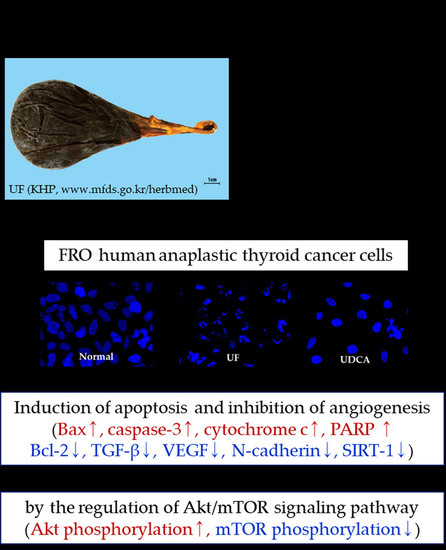
Anticancer Effects of Ursi Fel Extract and Its Active Compound, Ursodeoxycholic Acid, in FRO Anaplastic Thyroid Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of UF Extract
2.3. Cell Lines and Cell Culture
2.4. Cell Viability Assay
2.5. Flow Cytometry Analysis Using Annexin V and Propidium Iodide (PI) Staining
2.6. Hoechst Staining
2.7. Western Blot Analysis
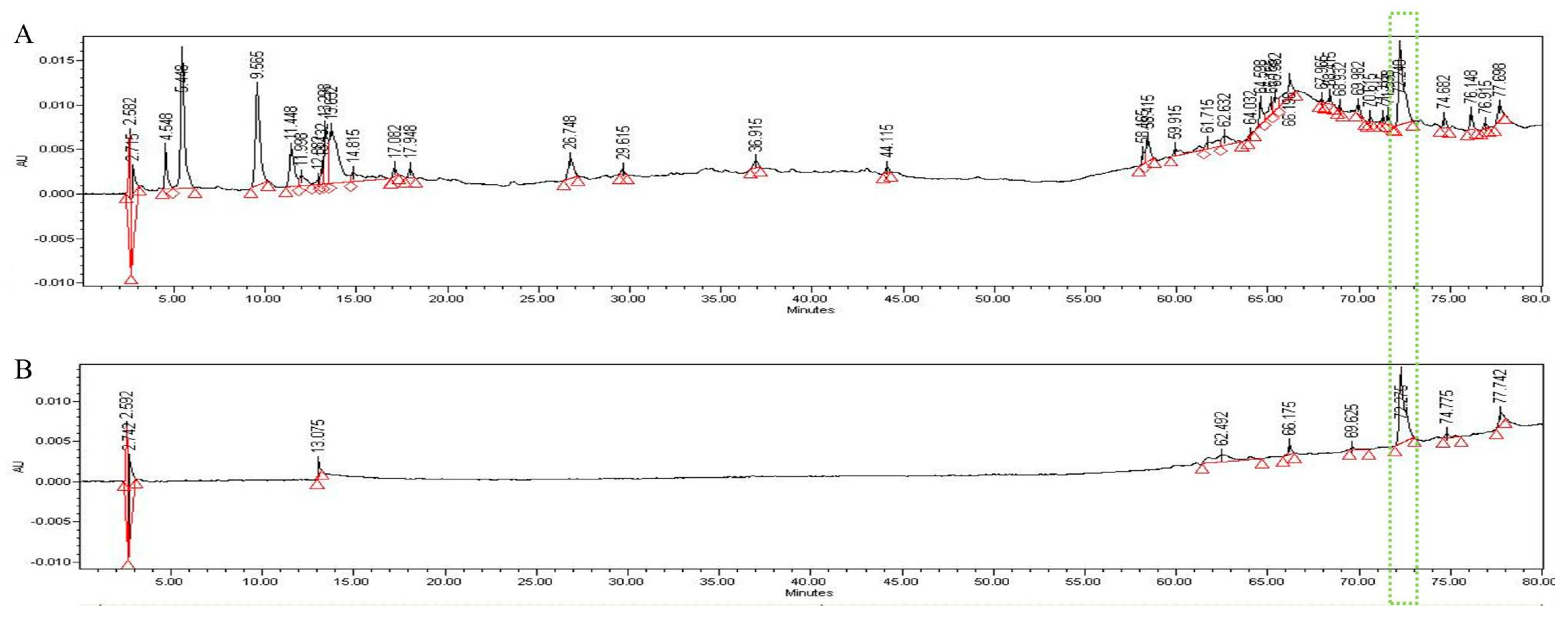
2.8. High-Performance Liquid Chromatography (HPLC) Analysis
2.9. Statistical Analysis
3. Results
3.1. Effects of UF Extract and UDCA on Cell Growth
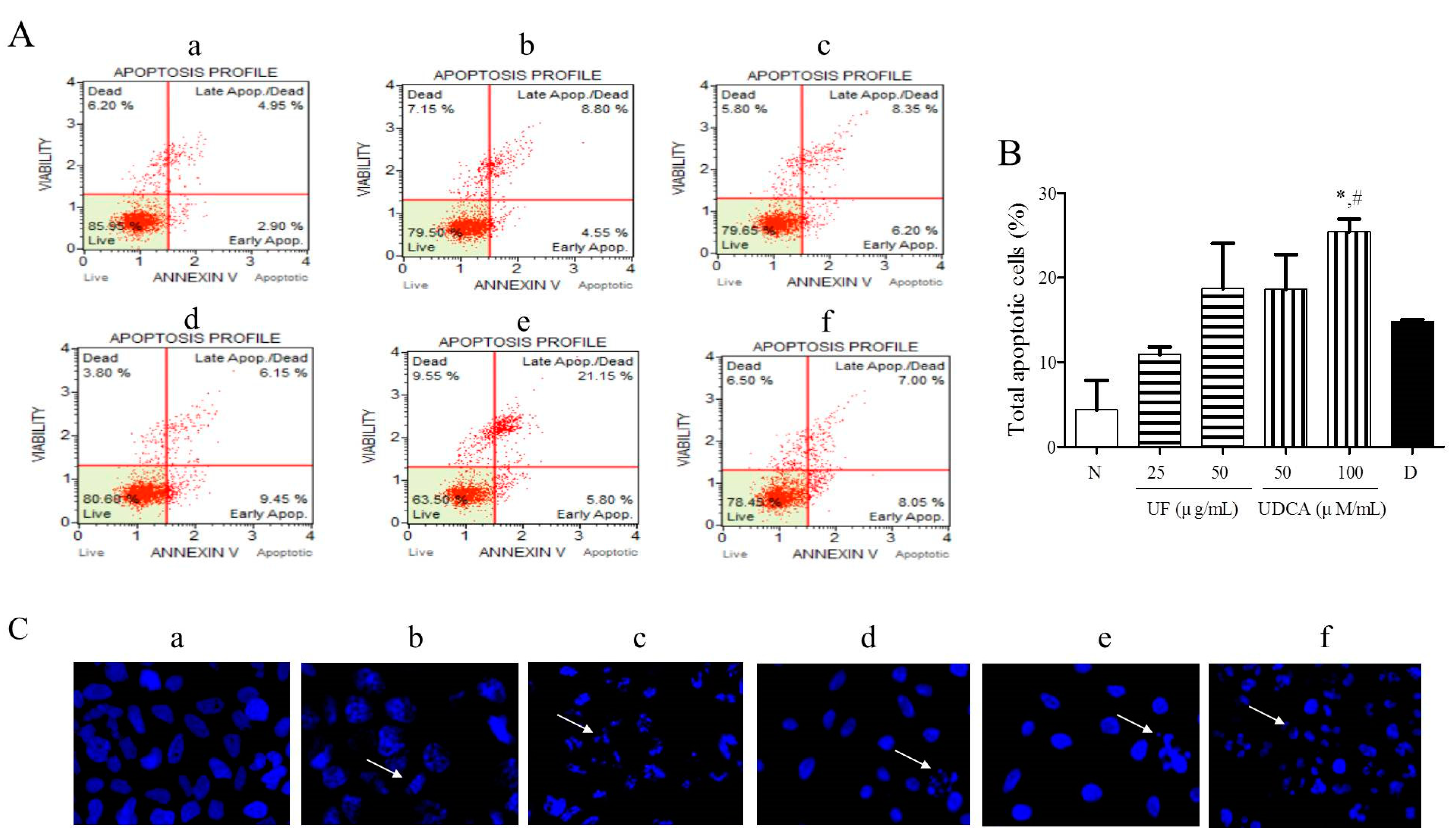
3.2. Effect of UF Extract and UDCA on Apoptosis
