Low-Field NMR Study of Shortcake Biscuits with Cricket Powder, and Their Nutritional and Physical Characteristics
Abstract
:1. Introduction
2. Results and Discussion
2.1. Consumer Study
2.2. Biscuits Appearance
2.3. Nutritional Value
2.4. Physical Properties
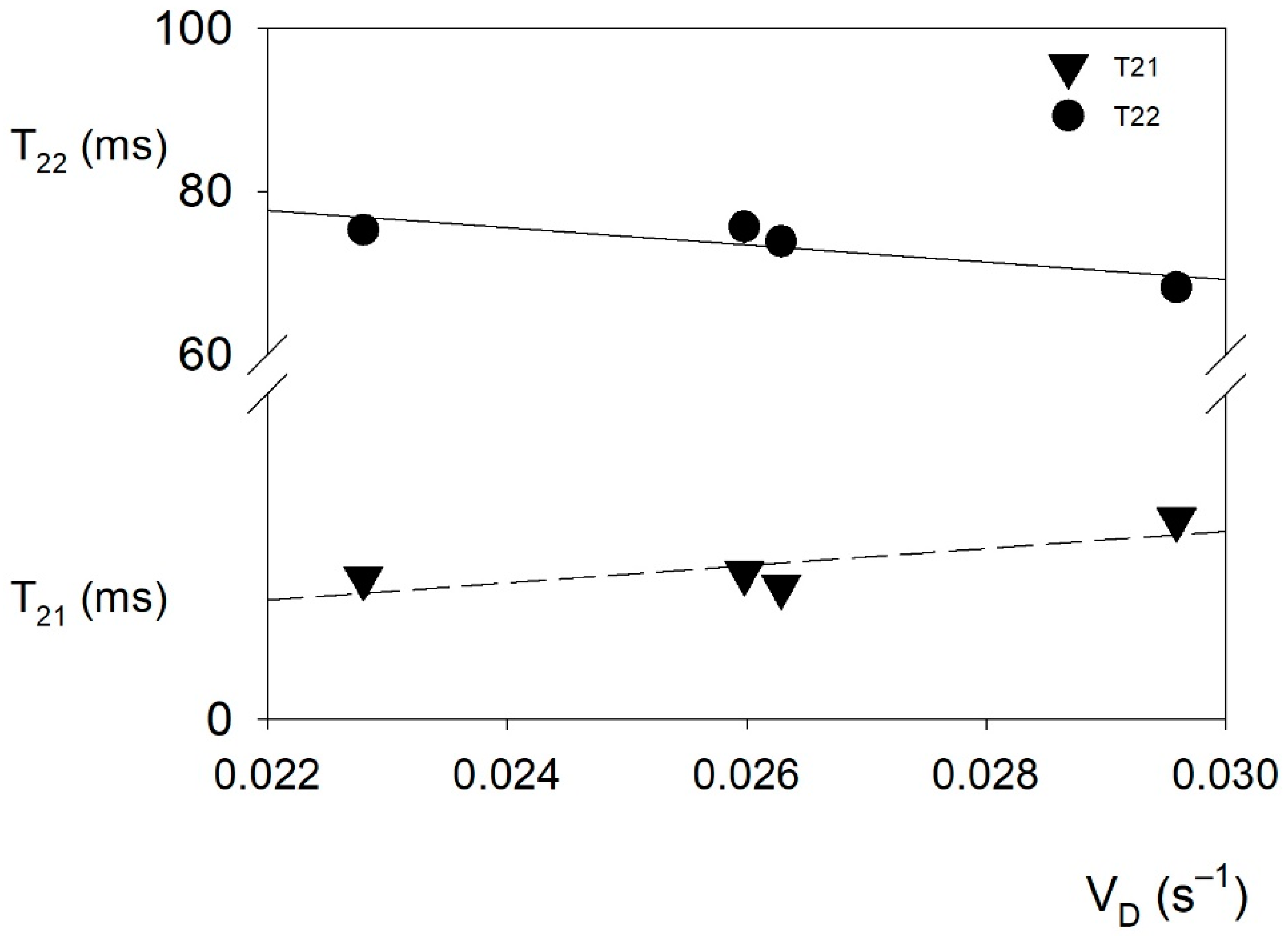
2.5. Water Behavior
3. Materials and Methods
3.1. Shortcake Biscuits Manufacturing
3.2. Consumer Acceptance
3.3. Color Measurements
3.4. Proximate Composition and Energy Value
3.5. Minerals Content
3.6. Amino Acid Composition
3.7. Fatty Acid Composition Analysis
3.8. Texture Analysis
3.9. LF NMR Relaxometry
3.10. Water Activity
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Metro, D.; Tardugno, R.; Papa, M.; Bisignano, C.; Manasseri, L.; Calabrese, G.; Gervasi, T.; Dugo, G.; Cicero, N. Adherence to the Mediterranean diet in a Sicilian student population. Nat. Prod. Res. 2018, 32, 1775–1781. [Google Scholar] [CrossRef]
- Metro, D.; Papa, M.; Manasseri, L.; Gervasi, T.; Campone, L.; Pellizzeri, V.; Tardugno, R.; Dugo, G. Mediterranean diet in a Sicilian student population. Second part: Breakfast and its nutritional profile. Nat. Prod. Res. 2020, 34, 2255–2261. [Google Scholar] [CrossRef]
- Cammilleri, G.; Vazzana, M.; Arizza, V.; Giunta, F.; Vella, A.; Lo Dico, G.; Giaccone, V.; Giofrè, S.V.; Giangrosso, G.; Cicero, N.; et al. Mercury in fish products: What’s the best for consumers between bluefin tuna and yellowfin tuna? Nat. Prod. Res. 2018, 32, 457–462. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for the Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [Green Version]
- Jakubowska, D.; Staniewska, K. Information on food fortification with bioactive compounds in observation andconsumer studies. Pol. J. Nat. Sci. 2015, 30, 307–318. [Google Scholar]
- Betoret, E.; Rosell, C.M. Enrichment of bread with fruits and vegetables: Trends and strategies to increase functionality. Cereal Chem. 2020, 97, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Phongnarisorn, B.; Orfila, C.; Holmes, M.; Marshall, L. Enrichment of Biscuits with Matcha Green Tea Powder: Its Impact on Consumer Acceptability and Acute Metabolic Response. Foods 2018, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Maner, S.; Sharma, A.K.; Banerjee, K. Wheat Flour Replacement by Wine Grape Pomace Powder Positively Affects Physical, Functional and Sensory Properties of Cookies. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 87, 109–113. [Google Scholar] [CrossRef]
- Statista Sugar Confectionary Retail Sales Value in Europe 2014–2018. Available online: https://www.statista.com/statistics/1134398/sugar-confectionary-retail-sales-value-in-europe/ (accessed on 12 July 2021).
- Cookie Report—TOP Agency. Available online: https://topagency.com/report/cookie-report/ (accessed on 12 July 2021).
- Statista Confectionery: Weekly Consumption UK 2006–2019. Available online: https://www.statista.com/statistics/284521/weekly-household-consumption-of-confectionery-in-the-united-kingdom-uk/ (accessed on 12 July 2021).
