Unraveling the Photodynamic Activity of Cationic Benzoporphyrin-Based Photosensitizers against Bladder Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
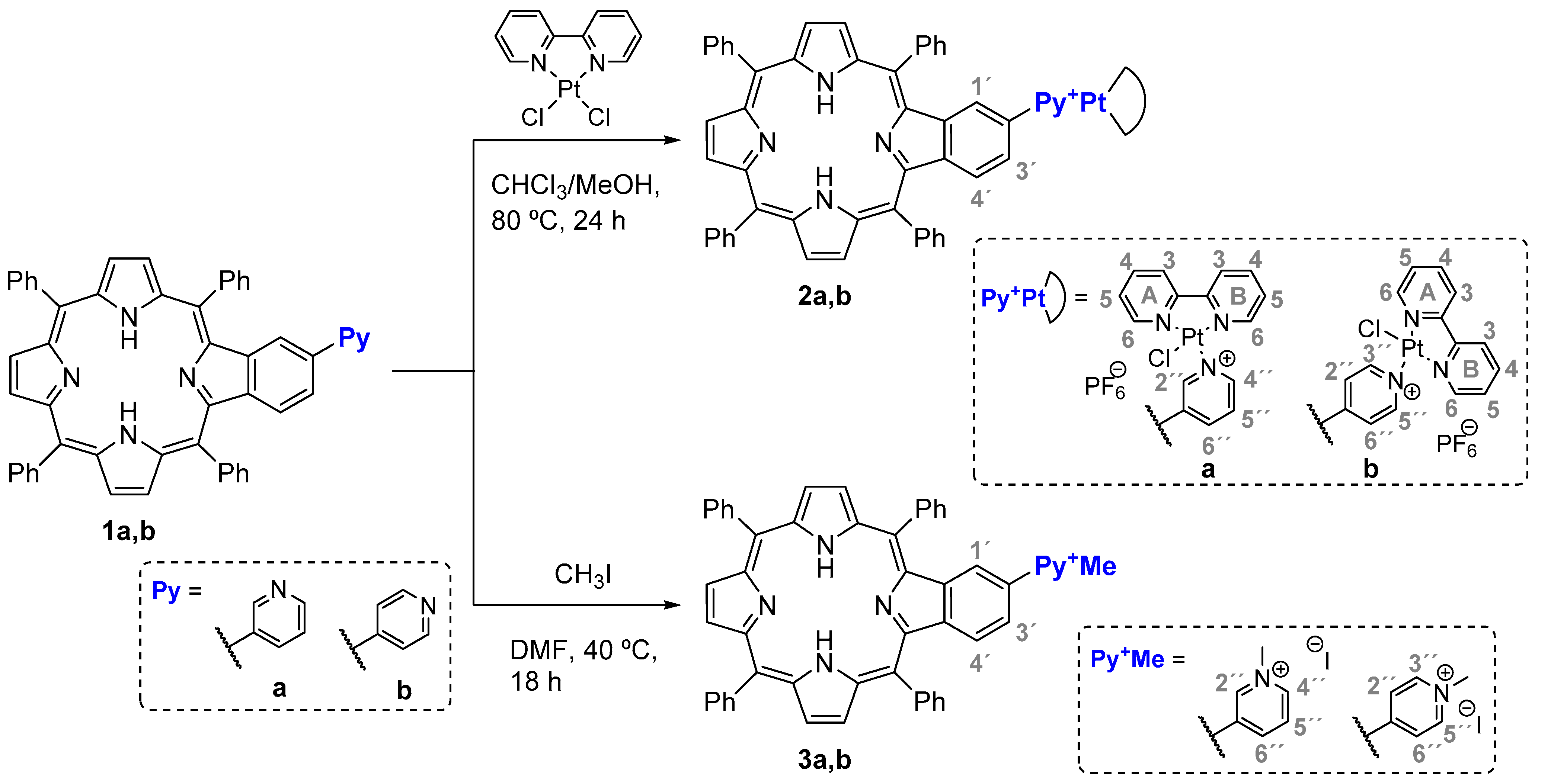
2.1. Synthesis
2.2. Incorporation into PVP Micelles
2.3. Photostability and Singlet Oxygen Generation
3. Photodynamic Activity of PVP-2a,b and PVP-3a,b Formulations against Human Bladder Cancer Cells
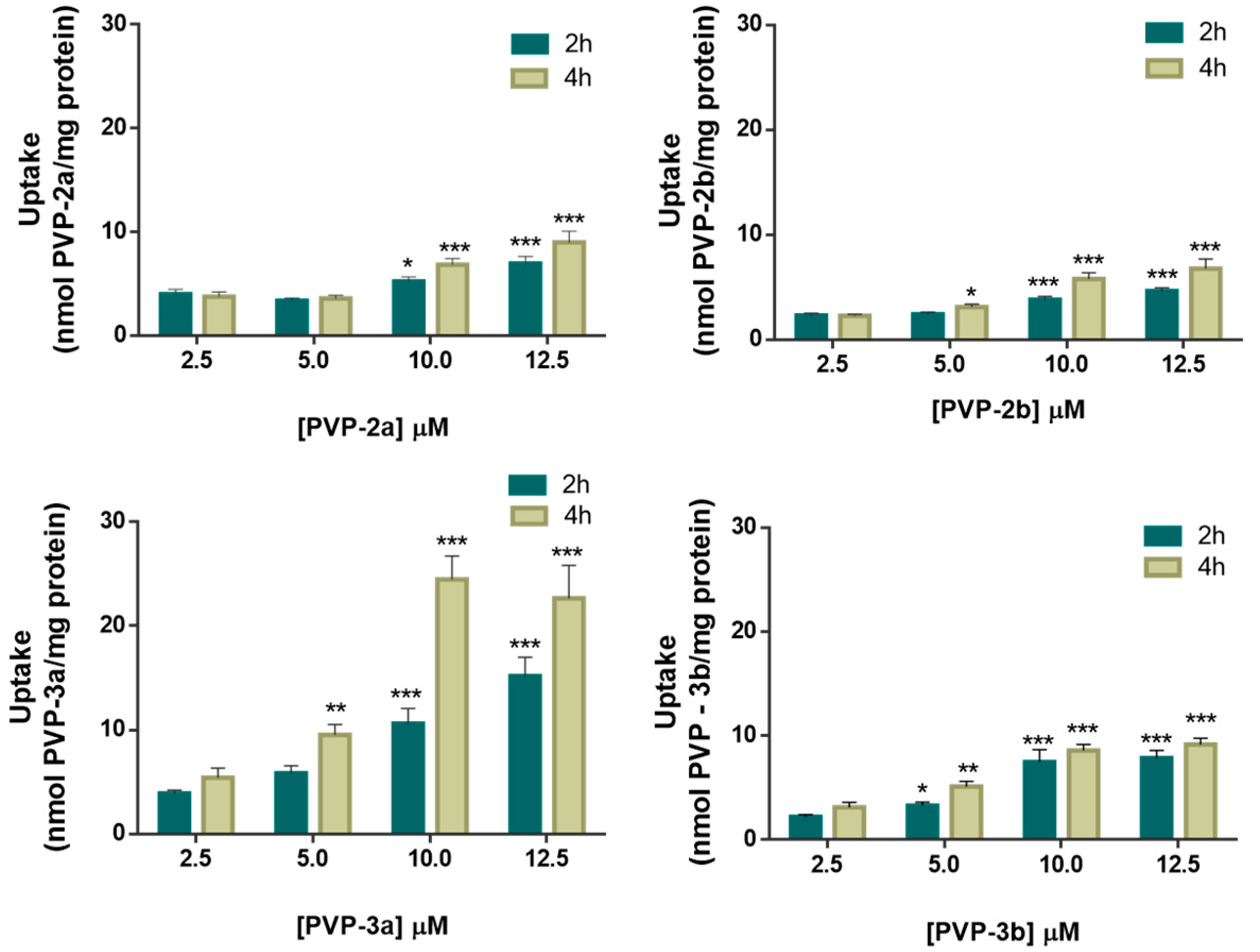
3.1. Cellular Uptake of PVP-2a,b and PVP-3a,b Formulations
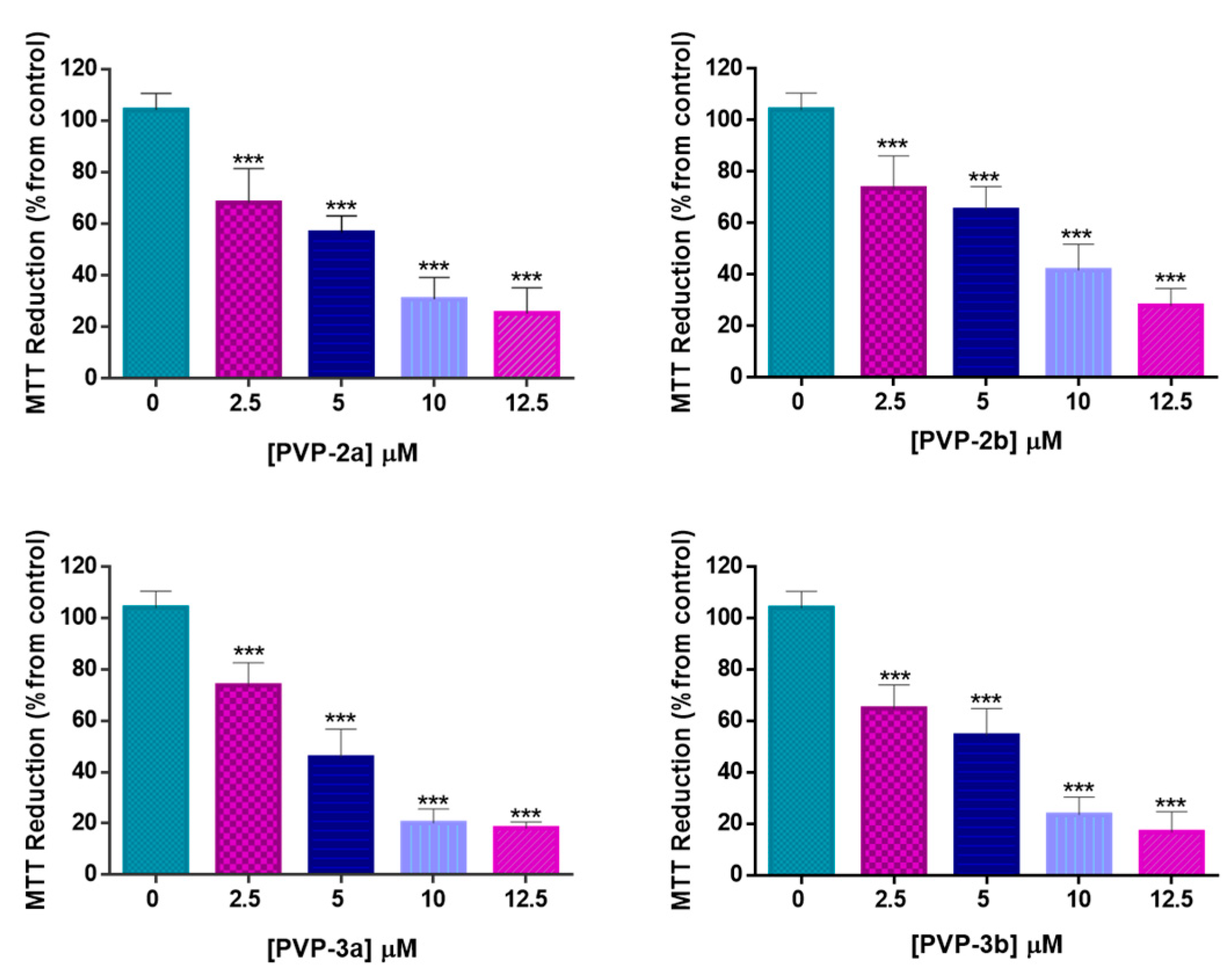
3.2. Cell Viability after PDT Treatment with PVP-2a,b and PVP-3a,b Formulations
4. Materials and Methods
4.1. General Remarks
4.2. Synthesis
4.2.1. Synthesis of the Benzoporphyrin Precursors 1
4.2.2. Synthesis of Porphyrin-Platinum(II) Complexes
4.2.3. Synthesis of Cationic Benzoporphyrins 3a,b
4.3. General Procedure to Prepare PVP-PS Micelles
4.4. Photostability Assays
4.5. Singlet Oxygen Generation
4.6. Photodynamic Activity of PVP-2a,b and PVP-3a,b Formulations against Human Bladder Cancer Cells
4.6.1. Cellular Uptake of PVP-2a,b and PVP-3a,b Formulations
4.6.2. Cell Viability after PDT Treatment with PVP-2a,b and PVP-3a,b Formulations
4.6.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Liu, H.; Lu, C.; Han, L.; Zhang, X.; Song, G. Optical—Magnetic probe for evaluating cancer therapy. Coord. Chem. Rev. 2021, 441, 213978. [Google Scholar] [CrossRef]
- Hang, Z.; Cooper, M.A.; Ziora, Z.M. Platinum-based anticancer drugs encapsulated liposome and polymeric micelle formulation in clinical trials. Biochem. Compd. 2016, 4, 2. [Google Scholar] [CrossRef]
- Tuo, W.; Xu, Y.; Fan, Y.; Li, J.; Qiu, M.; Xiong, X.; Li, X.; Sun, Y. Biomedical applications of Pt(II) metallacycle/metallacage-based agents: From mono-chemotherapy to versatile imaging contrasts and theranostic platforms. Coord. Chem. Rev. 2021, 443, 214017. [Google Scholar] [CrossRef]
- Gabano, E.; Ravera, M.; Osella, D. Pros and cons of bifunctional platinum(iv) antitumor prodrugs: Two are (not always) better than one. Dalton Trans. 2014, 43, 9813–9820. [Google Scholar] [CrossRef] [PubMed]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal complexes in cancer therapy—An update from drug design perspective. Drug Des. Dev. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef] [Green Version]
- Cocetta, V.; Ragazzi, E.; Montopoli, M. Mitochondrial Involvement in Cisplatin Resistance. Int. J. Mol. Sci. 2019, 20, 3384. [Google Scholar] [CrossRef] [Green Version]
- Facchetti, G.; Rimoldi, I. Anticancer platinum(II) complexes bearing N-heterocycle rings. Bioorgan. Med. Chem. Lett. 2019, 29, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wang, Z. Photoactivatable Platinum-Based Anticancer Drugs: Mode of Photoactivation and Mechanism of Action. Molecules 2020, 25, 5167. [Google Scholar] [CrossRef]
- Köberle, B.; Schoch, S. Platinum Complexes in Colorectal Cancer and Other Solid Tumors. Cancers 2021, 13, 2073. [Google Scholar] [CrossRef]
- Martinho, N.; Santos, T.C.B.; Florindo, H.F.; Silva, L.C. Cisplatin-Membrane Interactions and Their Influence on Platinum Complexes Activity and Toxicity. Front. Physiol. 2019, 9, 1898. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Deng, Z.; Zhu, G. Emerging platinum(iv) prodrugs to combat cisplatin resistance: From isolated cancer cells to tumor microenvironment. Dalton Trans. 2019, 48, 2536–2544. [Google Scholar] [CrossRef]
- Nayeem, N.; Contel, M. Exploring the Potential of Metallodrugs as Chemotherapeutics for Triple Negative Breast Cancer. Chem. Eur. J. 2021, 27, 8891–8917. [Google Scholar] [CrossRef]
