Antinociceptive Synergism of Pomegranate Peel Extract and Acetylsalicylic Acid in an Animal Pain Model
Abstract
:1. Introduction
2. Results
2.1. Time Course of PoPEx and ASA in the Formalin Test
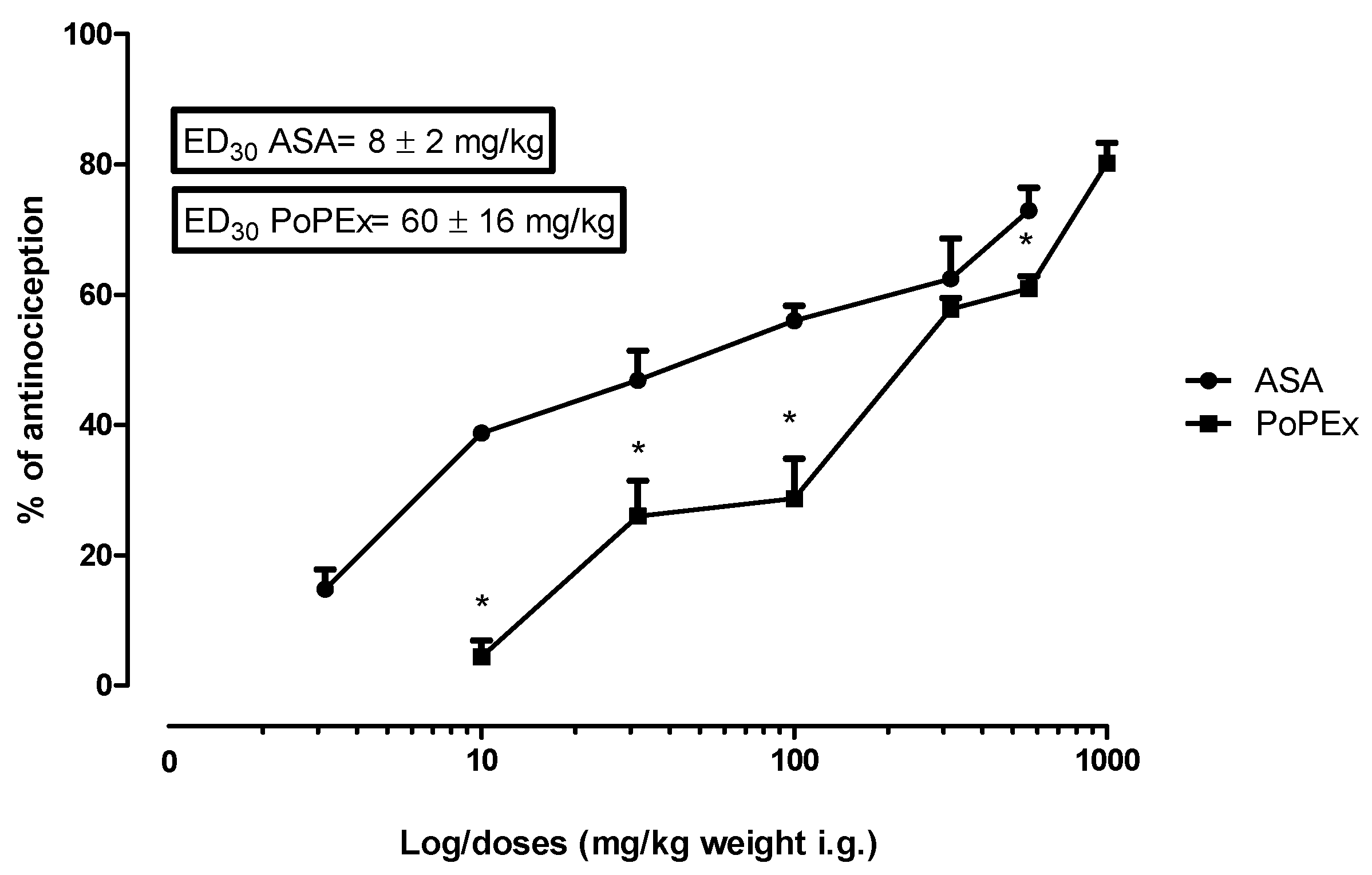
2.2. Phased and Overall Antinociceptive Effect of PoPEx and ASA in the Formalin Test
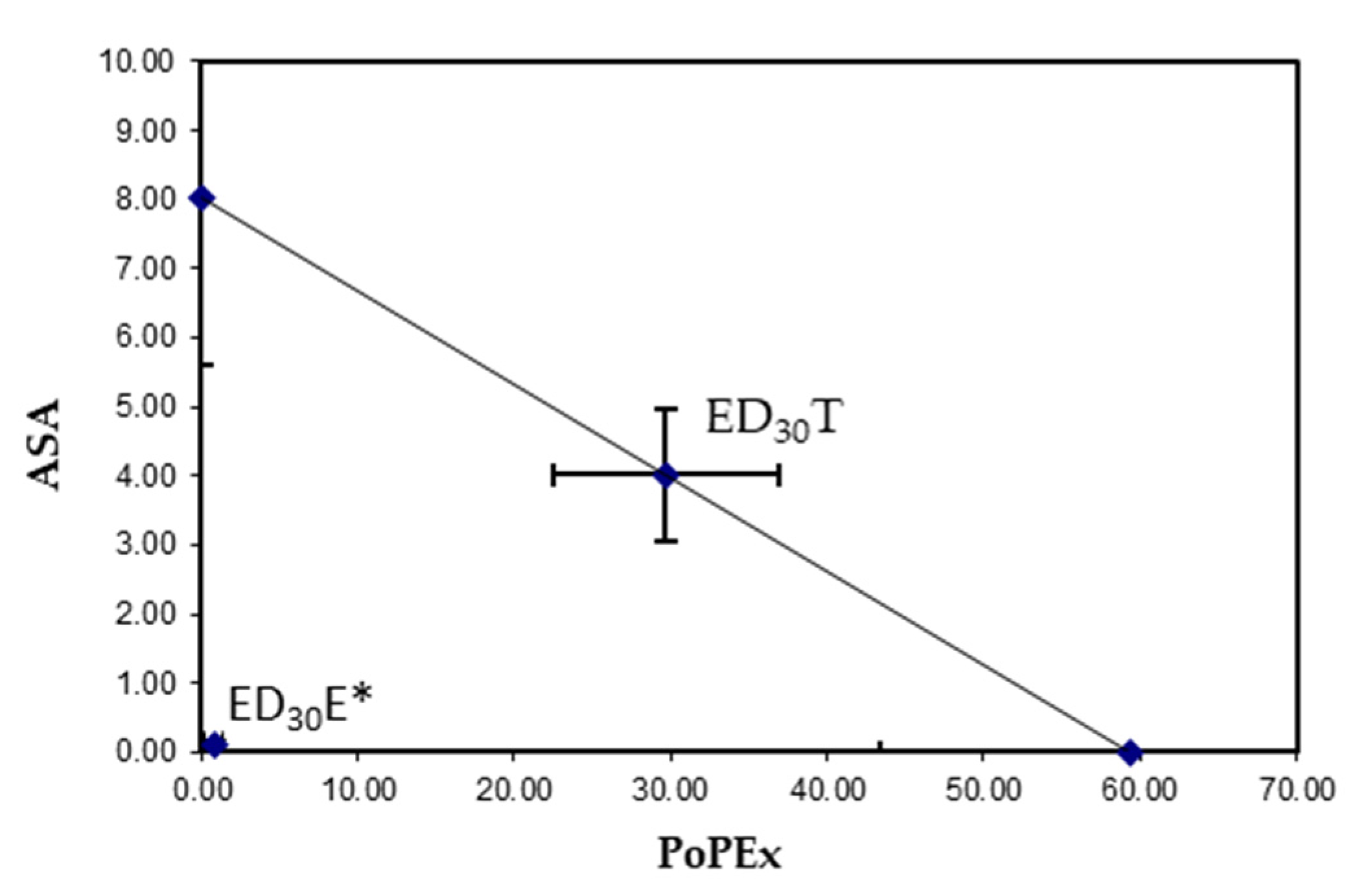
2.3. Isobologram
