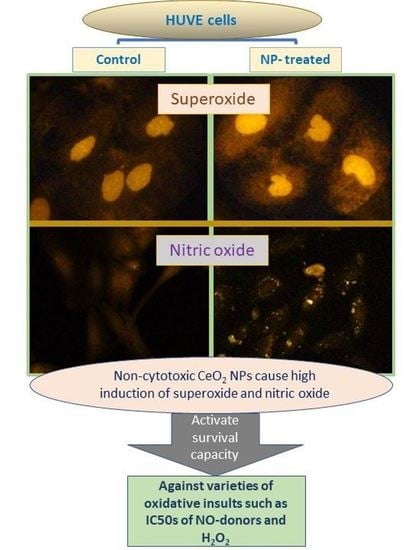
Anti-Inflammatory CeO2 Nanoparticles Prevented Cytotoxicity Due to Exogenous Nitric Oxide Donors via Induction Rather Than Inhibition of Superoxide/Nitric Oxide in HUVE Cells
Abstract
:1. Introduction
2. Results and Discussion
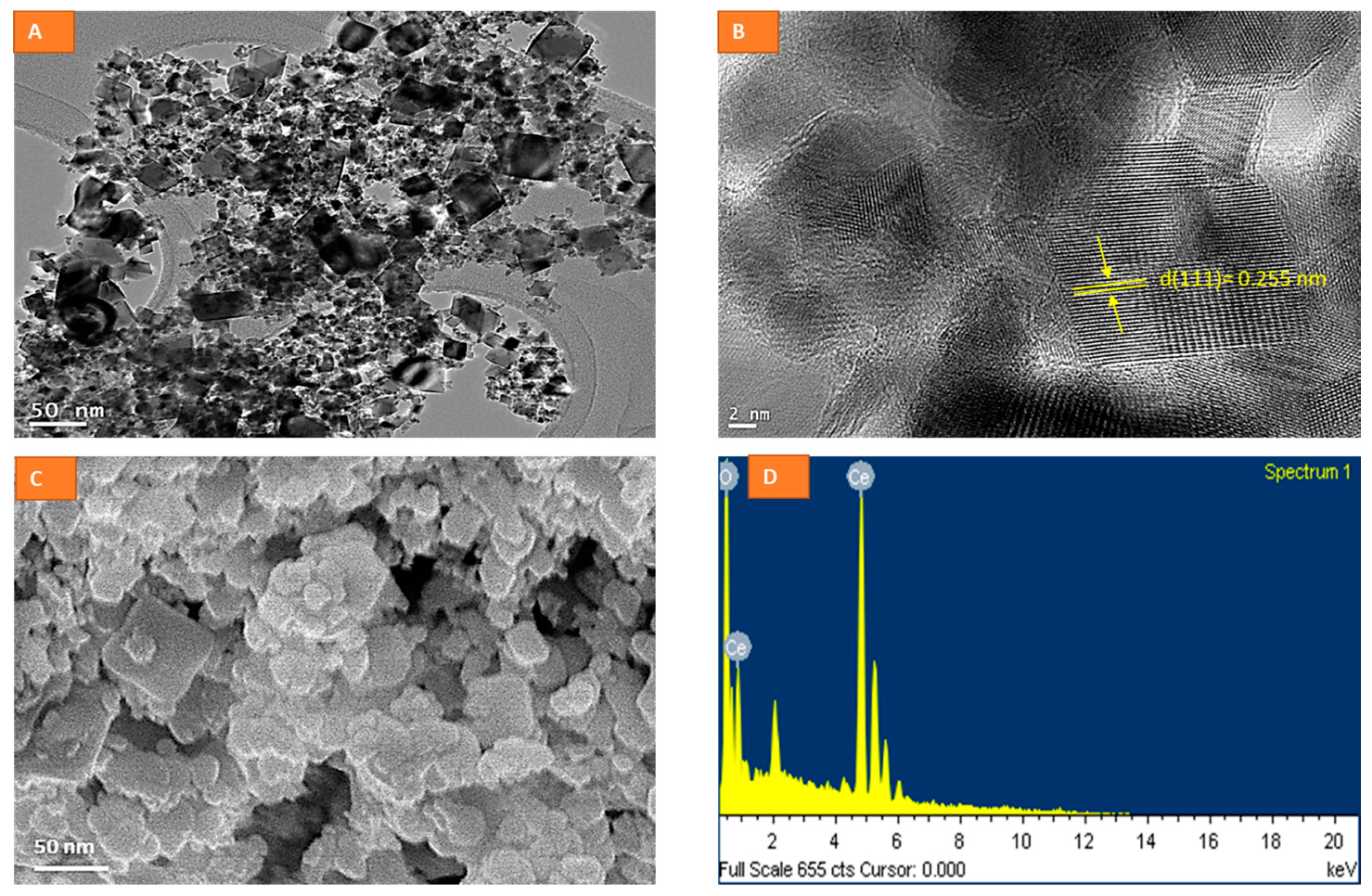
2.1. Physico-Chemical Characterization of CeO2 NPs
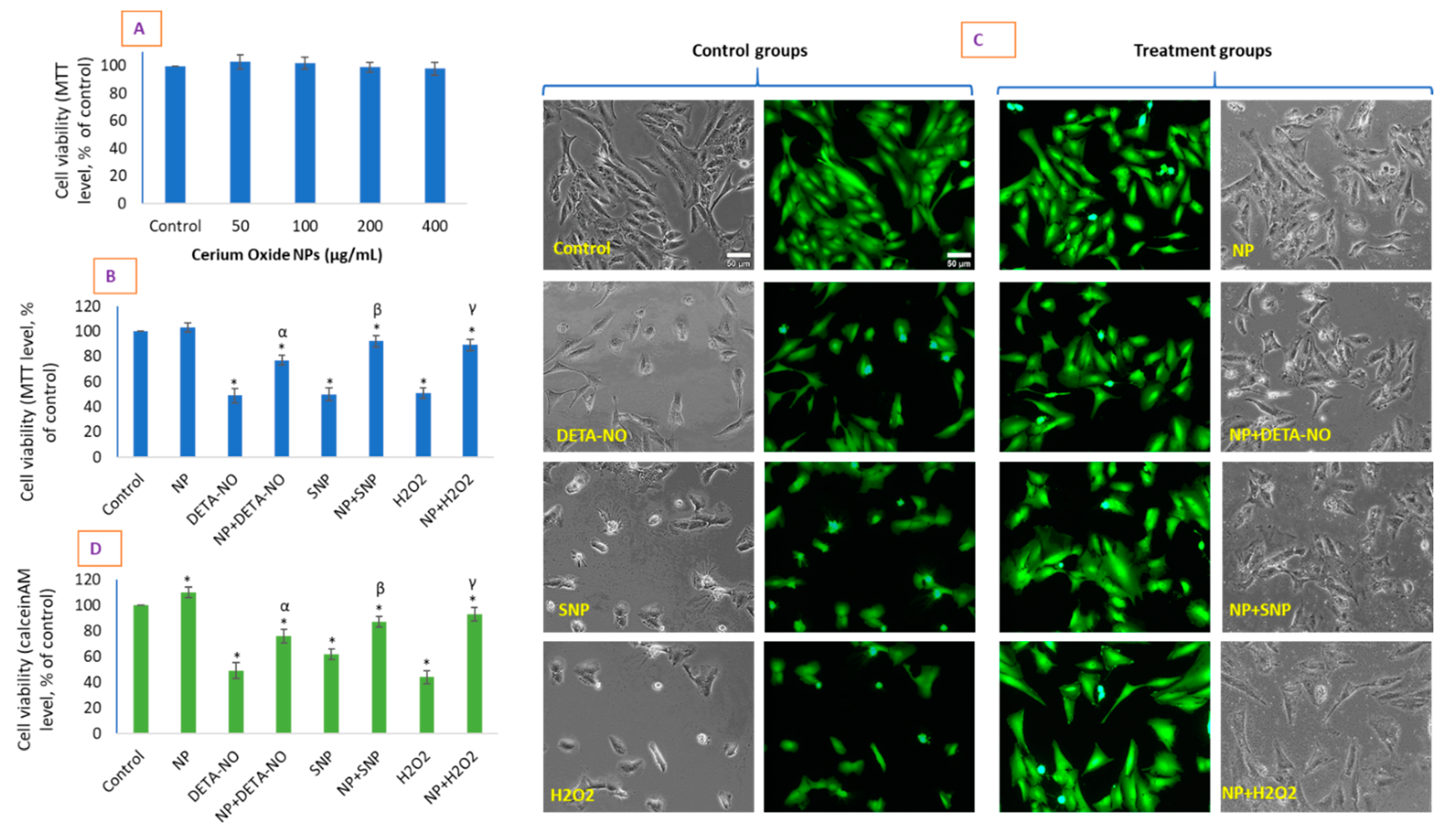
2.2. CeO2 NPs Did Not Exhibit Toxicity in HUVE Cell but Differentially Protected Cells against the IC50 of Two NO-Donors
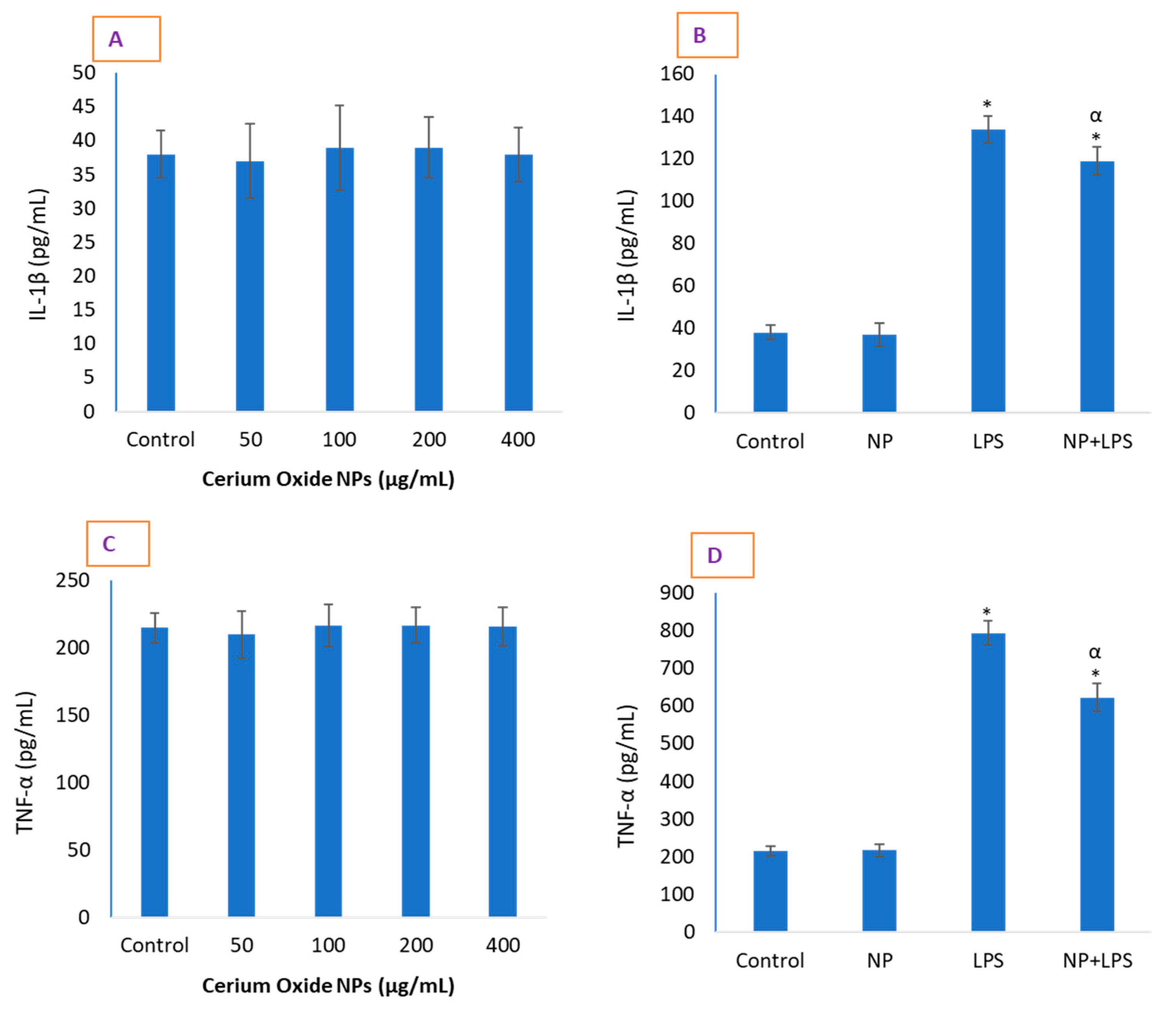
2.3. CeO2 NPs Significantly Reduced Inflammatory Markers in HUVE Cells
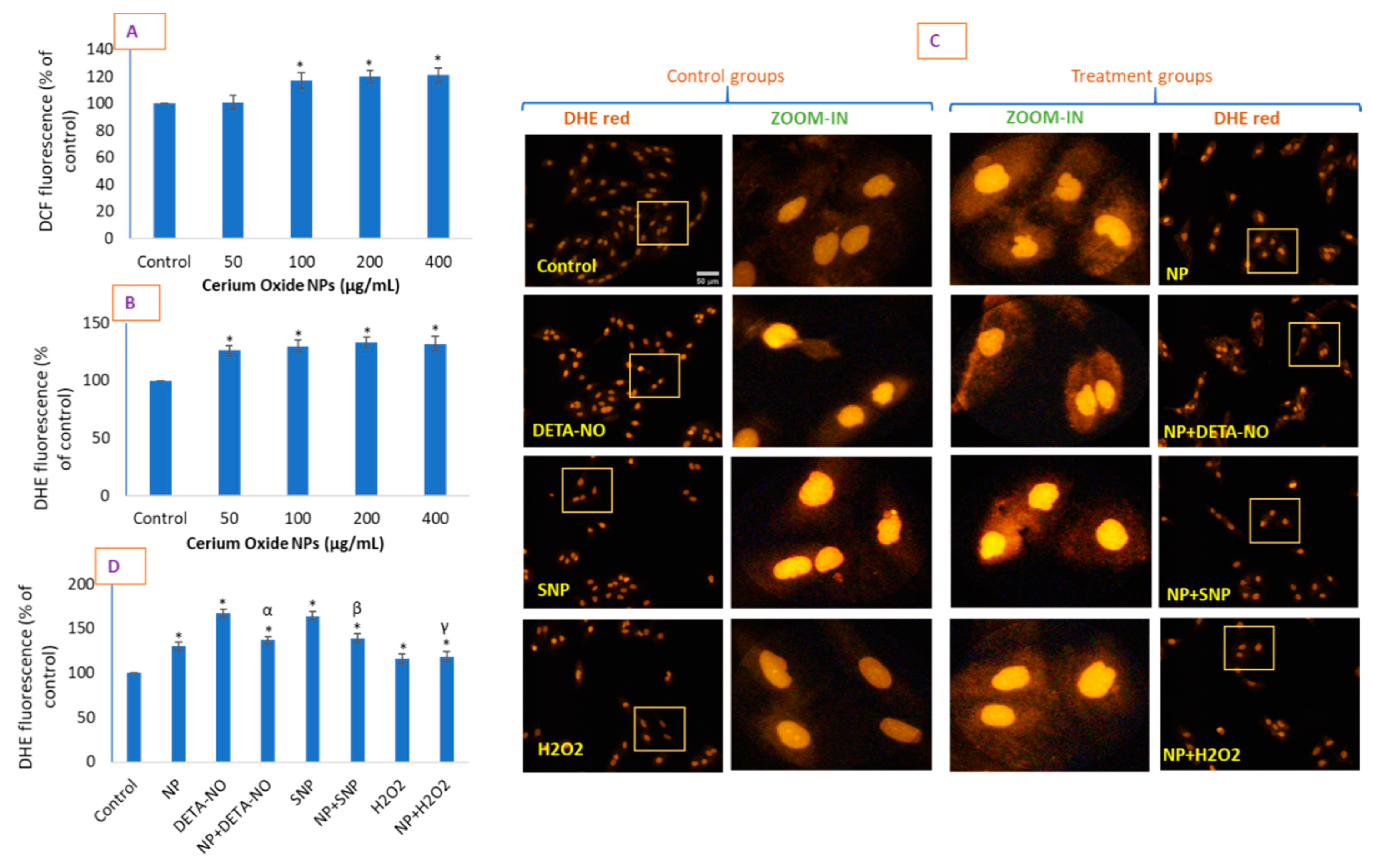
2.4. CeO2 NPs Increased Intracellular O2•− in HUVE Cells
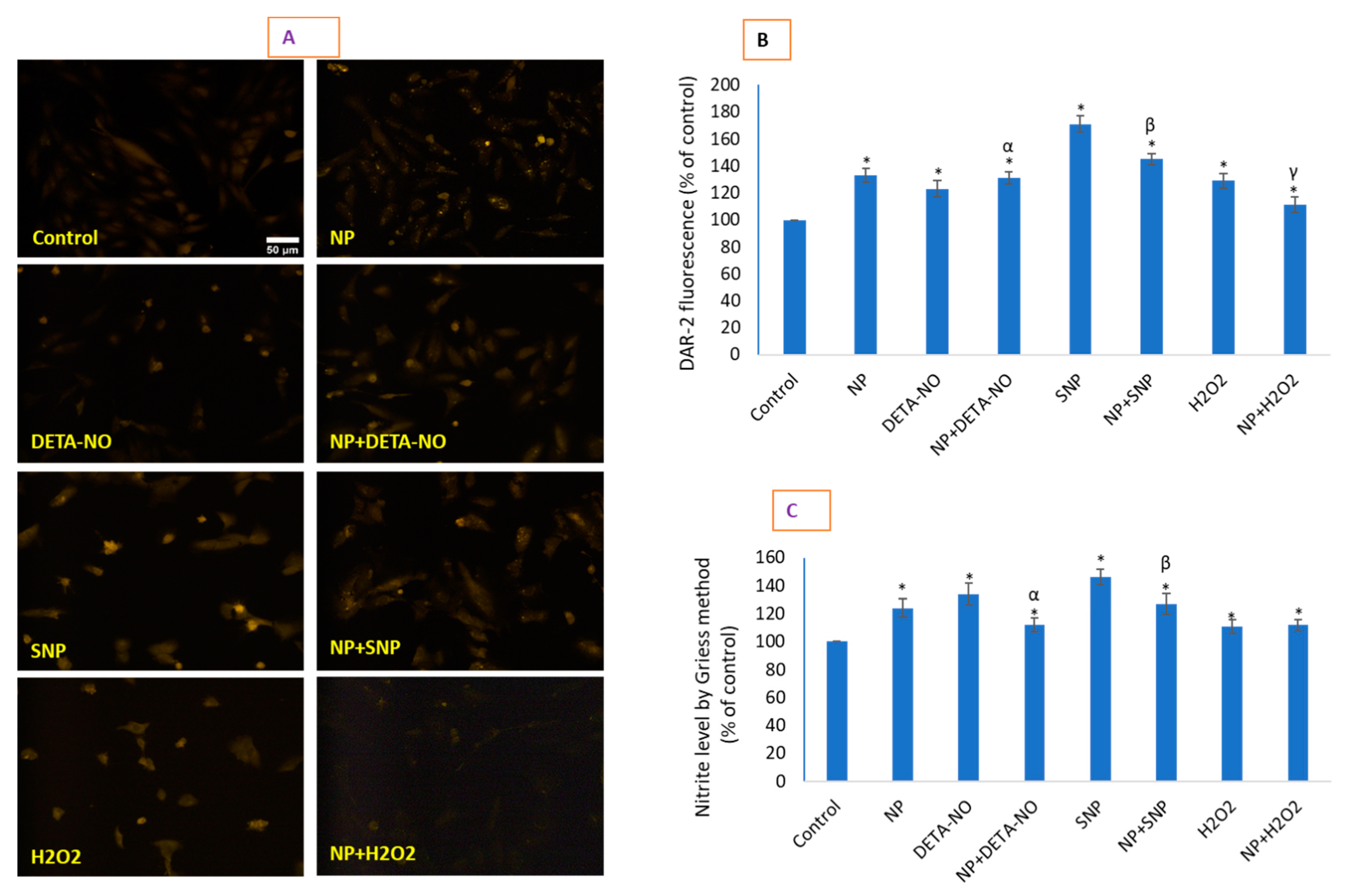
2.5. CeO2 NPs Increased Low Concentrations of Intracellular Nitric Oxide
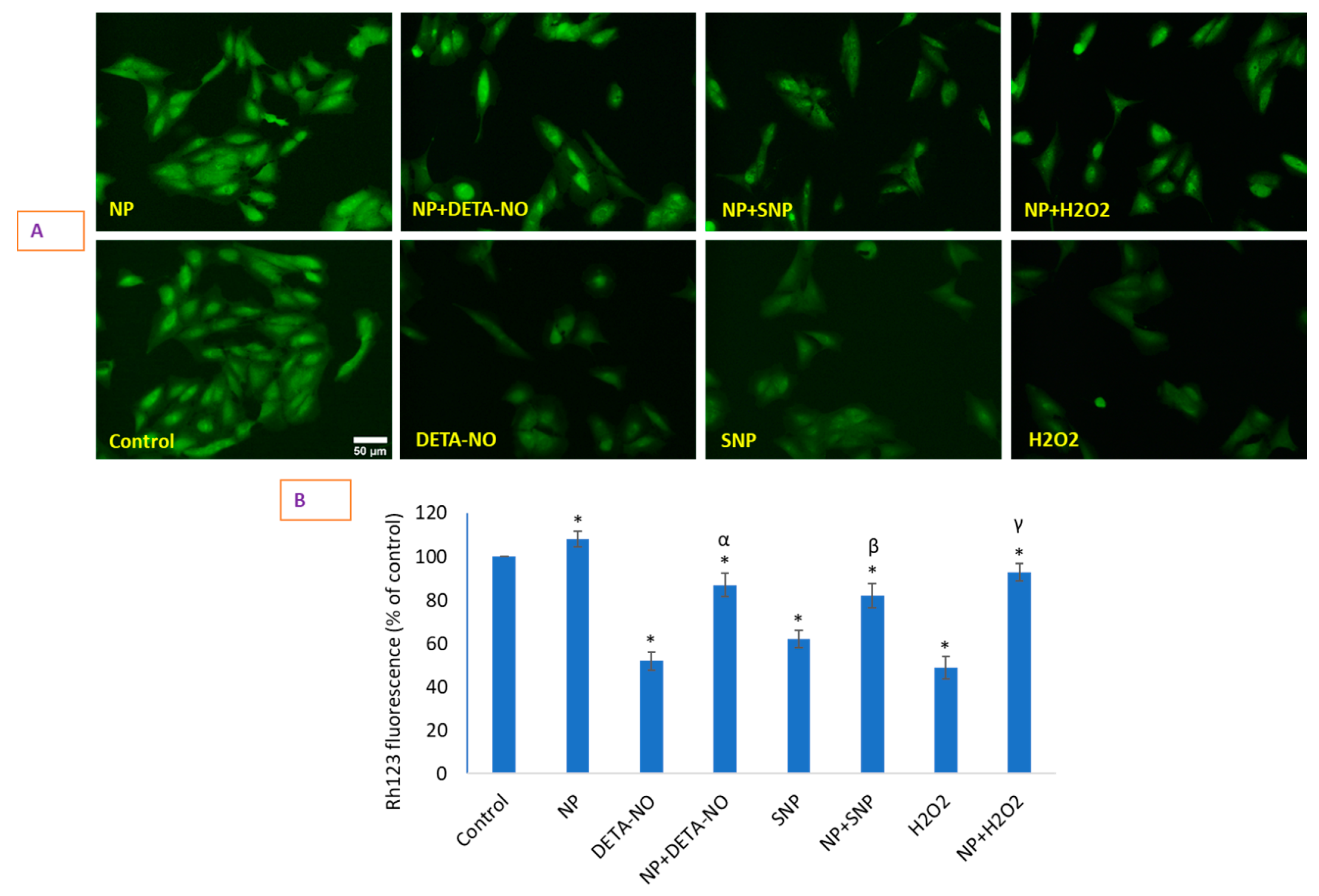
2.6. Loss of Mitochondrial Membrane Potential (MMP) That Occurred Due to NO Donors Was Significantly Recovered by CeO2 NPs
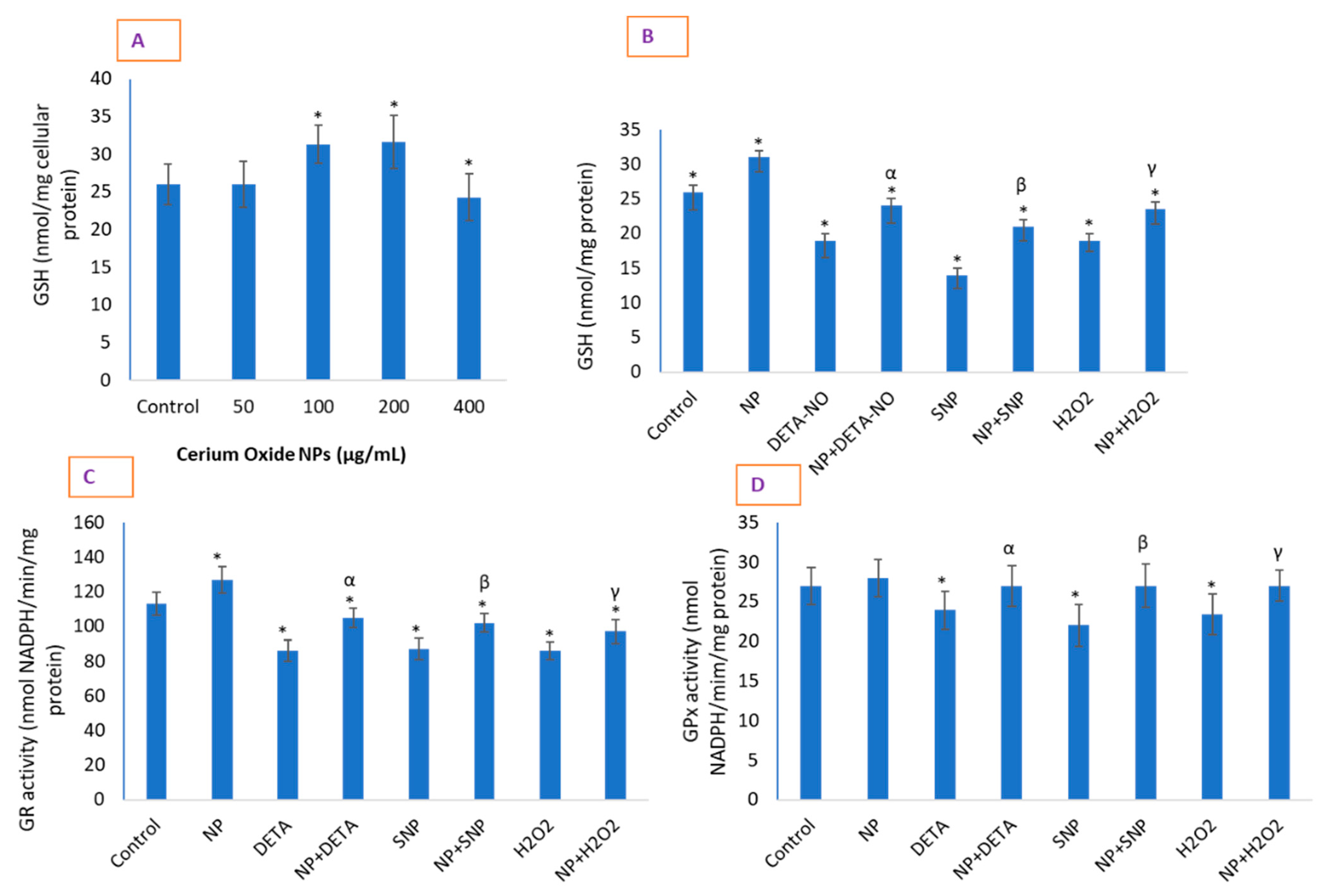
2.7. CeO2 NPs Significantly Restored Depleted GSH in Cells and Increased the Activity of Antioxidant Enzymes
3. Materials and Methods
3.1. Synthesis and Characterization of CeO2 Nanoparticles
3.2. Cell Culture and Cell Viability Due to CeO2 NP and NO-Donors
3.3. Determination of Cytokines IL-1β and TNF-α Levels
3.4. Determination of Intracellular ROS
3.5. Analysis of Intracellular NO
3.6. Determination of Mitochondrial Membrane Potential by Rh123
3.7. Determination of GSH and GSH-Related Antioxidant Enzymes
3.8. Protein Estimation
3.9. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Das, M.; Patil, S.; Bhargava, N.; Kang, J.F.; Riedel, L.M.; Seal, S.; Hickman, J.J. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials 2007, 28, 1918–1925. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Alshamsan, A. Mechanism of ROS scavenging and antioxidant signalling by redox metallic and fullerene nanomaterials: Potential implications in ROS associated degenerative disorders. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 802–813. [Google Scholar] [CrossRef]