- Li, J.; Chen, T. Transition metal complexes as photosensitizers for integrated cancer theranostic applications. Coord. Chem. Rev. 2020, 418, 213355. [Google Scholar] [CrossRef]
- Zhang, Q.; He, J.; Yu, W.; Li, Y.; Liu, Z.; Zhou, B.; Liu, Y. A promising anticancer drug: A photosensitizer based on the porphyrin skeleton. RSC Med. Chem. 2020, 11, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Kwon, N.; Kim, H.; Li, X.; Yoon, J. Supramolecular agents for combination of photodynamic therapy and other treatments. Chem. Sci. 2021, 12, 7248–7268. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, S.; Wu, J.; Yu, L.; Singh, N.; Yang, K.; Lan, M.; Wang, P.; Kim, J.S. Photodynamic therapy for hypoxic tumors: Advances and perspectives. Coord. Chem. Rev. 2021, 438, 213888. [Google Scholar] [CrossRef]
- Chen, D.; Xu, Q.; Wang, W.; Shao, J.; Huang, W.; Dong, X. Type I Photosensitizers Revitalizing Photodynamic Oncotherapy. Small 2021, 17, 2006742. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.T.P.D.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A. Cancer, Photodynamic Therapy and Porphyrin-Type Derivatives. An. Acad. Bras. Cienc. 2018, 90, 993–1026. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Antimicrobial photodynamic inactivation: A bright new technique to kill resistant microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Dąbrowski, J.M.; Pucelik, B.; Regiel-Futyra, A.; Brindell, M.; Mazuryk, O.; Kyzioł, A.; Stochel, G.; Macyk, W.; Arnaut, L. Engineering of relevant photodynamic processes through structural modifications of metallotetrapyrrolic photosensitizers. Coord. Chem. Rev. 2016, 325, 67–101. [Google Scholar] [CrossRef]
- Dharmaratne, P.; Sapugahawatte, D.N.; Wang, B.; Chan, C.L.; Lau, K.-M.; Lau, C.B.; Fung, K.P.; Ng, D.K.; Ip, M. Contemporary approaches and future perspectives of antibacterial photodynamic therapy (aPDT) against methicillin-resistant Staphylococcus aureus (MRSA): A systematic review. Eur. J. Med. Chem. 2020, 200, 112341. [Google Scholar] [CrossRef]
- Han, Q.; Lau, J.W.; Do, T.C.; Zhang, Z.; Xing, B. Near-infrared light brightens bacterial disinfection: Recent progress and perspectives. ACS Appl. Bio Mater. 2020, 4, 3937–3961. [Google Scholar] [CrossRef]
- Vallejo, M.; Moura, N.M.M.; Gomes, A.; Joaquinito, A.; Faustino, M.A.F.; Almeida, A.; Gonçalves, I.; Serra, V.V.; Neves, M.G.P.M.S. The Role of Porphyrinoid Photosensitizers for Skin Wound Healing. Int. J. Mol. Sci. 2021, 22, 4121. [Google Scholar] [CrossRef] [PubMed]
- Braz, M.; Salvador, D.; Gomes, A.T.; Mesquita, M.Q.; Faustino, M.A.F.; Neves, M.G.P.M.; Almeida, A. Photodynamic inactivation of methicillin-resistant Staphylococcus aureus on skin using a porphyrinic formulation. Photodiagn. Photodyn. Ther. 2020, 30, 101754. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, M.Q.; Dias, C.J.; Neves, M.G.P.M.S.; Almeida, A.; Faustino, M.A.F. Revisiting Current Photoactive Materials for Antimicrobial Photodynamic Therapy. Molecules 2018, 23, 2424. [Google Scholar] [CrossRef] [Green Version]
- Vieira, C.; Santos, A.; Mesquita, M.Q.; Gomes, A.T.P.D.C.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Advances in aPDT based on the combination of a porphyrinic formulation with potassium iodide: Effectiveness on bacteria and fungi planktonic/biofilm forms and viruses. J. Porphyr. Phthalocyanines 2019, 23, 534–545. [Google Scholar] [CrossRef]
