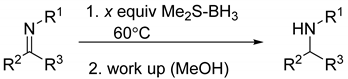Abstract
Although there exists a variety of different catalysts for hydroboration of organic substrates such as aldehydes, ketones, imines, nitriles etc., recent evidence suggests that tetra-coordinate borohydride species, formed by activation, redistribution, or decomposition of boron reagents, are the true hydride donors. We then proposed that Me2S-BH3 could also act as a hydride donor for the reduction of various imines, as similar compounds have been observed to reduce carbonyl substrates. This boron reagent was shown to be an effective and chemoselective hydroboration reagent for a wide variety of imines.
1. Introduction
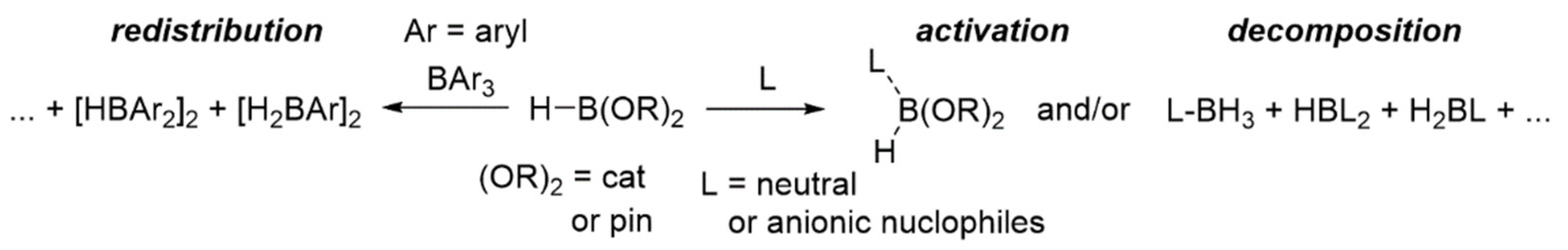
Hydroboration can be considered as one of the most powerful methods for reduction of various organic substrates such as aldehydes, ketones, imines, and nitriles under mild reaction conditions [1,2,3]. Pinacolborane (HBpin) or catecholborane (HBcat) has been predominantly used as the hydroborating agent in these particular transformations, but the boron fragment (i.e., Bcat/Bpin) was normally sacrificed to yield, for example, free alcohols (from carbonyls) or amines (from imines/nitriles). Furthermore, these reduction reactions were mainly performed in the presence of catalytic amounts of a diverse range of (in)organic/organometallic compounds [4,5,6,7,8,9]. Several of the described hydroboration reactions were efficient as catalyst loadings as low as 0.001 mol%, resulting in excellent substrate conversions [7]. Nevertheless, the exact role of these presumed (pre)catalytic species has been divisive, as several reports provided convincing evidence for the existence of hidden boron catalysis (HBC), i.e., the main role of the species that were introduced in “catalytic” amounts was the formation, via activation, redistribution, or decomposition (Scheme 1) of HBcat/HBipn, of boron-based compounds (e.g., hydroborates and boranes) that then acted as the true catalysts [10,11,12].
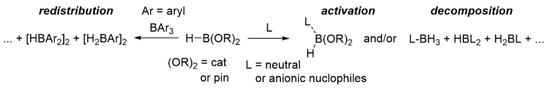
Scheme 1.
Observed activation, redistribution and/or decomposition of HBcat/HBpin in presence of nucleophiles (L) or other boranes (BAr3).
Four-coordinate borohydride compounds (e.g., HBR3−) were identified to serve as the (pre)catalysts for hydroboration of a hetero-atom containing unsaturated substrates such as aldehydes, ketones and imines [11,12]. This would then suggest that L-BH3 (L = THF, SMe2, NR3 (R = alkyl), N-heterocyclic carbene (NHC), etc.) could not only act as adequate reagents for reduction of these types of substrates but also deliver more cost-effective hydroboration protocol(s) as certain BH3-containing species act as synthetic precursors to HBpin/HBcat [13,14]. However, there appears to be a limited number of published works that use these particular reagents (i.e., L-BH3) for this specific purpose, with THF-BH3 being the preferred choice for hydroboration of mainly carbonyl substrates [15,16,17,18,19,20]. Furthermore, NHC-BH3 adducts were shown to be adequate reduction agents for C=X fragments (X=N, O, etc.), but an addition of an activator (e.g., silica gel, p-toluenesulfonic acid) was required [21,22]. The presence of protic activators (e.g., Al2O3) and/or protic solvent media (e.g., MeOH) were also required for efficient reduction of these substrates with, for example, NaBH4 and NaBH3CN [23,24,25,26,27]. Lastly, although ammonia borane (NH3-BH3) has been used for reduction of various aldehydes, ketones, imines, etc. [28,29,30,31], experimental and computational studies suggested that these particular reduction reactions underwent a concerted (double) hydrogen transfer mechanism [28,31], which is not typical for hydroboration reactions (see below). Therefore, herein we disclose chemoselective hydroboration of imines using solely Me2S-BH3 as the reducing agent in the absence of any activators and/or a protic solvent medium.