- Li, Y.; Hou, X.; Yang, C.; Pang, Y.; Li, X.; Jiang, G.; Liu, Y. Photoprotection of Cerium Oxide Nanoparticles against UVA radiation-induced Senescence of Human Skin Fibroblasts due to their Antioxidant Properties. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Pagliari, F.; Mandoli, C.; Forte, G.; Magnani, E.; Pagliari, S.; Nardone, G.; Licoccia, S.; Minieri, M.; Di Nardo, P.; Traversa, E. Cerium oxide nanoparticles protect cardiac progenitor cells from oxidative stress. ACS Nano 2012, 6, 3767–3775. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Khan, M.A.M.; Alrokayan, S.A. Glutathione replenishing potential of CeO2 nanoparticles in human breast and fibrosarcoma cells. J. Colloid Interface Sci. 2015, 453, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, S.; Perramón, M.; Casals, G.; Oró, D.; Ribera, J.; Morales-Ruiz, M.; Casals, E.; Casado, P.; Melgar-Lesmes, P.; Fernández-Varo, G.; et al. Cerium oxide nanoparticles protect against oxidant injury and interfere with oxidative mediated kinase signaling in human-derived hepatocytes. Int. J. Mol. Sci. 2019, 20, 5959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nedder, M.; Boland, S.; Devineau, S.; Zerrad-Saadi, A.; Rogozarski, J.; Lai-Kuen, R.; Baya, I.; Guibourdenche, J.; Vibert, F.; Chissey, A.; et al. Uptake of cerium dioxide nanoparticles and impact on viability, differentiation and functions of primary trophoblast cells from human placenta. Nanomaterials 2020, 10, 1309. [Google Scholar] [CrossRef] [PubMed]