- Almeida, A.; Faustino, M.A.F.; Tome, J.P.C. Photodynamic inactivation of bacteria: Finding the effective targets. Future Med. Chem. 2015, 7, 1221–1224. [Google Scholar] [CrossRef] [PubMed]
- Kadish, K.M.; Smith, K.M.; Guilard, R. Handbook of Porphyrin Science; World Scientific Publishing Company: Singapore, 2010. [Google Scholar]
- Lee, H.; Hong, K.-I.; Jang, W.-D. Design and applications of molecular probes containing porphyrin derivatives. Coord. Chem. Rev. 2018, 354, 46–73. [Google Scholar] [CrossRef]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for Chemical Sensor Applications. Chem. Rev. 2016, 117, 2517–2583. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Zhu, W.-H.; Xie, Y. Development of Ion Chemosensors Based on Porphyrin Analogues. Chem. Rev. 2016, 117, 2203–2256. [Google Scholar] [CrossRef]
- Moura, N.M.M.; Núñez, C.; Santos, S.M.; Faustino, M.A.F.; Cavaleiro, J.A.S.; Paz, F.A.; Neves, M.G.P.M.S.; Capelo, J.L.; Lodeiro, C. A New 3,5-Bisporphyrinylpyridine Derivative as a Fluorescent Ratiometric Probe for Zinc Ions. Chem. Eur. J. 2014, 20, 6684–6692. [Google Scholar] [CrossRef]
- Moura, N.M.M.; Núñez, C.; Santos, S.M.; Faustino, M.A.F.; Cavaleiro, J.A.S.; Neves, M.G.P.M.S.; Capelo, J.L.; Lodeiro, C. Synthesis, Spectroscopy Studies, and Theoretical Calculations of New Fluorescent Probes Based on Pyrazole Containing Porphyrins for Zn(II), Cd(II), and Hg(II) Optical Detection. Inorg. Chem. 2014, 53, 6149–6158. [Google Scholar] [CrossRef]
- Neves, C.; Filipe, O.M.; Mota, N.; Santos, S.; Silvestre, A.J.; Santos, E.; Neves, M.G.P.M.; Simões, M.M. Photodegradation of metoprolol using a porphyrin as photosensitizer under homogeneous and heterogeneous conditions. J. Hazard. Mater. 2018, 370, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Pegis, M.L.; Wise, C.F.; Martin, D.; Mayer, J.M. Oxygen Reduction by Homogeneous Molecular Catalysts and Electrocatalysts. Chem. Rev. 2017, 118, 2340–2391. [Google Scholar] [CrossRef]
- Da Silva, E.S.; Moura, N.M.M.; Neves, M.G.P.M.S.; Coutinho, A.; Prieto, M.; Silva, C.G.; Faria, J. Novel hybrids of graphitic carbon nitride sensitized with free-base meso-tetrakis(carboxyphenyl) porphyrins for efficient visible light photocatalytic hydrogen production. Appl. Catal. B Environ. 2018, 221, 56–69. [Google Scholar] [CrossRef]
- Zhang, W.; Lai, W.; Cao, R. Energy-Related Small Molecule Activation Reactions: Oxygen Reduction and Hydrogen and Oxygen Evolution Reactions Catalyzed by Porphyrin- and Corrole-Based Systems. Chem. Rev. 2016, 117, 3717–3797. [Google Scholar] [CrossRef]
- Nakagaki, S.; Mantovani, K.M.; Machado, G.S.; Castro, K.A.D.D.F.; Wypych, F. Recent Advances in Solid Catalysts Obtained by Metalloporphyrins Immobilization on Layered Anionic Exchangers: A Short Review and Some New Catalytic Results. Molecules 2016, 21, 291. [Google Scholar] [CrossRef]
- Costentin, C.; Robert, M.; Savéant, J.-M. Current Issues in Molecular Catalysis Illustrated by Iron Porphyrins as Catalysts of the CO2-to-CO Electrochemical Conversion. Acc. Chem. Res. 2015, 48, 2996–3006. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, D.; Gong, Z.; Li, Q.; Shi, R.; Yang, Z.; Lei, Z.; Li, J.; Wang, L.-N. Visible-light responsive organic nano-heterostructured photocatalysts for environmental remediation and H2 generation. J. Mater. Sci. Technol. 2019, 38, 93–106. [Google Scholar] [CrossRef]
- Radi, S.; El Abiad, C.; Moura, N.M.; Faustino, M.A.; Neves, M.G.P. New hybrid adsorbent based on porphyrin functionalized silica for heavy metals removal: Synthesis, characterization, isotherms, kinetics and thermodynamics studies. J. Hazard. Mater. 2019, 370, 80–90. [Google Scholar] [CrossRef]