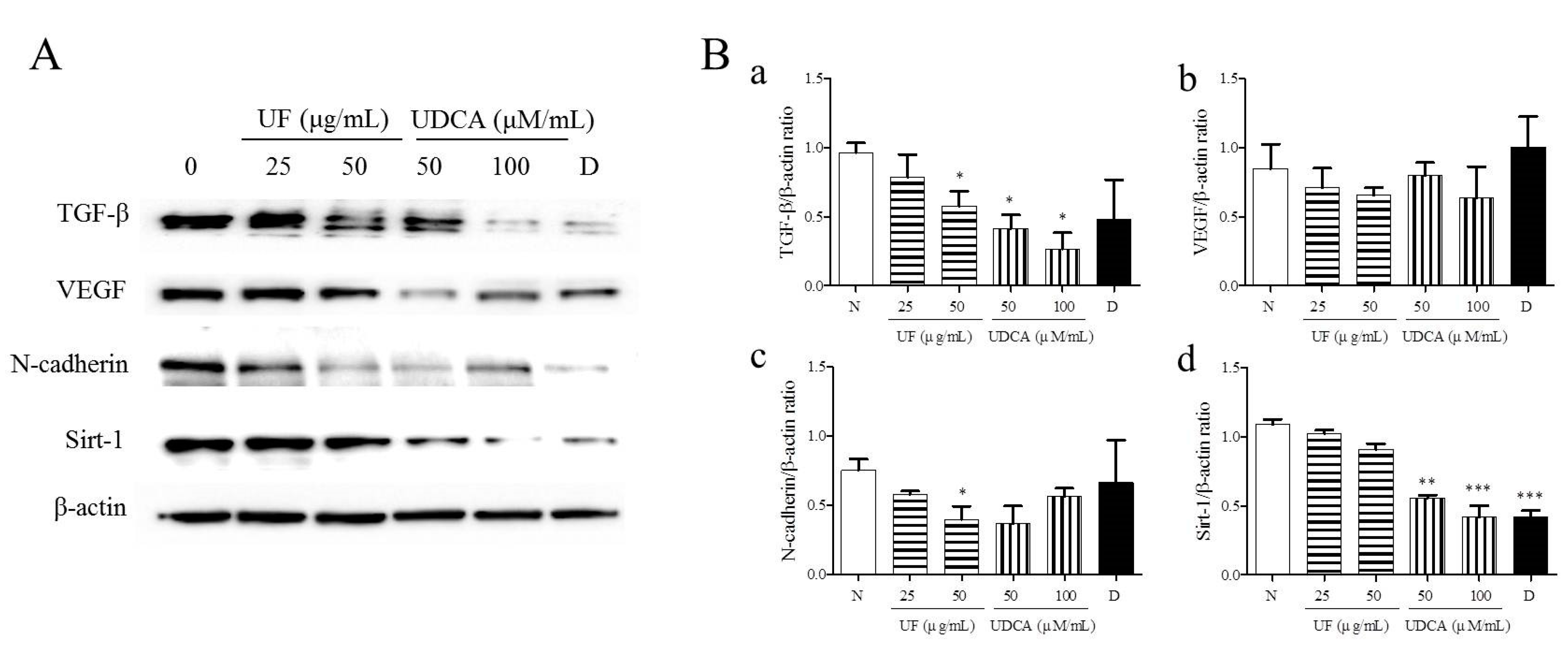
3.3. Effect of UF Extract and UDCA on the Expression of Angiogenic Regulators
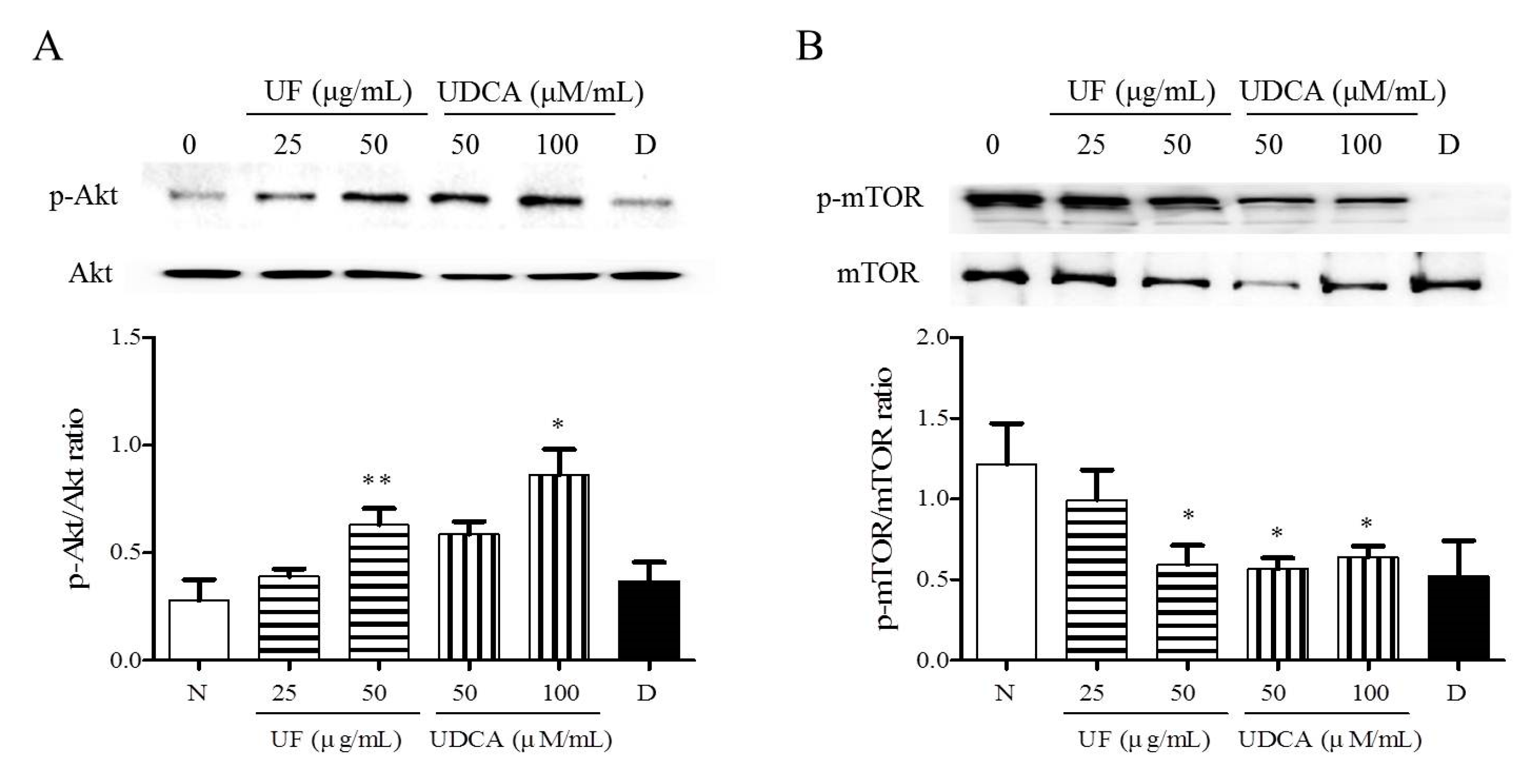
3.4. Effect of UF Extract and UDCA on the Akt/mTOR Pathway
3.5. HPLC Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gill, K.S.; Tassone, P.; Hamilton, J.; Hjelm, N.; Luginbuhl, A.; Cognetti, D.; Tuluc, M.; Martinez-Outschoorn, U.; Johnson, J.M.; Curry, J.M. Thyroid cancer metabolism: A review. J. Thyroid. Disord. Ther. 2016, 5, 200. [Google Scholar] [CrossRef]
- Lee, K.; Cassaro, S. Cancer, Thyroid. [Updated 2018 December 22]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459299/ (accessed on 1 March 2019).