- Nethi, S.K.; Nanda, H.S.; Steele, T.W.J.; Patra, C.R. Functionalized nanoceria exhibit improved angiogenic properties. J. Mater. Chem. B 2017, 5, 9371–9383. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, O.A.; Akinloye, O.; Adaramoye, O.A. Cerium Oxide Nanoparticles Attenuate Oxidative Stress and Inflammation in the Liver of Diethylnitrosamine-Treated Mice. Biol. Trace Elem. Res. 2020, 193, 214–225. [Google Scholar] [CrossRef]
- Corsi, F.; Caputo, F.; Traversa, E.; Ghibelli, L. Not Only Redox: The Multifaceted Activity of Cerium Oxide Nanoparticles in Cancer Prevention and Therapy. Front. Oncol. 2018, 8, 309. [Google Scholar] [CrossRef] [Green Version]
- Fisher, A.B. Redox signaling across cell membranes. Antioxidants Redox Signal. 2009, 11, 1349–1356. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A. Challenges facing nanotoxicology and nanomedicine due to cellular diversity. Clin. Chim. Acta 2018, 487, 186–196. [Google Scholar] [CrossRef]
- Forman, H.J.; Fukuto, J.M.; Torres, M. Redox signaling: Thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am. J. Physiol. Cell Physiol. 2004, 287. [Google Scholar] [CrossRef]
- Skonieczna, M.; Hejmo, T.; Poterala-Hejmo, A.; Cieslar-Pobuda, A.; Buldak, R.J. NADPH Oxidases (NOX): Insights into Selected Functions and Mechanisms of Action in Cancer and Stem Cells. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Petry, A.; Djordjevic, T.; Weitnauer, M.; Kietzmann, T.; Hess, J.; Görlach, A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxidants Redox Signal. 2006, 8, 1473–1484. [Google Scholar] [CrossRef]
- Gray, E.; Ginty, M.; Kemp, K.; Scolding, N.; Wilkins, A. Peroxisome proliferator-activated receptor-α agonists protect cortical neurons from inflammatory mediators and improve peroxisomal function. Eur. J. Neurosci. 2011, 33, 1421–1432. [Google Scholar] [CrossRef]
- Shi, H.Y.; Yan, S.M.; Guo, Y.M.; Zhang, B.Q.; Guo, X.Y.; Shi, B.L. Vitamin A pretreatment protects NO-induced bovine mammary epithelial cells from oxidative stress by modulating Nrf2 and NF-κB signaling pathways. J. Anim. Sci. 2018, 96, 1305–1316. [Google Scholar] [CrossRef]