- El Abiad, C.; Radi, S.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Moura, N.M.M. Supramolecular Hybrid Material Based on Engineering Porphyrin Hosts for an Efficient Elimination of Lead(II) from Aquatic Medium. Molecules 2019, 24, 669. [Google Scholar] [CrossRef] [Green Version]
- DI Carlo, G.; Biroli, A.O.; Pizzotti, M.; Tessore, F. Efficient Sunlight Harvesting by A4 β-Pyrrolic Substituted ZnII Porphyrins: A Mini-Review. Front. Chem. 2019, 7, 177. [Google Scholar] [CrossRef]
- DI Carlo, G.; Biroli, A.O.; Tessore, F.; Caramori, S.; Pizzotti, M. β-Substituted ZnII porphyrins as dyes for DSSC: A possible approach to photovoltaic windows. Coord. Chem. Rev. 2018, 358, 153–177. [Google Scholar] [CrossRef]
- Kundu, S.; Patra, A. Nanoscale Strategies for Light Harvesting. Chem. Rev. 2016, 117, 712–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbani, M.; Grätzel, M.; Nazeeruddin, M.K.; Torres, T. Meso-Substituted Porphyrins for Dye-Sensitized Solar Cells. Chem. Rev. 2014, 114, 12330–12396. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Marrero, V.; González-Delgado, J.A.; Torres, T. Emerging Perspectives on Applications of Porphyrinoids for Photodynamic Therapy and Photoinactivation of Microorganisms. Macroheterocycles 2019, 12, 8–16. [Google Scholar] [CrossRef]
- McKenzie, L.K.; Bryant, H.E.; Weinstein, J.A. Transition metal complexes as photosensitisers in one- and two-photon photodynamic therapy. Coord. Chem. Rev. 2019, 379, 2–29. [Google Scholar] [CrossRef] [Green Version]
- Sandland, J.; Malatesti, N.; Boyle, R. Porphyrins and related macrocycles: Combining photosensitization with radio- or optical-imaging for next generation theranostic agents. Photodiagn. Photodyn. Ther. 2018, 23, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Calvete, M.; Pinto, S.; Pereira, M.M.; Geraldes, C. Metal coordinated pyrrole-based macrocycles as contrast agents for magnetic resonance imaging technologies: Synthesis and applications. Coord. Chem. Rev. 2017, 333, 82–107. [Google Scholar] [CrossRef]
- Chang, K.P.; the New Light Group; Kolli, B.K. New “light” for one-world approach toward safe and effective control of animal diseases and insect vectors from leishmaniac perspectives. Parasites Vectors 2016, 9, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, C.; Gomes, A.T.P.D.C.; Mesquita, M.Q.; Moura, N.M.M.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. An Insight into the Potentiation Effect of Potassium Iodide on aPDT Efficacy. Front. Microbiol. 2018, 9, 2665. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Zhou, T.; Bai, R.; Xie, Y. Chemical approaches for the enhancement of porphyrin skeleton-based photodynamic therapy. J. Enzym. Inhib. Med. Chem. 2020, 35, 1080–1099. [Google Scholar] [CrossRef] [PubMed]
- Chelushkin, P.S.; Tunik, S.P. Phosphorescence Lifetime Imaging (PLIM): State of the Art and Perspectives. In Progress in Photon Science; Springer: Berlin/Heidelberg, Germany, 2019; pp. 109–128. [Google Scholar]
- Paul-Roth, C.O.; Drouet, S.; Merhi, A.; Williams, J.; Gildea, L.F.; Pearson, C.; Petty, M. Synthesis of platinum complexes of fluorenyl-substituted porphyrins used as phosphorescent dyes for solution-processed organic light-emitting devices. Tetrahedron 2013, 69, 9625–9632. [Google Scholar] [CrossRef] [Green Version]
- Lvova, L.; Verrelli, G.; Stefanelli, M.; Nardis, S.; Di Natale, C.; Amico, A.D.; Makarychev-Mikhailov, S.; Paolesse, R. Platinum porphyrins as ionophores in polymeric membrane electrodes. Analyst 2011, 136, 4966–4976. [Google Scholar] [CrossRef]