2. Results & Discussion
Instead of generating an “optimized” reaction condition with one of the examined imines and then implementing this procedure for the rest of the substrates, we decided to optimize each transformation in order to maximize the substrate conversions. Thus, the reactions were screened by varying the amount of Me2S-BH3 and the reaction temperature while the reactants were mixed in about 1 mL of CDCl3 in a sealed J. Young NMR tube. The most important outcomes and observations are summarized in Table 1. In a vast majority of examined transformations, heating to 60 °C was necessary to obtain quantitative substrates conversions with Me2S-BH3 loadings varying between 0.75 and 1.50 equiv. For example, most of the reaction mixtures showed negligible reactivity at room temperature, while imine substrates with enhanced steric properties (entries 5 and 6, Table 1) required, in general, 1.50 equiv loadings of Me2S-BH3 with respect to the imine. Reductions of imines that contain a 2,6-disopropylaniline fragment (e.g., entry 5) have been rarely examined, presumably due to low conversions of these particular substrates under the reported reaction conditions [32,33]. Furthermore, reduction of the imines that contained N-aryl substituents (entries 4 and 12) required not only a lower Me2S-BH3 loading (e.g., 0.75 vs. 1.10 mmol) but also a shorter reaction time (e.g., 6 vs. 12 h) in comparison to their N-alkyl containing analogues (e.g., entries 2 and 3 vs. entry 12). This can be potentially explained by the presence of the resonance structures involving the N-aryl fragment puling the electron density away from the N=C fragment and hence allowing hydride transfer to the carbon atom of this fragment (see below). It was then not surprising to observe that the presence of an electron withdrawing group (CF3) had a rate-enhancing effect (entry 7) while an electron donating group (OMe) had an opposite effect (entry 8). These observations suggested that the rate limiting step for the examined reactions was nucleophilic in nature (i.e., hydride transfer from a B-H fragment to the imine substrate; see below) and not electrophilic (i.e., formation of a imine-BH3 adduct) [20].

Table 1.
Summary of reaction conditions to achieve quantitative conversion of imines 1.