- Hooda, S.; Jood, S. Organoleptic and nutritional evaluation of wheat biscuits supplemented with untreated and treated fenugreek flour. Food Chem. 2005, 90, 427–435. [Google Scholar] [CrossRef]
- Tyagi, S.K.; Manikantan, M.R.; Oberoi, H.S.; Kaur, G. Effect of mustard flour incorporation on nutritional, textural and organoleptic characteristics of biscuits. J. Food Eng. 2007, 80, 1043–1050. [Google Scholar] [CrossRef]
- De Nogueira, A.C.; Steel, C.J. Protein enrichment of biscuits: A review. Food Rev. Int. 2018, 34, 796–809. [Google Scholar] [CrossRef]
- FAO. Assessing the Potential of Insects as Food and Feed in Assuring Food Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2012. [Google Scholar]
- Montowska, M.; Kowalczewski, P.Ł.; Rybicka, I.; Fornal, E. Nutritional value, protein and peptide composition of edible cricket powders. Food Chem. 2019, 289, 130–138. [Google Scholar] [CrossRef]
- Skotnicka, M.; Karwowska, K.; Kłobukowski, F.; Borkowska, A.; Pieszko, M. Possibilities of the Development of Edible Insect-Based Foods in Europe. Foods 2021, 10, 766. [Google Scholar] [CrossRef]
- Zielińska, E.; Pankiewicz, U.; Sujka, M. Nutritional, Physiochemical, and Biological Value of Muffins Enriched with Edible Insects Flour. Antioxidants 2021, 10, 1122. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, E.; Baraniak, B.; Karaś, M.; Rybczyńska, K.; Jakubczyk, A. Selected species of edible insects as a source of nutrient composition. Food Res. Int. 2015, 77, 460–466. [Google Scholar] [CrossRef]
- Pauter, P.; Różańska, M.; Wiza, P.; Dworczak, S.; Grobelna, N.; Sarbak, P.; Kowalczewski, P.Ł. Effects of the replacement of wheat flour with cricket powder on the characteristics of muffins. Acta Sci. Pol. Technol. Aliment. 2018, 17, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Walkowiak, K.; Kowalczewski, P.Ł.; Kubiak, P.; Baranowska, H.M. Effect of cricket powder addition on 1H NMR mobility and texture of pork pate. J. Microbiol. Biotechnol. Food Sci. 2019, 9, 191–194. [Google Scholar] [CrossRef]
- Kowalczewski, P.; Walkowiak, K.; Masewicz, Ł.; Bartczak, O.; Lewandowicz, J.; Kubiak, P.; Baranowska, H. Gluten-Free Bread with Cricket Powder—Mechanical Properties and Molecular Water Dynamics in Dough and Ready Product. Foods 2019, 8, 240. [Google Scholar] [CrossRef] [Green Version]
- Smarzyński, K.; Sarbak, P.; Musiał, S.; Jeżowski, P.; Piątek, M.; Kowalczewski, P.Ł. Nutritional analysis and evaluation of the consumer acceptance of pork pâté enriched with cricket powder-preliminary study. Open Agric. 2019, 4, 159–163. [Google Scholar] [CrossRef]
- Duda, A.; Adamczak, J.; Chełmińska, P.; Juszkiewicz, J.; Kowalczewski, P. Quality and Nutritional/Textural Properties of Durum Wheat Pasta Enriched with Cricket Powder. Foods 2019, 8, 46. [Google Scholar] [CrossRef] [Green Version]
- Bruneel, C.; Pareyt, B.; Brijs, K.; Delcour, J.A. The impact of the protein network on the pasting and cooking properties of dry pasta products. Food Chem. 2010, 120, 371–378. [Google Scholar] [CrossRef]
- Małyszek, Z.; Lewandowicz, J.; Le Thanh-Blicharz, J.; Walkowiak, K.; Kowalczewski, P.Ł.; Baranowska, H.M. Water Behavior of Emulsions Stabilized by Modified Potato Starch. Polymers 2021, 13, 2200. [Google Scholar] [CrossRef]
- Kamal, T.; Cheng, S.; Khan, I.A.; Nawab, K.; Zhang, T.; Song, Y.; Wang, S.; Nadeem, M.; Riaz, M.; Khan, M.A.U.; et al. Potential uses of LF-NMR and MRI in the study of water dynamics and quality measurement of fruits and vegetables. J. Food Process. Preserv. 2019, 43, e14202. [Google Scholar] [CrossRef]
- Ezeanaka, M.C.; Nsor-Atindana, J.; Zhang, M. Online Low-field Nuclear Magnetic Resonance (LF-NMR) and Magnetic Resonance Imaging (MRI) for Food Quality Optimization in Food Processing. Food Bioprocess Technol. 2019, 12, 1435–1451. [Google Scholar] [CrossRef]
- Lewandowicz, J.; Baranowska, H.M.; Szwengiel, A.; Le Thanh-Blicharz, J. Molecular structure vs. Functional properties of waxy and normal corn starch. In Proceedings of the 12th International Conference on Polysaccharides-Glycoscience, Prague, Czech Republic, 19–21 October 2016; Rapkova, R., Copikova, J., Sarka, E., Eds.; Czech Chemical Society: Prague, Czech Republic, 2016; pp. 53–57. [Google Scholar]
- Lewandowicz, J.; Ostrowska-Ligeza, E.; Baranowska, H.M. Gelatinization of Starch: A Comparative Study of Viscographic, Differential Scanning Calorimetry and Low Field NMR Analyses. In Proceedings of the 16th International Conference on Polysaccharides-Glycoscience, Prague, Czech Republic, 4–6 November 2020; Rapkova, R., Copikova, J., Sarka, E., Eds.; Czech Chemical Society: Prague, Czech Republic, 2020; pp. 9–14. [Google Scholar]
- Sikora, M.; Krystyjan, M.; Dobosz, A.; Tomasik, P.; Walkowiak, K.; Masewicz, Ł.; Kowalczewski, P.Ł.; Baranowska, H.M. Molecular Analysis of Retrogradation of Corn Starches. Polymers 2019, 11, 1764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masewicz, L.; Pers, K.; Le Thanh-Blicharz, J.; Lewandowicz, J.; Baranowska, H.M. The effect of degree of substitution on dynamics of molecules of hydration water in acetylated distarch adipate powders. In Proceedings of the 13th International Conference on Polysaccharides-Glycoscience, Prague, Czech Republic, 9–10 November 2017; Rapkova, R., Copikova, J., Sarka, E., Eds.; Czech Chemical Society: Prague, Czech Republic, 2017; pp. 29–32. [Google Scholar]
- Resende, M.T.; Osheter, T.; Linder, C.; Wiesman, Z. Proton Low Field NMR Relaxation Time Domain Sensor for Monitoring of Oxidation Stability of PUFA-Rich Oils and Emulsion Products. Foods 2021, 10, 1385. [Google Scholar] [CrossRef] [PubMed]
- Stangierski, J.; Rezler, R.; Baranowska, H.M.; Poliszko, S. Effect of enzymatic modification on chicken surimi. Czech J. Food Sci. 2012, 30, 404–411. [Google Scholar] [CrossRef] [Green Version]