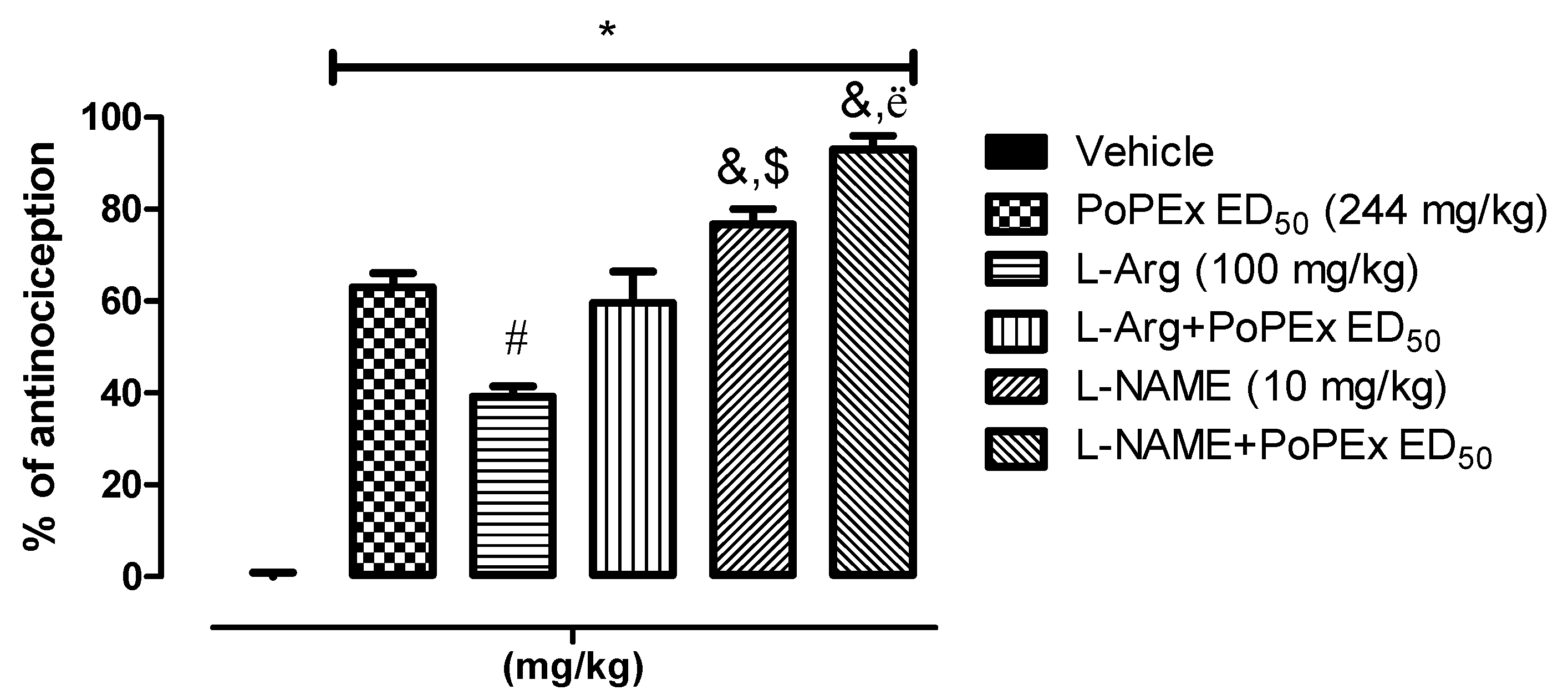
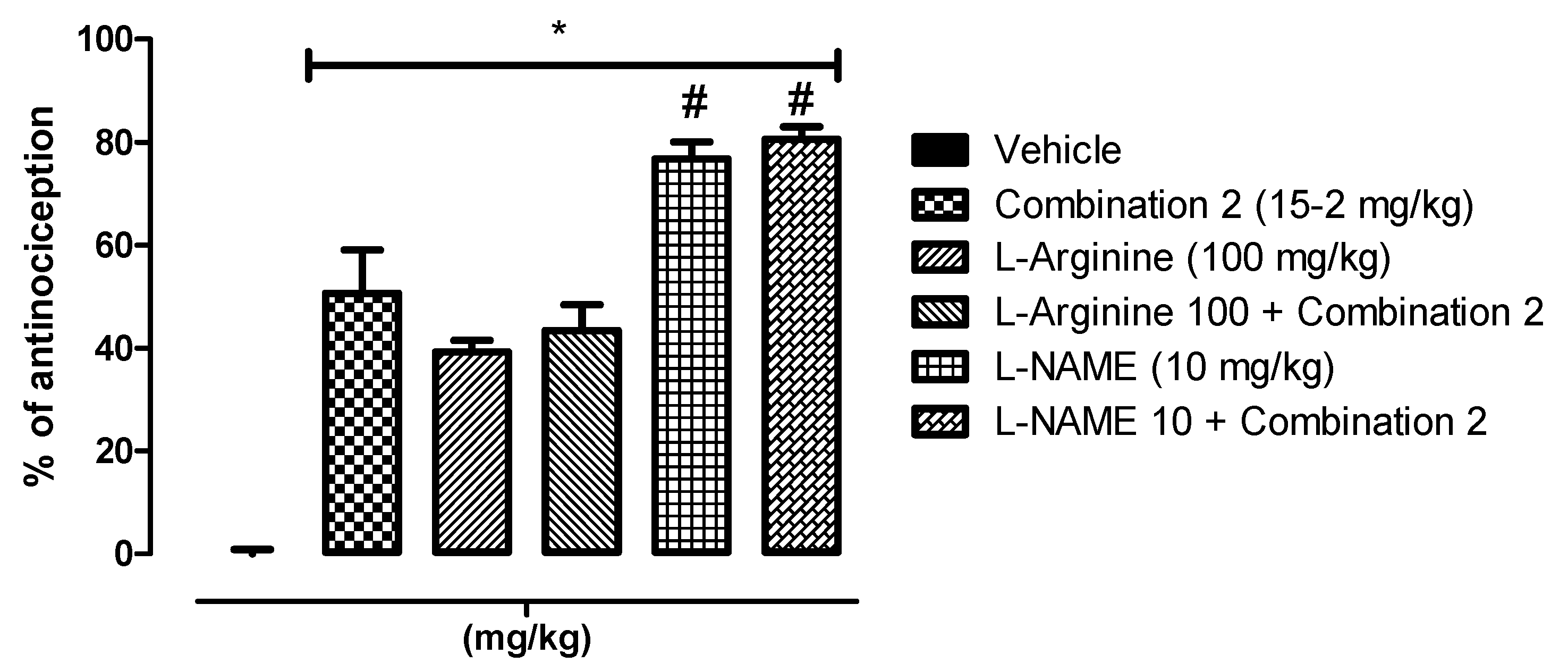
2.4. Involvement of the l-Arginine/NO/cGMP Pathway
2.5. Antioxidant Activity and Total Phenolic Content
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction
4.1.1. Collection of the Plant Materials
4.1.2. Preparation of Extracts
4.2. Animals
4.3. Compound
4.4. Formalin Test
Measurement of Pain Behavior
4.5. Isobolographic Analysis
4.6. Involvement of the l-Arginine/NO/cGMP Pathway
4.7. Total Phenolic Content and Antioxidant Activity
4.7.1. Total Phenolic Content
4.7.2. Antioxidant Activity
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- The Plant List Lythraceae-The Plant List. Available online: http://www.theplantlist.org/1.1/browse/A/Lythraceae/ (accessed on 20 August 2020).