More importantly, according to 1H-NMR spectroscopy, all reactions resulted exclusively or solely in the anticipated reduction of the C=N double bond. This was particularly important for the reduction of the imine substrates that also contained an alkenyl group (i.e., α,β-unsaturated imines; entries 13 and 14, Table 1). Quantitative substrate conversions with excellent chemoselectivities (>98%) were achieved with these particular imines, while, at the same time, generating the fastest reaction rates among the examined substrates, despite the transformations performing at −78 °C (for the selectivity purposes). Lastly, according to the results summarized in Table 1, it appeared that this reduction protocol favored, in terms of reaction rates, ketimines over aldimines, which is not typically observed in the literature [10,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. At the moment, the precise reason(s) for this observation is(are) not known but it may suggest that the electrophilic step i.e., coordination of imine to BH3 (see below) was rate determining, as one would expect that the hydride transfer (i.e., the nucleophilic step) would be less favored for ketimines over aldimines.
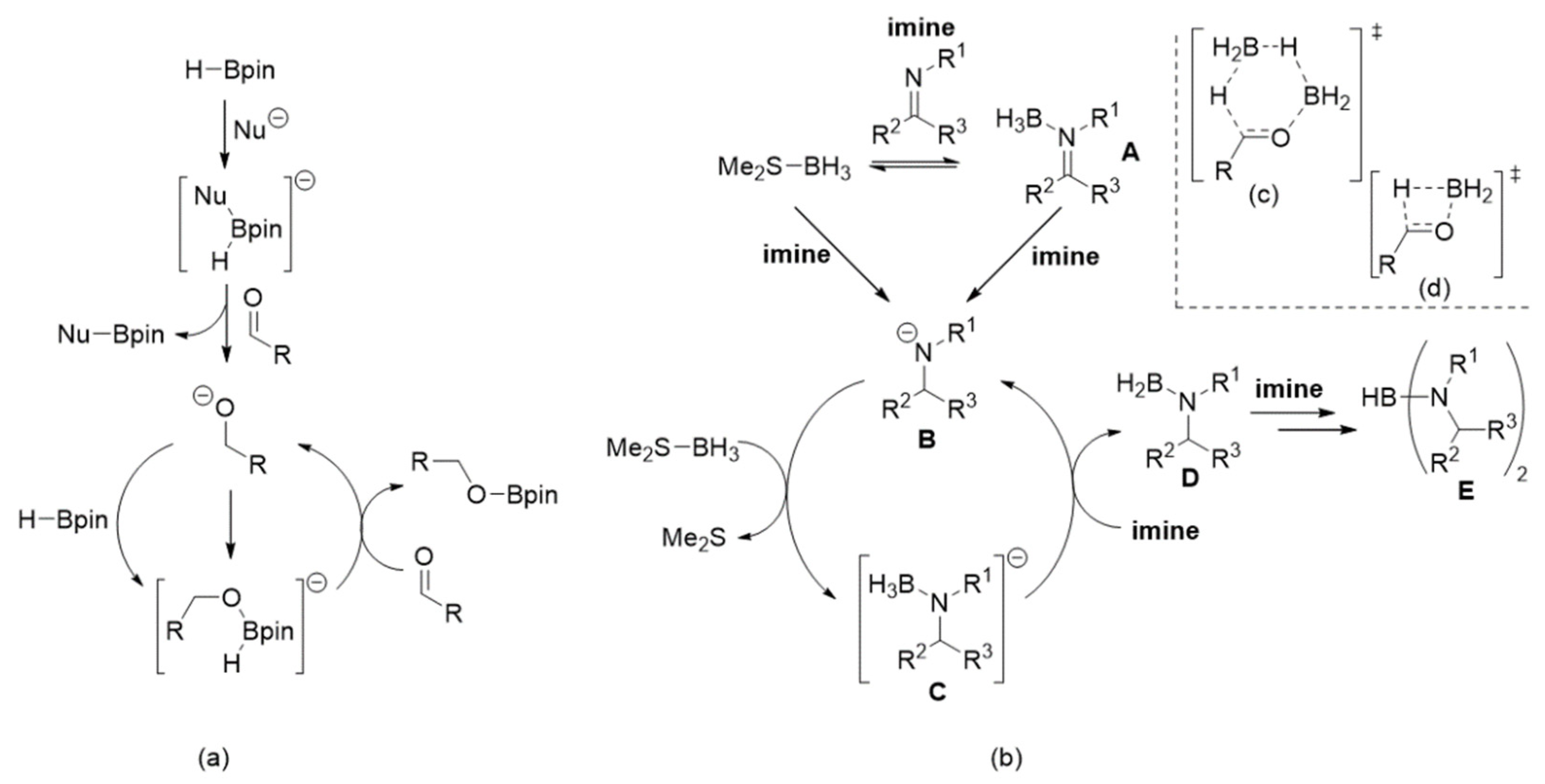
As mentioned in the introduction, catalytic hydroboration of unsaturated C=X fragments (X=O, N, etc., but X≠C) has been a controversial topic. However, there is a significant body of evidence suggesting that four-coordinate B-H containing compound(s) (usually anionic), generated by activation, decomposition, or redistribution of boron reagents, act as initial hydride donors and hence as initiators of catalytically active species [11,12]. Clark and co-workers suggested a mechanism (Scheme 2a) that involved “activation” of HBpin (or HBcat) by coordination of a nucleophile (the electrophilic step), followed by hydride transfer (the nucleophilic step) from boron to the substrate (e.g., aldehyde), to yield the corresponding anion (e.g., alkoxide) [46]. This anion would then bind to another molecule of H-Bpin to generate the catalytically active species (e.g., [HBpin(alkoxy)]−). Thus, we propose that for reduction of imines with Me2S-BH3, initial hydride transfer occurs from either Me2S-BH3 or imine-BH3 (A, Scheme 2b) to produce amide anion B. This anion then displaces Me2S from Me2S-BH3 to generate the catalytically active species C, which acts as a hydride donor to another imine completing the cycle while also yielding reduced species D.
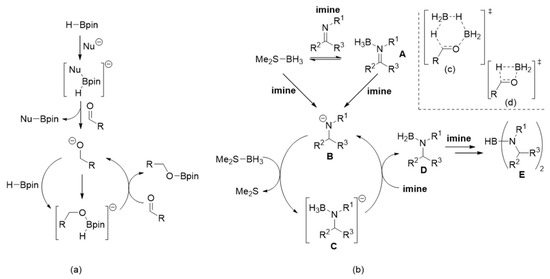
Scheme 2.
(a) The Clark mechanism; (b) the proposed mechanism for hydroboration of imines with MeS2-BH3; (c) and (d) proposed transition states for the hydride step in reduction of carbonyl compounds.
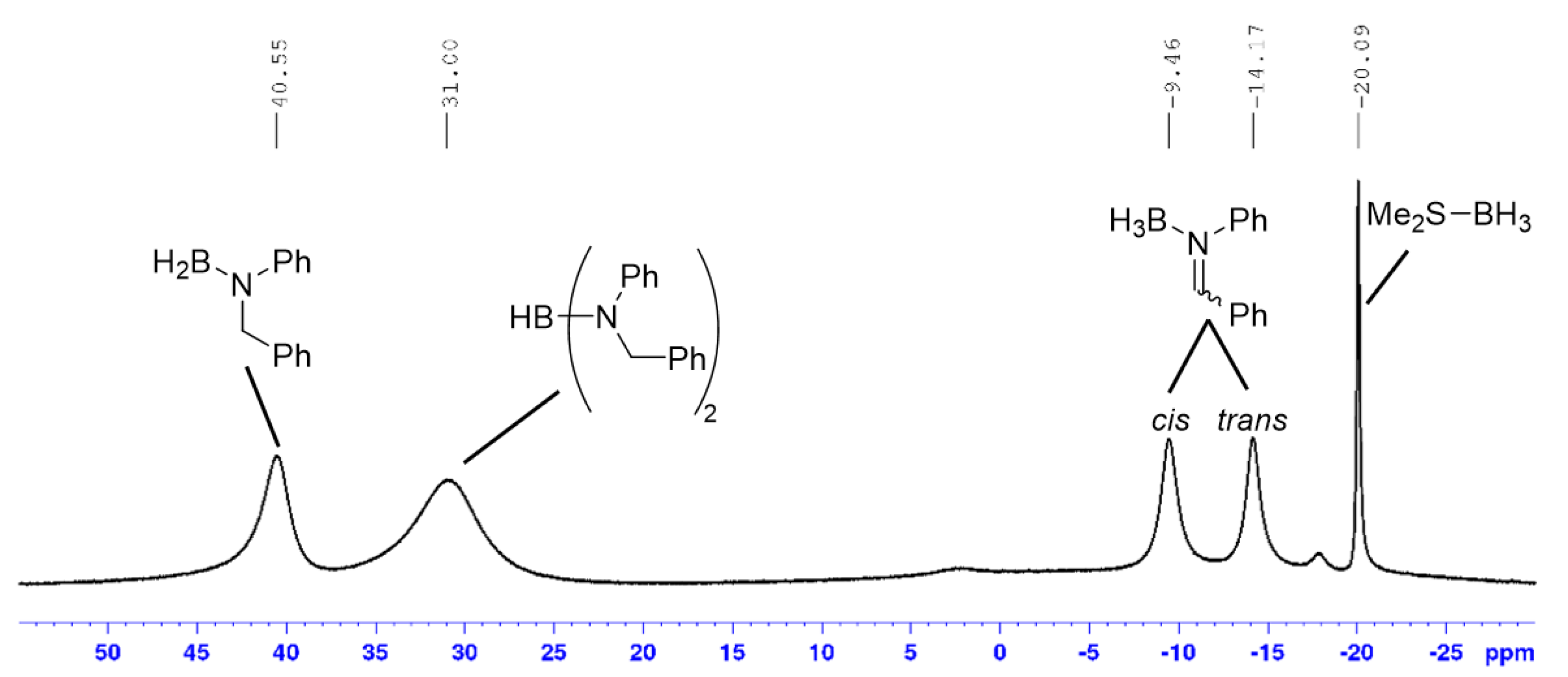