- Lee, A.Y.; Choi, J.W.; Yokozawa, T.; Cho, E.J. Preventive effect of oligonol on nitric oxide and reactive oxygen species production through regulation of nuclear factor kappa B signaling pathway in RAW 264.7 macrophage cells against sodium nitroprusside. RSC Adv. 2019, 9, 3987–3993. [Google Scholar] [CrossRef] [Green Version]
- Dominiak, A.; Wilkaniec, A.; Wroczyński, P.; Jęśko, H.; Adamczyk, A. Protective Effects of Selol Against Sodium Nitroprusside-Induced Cell Death and Oxidative Stress in PC12 Cells. Neurochem. Res. 2016, 41, 3215–3226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bewersdorff, T.; Gruber, A.; Eravci, M.; Dumbani, M.; Klinger, D.; Haase, A. Amphiphilic nanogels: Influence of surface hydrophobicity on protein corona, biocompatibility and cellular uptake. Int. J. Nanomed. 2019, 14, 7861–7878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.H.; Stephen Inbaraj, B. Various physicochemical and surface properties controlling the bioactivity of cerium oxide nanoparticles. Crit. Rev. Biotechnol. 2018, 38, 1003–1024. [Google Scholar] [CrossRef]
- You, G.; Hou, J.; Xu, Y.; Miao, L.; Ao, Y.; Xing, B. Surface Properties and Environmental Transformations Controlling the Bioaccumulation and Toxicity of Cerium Oxide Nanoparticles: A Critical Review. In Reviews of Environmental Contamination and Toxicology; Springer Nature: Cham, Switzerland, 2021; Volume 253, pp. 155–206. [Google Scholar]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Botta, M.; Distrutti, E.; Mencarelli, A.; Parlato, M.C.; Raffi, F.; Cipriani, S.; Fiorucci, S. Anti-inflammatory activity of a new class of nitric oxide synthase inhibitors that release nitric oxide. ChemMedChem 2008, 3, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Wang, K.; Kolattukudy, P.E. Cerium oxide nanoparticles inhibits oxidative stress and nuclear Factor-κB activation in H9c2 cardiomyocytes exposed to