- Selahvarzi, Y.; Zamani, Z.; Fatahi, R.; Talaei, A.-R. Effect of deficit irrigation on flowering and fruit properties of pomegranate (Punica granatum cv. Shahvar). Agric. Water Manag. 2017, 192, 189–197. [Google Scholar] [CrossRef]
- Kumari, I.; Kaurav, H.; Chaudhary, G.; Punica granatum, L. (Dadim), Therapeutic Importance of World’s Most Ancient Fruit Plant. J. Drug Deliv. Ther. 2021, 11, 113–121. [Google Scholar] [CrossRef]
- Holland, D.; Hatib, K.; Bar-Ya’Akov, I. Pomegranate: Botany, Horticulture, Breeding. Hortic. Rev. 2009, 35, 127–191. [Google Scholar] [CrossRef]
- Betanzos-Cabrera, G.; Montes-Rubio, P.Y.; Fabela-Illescas, H.E.; Belefant-Miller, H.; Cancino-Diaz, J.C. Antibacterial activity of fresh pomegranate juice against clinical strains of Staphylococcus epidermidis. Food Nutr. Res. 2015, 59, 27620. [Google Scholar] [CrossRef] [Green Version]
- Poyrazoğlu, E.; Gökmen, V.; Artιk, N. Organic Acids and Phenolic Compounds in Pomegranates (Punica granatum L.) Grown in Turkey. J. Food Compos. Anal. 2002, 15, 567–575. [Google Scholar] [CrossRef]
- Amin, G. Popular Medical Plants of Iran; Farhang Publication: Tehran, Iran, 1991; Volume 51–52. [Google Scholar]
- Lansky, E.; Shubert, S.; Neeman, I. Pharmacological and Therapeutic Properties of Pomegranate. In Production, Processing and Marketing of Pomegranate in the Mediterranean Region: Advances in Research and Technology; Martínez-Nicolás, J.J., Martínez-Tomé, J., Eds.; CIHEAM: Zaragoza, Spain, 2000; pp. 231–235. [Google Scholar]
- Tanveer, A.; Farooq, U.; Akram, K.; Hayat, Z.; Shafi, A.; Nazar, H.; Ahmad, Z. Pomegranate Extracts: A Natural Preventive Measure against Spoilage and Pathogenic Microorganisms. Food Rev. Int. 2014, 31, 29–51. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Van Hée, V.F.; Bindels, L.B.; De Backer, F.; Cani, P.D.; Delzenne, N.M. Polyphenol-rich extract of pomegranate peel alleviates tissue inflammation and hypercholesterolaemia in high-fat diet-induced obese mice: Potential implication of the gut microbiota. Br. J. Nutr. 2013, 109, 802–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajaikumar, K.; Asheef, M.; Babu, B.; Padikkala, J. The inhibition of gastric mucosal injury by Punicagranatum L. (pomegranate) methanolic extract. J. Ethnopharmacol 2005, 96, 171–176. [Google Scholar] [CrossRef]
- Ismail, T.; Sestili, P.; Akhtar, S. Pomegranate peel and fruit extracts: A review of potential anti-inflammatory and anti-infective effects. J. Ethnopharmacol 2012, 143, 397–405. [Google Scholar] [CrossRef]
- Karwasra, R.; Singh, S.; Sharma, D.; Sharma, S.; Sharma, N.; Khanna, K. Pomegranate supplementation attenuates inflammation, joint dysfunction via inhibition of NF-κB signaling pathway in experimental models of rheumatoid arthritis. J. Food Biochem. 2019, 43, e12959. [Google Scholar] [CrossRef]
- Labib, R.M.; El-Ahmady, S.H. Antinociceptive, anti-gastric ulcerogenic and anti-inflammatory activities of standardized egyptian pomegranate peel extract. J. Appl. Pharm. Sci. 2015, 5, 48–51. [Google Scholar] [CrossRef] [Green Version]
- Mo, J.; Panichayupakaranant, P.; Kaewnopparat, N.; Nitiruangjaras, A.; Reanmongkol, W. Topical anti-inflammatory and analgesic activities of standardized pomegranate rind extract in comparison with its marker compound ellagic acid in vivo. J. Ethnopharmacol 2013, 148, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Olapour, S.; Najafzadeh, H. Evaluation Analgesic, Anti-Inflammatory and Antiepileptic Effect of Hydro Alcoholic Peel Extract of” Punica granatum (pomegranate)”. Asian J. Med Sci. 2010, 2, 266–270. [Google Scholar]
- Ouachrif, A.; Khalki, H.; Chaib, S.; Mountassir, M.; Aboufatima, R.; Farouk, L.; Benharraf, A.; Chait, A. Comparative study of the anti-inflammatory and antinociceptive effects of two varieties of Punica granatum. Pharm. Biol. 2012, 50, 429–438. [Google Scholar] [CrossRef]
- Rafraf, M.; Hemmati, S.; Jafarabadi, M.A.; Moghaddam, A.; Haghighian, M.K. Pomegranate (Punica granatum L.) Peel Hydroalcoholic Extract Supplementation Reduces Pain and Improves Clinical Symptoms of Knee Osteoarthritis: A Randomized Double-Blind Placebo Controlled Study. Iran. Red Crescent Med. J. 2016, 19, 1. [Google Scholar] [CrossRef]