Recently, Abe and Yamataka proposed that reduction of carbonyl compounds using BH3 (in THF), the first step was H3B-carbonyl adduct formation (similar to A, Scheme 2b), followed by a hydride transfer step via a BH3-assisted transition state (Scheme 2c) [20]. However, although a majority of our examined hydroboration reactions require excess Me2S-BH3, it was still possible to achieve quantitative imine reduction with sub-stoichiometric amounts (0.75 mol%) of this boron reagent for several transformations (entries 1, 4, 13 and 14; Table 1). This suggested that, at least in certain instances, it was not only possible to reduce more than one imine substrate with one equivalent of Me2S-BH3 but also that the BH3-catalysed hydride transfer step (in our case going from A to D) step was less likely to occur. Furthermore, it was also suggested that the hydride transfer step (e.g., A → D in our case) occurred via a bimolecular transition state (Scheme 2d) [19]. This would help explain our observation that more than one equivalent of imine was reduced by MeS2-BH3 but a recent theoretical study indicated that a similar transition was high in energy [47]. Regardless of the nature of the hydride transfer step(s), it is still important to mention that we identified, via 11B[1H]-NMR spectroscopy, several proposed intermediates described in Scheme 2b. After mixing Me2S-BH3 and N-benzylideneaniline in a 1:1 mol ratio at room temperature for 6 h, it was possible to detect respective intermediates A (δB ~ −9 (cis) and −14 (trans) ppm, Figure 1; [48]), D (δB ~ 41 ppm; [49]) and E (δB ~ 31 ppm; [50,51]). The fact that unreacted Me2S-BH3 (δB ~ −20 ppm) was also present strongly suggested the existence of an equilibrium process between this reagent and intermediate A as indicated in Scheme 2b.
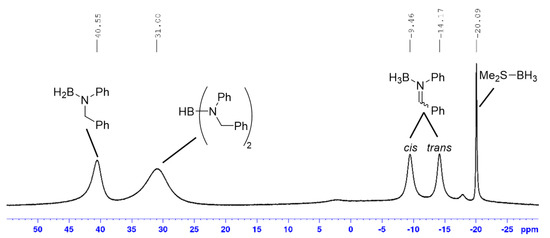
Figure 1.
11B[1H]-NMR spectrum of a mixture (1:1) between Me2S-BH3 and N-benzylideneaniline after 6 h.
In conclusion, we have shown that Me2S-BH3 could also be used for reduction of a number of imines under mild reaction conditions and excellent chemoselectivity control. We have also managed to detect several key intermediates in the overall reaction pathway, which should aid in a better understanding of the overall hydroboration mechanism.
3. Materials and Methods
All imines were synthesized according to the literature reports (Table 2), while Me2S-BH3 was purchased from a commercial source and used as received. CDCl3 was dried by distilling it over CaSO4, while CH2Cl2 was dried by distilling over CaH2. Reduction of imines was performed using standard Schlenk techniques, while subsequent work-up steps (with MeOH) were performed in on a benchtop.

Table 2.
Literature references for the synthesis of the examined imines and spectroscopic data for the corresponding amines.