cigarette smoke extract. J. Pharmacol. Exp. Ther. 2011, 338, 53–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, H.; Nassar, A.M.K.; Noreldin, A.E.; Samak, D.; Elshony, N.; Wasef, L.; Elewa, Y.H.A.; Hassan, S.M.A.; Saati, A.A.; Hetta, H.F.; et al. Chemo-protective potential of cerium oxide nanoparticles against fipronil-induced oxidative stress, apoptosis, inflammation and reproductive dysfunction in male white albino rats. Molecules 2020, 25, 3479. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, B.I.; Cave, G.W.V.; Barnett, Y.; Pierscionek, B. Treatment of human lens epithelium with high levels of Nanoceria leads to reactive oxygen species mediated apoptosis. Molecules 2020, 25, 441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horie, M.; Nishio, K.; Kato, H.; Fujita, K.; Endoh, S.; Nakamura, A.; Miyauchi, A.; Kinugasa, S.; Yamamoto, K.; Niki, E.; et al. Cellular responses induced by cerium oxide nanoparticles: Induction of intracellular calcium level and oxidative stress on culture cells. J. Biochem. 2011, 150, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Brüne, B. Nitric oxide: NO apoptosis or turning it ON? Cell Death Differ. 2003, 10, 864–869. [Google Scholar] [CrossRef]
- Kumari, M.; Singh, S.P.; Chinde, S.; Rahman, M.F.; Mahboob, M.; Grover, P. Toxicity study of cerium oxide nanoparticles in human neuroblastoma cells. Int. J. Toxicol. 2014, 33, 86–97. [Google Scholar] [CrossRef]
- Nemmar, A.; Yuvaraju, P.; Beegam, S.; Fahim, M.A.; Ali, B.H. Cerium Oxide Nanoparticles in Lung Acutely Induce Oxidative Stress, Inflammation, and DNA Damage in Various Organs of Mice. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Azad, M.B.; Gibson, S.B. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009, 16, 1040–1052. [Google Scholar] [CrossRef] [Green Version]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menon, S.G.; Sarsour, E.H.; Kalen, A.L.; Venkataraman, S.; Hitchler, M.J.; Domann, F.E.; Oberley, L.W.; Goswami, P.C. Superoxide signaling mediates N-acetyl-L-cysteine-induced G1 arrest: Regulatory role of cyclin D1 and manganese superoxide dismutase. Cancer Res. 2007, 67, 6392–6399. [Google Scholar] [CrossRef] [Green Version]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Shields, H.J.; Traa, A.; Van Raamsdonk, J.M. Beneficial and Detrimental Effects of Reactive Oxygen Species on Lifespan: A Comprehensive Review of Comparative and Experimental Studies. Front. Cell Dev. Biol. 2021, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Moellering, D.; Mc Andrew, J.; Patel, R.P.; Forman, H.J.; Mulcahy, R.T.; Jo, H.; Darley-Usmar, V.M. The induction of GSH synthesis by nanomolar concentrations of NO in endothelial cells: A role for γ-glutamylcysteine synthetase and γ-glutamyl transpeptidase. FEBS Lett. 1999, 448, 292–296. [Google Scholar] [CrossRef] [Green Version]
- Baldelli, S.; Ciccarone, F.; Limongi, D.; Checconi, P.; Palamara, A.T.; Ciriolo, M.R. Glutathione and nitric oxide: Key team players in use and disuse of skeletal muscle. Nutrients 2019, 11, 2318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortese-Krott, M.M.; Suschek, C.V.; Wetzel, W.; Kröncke, K.D.; Kolb-Bachofen, V. Nitric oxide-mediated protection of endothelial cells from hydrogen peroxide is mediated by intracellular zinc and glutathione. Am. J. Physiol. Cell Physiol. 2009, 296, 811–820. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.X.; Chen, W.F.; Zhou, X.Z.; Gu, Z.Y.; Chen, C.M. Synthesis of CeO2 nanoparticles by mechanochemical processing and the inhibiting action of NaCl on particle agglomeration. Mater. Lett. 2005, 59, 48–52. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Peshavariya, H.M.; Dusting, G.J.; Selemidis, S. Analysis of dihydroethidium fluorescence for the detection of intracellular and extracellular superoxide produced by NADPH oxidase. Free Radic. Res. 2007, 41, 699–712. [Google Scholar] [CrossRef]