- Argov-Argaman, N.; Cohen-Zinder, M.; Leibovich, H.; Yishay, M.; Eitam, H.; Agmon, R.; Hadaya, O.; Mesilati-Stahy, R.; Miron, J.; Shabtay, A. Dietary pomegranate peel improves milk quality of lactating ewes: Emphasis on milk fat globule membrane properties and antioxidative traits. Food Chem. 2020, 313, 125822. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD–ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Nainwani, R.; Singh, D.; Soni, P.K.; Gupta, A.; Gautam, R. Chemical constituents of various parts of Punica granatum and their anti-inflammatory activity: A review. Asian J. Pharm. Res. Dev. 2013, 48–53, 48–53. [Google Scholar]
- BenSaad, L.A.; Kim, K.H. Phytochemical constituents and analgesic activity of ethyl acetate fraction of Punicagranatum L (Punicaceae). Trop. J. Pharm. Res. 2015, 14, 87–93. [Google Scholar] [CrossRef] [Green Version]
- BenSaad, L.A.; Kim, K.H.; Quah, C.C.; Kim, W.R.; Shahimi, M. Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum. BMC Complementary Altern. Med. 2017, 17, 47. [Google Scholar] [CrossRef] [Green Version]
- Giménez-Bastida, J.A.; González-Sarrías, A.; Larrosa, M.; Tomás-Barberán, F.; Espín, J.C.; García-Conesa, M.T. Ellagitannin metabolites, urolithin A glucuronide and its aglycone urolithin A, ameliorate TNF-α-induced inflammation and associated molecular markers in human aortic endothelial cells. Mol. Nutr. food Res. 2012, 56, 784–796. [Google Scholar] [CrossRef]
- Hollebeeck, S.; Winand, J.; Hérent, M.-F.; During, A.; Leclercq, J.; Larondelle, Y.; Schneider, Y.-J. Anti-inflammatory effects of pomegranate (Punica granatum L.) husk ellagitannins in Caco-2 cells, an in vitro model of human intestine. Food Funct. 2012, 3, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-J.; Chen, L.-G.; Liang, W.-L.; Wang, C.-C. Anti-inflammatory effects of Punica granatum Linne in vitro and in vivo. Food Chem. 2010, 118, 315–322. [Google Scholar] [CrossRef]
- Mansouri, M.T.; Naghizadeh, B.; Ghorbanzadeh, B. Ellagic acid enhances morphine analgesia and attenuates the development of morphine tolerance and dependence in mice. Eur. J. Pharmacol. 2014, 741, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.T.; Naghizadeh, B.; Ghorbanzadeh, B. Involvement of opioid receptors in the systemic and peripheral antinociceptive actions of ellagic acid in the rat formalin test. Pharmacol. Biochem. Behav. 2014, 120, 43–49. [Google Scholar] [CrossRef]
- Mansouri, M.T.; Naghizadeh, B.; Ghorbanzadeh, B. Ellagic acid enhances the antinociceptive action of venlafaxine in mouse acetic acid-induced pain: An isobolographic analysis. Pharmacol. Rep. 2015, 67, 473–477. [Google Scholar] [CrossRef]
- O’Leary, K.A.; de Pascual-Tereasa, S.; Needs, P.W.; Bao, Y.-P.; O’Brien, N.M.; Williamson, G. Effect of flavonoids and vitamin E on cyclooxygenase-2 (COX-2) transcription. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2004, 551, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Olajide, O.A.; Kumar, A.; Velagapudi, R.; Okorji, U.P.; Fiebich, B.L. Punicalagin inhibits neuroinflammation in LPS-activated rat primary microglia. Mol. Nutr. food Res. 2014, 58, 1843–1851. [Google Scholar] [CrossRef]
- Kamali, M.; Tavakoli, H.; Khodadoost, M.; Daghaghzadeh, H.; Kamalinejad, M.; Gachkar, L.; Mansourian, M.; Adibi, P. Efficacy of the Punica granatum peels aqueous extract for symptom management in ulcerative colitis patients. A randomized, placebo-controlled, clinical trial. Complementary Ther. Clin. Pract. 2015, 21, 141–146. [Google Scholar] [CrossRef]
- Marín, M.; Giner, R.M.; Ríos, J.-L.; Recio, M.C. Intestinal anti-inflammatory activity of ellagic acid in the acute and chronic dextrane sulfate sodium models of mice colitis. J. Ethnopharmacol. 2013, 150, 925–934. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information, PubChem Database. Aspirin, CID = 2244. National Center for Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Aspirin (accessed on 20 August 2020).
- Montinari, M.R.; Minelli, S.; De Caterina, R. The first 3500 years of aspirin history from its roots–A concise summary. Vasc. Pharmacol. 2019, 113, 1–8. [Google Scholar] [CrossRef]
- American Society of Health-System Pharmacists. In Aspirin Monograph for Professionals, 28/02/20 ed.; American Society of Health-System Pharmacists: Bethesda, MD, USA; Drugs.com: Dallas, TX, USA, 2020.