- Baranowska, H.M.; Rezler, R. Water binding analysis of fat-water emulsions. Food Sci. Biotechnol. 2015, 24, 1921–1925. [Google Scholar] [CrossRef]
- Le Thanh-Blicharz, J. The Influence of the Physicochemical Properties and Structure of Modified Starches on Their Functionality in the Formation and Stabilization of Food Emulsions; Wydawnictwo Uniwerystetu Przyrodniczego w Poznaniu: Poznań, Poland, 2018; ISBN 978-83-7160-903-9. [Google Scholar]
- Kowalczewski, P.Ł.; Walkowiak, K.; Masewicz, Ł.; Smarzyński, K.; Le Thanh-Blicharz, J.; Kačániová, M.; Baranowska, H.M. LF NMR spectroscopy analysis of water dynamics and texture of gluten-free bread with cricket powder during storage. Food Sci. Technol. Int. 2021, 108201322098791. [Google Scholar] [CrossRef]
- Li, J.; Hou, G.G.; Chen, Z.; Gehring, K. Effects of endoxylanases, vital wheat gluten, and gum Arabic on the rheological properties, water mobility, and baking quality of whole-wheat saltine cracker dough. J. Cereal Sci. 2013, 58, 437–445. [Google Scholar] [CrossRef]
- Baranowska, H.M.; Masewicz, Ł.; Kowalczewski, P.Ł.; Lewandowicz, G.; Piątek, M.; Kubiak, P. Water properties in pâtés enriched with potato juice. Eur. Food Res. Technol. 2018, 244, 387–393. [Google Scholar] [CrossRef] [Green Version]
- Colnago, L.A.; Wiesman, Z.; Pages, G.; Musse, M.; Monaretto, T.; Windt, C.W.; Rondeau-Mouro, C. Low field, time domain NMR in the agriculture and agrifood sectors: An overview of applications in plants, foods and biofuels. J. Magn. Reson. 2021, 323, 106899. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Chambers, E. Consumer Avoidance of Insect Containing Foods: Primary Emotions, Perceptions and Sensory Characteristics Driving Consumers Considerations. Foods 2019, 8, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piha, S.; Pohjanheimo, T.; Lähteenmäki-Uutela, A.; Křečková, Z.; Otterbring, T. The effects of consumer knowledge on the willingness to buy insect food: An exploratory cross-regional study in Northern and Central Europe. Food Qual. Prefer. 2018, 70, 1–10. [Google Scholar] [CrossRef]
- House, J. Consumer acceptance of insect-based foods in the Netherlands: Academic and commercial implications. Appetite 2016, 107, 47–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishyna, M.; Chen, J.; Benjamin, O. Sensory attributes of edible insects and insect-based foods—Future outlooks for enhancing consumer appeal. Trends Food Sci. Technol. 2020, 95, 141–148. [Google Scholar] [CrossRef]
- Melgar-Lalanne, G.; Hernández-Álvarez, A.; Salinas-Castro, A. Edible Insects Processing: Traditional and Innovative Technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1166–1191. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.; Kim, H.R.; Cho, I.H. Aroma Characteristics of Raw and Cooked Tenebrio molitor Larvae (Mealworms). Food Sci. Anim. Resour. 2020, 40, 649–658. [Google Scholar] [CrossRef]
- Grossmann, K.K.; Merz, M.; Appel, D.; De Araujo, M.M.; Fischer, L. New insights into the flavoring potential of cricket (Acheta domesticus) and mealworm (Tenebrio molitor) protein hydrolysates and their Maillard products. Food Chem. 2021, 364, 130336. [Google Scholar] [CrossRef]
- Hazard, B.; Trafford, K.; Lovegrove, A.; Griffiths, S.; Uauy, C.; Shewry, P. Strategies to improve wheat for human health. Nat. Food 2020, 1, 475–480. [Google Scholar] [CrossRef]
- Blanshard, J.M.V. Elements of cereal product structure. In Food Structure; Elsevier: Amsterdam, The Netherlands, 1988; pp. 313–330. [Google Scholar]
- Burt, K.G.; Kotao, T.; Lopez, I.; Koeppel, J.; Goldstein, A.; Samuel, L.; Stopler, M. Acceptance of Using Cricket Flour as a Low Carbohydrate, High Protein, Sustainable Substitute for All-Purpose Flour in Muffins. J. Culin. Sci. Technol. 2020, 18, 201–213. [Google Scholar] [CrossRef]
- Kowalski, S.; Lukasiewicz, M.; Juszczak, L.; Kutyła-Kupidura, E.M. Dynamics of 5-hydroxymethylfurfural formation in shortbreads during thermal processing. Czech J. Food Sci. 2013, 31, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Zanoni, B.; Peri, C.; Bruno, D. Modelling of browning kinetics of bread crust during baking. LWT-Food Sci. Technol. 1995, 28, 604–609. [Google Scholar] [CrossRef]
- Zielińska, E.; Pankiewicz, U. Nutritional, Physiochemical, and Antioxidative Characteristics of Shortcake Biscuits Enriched with Tenebrio molitor Flour. Molecules 2020, 25, 5629. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewski, P.Ł.; Gumienna, M.; Rybicka, I.; Górna, B.; Sarbak, P.; Dziedzic, K.; Kmiecik, D. Nutritional Value and Biological Activity of Gluten-Free Bread Enriched with Cricket Powder. Molecules 2021, 26, 1184. [Google Scholar] [CrossRef]
- Mokrzycki, W.; Tatol, M. Color difference Delta E—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Van Huis, A. New Sources of Animal Proteins: Edible Insects. In New Aspects of Meat Quality; Elsevier: Amsterdam, The Netherlands, 2017; pp. 443–461. [Google Scholar]
- Van Huis, A. Nutrition and health of edible insects. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 228–231. [Google Scholar] [CrossRef]
- Jantzen da Silva Lucas, A.; Menegon de Oliveira, L.; da Rocha, M.; Prentice, C. Edible insects: An alternative of nutritional, functional and bioactive compounds. Food Chem. 2020, 311, 126022. [Google Scholar] [CrossRef]
- Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers, Amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32011R1169 (accessed on 12 July 2021).
- Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32006R1924 (accessed on 12 July 2021).
- World Health Organization. Salt Reduction. Available online: https://www.who.int/news-room/fact-sheets/detail/salt-reduction (accessed on 12 July 2021).
- Public Health England. Salt Reduction Targets for 2024; 2020. Available online: https://www.gov.uk/government/publications/salt-reduction-targets-for-2024 (accessed on 12 July 2021).