- Luciano, M.; Br ckner, C. Modifications of porphyrins and hydroporphyrins for their solubilization in aqueous media. Molecules 2017, 22, 980. [Google Scholar] [CrossRef] [Green Version]
- Pushpanandan, P.; Maurya, Y.K.; Omagari, T.; Hirosawa, R.; Ishida, M.; Mori, S.; Yasutake, Y.; Fukatsu, S.; Mack, J.; Nyokong, T.; et al. Singly and Doubly N-Confused Calix[4]phyrin Organoplatinum(II) Complexes as Near-IR Triplet Sensitizers. Inorg. Chem. 2017, 56, 12572–12580. [Google Scholar] [CrossRef]
- Moura, N.M.M.; Cuerva, C.; Cavaleiro, J.; Mendes, R.F.; Paz, F.A.; Cano, M.; Neves, M.G.P.M.S.; Lodeiro, C. Metallomesogens with Luminescent Behaviour: Palladium Complexes Derived from Alkylamide Tetraarylporphyrins. ChemPlusChem 2016, 81, 262–273. [Google Scholar] [CrossRef]
- Ito, S.; Makihata, D.; Ishii, Y.; Saito, Y.; Oba, T. Synthesis of π-extended platinum porphyrins. Tetrahedron Lett. 2015, 56, 7043–7045. [Google Scholar] [CrossRef]
- Chen, H.-C.; Hetterscheid, D.G.H.; Williams, R.M.; Van Der Vlugt, J.I.; Reek, J.N.H.; Brouwer, A.M. Platinum(ii)–porphyrin as a sensitizer for visible-light driven water oxidation in neutral phosphate buffer. Energy Environ. Sci. 2015, 8, 975–982. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, S.; Labuta, J.; Van Rossom, W.; Ishikawa, D.; Minami, K.; Hill, J.; Ariga, K. Porphyrin-based sensor nanoarchitectonics in diverse physical detection modes. Phys. Chem. Chem. Phys. 2014, 16, 9713–9746. [Google Scholar] [CrossRef] [PubMed]
- Moiseev, A.G.; Margulies, E.A.; Schneider, J.A.; Bélanger-Gariépy, F.; Perepichka, D.F. Protecting the triplet excited state in sterically congested platinum porphyrin. Dalton Trans. 2014, 43, 2676–2683. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kurahashi, T.; Matsubara, S. Dicationic platinum porphyrin catalyzed cycloisomerization of enynes. Tetrahedron Lett. 2013, 54, 6196–6198. [Google Scholar] [CrossRef]
- Onitsuka, K.; Kitajima, H.; Fujimoto, M.; Iuchi, A.; Takei, F.; Takahashi, S. Platinum–acetylide dendrimers possessing a porphyrin core. Chem. Commun. 2002, 21, 2576–2577. [Google Scholar] [CrossRef]
- Xu, X.-D.; Zhang, J.; Chen, L.-J.; Guo, R.; Wang, D.-X.; Yang, H.-B. Design and synthesis of branched platinum–acetylide complexes possessing a porphyrin core and their self-assembly behaviour. Chem. Commun. 2012, 48, 11223–11225. [Google Scholar] [CrossRef]
- Jana, A.; McKenzie, L.; Wragg, A.B.; Ishida, M.; Hill, J.P.; Weinstein, J.A.; Baggaley, E.; Ward, M.D. Porphyrin/Platinum(II) C^N^N Acetylide Complexes: Synthesis, Photophysical Properties, and Singlet Oxygen Generation. Chem. Eur. J. 2016, 22, 4164–4174. [Google Scholar] [CrossRef]
- Couto, G.K.; Pacheco, B.S.; Borba, V.M.; Junior, J.C.R.; Oliveira, T.L.; Segatto, N.V.; Seixas, F.K.; Acunha, T.V.; Iglesias, B.A.; Collares, T. Tetra-cationic platinum(II) porphyrins like a candidate photosensitizers to bind, selective and drug delivery for metastatic melanoma. J. Photochem. Photobiol. B Biol. 2019, 202, 111725. [Google Scholar] [CrossRef]
- Rubbiani, R.; Wu, W.; Naik, A.; Larocca, M.; Schneider, L.; Padrutt, R.; Babu, V.; König, C.; Hinger, D.; Maake, C.; et al. Studying the cellular distribution of highly phototoxic platinated metalloporphyrins using isotope labelling. Chem. Commun. 2020, 56, 14373–14376. [Google Scholar] [CrossRef]
- Naik, A.; Rubbiani, R.; Gasser, G.; Spingler, B. Visible-Light-Induced Annihilation of Tumor Cells with Platinum-Porphyrin Conjugates. Angew. Chem. 2014, 126, 7058–7061. [Google Scholar] [CrossRef]