- Keutgen, X.M.; Sadowski, S.M.; Kebebew, E. Management of anaplastic thyroid cancer. Gland. Surg. 2015, 4, 44–51. [Google Scholar]
- Lim, S.M.; Shin, S.J.; Chung, W.Y.; Park, C.S.; Nam, K.H.; Kang, S.W.; Keum, K.C.; Kim, J.H.; Cho, J.Y.; Hong, Y.K.; et al. Treatment outcome of patients with anaplastic thyroid cancer: A single center experience. Yonsei Med. J. 2012, 53, 352–357. [Google Scholar] [CrossRef]
- Matuszczyk, A.; Petersenn, S.; Voigt, W.; Kegel, T.; Dralle, H.; Schmoll, H.; Bockisch, A.; Mann, K. Chemotherapy with paclitaxel and gemcitabine in progressive medullary and thyroid carcinoma of the follicular epithelium. Horm. Metab. Res. 2010, 42, 61–64. [Google Scholar] [CrossRef]
- Bishayee, A.; Sethi, G. Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin. Cancer Biol. 2016, 40, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.M. Chinese Materia Medica Xiongdan; China Chinese Medicine Publisher: Beijing, China, 2002; pp. 160–161. [Google Scholar]
- The National College of Oriental Medicine Herbology Classroom. Herbology; Youngrimsa: Seoul, Korea, 2016. [Google Scholar]
- Feng, Y.; Siu, K.; Wang, N.; Ng, K.M.; Tsao, S.W.; Nagamatsu, T.; Tong, Y. Bear bile: Dilemma of traditional medicinal use and animal protection. J. Ethnobiol. Ethnomed. 2019, 5, 1–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, A.A.; Titorenko, V.I.; Beach, A.; Sanderson, J.T. Bile acids induce apoptosis selectively in androgen-dependent and -independent prostate cancer cells. PeerJ 2013, 1, e122. [Google Scholar] [CrossRef]
- Lim, S.C.; Choi, J.E.; Kang, H.S.; Han, S.I. Ursodeoxycholic acid switches oxaliplatin-induced necrosis to apoptosis by inhibiting reactive oxygen species production and activating p53-caspase 8 pathway in HepG2 hepatocellular carcinoma. Int. J. Cancer 2010, 126, 1582–1595. [Google Scholar] [CrossRef]
- Lim, S.C.; Duong, H.Q.; Choi, J.E.; Lee, T.B.; Kang, J.H.; Oh, S.H.; Han, S.I. Lipid raft-dependent death receptor 5 (DR5) expression and activation are critical for ursodeoxycholic acid-induced apoptosis in gastric cancer cells. Carcinogenesis 2011, 32, 723–731. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.S.; Jung, J.H.; Panchanathan, R.; Yun, J.W.; Kim, D.H.; Kim, H.J.; Kim, G.S.; Rhu, C.H.; Shin, S.C.; Hong, S.C.; et al. Ursodeoxycholic acid induces death receptor-mediated apoptosis in prostate cancer cells. J. Cancer Prev. 2017, 22, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessel, D.; Caruso, J.A.; Reiners, J.J., Jr. Potentiation of photodynamic therapy by ursodeoxycholic acid. Cancer 2000, 60, 6985–6988. [Google Scholar]
- Are, C.; Shaha, A.R. Anaplastic thyroid carcinoma: Biology, pathogenesis, prognostic factors, and treatment approaches. Ann. Surg. Oncol. 2016, 13, 453–464. [Google Scholar] [CrossRef]
- Park, J.W.; Choi, S.H.; Yoon, H.I.; Lee, J.; Kim, T.H.; Kim, J.W.; Lee, I.J. Treatment outcomes of radiotherapy for anaplastic thyroid cancer. Radiat. Oncol. J. 2018, 36, 103. [Google Scholar] [CrossRef] [Green Version]
- Onoda, N.; Kashiwagi, S.; Noda, S.; Kawajiri, H.; Takashima, T.; Ishikawa, T.; Hirakawa, K. High efficacy of chemoradiation therapy sensitized by weekly docetaxel for anaplastic thyroid cancer. Anticancer. Res. 2013, 33, 3445–3448. [Google Scholar]