- Udomsil, N.; Imsoonthornruksa, S.; Gosalawit, C.; Ketudat-Cairns, M. Nutritional Values and Functional Properties of House Cricket (Acheta domesticus) and Field Cricket (Gryllus bimaculatus). Food Sci. Technol. Res. 2019, 25, 597–605. [Google Scholar] [CrossRef]
- Grapes, M.; Whiting, P.; Dinan, L. Fatty acid and lipid analysis of the house cricket, Acheta domesticus. Insect Biochem. 1989, 19, 767–774. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Peñas, E.; Hernández-Ledesma, B. Pseudocereal grains: Nutritional value, health benefits and current applications for the development of gluten-free foods. Food Chem. Toxicol. 2020, 137, 111178. [Google Scholar] [CrossRef]
- Mathai, J.K.; Liu, Y.; Stein, H.H. Values for digestible indispensable amino acid scores (DIAAS) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores (PDCAAS). Br. J. Nutr. 2017, 117, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Taylor, C.; Nebl, T.; Ng, K.; Bennett, L.E. Effects of chemical composition and baking on in vitro digestibility of proteins in breads made from selected gluten-containing and gluten-free flours. Food Chem. 2017, 233, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Andrade, C. Maillard reaction products: Some considerations on their health effects. Clin. Chem. Lab. Med. 2014, 52. [Google Scholar] [CrossRef]
- Lund, M.N.; Ray, C.A. Control of Maillard Reactions in Foods: Strategies and Chemical Mechanisms. J. Agric. Food Chem. 2017, 65, 4537–4552. [Google Scholar] [CrossRef] [Green Version]
- Zheng, B.; Zhao, H.; Zhou, Q.; Cai, J.; Wang, X.; Cao, W.; Dai, T.; Jiang, D. Relationships of protein composition, gluten structure, and dough rheological properties with short biscuits quality of soft wheat varieties. Agron. J. 2020, 112, 1921–1930. [Google Scholar] [CrossRef]
- Chevallier, S.; Colonna, P.; Buléon, A.; Della Valle, G. Physicochemical Behaviors of Sugars, Lipids, and Gluten in Short Dough and Biscuit. J. Agric. Food Chem. 2000, 48, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Cookie texture, spread ratio and sensory acceptability of cookies as a function of soluble dietary fiber, baking time and different water levels. LWT 2017, 80, 537–542. [Google Scholar] [CrossRef]
- Nanyen, D. Nutritional Composition, Physical and Sensory Properties of Cookies from Wheat, Acha and Mung Bean Composite Flours. Int. J. Nutr. Food Sci. 2016, 5, 401. [Google Scholar] [CrossRef] [Green Version]
- Ho, L.-H.; Abdul Latif, N.W.B. Nutritional composition, physical properties, and sensory evaluation of cookies prepared from wheat flour and pitaya (Hylocereus undatus) peel flour blends. Cogent Food Agric. 2016, 2, 1136369. [Google Scholar] [CrossRef]
- Zaker, A.; Genitha, T.R.; Hashmi, S.I. Effects of Defatted Soy Flour Incorporation on Physical, Sensorial and Nutritional Properties of Biscuits. J. Food Process. Technol. 2012, 3, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, A.S.; Joshi, D.C. Effect of replacement of wheat flour with pumpkin powder on textural and sensory qualities of biscuit. Int. Food Res. J. 2013, 20, 587. [Google Scholar]
- Gaines, C.S. Influence of chemical and physical modification of soft wheat protein on sugar-snap cookie dough consistency, cookie size, and hardness. Cereal Chem. 1990, 67, 73–77. [Google Scholar]
- Pareyt, B.; Delcour, J.A. The Role of Wheat Flour Constituents, Sugar, and Fat in Low Moisture Cereal Based Products: A Review on Sugar-Snap Cookies. Crit. Rev. Food Sci. Nutr. 2008, 48, 824–839. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Saxena, D.C.; Singh, S. Physical, textural, and sensory characteristics of wheat and amaranth flour blend cookies. Cogent Food Agric. 2016, 2, 1125773. [Google Scholar] [CrossRef]
- Walkowiak, K.; Lewandowicz, J.; Masewicz, L.; Le Thanh-Blicharz, J.; Baranowska, H.M. Application of empirical model of relationship of spin-lattice relaxation times and values of critical hydration for analysis of potato starch aerogels. In Proceedings of the 14th International Conference on Polysaccharides-Glycoscience, Prague, Czech Republic, 7–9 November 2018; Rapkova, R., Hinkova, A., Copikova, J., Sarka, E., Eds.; Czech Chemical Society: Prague, Czech Republic, 2018; pp. 90–92. [Google Scholar]
- Zayas, J.F. Solubility of Proteins. In Functionality of Proteins in Food; Springer: Berlin/Heidelberg, Germany, 1997; pp. 6–75. [Google Scholar]
- Bassett, F. Comparison of Functional, Nutritional, and Sensory Properties of Spray-Dried and Oven-Dried Cricket (Acheta domesticus) Powder. Master’s Thesis, Brigham Young University, Provo, UT, USA, 2018. [Google Scholar]
- Bosmans, G.M.; Lagrain, B.; Ooms, N.; Fierens, E.; Delcour, J.A. Biopolymer Interactions, Water Dynamics, and Bread Crumb Firming. J. Agric. Food Chem. 2013, 61, 4646–4654. [Google Scholar] [CrossRef]
- Berendsen, H.J.C. Specific Interactions of Water with Biopolymers. In Water in Disperse Systems; Springer: Boston, MA, USA, 1975; pp. 293–330. [Google Scholar]
- Villanueva, N.D.; Petenate, A.J.; Da Silva, M.A.A. Performance of three affective methods and diagnosis of the ANOVA model. Food Qual. Prefer. 2002, 11, 363–370. [Google Scholar] [CrossRef]
- AACC 44-19.01 Moisture--Air-Oven Method, Drying at 135 degrees. In AACC International Approved Methods; AACC International: Washington, DC, USA, 2009.
- ISO 20483:2013 Cereals and Pulses-Determination of the Nitrogen Content and Calculation of the Crude Protein Content-Kjeldahl Method; International Organization for Standardization: Geneva, Switzerland, 2013.
- Tkachuk, R. Note on the nitrogen-to-protein conversion factor for wheat flour. Cereal Chem. 1966, 43, 223–225. [Google Scholar]
- Ritvanen, T.; Pastell, H.; Welling, A.; Raatikainen, M. The nitrogen-to-protein conversion factor of two cricket species-Acheta domesticus and Gryllus bimaculatus. Agric. Food Sci. 2020, 29. [Google Scholar] [CrossRef]
- AACC Crude Fat in Wheat, Corn, and Soy Flour, Feeds, and Mixed Feeds. AACC Approved Methods of Analysis. 2009. Available online: http://methods.aaccnet.org/summaries/30-25-01.aspx (accessed on 12 July 2021). [CrossRef]
- AACC Ash in Farina and Semolina. AACC Approved Methods of Analysis. 2009. Available online: http://methods.aaccnet.org/summaries/08-12-01.aspx (accessed on 12 July 2021). [CrossRef]
- Rybicka, I.; Gliszczyńska-Świgło, A. Minerals in grain gluten-free products. The content of calcium, potassium, magnesium, sodium, copper, iron, manganese, and zinc. J. Food Compos. Anal. 2017, 59, 61–67. [Google Scholar] [CrossRef]
- European Food Safety Authority. Dietary Reference Values for nutrients Summary report. EFSA Support. Publ. 2017, 14, e15121. [Google Scholar] [CrossRef] [Green Version]
- Kwanyuen, P.; Burton, J.W. A Modified Amino Acid Analysis Using PITC Derivatization for Soybeans with Accurate Determination of Cysteine and Half-Cystine. J. Am. Oil Chem. Soc. 2010, 87, 127–132. [Google Scholar] [CrossRef]
- Polanowska, K.; Grygier, A.; Kuligowski, M.; Rudzińska, M.; Nowak, J. Effect of tempe fermentation by three different strains of Rhizopus oligosporus on nutritional characteristics of faba beans. LWT 2020, 122, 109024. [Google Scholar] [CrossRef]
- Çevikkalp, S.A.; Löker, G.B.; Yaman, M.; Amoutzopoulos, B. A simplified HPLC method for determination of tryptophan in some cereals and legumes. Food Chem. 2016, 193, 26–29. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane, S.G.H.; Stanley, G.H.S.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- AOCS Official Method Ce 1h-05. Determination of cis-, trans-, Saturated, Monounsaturated and Polyunsaturated Fatty Acids in Vegetable or Non-Ruminant Animal Oils and Fats by Capillary GLC. 2009. Available online: https://www.aocs.org/attain-lab-services/methods/methods/search-results?method=111777 (accessed on 12 July 2021).