- Tasso, T.T.; Tsubone, T.M.; Baptista, M.S.; Mattiazzi, L.M.; Acunha, T.V.; Iglesias, B.A. Isomeric effect on the properties of tetraplatinated porphyrins showing optimized phototoxicity for photodynamic therapy. Dalton Trans. 2017, 46, 11037–11045. [Google Scholar] [CrossRef] [PubMed]
- Alemayehu, A.B.; Vazquez-Lima, H.; Beavers, C.M.; Gagnon, K.J.; Bendix, J.; Ghosh, A. Platinum corroles. Chem. Commun. 2014, 50, 11093–11096. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, B.A.; Barata, J.F.; Pereira, P.M.; Girão, H.; Fernandes, R.; Tome, J.; Neves, M.G.P.M.S.; Cavaleiro, J.A. New platinum(II)–bipyridyl corrole complexes: Synthesis, characterization and binding studies with DNA and HSA. J. Inorg. Biochem. 2015, 153, 32–41. [Google Scholar] [CrossRef]
- Volov, A.N.; Volov, N.A.; Burtsev, I.D. New amphiphilic platinum(II) phthalocyanine with peracetylated β-galactose moiety–synthesis and photophysical properties. Polyhedron 2021, 206, 115331. [Google Scholar] [CrossRef]
- Volov, A.N.; Burtsev, I.D. New glycosylated platinum(II) phthalocyanine containing ribose moiety–synthesis and photophysical properties. J. Organomet. Chem. 2020, 922, 121372. [Google Scholar] [CrossRef]
- Che, Y.; Yang, W.; Tang, G.; Dumoulin, F.; Zhao, J.; Liu, L.; Isci, U. Photophysical properties of palladium/platinum tetrasulfonyl phthalocyanines and their application in triplet–triplet annihilation upconversion. J. Mater. Chem. C 2018, 6, 5785–5793. [Google Scholar] [CrossRef]
- Zorlu, Y.; Dumoulin, F.; Durmuş, M.; Ahsen, V. Comparative studies of photophysical and photochemical properties of solketal substituted platinum(II) and zinc(II) phthalocyanine sets. Tetrahedron 2010, 66, 3248–3258. [Google Scholar] [CrossRef]
- Lokesh, K.; Uma, N.; Achar, B. The Microwave-assisted syntheses and a conductivity study of a platinum phthalocyanine and its derivatives. Polyhedron 2009, 28, 1022–1028. [Google Scholar] [CrossRef]
- Vallejo, M.C.; Reis, M.J.; Pereira, A.M.; Serra, V.V.; Cavaleiro, J.A.S.; Moura, N.M.M.; Neves, M.G.P.M.S. Merging pyridine(s) with porphyrins and analogues: An overview of synthetic approaches. Dye. Pigment. 2021, 191, 109298. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Katoh, T.; Shinokubo, H.; Osuka, A. Pt(II)- and Pt(IV)-Bridged Cofacial Diporphyrins via Carbon−Transition Metal σ-Bonds. J. Am. Chem. Soc. 2008, 130, 14440–14441. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Shinokubo, H.; Osuka, A. Double Cleavage of sp2 C−H and sp3 C−H Bonds on One Metal Center: DMF-Appended Cyclometalated Platinum(II) and -(IV) Porphyrins. Inorg. Chem. 2009, 48, 795–797. [Google Scholar] [CrossRef]
- Yoshida, K.; Yamaguchi, S.; Osuka, A.; Shinokubo, H. Platinum(II) and Platinum(IV) Porphyrin Pincer Complexes: Synthesis, Structures, and Reactivity. Organometallics 2010, 29, 3997–4000. [Google Scholar] [CrossRef]
- Jiang, H.-W.; Tanaka, T.; Osuka, A. Singly and doubly β-to-β platinum-bridged porphyrin dimers and their reductive eliminations. Chem. Sci. 2015, 6, 6102–6105. [Google Scholar] [CrossRef] [Green Version]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; MSHS. Bladder Cancer. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Moura, N.M.M.; Ramos, C.I.V.; Linhares, I.; Santos, S.M.; Faustino, M.A.F.; Almeida, A.; Cavaleiro, J.A.S.; Amado, F.M.L.; Lodeiro, C.; Neves, M.G.P.M.S. Synthesis, characterization and biological evaluation of cationic porphyrin–terpyridine derivatives. RSC Adv. 2016, 6, 110674–110685. [Google Scholar] [CrossRef]