- Von Bohlen und Halbach, O. Nitric oxide imaging in living neuronal tissues using fluorescent probes. Nitric Oxide Biol. Chem. 2003, 9, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Hirotani, M.; Nakatsubo, N.; Kikuchi, K.; Urano, Y.; Higuchi, T.; Hirata, Y.; Nagano, T. Bioimaging of nitric oxide with fluorescent indicators based on the rhodamine chromophore. Anal. Chem. 2001, 73, 1967–1973. [Google Scholar] [CrossRef]
- Li, H.; Wan, A. Fluorescent probes for real-time measurement of nitric oxide in living cells. Analyst 2015, 140, 7129–7141. [Google Scholar] [CrossRef]
- Tatsutomi, M.; Jo, J.I.; Tabata, Y. Preparation of a nitric oxide imaging agent from gelatin derivative micelles. Regen. Ther. 2016, 5, 64–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, X.; Rubakhin, S.S.; Sweedler, J.V. Simultaneous nitric oxide and dehydroascorbic acid imaging by combining diaminofluoresceins and diaminorhodamines. J. Neurosci. Methods 2008, 168, 373–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousfi, N.; Pruvot, B.; Lopez, T.; Magadoux, L.; Franche, N.; Pichon, L.; Salvadori, F.; Solary, E.; Garrido, C.; Laurens, V.; et al. The Impact of Tumor Nitric Oxide Production on VEGFA Expression and Tumor Growth in a Zebrafish Rat Glioma Xenograft Model. PLoS ONE 2015, 10, e0120435. [Google Scholar] [CrossRef] [PubMed]
- Hassani, A.; Azarian, M.M.S.; Ibrahim, W.N.; Hussain, S.A. Preparation, characterization and therapeutic properties of gum arabic-stabilized gallic acid nanoparticles. Sci. Rep. 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, H.; Wu, J. Effects of nitric oxide on the biological behavior of HepG2 human hepatocellular carcinoma cells. Exp. Ther. Med. 2016, 11, 1875–1880. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.V.; Walsh, M.L.; Chen, L.B. Localization of mitochondria in living cells with rhodamine 123. Proc. Natl. Acad. Sci. USA 1980, 77, 990–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baracca, A.; Sgarbi, G.; Solaini, G.; Lenaz, G. Rhodamine 123 as a probe of mitochondrial membrane potential: Evaluation of proton flux through F0 during ATP synthesis. Biochim. Biophys. Acta Bioenerg. 2003, 1606, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Seefeldt, T.; Dwivedi, C.; Peitz, G.; Herman, J.; Carlson, L.; Zhang, Z.; Guan, X. 2-Acetylamino-3-[4-(2-acetylamino-2-carboxyethylsulfanylcarbonylamino) phenylcarbamoylsulfanyl]propionic acid and its derivatives as a novel class of glutathione reductase inhibitors. J. Med. Chem. 2005, 48, 5224–5231. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtar, M.J.; Ahamed, M.; Alhadlaq, H. Anti-Inflammatory CeO2 Nanoparticles Prevented Cytotoxicity Due to Exogenous Nitric Oxide Donors via Induction Rather Than Inhibition of Superoxide/Nitric Oxide in HUVE Cells. Molecules 2021, 26, 5416. https://doi.org/10.3390/molecules26175416
Akhtar MJ, Ahamed M, Alhadlaq H. Anti-Inflammatory CeO2 Nanoparticles Prevented Cytotoxicity Due to Exogenous Nitric Oxide Donors via Induction Rather Than Inhibition of Superoxide/Nitric Oxide in HUVE Cells. Molecules. 2021; 26(17):5416. https://doi.org/10.3390/molecules26175416
Chicago/Turabian StyleAkhtar, Mohd Javed, Maqusood Ahamed, and Hisham Alhadlaq. 2021. "Anti-Inflammatory CeO2 Nanoparticles Prevented Cytotoxicity Due to Exogenous Nitric Oxide Donors via Induction Rather Than Inhibition of Superoxide/Nitric Oxide in HUVE Cells" Molecules 26, no. 17: 5416. https://doi.org/10.3390/molecules26175416
APA StyleAkhtar, M. J., Ahamed, M., & Alhadlaq, H. (2021). Anti-Inflammatory CeO2 Nanoparticles Prevented Cytotoxicity Due to Exogenous Nitric Oxide Donors via Induction Rather Than Inhibition of Superoxide/Nitric Oxide in HUVE Cells. Molecules, 26(17), 5416. https://doi.org/10.3390/molecules26175416