- Smallridge, R.C.; Marlow, L.A.; Copland, J.A. Anaplastic thyroid cancer: Molecular pathogenesis and emerging therapies. Endocr.-Relat. Cancer 2009, 16, 17–44. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.Y.; Gao, X.; Tong, Z.L.; Wang, X.G. Summary on pharmacological action and clinical study of the chemical composition of bear bile. Inf. Tradit. Chin. Med. 2005, 22, 30–33. [Google Scholar]
- You, Y.Y.; Wan, D.G. Advance in research on medical use of animal bile. J. Guiyang Coll. Tradit. Chin. Med. 2007, 29, 58–61. [Google Scholar]
- Kaplan, M.M. The use of methotrexate, colchicine, and other immunomodulatory drugs in the treatment of primary biliary cirrhosis. Semin. Liver Dis. 1997, 17, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Attardi, L.D. The role of apoptosis in cancer de velopment and treatment response. Nat. Rev. Cancer 2005, 5, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.M.; Wang, S.; Zhang, H.; Lu, Y.Y.; Wang, X.F.; Motoo, Y.; Su, S.B. The combination of baicalin and baicalein enhances apoptosis via the ERK/p38 MAPK pathway in human breast cancer cells. Acta. Pharmacol. Sin. 2009, 30, 1648–1658. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Bruncko, M.; Oost, T.K.; Belli, B.A.; Ding, H.; Joseph, M.K.; Kunzer, A.; Martineau, D.; McClellan, W.J.; Mitten, M.; Ng, S.C.; et al. Studies leading to potent, dual inhibitors of Bcl- 2 and Bcl-xL. J. Med. Chem. 2007, 50, 641–662. [Google Scholar] [CrossRef] [PubMed]
- Majno, G.; Joris, I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am. J. Pathol. 1995, 146, 3–15. [Google Scholar] [PubMed]
- Mincione, G.; Tarantelli, C.; Vianale, G.; Di Marcantonio, M.C.; Cotellese, R.; Francomano, F.; Nicola, M.D.; Costantini, E.; Cichella, A.; Muraro, R. Mutual regulation of TGF-β1, TβRII and ErbB receptors expression in human thyroid carcinomas. Exp. Cell Res. 2014, 327, 24–36. [Google Scholar] [CrossRef]
- Sun, W.; Xu, Y.; Zhao, C.; Hao, F.; Chen, D.; Guan, J.; Zhang, K. Targeting TGF-β1 suppresses survival of and invasion by anaplastic thyroid carcinoma cells. Am. J. Transl. Res. 2017, 9, 1418–1425. [Google Scholar] [PubMed]
- Gulubova, M.; Ivanova, K.; Ananiev, J.; Gerenova, J.; Zdraveski, A.; Stoyanov, H.; Vlaykova, T. VEGF expression, microvessel density and dendritic cell decrease in thyroid cancer. Biotechnol. Biotechnol. Equip. 2014, 28, 508–517. [Google Scholar] [CrossRef] [Green Version]
- Araki, K.; Shimura, T.; Suzuki, H.; Tsutsumi, S.; Wada, W.; Yajima, T.; Kobayahi, T.; Kubo, N.; Kuwano, H. E/N-cadherin switch mediates cancer progression via TGF-β-induced epithelial-to-mesenchymal transition in extrahepatic cholangiocarcinoma. Br. J. Cancer 2011, 105, 1885–1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.T.; Gu, W. SIRT1: Regulator of p53 deacetylation. Genes Cancer 2013, 4, 112–117. [Google Scholar] [CrossRef] [Green Version]