- Raffa, R. Pharmacology of oral combination analgesics: Rational therapy for pain. J. Clin. Pharm. Ther. 2001, 26, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Martínez, I.; Garcia, A.I.; Rodeiro, I.; Morón, F. Plantas medicinales reportadas con reacciones adversas en Cuba: Potenciales interacciones con fármacos de uso convencional. J. Pharm. Pharmacogn. Res. 2015, 3, 37–44. [Google Scholar]
- Tres, J. Interacción Entre Fármacos y Plantas Medicinales; Anales del Sistema Sanitario de Navarra: SciELO, Espana, 2006; pp. 233–252. [Google Scholar]
- Vierck, C.J. Modelos animales del dolor. In Wall y Melzack. Tratado del Dolor; Elsevier: Madrid, Spain, 2007; pp. 177–187. [Google Scholar] [CrossRef]
- Meunier, C.J.; Burton, J.; Cumps, J.; Verbeeck, R.K. Evaluation of the formalin test to assess the analgesic activity of diflunisal in the rat. Eur. J. Pharm. Sci. 1998, 6, 307–312. [Google Scholar] [CrossRef]
- González-Trujano, M.E.; Pellicer, F.; Mena, P.; Moreno, D.A.; García-Viguera, C. Antinociceptive and anti-inflammatory activities of a pomegranate (Punica granatum L.) extract rich in ellagitannins. Int. J. Food Sci. Nutr. 2015, 66, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-S.; Lee, J.-K.; Suh, H.-W. Antinociceptive profiles of aspirin and acetaminophen in formalin, substance P and glutamate pain models. Brain Res. 2001, 921, 233–239. [Google Scholar] [CrossRef]
- Ortiz, M.I.; Fernández-Martínez, E.; Soria-Jasso, L.E.; Lucas-Gómez, I.; Villagómez-Ibarra, R.; González-García, M.P.; Castañeda-Hernández, G.; Salinas-Caballero, M. Isolation, identification and molecular docking as cyclooxygenase (COX) inhibitors of the main constituents of Matricaria chamomilla L. extract and its synergistic interaction with diclofenac on nociception and gastric damage in rats. Biomed. Pharmacother. 2016, 78, 248–256. [Google Scholar] [CrossRef]
- Vane, J.; Botting, R. The mechanism of action of aspirin. Thromb. Res. 2003, 110, 255–258. [Google Scholar] [CrossRef]
- Saad, L.B.; Hwi, K.K.; Quah, T. Evaluation of the antinociceptive effect of the ethanolic extract of Punica granatum. Afr. J. Tradit. Complementary Altern. Med. 2014, 11, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Ding, Y.; Liu, R.; Xiang, L.; Du, L. Pomegranate: Constituents, bioactivities and pharmacokinetics. Fruit Veg. Cereal Sci. Biotechnol. 2010, 4, 77–87. [Google Scholar]
- Parisio, C.; Lucarini, E.; Micheli, L.; Toti, A.; Khatib, M.; Mulinacci, N.; Calosi, L.; Bani, D.; Di Cesare Mannelli, L.; Ghelardini, C. Pomegranate Mesocarp against Colitis-Induced Visceral Pain in Rats: Effects of a Decoction and Its Fractions. Int. J. Mol. Sci. 2020, 21, 4304. [Google Scholar] [CrossRef] [PubMed]
- Zeghad, N.; Madi, A.; Helmi, S.; Belkhiri, A. In vivo analgesic activity and safety assessment of Vitis vinifera L and Punica granatum L fruits extracts. Trop. J. Pharm. Res. 2016, 15, 1915–1921. [Google Scholar] [CrossRef] [Green Version]
- Miclescu, A.; Gordh, T. Nitric oxide and pain:‘Something old, something new’. Acta Anaesthesiol. Scand. 2009, 53, 1107–1120. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.; Klein-Júnior, L.C.; Cechinel Filho, V.; de Campos Buzzi, F. Anti-hyperalgesic activity of corilagin, a tannin isolated from Phyllanthus niruri L.(Euphorbiaceae). J. Ethnopharmacol. 2013, 146, 318–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takano-Ishikawa, Y.; Goto, M.; Yamaki, K. Structure–activity relations of inhibitory effects of various flavonoids on lipopolysaccharide-induced prostaglandin E2 production in rat peritoneal macrophages: Comparison between subclasses of flavonoids. Phytomedicine 2006, 13, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zan, Y.; Wang, Z.-J.J.; Hu, X.-Y.; Huang, F. Quercetin ameliorates paclitaxel-induced neuropathic pain by stabilizing mast cells, and subsequently blocking PKCε-dependent activation of TRPV1. Acta Pharmacol. Sin. 2016, 37, 1166–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Shitany, N.A.; El-Bastawissy, E.A.; El-desoky, K. Ellagic acid protects against carrageenan-induced acute inflammation through inhibition of nuclear factor kappa B, inducible cyclooxygenase and proinflammatory cytokines and enhancement of interleukin-10 via an antioxidant mechanism. Int. Immunopharmacol. 2014, 19, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanzadeh, B.; Mansouri, M.T.; Hemmati, A.A.; Naghizadeh, B.; Mard, S.A.; Rezaie, A. Involvement of l-arginine/NO/cGMP/KATP channel pathway in the peripheral antinociceptive actions of ellagic acid in the rat formalin test. Pharmacol. Biochem. Behav. 2014, 126, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, A.; Manabe, S.; Manabe, Y.; Takagi, H. Effect of topical administration of l-arginine on formalin-induced nociception in the mouse: A dual role of peripherally formed NO in pain modulation. Br. J. Pharmacol. 1994, 112, 547–550. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.R.; De Campos, R.O.; Miguel, O.G.; Cechinel-Filho, V.; Yunes, R.A.; Calixto, J.B. The involvement of K+ channels and Gi/o protein in the antinociceptive action of the gallic acid ethyl ester. Eur. J. Pharmacol. 1999, 379, 7–17. [Google Scholar] [CrossRef]