- Moura, N.M.M.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Paz, F.A.; Silva, A.; Tomé, A.C.; Cavaleiro, J.A.S. New synthetic approach to benzoporphyrins and Kröhnke type porphyrin-2-ylpyridines. Chem. Commun. 2012, 48, 6142. [Google Scholar] [CrossRef]
- Moura, N.M.M.; Nuñez, C.; Santos, S.M.; Faustino, M.A.; Cavaleiro, J.; Neves, M.G.P.M.S.; Capelo, J.L.; Lodeiro, C. Functionalized Porphyrins as Red Fluorescent Probes for Metal Cations: Spectroscopic, MALDI-TOF Spectrometry, and Doped-Polymer Studies. ChemPlusChem 2013, 78, 1230–1243. [Google Scholar] [CrossRef]
- Hashimoto, T.; Choe, Y.-K.; Nakano, A.H.; Hirao, K. Theoretical Study of the Q and B Bands of Free-Base, Magnesium, and Zinc Porphyrins, and Their Derivatives. J. Phys. Chem. A 1999, 103, 1894–1904. [Google Scholar] [CrossRef]
- Maximiano, R.; Piovesan, E.; Zilio, S.; Machado, A.; De Paula, R.; Cavaleiro, J.A.S.; Borissevitch, I.; Ito, A.; Gonçalves, P.; Neto, N.B. Excited-state absorption investigation of a cationic porphyrin derivative. J. Photochem. Photobiol. A Chem. 2010, 214, 115–120. [Google Scholar] [CrossRef]
- Baskin, J.S.; Yu, H.-Z.; Zewail, A.H. Ultrafast Dynamics of Porphyrins in the Condensed Phase: I. Free Base Tetraphenylporphyrin†. J. Phys. Chem. A 2002, 106, 9837–9844. [Google Scholar] [CrossRef]
- Ohno, O.; Kaizu, Y.; Kobayashi, H. Luminescence of some metalloporphins including the complexes of the IIIb metal group. J. Chem. Phys. 1985, 82, 1779–1787. [Google Scholar] [CrossRef]
- Seybold, P.G.; Gouterman, M. Porphyrins. J. Mol. Spectrosc. 1969, 31, 1–13. [Google Scholar] [CrossRef]
- Molinspiration Cheminformatics. The n-Octanol:Water Partition Coefficients (miLog P) Were Evaluated Using the MolinspirationWebME Editor 3.81. Available online: http://www.molinspiration.com (accessed on 16 August 2021).
- Isakau, H.; Parkhats, M.; Knyukshto, V.; Dzhagarov, B.; Petrov, E.; Petrov, P. Toward understanding the high PDT efficacy of chlorin e6–polyvinylpyrrolidone formulations: Photophysical and molecular aspects of photosensitizer–polymer interaction in vitro. J. Photochem. Photobiol. B Biol. 2008, 92, 165–174. [Google Scholar] [CrossRef]
- Kashef, N.; Huang, Y.; Hamblin, M.R. Advances in antimicrobial photodynamic inactivation at the nanoscale. Nanophotonics 2017, 6, 853–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwach-Abdellaoui, K.; Vivien-Castioni, N.; Gurny, R. Local delivery of antimicrobial agents for the treatment of periodontal diseases. Eur. J. Pharm. Biopharm. 2000, 50, 83–99. [Google Scholar] [CrossRef]
- Cardoso, M.F.D.C.; Gomes, A.T.P.C.; Moreira, C.D.S.; Simões, M.M.Q.; Neves, M.G.P.M.S.; Da Rocha, D.R.; Silva, F.D.C.D.; Moreirinha, C.; Almeida, A.; Ferreira, V.F.; et al. Efficient Catalytic Oxidation of 3-Arylthio- and 3-Cyclohexylthio-lapachone Derivatives to New Sulfonyl Derivatives and Evaluation of Their Antibacterial Activities. Molecules 2017, 22, 302. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.T.P.C.; Fernandes, R.; Ribeiro, C.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Silva, F.D.C.D.; Ferreira, V.F.; Cavaleiro, J.A.S. Synthesis, Characterization and Photodynamic Activity against Bladder Cancer Cells of Novel Triazole-Porphyrin Derivatives. Molecules 2020, 25, 1607. [Google Scholar] [CrossRef] [Green Version]