General procedure for reduction of imines: After 1.0 mmol of an imine (entries 1–12) and Me2S-BH3 (amounts according to Table 1) were mixed in a sealed J. Young NMR tube using about 1 mL of CDCl3, the reaction mixture was left at 60 °C for the time duration indicated in Table 1. For α,β-unsaturated imines (entries 13 and 14), the reactants were mixed in CH2Cl2 at −78 °C. After the reaction was completed (via 1H-NMR spectroscopy), it was quenched with 5 mL of MeOH, followed by removal of all volatiles under reduced pressure. The crude product mixture was then dissolved in 10 mL ethyl acetate, washed three times with 10 mL of water/brine, and dried with MgSO4. All amine samples were collected as oils after removal of solvent apart from benzylmethylamine (entry 1) and N-benzylaniline (entry 4), which were obtained as solids. The spectroscopic data for all amines matched those reported (Table 2).
Purity was assessed by 1H and 13C[1H]-NMR spectroscopy and all samples were >95% pure. 1H (400.2 MHz), 13C[1H] (100.6 MHz), and/or 11B[1H] (128.6 MHz) NMR spectra of reactions and/or isolated amines in CDCl3 were recorded on a Bruker Avance III 400.
1H and 13C[1H]-NMR spectroscopic data for isolated amines (Supplementary Materials):
N-methyl-1-phenylmethanamine (Entry 1): 1H-NMR (400.2 MHz, CDCl3): δ 7.31 (m, 2H), 7.25 (m, 3H), 3.86 (s, 2H), 3.05 (s, br, 1H), 2.32 (s, 1H), 2.31 (s, 2H). 13C-NMR (CDCl3, 100.6 MHz): δ 136.5, 136.3, 129.8, 129.5, 128.3, 128.2, 127.7, 127.7, 66.8, 66.2, 48.4, 47.7.
N-benzylpropan-2-amine (Entry 2): 1H-NMR (400.2 MHz, CDCl3): δ 7.25 (m, 4H), 7.17 (m, 1H), 3.71 (s, 2H), 2.79 (hept, 3J = 6.2 Hz, 1H), 1.57 (s, br, 1H), 1.03 (d, 3J = 6.2 Hz, 6H). 13C-NMR (CDCl3, 100.6 MHz): δ 140.6, 128.4, 128.2, 126.9, 51.6, 48.1, 22.9.
N-benzyl-2-methylpropan-2-amine (Entry 3): 1H-NMR (400.2 MHz, CDCl3): δ 7.25 (m, 4H), 7.15 (m, 1H), 3.66 (s, 2H), 1.11 (s, 9H). 13C-NMR (CDCl3, 100.6 MHz): δ 140.1, 127.4, 127.3, 125.8, 49.9, 46.2, 28.0, 27.3.
N-benzylaniline (Entry 4): 1H-NMR (400.2 MHz, CDCl3): δ 7.26 (m, 5H), 7.10 (m, 2H), 6.67 (m, 1H), 6.59 (m, 2H), 4.64 (s, br, 1H), 4.25 (s, 2H). 13C NMR (CDCl3, 100.6 MHz): δ 146.4, 137.9, 128.3, 127.6, 126.7, 126.3, 117.1, 112.4, 47.7.
N-benzyl-2,6-diisopropylaniline (Entry 5): 1H-NMR (400.2 MHz, CDCl3): δ 7.29 (m, 5H), 7.04 (m, 3H), 4.00 (s, 2H), 3.24 (hept, 3J = 6.8 Hz, 2H), 1.16 (d, 3J = 6.8Hz, 12H). 13C NMR (CDCl3, 100.6 MHz): δ 142.9, 128.6, 128.1, 127.5, 123.7, 56.0, 27.8, 24.3.
N-(2,6-dimethylbenzyl)propan-2-amine (Entry 6): 1H-NMR (400.2 MHz, CDCl3): δ 6.93 (m, 3H), 3.66 (s, 2H), 2.84 (hept, 3J = 6.2 Hz, 1H), 2.32 (s, 6H), 1.05 (d, 3J = 6.2 Hz, 6H). 13C-NMR (CDCl3, 100.6 MHz): δ 135.9, 127.2, 125.9, 48.6, 44.6, 21.9, 18.5.
N-(4-(trifluoromethyl)benzyl)propan-2-amine (Entry 7): 1H-NMR (400.2 MHz, CDCl3): δ 7.59 (d, 3J = 8.0 Hz, 2H), 7.47 (d, 3J = 8.0 Hz, 2H), 3.86 (s, 2H), 2.87 (hept, 3J = 6.2 Hz, 1H), 1.78 (s, br. 1H), 1.13 (d, 3J = 6.2Hz, 6H). 13C-NMR (CDCl3, 100.6 MHz): δ 144.7, 129.3, 129.0, 128.3, 125.6, 125.3, 122.9, 50.9, 48.2, 22.8.
N-(4-methoxybenzyl)propan-2-amine (Entry 8): 1H-NMR (400.2 MHz, CDCl3): δ 7.18 (d, 3J = 8.4 Hz, 2H), 6.78 (d, 3J = 8.4 Hz, 2H), 3.71 (s, 3H), 3.64 (s, 2H), 2.78 (hept, 3J = 6.2 Hz, 1H), 1.66 (s, broad, 1H), 1.02 (d, 3J = 6.2 Hz, 6H). 13C-NMR (CDCl3, 100.6 MHz): δ 158.6, 132.6, 129.4, 113.8, 55.3, 50.9, 48.0, 22.8.