- Canali, G.; Balestra, F.; Glicerina, V.; Pasini, F.; Caboni, M.F.; Romani, S. Influence of different baking powders on physico-chemical, sensory and volatile compounds in biscuits and their impact on textural modifications during soaking. J. Food Sci. Technol. 2020, 57, 3864–3873. [Google Scholar] [CrossRef]
- Brosio, E.; Gianferri, R.R. An analytical tool in foods characterization and traceability. In Basic NMR in Foods Characterization; Research Signpost: Kerala, India, 2009; pp. 9–37. [Google Scholar]
- Weglarz, W.P.; Haranczyk, H. Two-dimensional analysis of the nuclear relaxation function in the time domain: The program CracSpin. J. Phys. D Appl. Phys. 2000, 33, 1909–1920. [Google Scholar] [CrossRef]

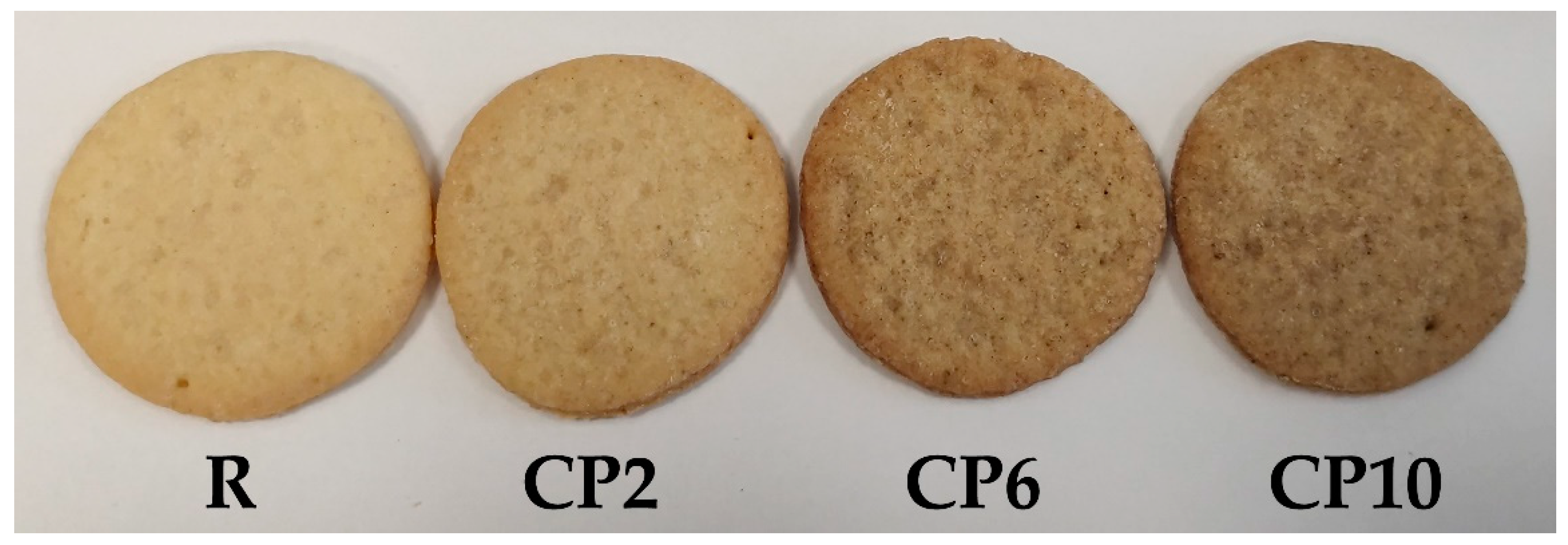
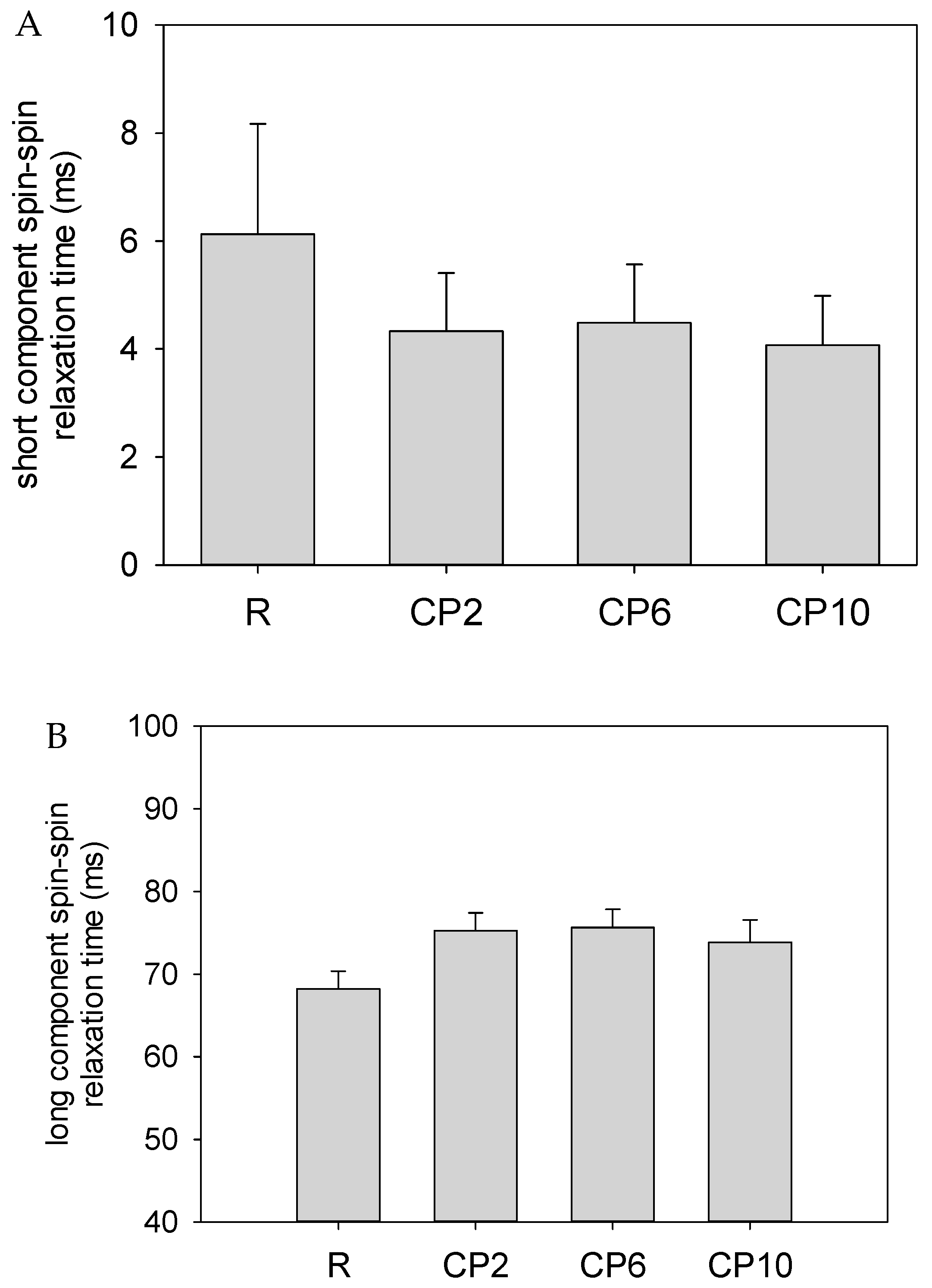
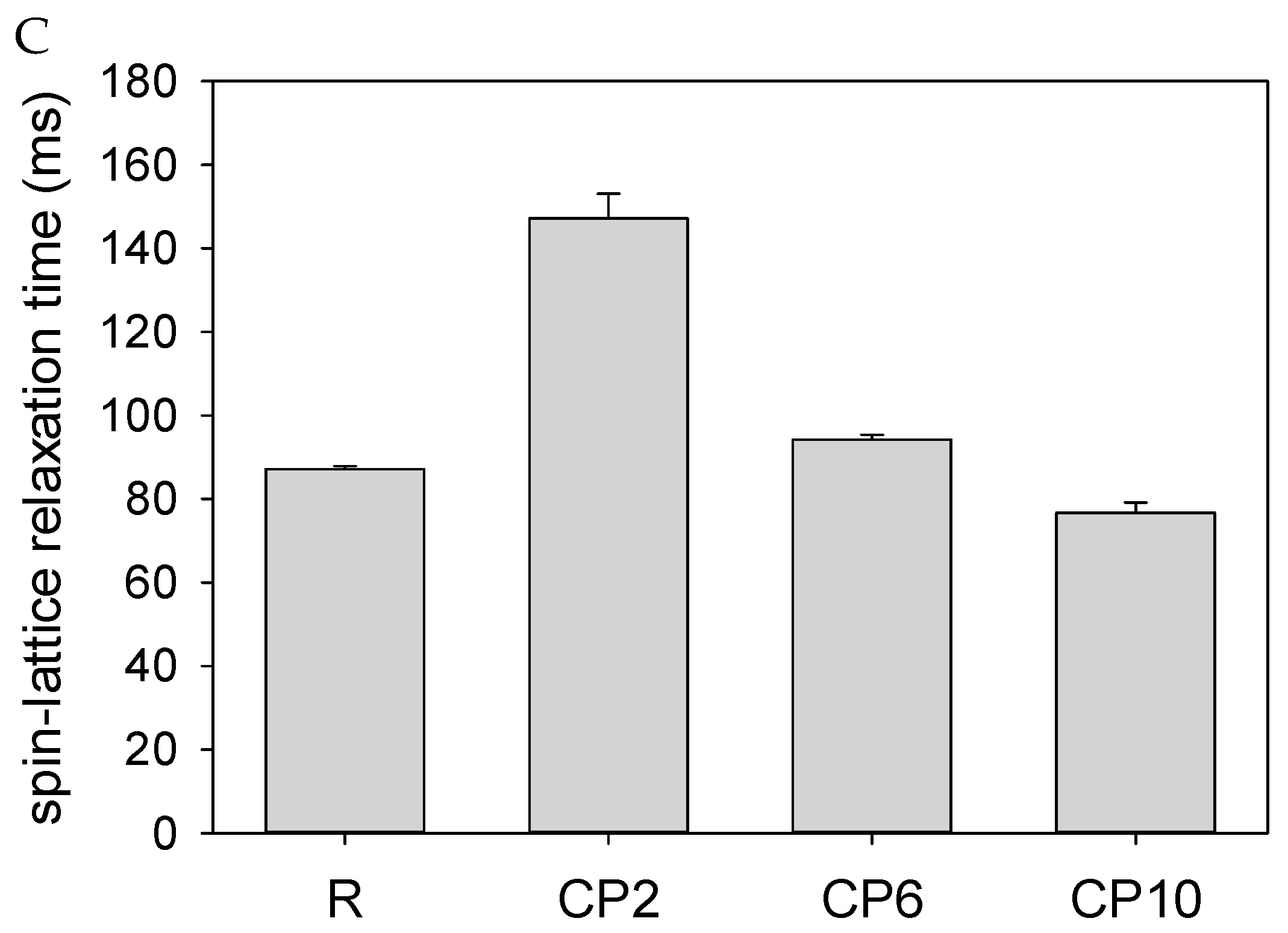
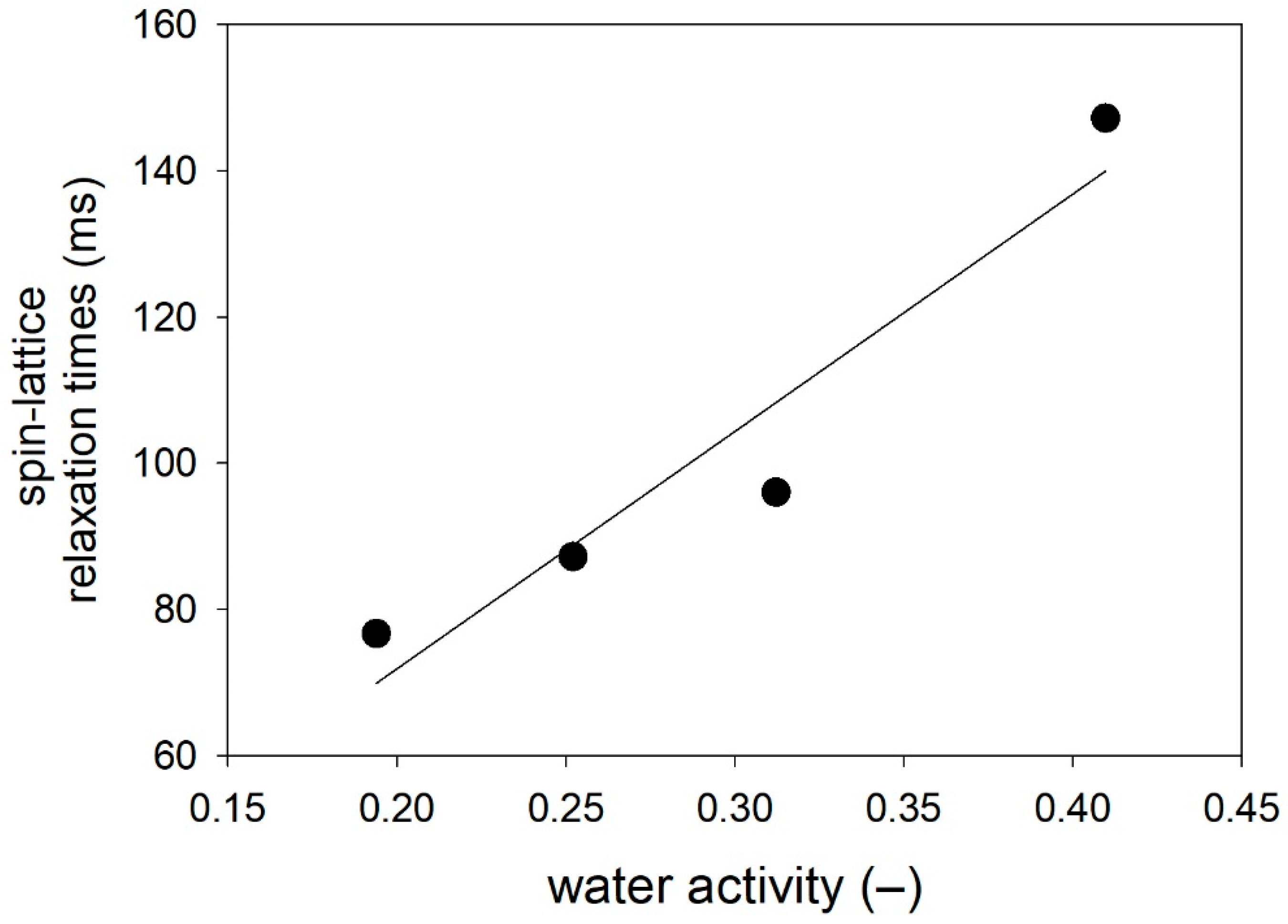
| Parameter | CP | R | CP2 | CP6 | CP10 |
|---|---|---|---|---|---|
| L* | 52.04 ± 0.70 | 75.53 ± 0.28 a | 73.90 ± 0.28 b | 65.98 ± 0.29 c | 63.29 ± 0.16 d |
| a* | 6.02 ± 0.20 | 3.12 ± 0.05 d | 4.17 ± 0.08 c | 4.96 ± 0.02 b | 5.17 ± 0.06 a |
| b* | 14.65 ± 1.77 | 25.19 ± 0.93 a | 22.86 ± 0.37 b | 22.42 ± 0.53 b | 20.47 ± 0.12 c |
| ΔE | - | - | 3.03 | 10.11 | 13.28 |
| WI | 49.49 | 64.74 | 65.05 | 58.96 | 57.65 |
| Parameter | R | CP2 | CP6 | CP10 |
|---|---|---|---|---|
| Moisture (%) | 1.75 ± 0.49 a | 1.65 ± 0.21 a | 2.08 ± 0.31 a | 1.75 ± 0.19 a |
| Protein (%) | 6.08 ± 0.08 d | 7.80 ± 0.24 d | 9.24 ± 0.18 b | 10.30 ± 0.09 a |
| Fat (%) | 14.7 ± 0.4 d | 16.2 ± 0.1 b | 16.8 ± 0.2 b | 17.5 ± 0.4 a |
| Ash (%) | 1.01 ± 0.12 b | 1.03 ± 0.03 b | 1.09 ± 0.15 b | 1.35 ± 0.09 a |
| Carbohydrates 1 (%) | 76.5 ± 1.14 a | 73.3 ± 1.03 c | 70.8 ± 1.01 b | 69.1 ± 1.15 d |
| Energy value 2 (kcal/100 g) | 454.5 d | 462.4 b | 461.5 b | 466.8 a |
| Mineral | NRV/AI (mg/Day) | R (mg/100) | CP2 (mg/100 g) | CP6 (mg/100 g) | CP10 (mg/100 g) |
|---|---|---|---|---|---|
