubstituted 4-haloimidazo[1,2-a:4,5-c′]dipyridines. Synthesis 2015, 24, 3983–3989. [Google Scholar] [CrossRef] [Green Version]
- Jana, R.; Pathak, T.P.; Sigman, M.S. Advances in transition metal (Pd,Ni,Fe)-catalyzed cross-coupling reactions using alkyl-organometallics as reaction partners. Chem. Rev. 2011, 111, 1417–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaumont, S.; Retailleau, P.; Dauban, P.; Dodd, R.H. Synthesis of indolobenzazepinones by application of an isocyanide-based multicomponent reaction. Eur. J. Org. Chem. 2008, 2008, 5162–5175. [Google Scholar] [CrossRef]
- Santra, S.; Hota, P.K.; Bhattacharyya, R.; Bera, P.; Ghosh, P.; Mandal, S.K. Palladium nanoparticles on graphite oxide: A recyclable catalyst for the synthesis of biaryl cores. ACS Catal. 2013, 3, 2776–2789. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Cheprakov, A.V. The Heck reaction as a sharpening stone of palladium catalysis. Chem. Rev. 2000, 100, 3009–3066. [Google Scholar] [CrossRef]
- Stille, J.K. The palladium-catalyzed cross-coupling reactions of organotin reagents with organic electrophiles. Angew. Chem. Int. Ed. 1986, 25, 508–524. [Google Scholar] [CrossRef]
- Hammoud, S.; Anselmi, E.; Cherry, K.; Kizirian, J.-C.; Thibonnet, J. Synthesis and reactivity of oxazinoindolones via regioselective 6-exo-dig iodolactonization. Eur. J. Org. Chem. 2018, 2018, 6314–6327. [Google Scholar] [CrossRef]
- Harrowven, D.C.; Curran, D.P.; Kostiuk, S.L.; Wallis-Guy, I.L.; Whiting, S.; Stenning, K.J.; Tang, B.; Packard, E.; Nanson, L. Potassium carbonate-silica: A highly effective stationary phase for the chromatographic removal of organotin impurities. Chem. Commun. 2010, 46, 6335–6337. [Google Scholar] [CrossRef] [PubMed]
- Armarego, W.L.F. Chai. In Purification of Laboratory Chemicals, 7th ed.; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Seyferth, D.; Stone, F.G.A. Vinyl derivatives of the metals. I. Synthesis of vinyltin compounds. J. Am. Chem. Soc. 1957, 79, 515–517. [Google Scholar] [CrossRef]
- Saihi, M.L.; Pereyre, M. Reactivity of vinylic organotin compounds towards acid chlorides and anhydrides and α-halo esters. Bulletin de la Société Chimique de France 1977, 9, 1251–1255. [Google Scholar] [CrossRef]
- Cunico, R.F.; Clayton, F.J. Trans-.beta.-trimethylsilylvinyllithium. J. Org. Chem. 1976, 41, 1480–1482. [Google Scholar] [CrossRef]
- Chong, J.M.; Park, S.B. Stereoselective synthesis of (E)-vinylstannanes from aldehydes. J. Org. Chem. 1993, 58, 523–527. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).