N-benzhydrylpropan-2-amine (Entry 9): 1H-NMR (400.2 MHz, CDCl3): δ 7.21 (m, 10H), 4.89 (s, 1H), 2.67 (hept, 3J = 6.2 Hz, 1H), 1.40 (s, br, 1H), 1.00 (d, 3J = 6.2 Hz, 6H). 13C-NMR (CDCl3, 100.6 MHz): δ 144.5, 128.4, 127.4, 126.9, 64.3, 46.2, 23.1.
N-(1-phenylethyl)propan-2-amine (Entry 10): 1H-NMR (400.2 MHz, CDCl3): δ 7.20 (m, 4H), 7.13 (m, 1H), 3.80 (q, 3J = 6.4 Hz, 1H), 2.54 (hept, 3J = 6.2 Hz, 1H), 1.25 (d, 3J = 6.4 Hz, 3H), 0.94 (d, 3J = 6.2 Hz, 3H), 0.91 (d, 3J = 6.2 Hz, 3H). 13C-NMR (CDCl3, 100.6 MHz): δ 145.1, 127.4, 125.7, 125.4, 54.0, 44.5, 23.8, 23.0, 21.1.
N-isopropylcyclohexanamine (Entry 11): 1H-NMR (400.2 MHz, CDCl3): δ 2.89 (hept, 3J = 6.2 Hz, 1H), 2.43 (m, 1H), 1.81 (m, 2H), 1.65 (m, 2H), 1.54 (m, 2H), 1.10 (m, 4H), 0.97 (d, 3J = 6.2 Hz, 6H), 0.95 (m, 1H). 13C-NMR (CDCl3, 100.6 MHz): δ 53.5, 44.8, 34.0, 26.2, 25.3, 23.4.
N-cyclohexylaniline (Entry 12): 1H-NMR (400.2 MHz, CDCl3): δ 7.08 (t, 3J = 7.8 Hz, 2H), 6.55 (m, 3H), 3.75 (br s, 1H), 3.17 (m, 1H), 1.98 (m, 2H), 1.67 (m, 2H), 1.57 (m, 1H), 1.27 (m, 2H), 1.10 (m, 3H). 13C-NMR (CDCl3, 100.6 MHz): δ 147.1, 129.3, 117.1, 113, 4, 52.0, 33.4, 25.9, 25.0.
(E)-N-isopropyl-3-phenylprop-2-en-1-amine (Entry 13): 1H-NMR (400.2 MHz, CDCl3): δ 7.24 (m, 5H, Phenyl), 6.50 (d, 3J = 15.8 Hz, 1H), 6.29 (dt, 3J = 15.8 Hz, 3J = 6.4 Hz, 1H), 3.39 (d, 3J = 6.4 Hz, 2H), 2.87 (hept, 3J = 6.2 Hz, 1H), 1.39 (s, broad, 1H), 1.08 (d, 3J = 6.2Hz, 6H). 13C-NMR (CDCl3, 100.6 MHz): δ 137.1, 131.1, 128.6, 128.5, 127.3, 126.2, 48.4, 48.1, 22.9.
(E)-N-isopropyl-1,3-diphenylprop-2-en-1-amine (Entry 14): 1H-NMR (400.2 MHz, CDCl3): δ 7.26 (m, 10H, phenyl), 6.53 (d, 3J = 15.8 Hz, 1H), 6.31 (dd, 3J = 15.8 Hz, 3J = 7.3 Hz, 1H), 4.51 (d, 3J = 7.3 Hz, 1H), 2.83 (hept, 3J = 6.2 Hz, 1H), 1.11 (d, 3J = 6.2 Hz, 3H), 1.08 (d, 3J = 6.2 Hz, 3H). 13C-NMR (CDCl3, 100.6 MHz): δ 143.1, 136.98, 132.9, 130.0, 128.5, 128.4, 127.4, 127.3, 127.1, 126.4, 62.4, 45.6, 23.2, 22.8.
Supplementary Materials
This information is available online. Copies of 1H and 13C-NMR spectra of isolated amines (Figures S1–S28).