| Ca | 800 | 31.4 ± 1.8 d | 38.5 ± 2.0 c | 53.2 ± 0.9 b | 67.0 ± 3.2 a |
| Mg | 375 | 10.4 ± 0.1 d | 11.1 ± 0.2 c | 13.6 ± 0.1 b | 17.6 ± 1.0 a |
| K | 3500 | 102.6 ± 1.0 d | 109.8 ± 2.2 c | 137.8 ± 4.4 b | 152.3 ± 11.0 a |
| Na | 1500 | 323.1 ± 10.3 a | 310.2 ± 9.1 b | 302.0 ± 8.3 b | 310.6 ± 11.9 b |
| Cu | 1 | 0.021 ± 0.001 d | 0.044 ± 0.004 c | 0.106 ± 0.007 b | 0.196 ± 0.006 a |
| Fe | 14 | 0.536 ± 0.017 d | 0.602 ± 0.014 c | 0.662 ± 0.027 b | 0.786 ± 0.039 a |
| Mn | 2 | 0.191 ± 0.004 d | 0.216 ± 0.009 c | 0.310 ± 0.010 b | 0.365 ± 0.008 a |
| Zn | 10 | 0.706 ± 0.003 d | 0.819 ± 0.051 c | 1.23 ± 0.08 b | 1.61 ± 0.07 a |
| Fatty Acid | R | CP2 | CP6 | CP10 |
|---|---|---|---|---|
| C 8:0 | 0.482 ± 0.002 a | 0.477 ± 0.016 b | 0.479 ± 0.013 b | 0.487 ± 0.001 a |
| C 10:0 | 0.463 ± 0.005 b | 0.453 ± 0.013 b | 0.448 ± 0.009 a | 0.448 ± 0.005 a |
| C 12:0 | 6.340 ± 0.002 a | 6.179 ± 0.064 b | 6.102 ± 0.085 b | 6.100 ± 0.015 b |
| C 14:0 | 2.863 ± 0.004 a | 2.837 ± 0.008 b | 2.810 ± 0.013 b | 2.803 ± 0.002 b |
| C 16:0 | 31.223 ± 0.019 b | 31.629 ± 0.063 a | 31.560 ± 0.033 a | 31.343 ± 0.197 b |
| C 16:1 | 0.159 ± 0.045 a | 0.130 ± 0.001 b | 0.129 ± 0.003 b | 0.133 ± 0.001 b |
| C 18:0 | 3.855 ± 0.029 c | 3.956 ± 0.004 b | 4.014 ± 0.027 b | 4.044 ± 0.001 a |
| C 18:1 | 31.401 ± 0.024 a | 31.218 ± 0.025 b | 31.123 ± 0.080 b | 31.146 ± 0.120 b |
| C 18:2 | 21.311 ± 0.032 a | 21.288 ± 0.061 a | 21.502 ± 0.049 b | 21.655 ± 0.042 c |
| C 18:3 | 1.058 ± 0.021 c | 1.055 ± 0.046 c | 1.093 ± 0.006 b | 1.126 ± 0.008 a |
| C 20:0 | 0.667 ± 0.010 a | 0.603 ± 0.005 b | 0.556 ± 0.007 c | 0.548 ± 0.065 c |
| C 22:0 | 0.175 ± 0.016 b | 0.175 ± 0.025 b | 0.182 ± 0.030 a | 0.170 ± 0.002 b |
| Σ SFA | 46.069 ± 0.016 | 46.308 ± 0.131 | 46.151 ± 0.023 | 45.941 ± 0.152 |
| Σ MUFA | 31.561 ± 0.069 | 31.347 ± 0.024 | 31.253 ± 0.077 | 31.278 ± 0.118 |
| Σ PUFA | 22.370 ± 0.053 | 22.344 ± 0.107 | 22.596 ± 0.055 | 22.781 ± 0.034 |
| Amino Acid | R | CP2 | CP6 | CP10 |