- Nuamsetti, T.; Dechayuenyong, P.; Tantipaibulvut, S. Antibacterial activity of pomegranate fruit peels and arils. Sci. Asia 2012, 38, 319–322. [Google Scholar] [CrossRef] [Green Version]
- Pande, G.; Akoh, C.C. Antioxidant capacity and lipid characterization of six Georgia-grown pomegranate cultivars. J. Agric. Food Chem. 2009, 57, 9427–9436. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.K.; Bhardwaj, T. Therapeutic role of nitric oxide as emerging molecule. Biomed. Pharmacother. 2017, 85, 182–201. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.M.; Prado, W.A. The dual effect of a nitric oxide donor in nociception. Brain Res. 2001, 897, 9–19. [Google Scholar] [CrossRef]
- Chen, X.; Levine, J.D. NOS inhibitor antagonism of PGE2-induced mechanical sensitization of cutaneous C-fiber nociceptors in the rat. J. Neurophysiol. 1999, 81, 963–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, I.; Ferreira, S. L-NAME causes antinociception by stimulation of the arginine-NO-cGMP pathway. Mediat. Inflamm. 2000, 9, 25–30. [Google Scholar] [CrossRef]
- Aley, K.; McCarter, G.; Levine, J.D. Nitric oxide signaling in pain and nociceptor sensitization in the rat. J. Neurosci. 1998, 18, 7008–7014. [Google Scholar] [CrossRef] [Green Version]
- Mansouri, M.T.; Naghizadeh, B.; Ghorbanzadeh, B.; Rajabi, H.; Pashmforoush, M. Pharmacological evidence for systemic and peripheral antinociceptive activities of pioglitazone in the rat formalin test: Role of PPARγ and nitric oxide. Eur. J. Pharmacol. 2017, 805, 84–92. [Google Scholar] [CrossRef]
- Archer, S.L.; Hampl, V. NG-monomethyl-l-arginine causes nitric oxide synthesis in isolated arterial rings: Trouble in paradise. Biochem. Biophys. Res. Commun. 1992, 188, 590–596. [Google Scholar] [CrossRef]
- Chen, L.; Salafranca, M.N.; Mehta, J.L. Cyclooxygenase inhibition decreases nitric oxide synthase activity in human platelets. Am. J. Physiol. -Heart Circ. Physiol. 1997, 273, H1854–H1859. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Ohkubo, T.; Takahashi, H.; Inoki, R. Modified formalin test: Characteristic biphasic pain response. Pain 1989, 38, 347–352. [Google Scholar] [CrossRef]
- Wheeler-Aceto, H.; Cowan, A. Standardization of the rat paw formalin test for the evaluation of analgesics. Psychopharmacology. 1991, 104, 35–44. [Google Scholar] [CrossRef]
- Usha, T.; Goyal, A.K.; Lubna, S.; Prashanth, H.; Mohan, T.M.; Pande, V.; Middha, S.K. Identification of anti-cancer targets of eco-friendly waste Punica granatum peel by dual reverse virtual screening and binding analysis. Asian Pac. J. Cancer Prev. 2015, 15, 10345–10350. [Google Scholar] [CrossRef] [Green Version]
- Cerdá, B.; Cerón, J.J.; Tomás-Barberán, F.A.; Espín, J.C. Repeated oral administration of high doses of the pomegranate ellagitannin punicalagin to rats for 37 days is not toxic. J. Agric. Food Chem. 2003, 51, 3493–3501. [Google Scholar] [CrossRef]
- El Deeb, K.S.; Eid, H.H.; Ali, Z.Y.; Shams, M.M.; Elfiky, A.M. Bioassay-guided fractionation and identification of antidiabetic compounds from the rind of Punica Granatum Var. nana. Nat. Prod. Res. 2019, 35, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.K.; Sharma, S.; Sharma, K.; Gupta, G. Evaluation of Antiarthritic Activity of Butanol Fraction of Punica granatum Linn. Rind Extract Against Freund’s Complete Adjuvant-Induced Arthritis in Rats. J. Environ. Pathol., Toxicol. Oncol. 2018, 37, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, M.; Lanas, A. NSAIDs-induced gastrointestinal damage. Review. Minerva Gastroenterol. Dietol. 2006, 52, 249–259. [Google Scholar]
- García-Rayado, G.; Navarro, M.; Lanas, A. NSAID induced gastrointestinal damage and designing GI-sparing NSAIDs. Expert Rev. Clin. Pharmacol. 2018, 11, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Boyd, E.M. The acute oral toxicity of acetylsalicylic acid. Toxicol. Appl. Pharmacol. 1959, 1, 229–239. [Google Scholar] [CrossRef]