Author Contributions
Supervision, conceptualization and writing-original draft preparation, D.V.; Initial methodology development, Z.L. and S.Z.; Experimental methodology, M.M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Raw data for this study are not required to be submitted to any archived datasets.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chong, C.C.; Kinjo, R. Catalytic Hydroboration of Carbonyl Derivatives, Imines, and Carbon Dioxide. ACS Catal. 2015, 5, 3238–3259. [Google Scholar] [CrossRef]
- Geier, S.J.; Vogels, C.M.; Westcott, S.A. Current developments in the catalyzed hydroboration reaction. ACS Symp. Ser. 2016, 1236, 209–225. [Google Scholar]
- Tamang, S.R.; Findlater, M. Emergence and applications of base metals (Fe, Co, and Ni) in hydroboration and hydrosilylation. Molecules 2019, 24, 3194. [Google Scholar] [CrossRef] [Green Version]
- Hadlington, T.J.; Hermann, M.; Frenking, G.; Jones, C. Low Coordinate Germanium(II) and Tin(II) Hydride Complexes: Efficient Catalysts for the Hydroboration of Carbonyl Compounds. J. Am. Chem. Soc. 2014, 136, 3028–3031. [Google Scholar] [CrossRef] [PubMed]
- Bagherzadeh, S.; Mankad, N.P. Extremely efficient hydroboration of ketones and aldehydes by copper carbene catalysis. Chem. Commun. 2016, 52, 3844–3846. [Google Scholar] [CrossRef] [PubMed]
- King, A.E.; Stieber, S.C.E.; Henson, N.J.; Kozimor, S.A.; Scott, B.L.; Smythe, N.C.; Sutton, A.D.; Gordon, J.C. Ni(bpy)(cod): A Convenient Entryway into the Efficient Hydroboration of Ketones, Aldehydes, and Imines. Eur. J. Inorg. Chem. 2016, 2016, 1635–1640. [Google Scholar] [CrossRef]
- Mukherjee, D.; Osseili, H.; Spaniol, T.P.; Okuda, J. Alkali metal hydridotriphenylborates [(L)M][HBPh3] (M = Li, Na, K): Chemoselective catalysts for carbonyl and CO2 hydroboration. J. Am. Chem. Soc. 2016, 138, 10790–10793. [Google Scholar] [CrossRef] [PubMed]
- Weidner, V.L.; Barger, C.J.; Delferro, M.; Lohr, T.L.; Marks, T.J. Rapid, Mild, and Selective Ketone and Aldehyde Hydroboration/Reduction Mediated by a Simple Lanthanide Catalyst. ACS Catal. 2017, 7, 1244–1247. [Google Scholar] [CrossRef]
- Tamang, S.R.; Singh, A.; Bedi, D.; Bazkiaei, A.R.; Warner, A.A.; Glogau, K.; McDonald, C.; Unruh, D.K.; Findlater, M. Polynuclear lanthanide-diketonato clusters for the catalytic hydroboration of carboxamides and esters. Nat. Catal. 2020, 3, 154–162. [Google Scholar] [CrossRef]
- Huchenski, B.S.N.; Speed, A.W.H. Protic additives or impurities promote imine reduction with pinacolborane. Org. Biomol. Chem. 2019, 17, 1999–2004. [Google Scholar] [CrossRef]
- Bage, A.D.; Hunt, T.A.; Thomas, S.P. Hidden Boron Catalysis: Nucleophile-Promoted Decomposition of HBpin. Org. Lett. 2020, 22, 4107–4112. [Google Scholar] [CrossRef] [PubMed]
- Bage, A.D.; Nicholson, K.; Hunt, T.A.; Langer, T.; Thomas, S.P. The Hidden Role of Boranes and Borohydrides in Hydroboration Catalysis. ACS Catal. 2020, 10, 13479–13486. [Google Scholar] [CrossRef]
- Arase, A.; Nunokawa, Y.; Masuda, Y.; Hoshi, M. Lithium borohydride promoted hydroboration of alkenes with 1,3,2-benzodioxaborole. J. Chem. Soc. Chem. Commun. 1991, 4, 205. [Google Scholar] [CrossRef]
- Tucker, C.E.; Davidson, J.; Knochel, P. Mild and stereoselective hydroborations of functionalized alkynes and alkenes using pinacolborane. J. Org. Chem. 1992, 57, 3482. [Google Scholar] [CrossRef]
- Yamataka, H.; Hanafusa, T. Reduction of benzophenone with metal hydrides. A kinetic isotope effect and substituent effect study. J. Org. Chem. 1988, 53, 772. [Google Scholar] [CrossRef]
- Lindsley, C.W.; DiMare, M. Metal alkoxide catalysis of catecholborane and borane reductions. Mechanistic studies. Tetrahedron Lett. 1994, 35, 5141. [Google Scholar] [CrossRef]
- Jockel, H.; Schmidt, R. Kinetics of the direct borane reduction of pinacolone in THF. J. Chem. Soc. Perkin Trans. 2 1997, 12, 2719–2723. [Google Scholar] [CrossRef]
- Cha, J.S.; Moon, S.J.; Park, J.H. A Solution of Borane in Tetrahydrofuran. A Stereoselective Reducing Agent for Reduction of Cyclic Ketones to Thermodynamically More Stable Alcohols. J. Org. Chem. 2001, 66, 7514–7515. [Google Scholar] [CrossRef]
- Kudo, T.; Higashide, T.; Ikedate, S.; Yamataka, H. Reaction Profiles and Substituent Effects for the Reduction of Carbonyl Compounds with Monomer and Dimer Simple Metal Hydride Reagents. J. Org. Chem. 2005, 70, 5157–5163. [Google Scholar] [CrossRef]