|---|---|---|---|---|
| Essential amino acids | ||||
| Histidine | 1.48 ± 0.01 d | 1.64 ± 0.03 c | 1.90 ± 0.01 b | 2.05 ± 0.02 a |
| Isoleucine | 2.65 ± 0.17 c | 2.78 ± 0.20 c | 3.43 ± 0.08 b | 3.70 ± 0.06 a |
| Leucine | 4.91 ± 0.07 d | 5.44 ± 0.21 c | 6.37 ± 0.11 b | 6.65 ± 0.07 a |
| Lysine | 1.33 ± 0.05 d | 1.86 ± 0.03 c | 2.44 ± 0.05 b | 2.77 ± 0.01 a |
| Cysteine | 3.75 ± 0.08 d | 4.36 ± 0.01 a | 4.10 ± 0.05 b | 3.93 ± 0.02 c |
| Methionine | 1.12 ± 0.05 c | 1.32 ± 0.14 b | 1.51 ± 0.02 a | 1.57 ± 0.03 a |
| Phenylalanine | 3.31 ± 0.13 c | 3.79 ± 0.11 b | 4.04 ± 0.17 ab | 4.30 ± 0.11 a |
| Tyrosine | 2.27 ± 0.02 d | 2.65 ± 0.03 c | 3.12 ± 0.01 b | 3.39 ± 0.01 a |
| Threonine | 2.33 ± 0.02 d | 2.58 ± 0.07 c | 3.15 ± 0.01 b | 3.45 ± 0.02 a |
| Tryptophan | 0.102 ± 0.003 d | 0.138 ± 0.003 c | 0.356 ± 0.002 b | 0.399 ± 0.005 a |
| Valine | 2.93 ± 0.04 d | 3.18 ± 0.09 c | 4.07 ± 0.01 b | 4.48 ± 0.04 a |
| Σ EAA * | 26.18 | 29.74 | 34.49 | 36.69 |
| Dispensable amino acids | ||||
| Alanine | 1.23 ± 0.01 d | 1.78 ± 0.04 c | 2.30 ± 0.01 b | 2.94 ± 0.01 a |
| Arginine | 2.13 ± 0.06 d | 2.48 ± 0.05 c | 3.26 ± 0.02 b | 3.79 ± 0.02 a |
| Aspartic acid | 4.70 ± 0.11 d | 6.05 ± 0.04 c | 6.84 ± 0.05 b | 8.89 ± 0.06 a |
| Glutamic acid | 22.02 ± 0.12 d | 23.19 ± 0.28 c | 22.70 ± 0.08 b | 24.79 ± 0.08 a |
| Glycine | 2.14 ± 0.01 d | 2.53 ± 0.04 c | 3.16 ± 0.02 b | 3.64 ± 0.02 a |
| Proline | 6.86 ± 0.04 d | 7.23 ± 0.03 c | 7.87 ± 0.06 b | 8.08 ± 0.02 a |
| Serine | 3.76 ± 0.01 d | 4.41 ± 0.12 c | 4.73 ± 0.03 b | 5.12 ± 0.01 a |
| Σ DAA * | 42.84 | 47.67 | 50.86 | 57.25 |
| Parameter | R | CP2 | CP6 | CP10 |
|---|---|---|---|---|
| Weight (g) | 7.21 ± 0.30 a | 7.55 ± 0.37 a | 7.54 ± 0.45 a | 7.46 ± 0.29 a |
| Diameter (cm) | 4.54 ± 0.21 b | 4.63 ± 0.30 b | 4.79 ± 0.17 ab | 4.88 ± 0.18 a |
| Thickness (cm) | 0.72 ± 0.06 a | 0.65 ± 0.05 a | 0.65 ± 0.07 a | 0.63 ± 0.06 a |
| Spread Ratio (–) | 6.30 ± 0.12 | 7.12 ± 0.03 | 7.40 ± 0.08 | 7.75 ± 0.09 |