- Gibaldi, M. Estimation of area under the curve. In Biopharmaceutics and Clinical Pharmacokinetics, 4th ed.; Gibaldi, M., Ed.; Lea and Febiger: Philadelphia, PA, USA, 1991; pp. 377–378. [Google Scholar]
- Tallarida, R.J. Drug Synergism and Dose-Effect Data Analysis; Chapman and Hall/CRC: New York, NY, USA, 2000. [Google Scholar] [CrossRef]
- Tallarida, R.J. The interaction index: A measure of drug synergism. Pain 2002, 98, 163–168. [Google Scholar] [CrossRef]
- Zakaria, Z.A.; Roosli, R.A.J.; Marmaya, N.H.; Omar, M.H.; Basir, R.; Somchit, M.N. Methanol extract of Dicranopteris linearis leaves attenuate pain via the modulation of opioid/NO-mediated pathway. Biomolecules 2020, 10, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz-Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Kuskoski, E.M.; Asuero, A.G.; Troncoso, A.M.; Mancini-Filho, J.; Fett, R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Food Sci. Technol. 2005, 25, 726–732. [Google Scholar] [CrossRef] [Green Version]
- Morales, F.J.; Jiménez-Pérez, S. Free radical scavenging capacity of Maillard reaction products as related to colour and fluorescence. Food Chem. 2001, 72, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ”antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]

| Dose (mg/kg) | Log (D) | Antinociceptive Effect (%) | ||
|---|---|---|---|---|
| PoPEx Phase 1 | PoPEx Phase 2 | PoPEx Overall effect | ||
| 10.00 | 1.00 | 1.62 | 3.23 * | 4.83 * |
| 31.62 | 1.50 | 6.01 * | 19.92 * | 25.99 * |
| 100.00 | 2.00 | 6.99 * | 22.09 * | 28.69 * |
| 316.22 | 2.50 | 7.22 * | 50.23 * | 57.81 * |
| 562.34 | 2.75 | 7.32 * | 55.58 * | 60.92 * |
| 1000.00 | 3.00 | 7.03 * | 72.8 * | 80.22 * |
| ASA Phase 1 | ASA Phase 2 | ASA Overall effect | ||
| 3.16 | 0.50 | 3.42 * | 10.71 * | 14.78 * |
| 10.00 | 1.00 | 4.47 * | 33.94 * | 38.77 * |
| 31.62 | 1.50 | 4.99 * | 41.73 * | 46.85 * |
| 100.00 | 2.00 | 6.47 * | 49.60 * | 56.02 * |
| 316.22 | 2.50 | 7.26 * | 55.04 * | 62.43 * |
| 562.34 | 2.75 | 9.94 * | 62.55 * | 72.94 * |
| Group | Doses mg/kg (Combination) | Log (D) | Antinociceptive Effect (%) | ||
|---|---|---|---|---|---|
| Combination Phase 1 | Combination Phase 2 | Overall Effect | |||
| (1) | 34.00 | 1.53 | 25.52 * | 36.31 * | 62.80 * |
| (2) | 17.00 | 1.23 | 10.93 * | 39.21 * | 50.60 * |
| (3) | 8.51 | 0.93 | 12.99 * | 35.09 * | 48.70 * |
| (4) | 4.27 | 0.62 | 10.35 * | 33.17 * | 43.51 * |
| (5) | 2.12 | 0.32 | 8.96 * | 24.47 * | 33.48 * |
| (6) | 1.06 | 0.02 | 7.83 * | 22.57 * | 30.41 * |
| Assay | DPPH· | ABTS·+ | FRAP | Total Phenolic Content |
|---|---|---|---|---|
| Units Standard curve | (µmol TE/g) Trolox (R2 = 0.99) | (µmol TE/g) Trolox (R2 = 0.99) | (µmol Eq FeSO4) FeSO4 (R2 = 0.99) | (mg 100 g-1 GAE) Gallic acid (R2 = 0.99) |
| PoPEx | 11805.08 ± 03 | 11105.72 ± 03 | 568.82 ± 0.08 | 2591.01 ± 0.06 |
| Dose (mg/kg Body Weight) | |||
|---|---|---|---|
| ASA | PoPEx | ||
| Log (D) | (mg/kg) | Log (D) | (mg/kg) |
| 0.50 | 3.16 | 1.00 | 10.00 |
| 1.00 | 10.00 | 1.50 | 31.62 |
| 1.50 | 31.62 | 2.00 | 100.00 |
| 2.00 | 100.00 | 2.50 | 316.22 |
| 2.50 | 316.00 | 2.75 | 562.34 |
| 2.75 | 562.34 | 3.00 | 1000.00 |
| Dose (mg/kg Body Weight) | |||
|---|---|---|---|
| Groups (n = 6) | ASA | PoPEx | Total |
| (1) | 4.00 | 30.00 | 34.00 |
| (2) | 2.00 | 15.00 | 17.00 |
| (3) | 1.00 | 7.50 | 8.51 |
| (4) | 0.50 | 3.75 | 4.25 |
| (5) | 0.25 | 1.87 | 2.12 |
| (6) | 0.12 | 0.93 | 1.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero-Solano, J.A.; Bautista, M.; Velázquez-González, C.; De la O-Arciniega, M.; González-Olivares, L.G.; Fernández-Moya, M.; Jaramillo-Morales, O.A. Antinociceptive Synergism of Pomegranate Peel Extract and Acetylsalicylic Acid in an Animal Pain Model. Molecules 2021, 26, 5434. https://doi.org/10.3390/molecules26185434
Guerrero-Solano JA, Bautista M, Velázquez-González C, De la O-Arciniega M, González-Olivares LG, Fernández-Moya M, Jaramillo-Morales OA. Antinociceptive Synergism of Pomegranate Peel Extract and Acetylsalicylic Acid in an Animal Pain Model. Molecules. 2021; 26(18):5434. https://doi.org/10.3390/molecules26185434
Chicago/Turabian StyleGuerrero-Solano, José Antonio, Mirandeli Bautista, Claudia Velázquez-González, Minarda De la O-Arciniega, Luis Guillermo González-Olivares, Monserrat Fernández-Moya, and Osmar Antonio Jaramillo-Morales. 2021. "Antinociceptive Synergism of Pomegranate Peel Extract and Acetylsalicylic Acid in an Animal Pain Model" Molecules 26, no. 18: 5434. https://doi.org/10.3390/molecules26185434