- Potente, M.; Ghaeni, L.; Baldessari, D.; Mostoslavsky, R.; Rossig, L.; Dequiedt, F.; Haendeler, J.; Mione, M.; Dejana, E.; Alt, F.W.; et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007, 21, 2644–2658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapia, O.; Riquelme, I.; Leal, P.; Sandoval, A.; Aedo, S.; Weber, H.; Letelier, P.; Bellolio, E.; Villaseca, M.; Garcia, P.; et al. The PI3K/AKT/mTOR pathway is activated in gastric cancer with potential prognostic and predictive significance. Int. J. Pathol. 2014, 465, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Kumar, D.; Shankar, S.; Srivastava, R.K. Rottlerin induces autophagy which leads to apoptotic cell death through inhibition of PI3K/Akt/mTOR pathway in human pancreatic cancer stem cells. Biochem. Pharmacol. 2012, 84, 1154–1163. [Google Scholar] [CrossRef]
- Janku, F.; McConkey, D.J.; Hong, D.S.; Kurzrock, R. Autophagy as a target for anticancer therapy. Nat. Rev. Clin. Oncol. 2011, 8, 528–539. [Google Scholar] [CrossRef]
- Edinger, A.L.; Thompson, C.B. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol. Biol. Cell 2002, 13, 2276–2288. [Google Scholar] [CrossRef] [Green Version]
- Yap, T.A.; Garrett, M.D.; Walton, M.I.; Raynaud, F.; de Bono, J.S.; Workman, P. Targeting the PI3K-AKT-mTOR pathway: Progress, pitfalls, and promises. Curr. Opin. Pharmacol. 2008, 8, 393–412. [Google Scholar] [CrossRef] [PubMed]
- Vander Haar, E.; Lee, S.I.; Bandhakavi, S.; Griffin, T.J.; Kim, D.H. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat. Cell Biol. 2007, 9, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.H.; Zhou, X.Y. Advances on PI3K/Akt/mTOR signalling pathway in malignancies. Chin. J. Pathol. 2005, 34, 674–676. [Google Scholar]
- West, K.A.; Brognard, J.; Clark, A.S.; Linnoila, I.R.; Yang, X.; Swain, S.M.; Harris, C.; Belinsky, S.; Dennis, P.A. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J. Clin. Investig. 2003, 111, 81–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, Y.; Corum, L.; Meng, Q.; Blenis, J.; Zheng, J.Z.; Shi, X.; Flynn, D.C.; Jiang, B.H. PI3K induced actin filament remodeling through Akt and p70S6K1: Implication of essential role in cell migration. Am. J. Physiol.-Cell Physiol. 2004, 286, C153–C163. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.H.; Koh, H.J.; Yoon, S.O.; Chung, A.S.; Cho, K.S.; Chung, J.K. Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J. 2001, 15, 1953–1962. [Google Scholar]
- Petrulea, M.S.; Plantinga, T.S.; Smit, J.W.; Georgescu, C.E.; Netea-Maier, R.T. PI3K/Akt/mTOR: A promising therapeutic target for non-medullary thyroid carcinoma. Cancer Treat. Rev. 2015, 41, 707–713. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.W.; Hwang, J.H. Anticancer Effects of Ursi Fel Extract and Its Active Compound, Ursodeoxycholic Acid, in FRO Anaplastic Thyroid Cancer Cells. Molecules 2021, 26, 5309. https://doi.org/10.3390/molecules26175309
Jung HW, Hwang JH. Anticancer Effects of Ursi Fel Extract and Its Active Compound, Ursodeoxycholic Acid, in FRO Anaplastic Thyroid Cancer Cells. Molecules. 2021; 26(17):5309. https://doi.org/10.3390/molecules26175309
Chicago/Turabian StyleJung, Hyo Won, and Ji Hye Hwang. 2021. "Anticancer Effects of Ursi Fel Extract and Its Active Compound, Ursodeoxycholic Acid, in FRO Anaplastic Thyroid Cancer Cells" Molecules 26, no. 17: 5309. https://doi.org/10.3390/molecules26175309
APA StyleJung, H. W., & Hwang, J. H. (2021). Anticancer Effects of Ursi Fel Extract and Its Active Compound, Ursodeoxycholic Acid, in FRO Anaplastic Thyroid Cancer Cells. Molecules, 26(17), 5309. https://doi.org/10.3390/molecules26175309