- Tavares, A.; Carvalho, C.M.B.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, J.P.C.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, A.; Gomes, N.C.M.; Alves, E.; et al. Antimicrobial Photodynamic Therapy: Study of Bacterial Recovery Viability and Potential Development of Resistance after Treatment. Mar. Drugs 2010, 8, 91–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimcik, P.; Miletin, M.; Radilova, H.; Novakova, V.; Kopecky, K.; Svec, J.; Rudolf, E. Synthesis, Properties andIn VitroPhotodynamic Activity of Water-soluble Azaphthalocyanines and Azanaphthalocyanines. Photochem. Photobiol. 2010, 86, 168–175. [Google Scholar] [CrossRef]
- Spesia, M.B.; Milanesio, M.E.; Durantini, E.N. Synthesis, properties and photodynamic inactivation of Escherichia coli by novel cationic fullerene C60 derivatives. Eur. J. Med. Chem. 2008, 43, 853–861. [Google Scholar] [CrossRef]
- Spiller, W.; Kliesch, H.; Wöhrle, D.; Hackbarth, S.; Röder, B.; Schnurpfeil, G. Singlet Oxygen Quantum Yields of Different Photosensitizers in Polar Solvents and Micellar Solutions. J. Porphyr. Phthalocyanines 1998, 2, 145–158. [Google Scholar] [CrossRef]
- Zenkevich, E.; Sagun, E.; Knyukshto, V.; Shulga, A.; Mironov, A.F.; Efremova, O.; Bonnett, R.; Songca, S.P.; Kassem, M. Photophysical and photochemical properties of potential porphyrin and chlorin photosensitizers for PDT. J. Photochem. Photobiol. B Biol. 1996, 33, 171–180. [Google Scholar] [CrossRef]
- De Almeida, D.R.Q.; Terra, L.F.; Labriola, L. Photodynamic therapy in cancer treatment—An update review. J. Cancer Metastasis Treat. 2019, 5, 25. [Google Scholar] [CrossRef] [Green Version]
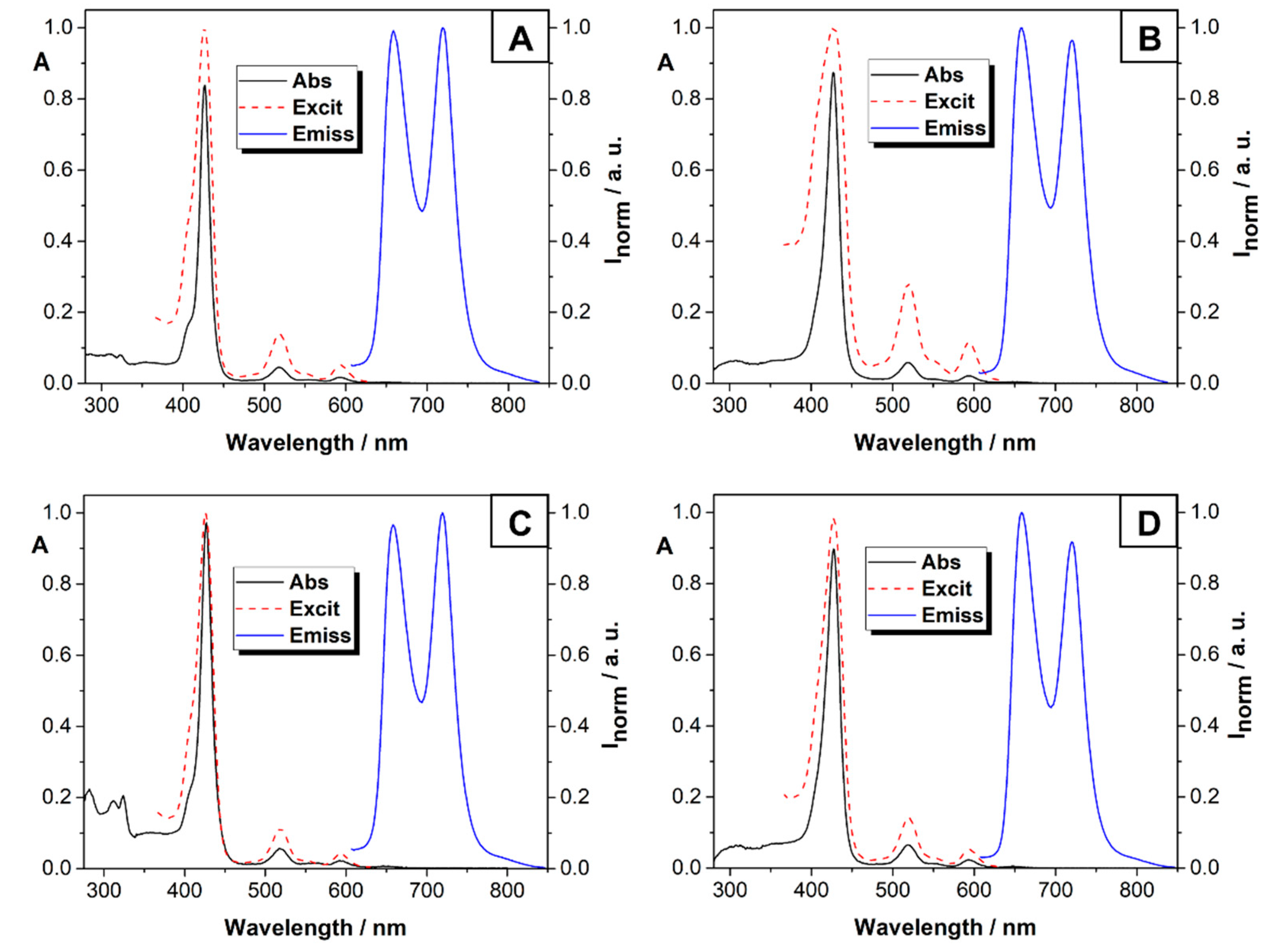

| Compd | λmax(nm):log ε | λem (nm) | Stokes Shift (cm−1) | ΦF |
|---|---|---|---|---|
| 2a | 286:4.44 312:4.43 323:4.45 427:5.45 518:4.01 593:3.57 | 659, 719 | 151,515.2 | 0.07 |
| 2b | 312:4.67 324:4.43 427:5.49 520:4.32 594:3.87 | 660, 720 | 3,151,515.2 | 0.06 |
| 3a | 427:5.39 518:4.07 593:3.72 | 659, 720 | 151,515.2 | 0.06 |
| 3b | 429:5.06 522:4.15 597:3.74 | 662, 723 | 153,846.2 | 0.05 |
| PVP-2a | 282:4.87 312:4.80 324:4.84 426:5.45 518:3.95 595:3.52 | 658, 719 | 158,730.2 | 0.07 |
| PVP-2b | 285:4.53 309:4.57 323:4.56 426:5.08 519:4.04 594:3.58 | 661, 720 | 149,253.7 | 0.04 |
| PVP-3a | 427:4.99 520:3.82 595:3.47 | 658, 720 | 158,730.2 | 0.05 |
| PVP-3b | 428:3.91 523:3.91 597:3.52 | 662, 725 | 153,846.2 | 0.04 |
| PVP Formulation | IC50PDT * |
|---|---|
| PVP-2a | 6.42 μM |
| PVP-2b | 8.14 μM |
| PVP-3a | 5.58 μM |
| PVP-3b | 5.51 μM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, A.T.P.C.; Neves, M.G.P.M.S.; Fernandes, R.; Ribeiro, C.F.; Cavaleiro, J.A.S.; Moura, N.M.M. Unraveling the Photodynamic Activity of Cationic Benzoporphyrin-Based Photosensitizers against Bladder Cancer Cells. Molecules 2021, 26, 5312. https://doi.org/10.3390/molecules26175312
Gomes ATPC, Neves MGPMS, Fernandes R, Ribeiro CF, Cavaleiro JAS, Moura NMM. Unraveling the Photodynamic Activity of Cationic Benzoporphyrin-Based Photosensitizers against Bladder Cancer Cells. Molecules. 2021; 26(17):5312. https://doi.org/10.3390/molecules26175312
Chicago/Turabian StyleGomes, Ana T. P. C., M. Graça P. M. S. Neves, Rosa Fernandes, Carlos F. Ribeiro, José A. S. Cavaleiro, and Nuno M. M. Moura. 2021. "Unraveling the Photodynamic Activity of Cationic Benzoporphyrin-Based Photosensitizers against Bladder Cancer Cells" Molecules 26, no. 17: 5312. https://doi.org/10.3390/molecules26175312
APA StyleGomes, A. T. P. C., Neves, M. G. P. M. S., Fernandes, R., Ribeiro, C. F., Cavaleiro, J. A. S., & Moura, N. M. M. (2021). Unraveling the Photodynamic Activity of Cationic Benzoporphyrin-Based Photosensitizers against Bladder Cancer Cells. Molecules, 26(17), 5312. https://doi.org/10.3390/molecules26175312