| Firmness (N) | 29.44 ± 3.07 a | 25.44 ± 6.80 ab | 25.22 ± 5.16 ab | 24.50 ± 2.56 b |
| Parameter | R | CP2 | CP6 | CP10 |
|---|---|---|---|---|
| water activity ar (-) | 0.3123 ± 0.0012 b | 0.4098 ± 0.0012 a | 0.2522 ± 0.0038 c | 0.1940 ± 0.0008 d |
| transport rate VD (s−1) | 0.0296 ± 0.0022 a | 0.0228 ± 0.0023 c | 0.0260 ± 0.0031 b | 0.0263 ± 0.0023 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smarzyński, K.; Sarbak, P.; Kowalczewski, P.Ł.; Różańska, M.B.; Rybicka, I.; Polanowska, K.; Fedko, M.; Kmiecik, D.; Masewicz, Ł.; Nowicki, M.; et al. Low-Field NMR Study of Shortcake Biscuits with Cricket Powder, and Their Nutritional and Physical Characteristics. Molecules 2021, 26, 5417. https://doi.org/10.3390/molecules26175417
Smarzyński K, Sarbak P, Kowalczewski PŁ, Różańska MB, Rybicka I, Polanowska K, Fedko M, Kmiecik D, Masewicz Ł, Nowicki M, et al. Low-Field NMR Study of Shortcake Biscuits with Cricket Powder, and Their Nutritional and Physical Characteristics. Molecules. 2021; 26(17):5417. https://doi.org/10.3390/molecules26175417
Chicago/Turabian StyleSmarzyński, Krzysztof, Paulina Sarbak, Przemysław Łukasz Kowalczewski, Maria Barbara Różańska, Iga Rybicka, Katarzyna Polanowska, Monika Fedko, Dominik Kmiecik, Łukasz Masewicz, Marcin Nowicki, and et al. 2021. "Low-Field NMR Study of Shortcake Biscuits with Cricket Powder, and Their Nutritional and Physical Characteristics" Molecules 26, no. 17: 5417. https://doi.org/10.3390/molecules26175417
APA StyleSmarzyński, K., Sarbak, P., Kowalczewski, P. Ł., Różańska, M. B., Rybicka, I., Polanowska, K., Fedko, M., Kmiecik, D., Masewicz, Ł., Nowicki, M., Lewandowicz, J., Jeżowski, P., Kačániová, M., Ślachciński, M., Piechota, T., & Baranowska, H. M. (2021). Low-Field NMR Study of Shortcake Biscuits with Cricket Powder, and Their Nutritional and Physical Characteristics. Molecules, 26(17), 5417. https://doi.org/10.3390/molecules26175417