- Abe, M.; Yamataka, H. Kinetic study of BH3 reduction of benzaldehydes: Identification of effective reducing species. J. Phys. Org. Chem. 2012, 25, 502–505. [Google Scholar] [CrossRef]
- Taniguchi, T.; Curran, D.P. Silica Gel Promotes Reductions of Aldehydes and Ketones by N-Heterocyclic Carbene Boranes. Org. Lett. 2012, 14, 4540–4543. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, L.-Y.; Sun, Z. Asymmetric Reduction of tert-Butanesulfinyl Ketimines by N-Heterocyclic Carbene Boranes. J. Org. Chem. 2015, 80, 11441–11446. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.T.; Kang, S.K. Clean and simple chemoselective reduction of imines to amines using boric acid-activated sodium borohydride under solvent-free conditions. Synlett 2004, 2004, 1484–1488. [Google Scholar] [CrossRef] [Green Version]
- Bolton, R.; Danks, T.N.; Paul, J.M. Preparation and reduction of some camphor imines. Tetrahedron Lett. 1994, 35, 3411. [Google Scholar] [CrossRef]
- Kazemi, F.; Kiasat, A.R.; Sarvestani, E. Practical reduction of imines by NaBH4/alumina under solvent-free conditions: An efficient route to secondary amine. Chin. Chem. Lett. 2008, 19, 1167–1170. [Google Scholar] [CrossRef]
- Kison, C.; Meyer, N.; Opatz, T. An aldimine cross-coupling for the diastereoselective synthesis of unsymmetrical 1,2-diamines. Angew. Chem. Int. Ed. 2005, 44, 5662–5664. [Google Scholar] [CrossRef] [PubMed]
- Salter, M.M.; Kobayashi, J.; Shimizu, Y.; Kobayashi, S. Direct-Type Catalytic Three-Component Mannich Reactions Leading to an Efficient Synthesis of α,β-Diamino Acid Derivatives. Org. Lett. 2006, 8, 3533–3536. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, L.; Fox, T.; Wang, Z.-X.; Berke, H. Transfer Hydrogenation of Imines with Ammonia-Borane: A Concerted Double-Hydrogen-Transfer Reaction. Angew. Chem. Int. Ed. 2010, 49, 2058. [Google Scholar] [CrossRef]
- Yang, X.; Fox, T.; Berke, H. Ammonia borane as a metal free reductant for ketones and aldehydes: A mechanistic study. Tetrahedron 2011, 67, 7121–7127. [Google Scholar] [CrossRef]
- Xu, W.; Fan, H.; Wu, G.; Chen, P. Comparative study on reducing aromatic aldehydes by using ammonia borane and lithium amidoborane as reducing reagents. New J. Chem. 2012, 36, 1496–1501. [Google Scholar] [CrossRef]
- Wang, X.; Yao, W.; Zhou, D.; Fan, H. Theoretical study on the mechanism for NH3BH3 reduction of ketones and imines. Mol. Phys. 2013, 111, 3014–3024. [Google Scholar] [CrossRef]
- Adams, M.R.; Tien, C.-H.; Huchenski, B.S.N.; Ferguson, M.J.; Speed, A.W.H. Diazaphospholene Precatalysts for Imine and Conjugate Reductions. Angew. Chem. Int. Ed. 2017, 56, 6268–6271. [Google Scholar] [CrossRef]
- Arrowsmith, M.; Hill, M.S.; Kociok-Koehn, G. Magnesium Catalysis of Imine Hydroboration. Chem. Eur. J. 2013, 19, 2776–2783. [Google Scholar] [CrossRef]
- Eisenberger, P.; Bailey, A.M.; Crudden, C.M. Taking the F out of FLP: Simple Lewis Acid-Base Pairs for Mild Reductions with Neutral Boranes via Borenium Ion Catalysis. J. Am. Chem. Soc. 2012, 134, 17384–17387. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Hatzakis, E.; McCarthy, S.M.; Reichl, K.D.; Lai, T.-Y.; Yennawar, H.P.; Radosevich, A.T. P-N Cooperative Borane Activation and Catalytic Hydroboration by a Distorted Phosphorous Triamide Platform. J. Am. Chem. Soc. 2017, 139, 6008–6016. [Google Scholar] [CrossRef]
- Yin, Q.; Soltani, Y.; Melen, R.L.; Oestreich, M. BArF3-Catalyzed Imine Hydroboration with Pinacolborane Not Requiring the Assistance of an Additional Lewis Base. Organometallics 2017, 36, 2381–2384. [Google Scholar] [CrossRef] [Green Version]
- Carden, J.L.; Gierlichs, L.J.; Wass, D.F.; Browne, D.L.; Melen, R.L. Unlocking the catalytic potential of tris(3,4,5-trifluorophenyl)borane with microwave irradiation. Chem. Commun. 2019, 55, 318–321. [Google Scholar] [CrossRef] [Green Version]
- Jaladi, A.K.; Kim, H.; Lee, J.H.; Shin, W.K.; Hwang, H.; An, D.K. Lithium diisobutyl-tert-butoxyaluminum hydride (LDBBA) catalyzed hydroboration of alkynes and imines with pinacolborane. New J. Chem. 2019, 43, 16524–16529. [Google Scholar] [CrossRef]
- Pandey, V.K.; Donthireddy, S.N.R.; Rit, A. Catalyst-Free and Solvent-Free Facile Hydroboration of Imines. Chem. Asian J. 2019, 14, 3255–3258. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Eisen, M.S. Catalytic Recycling of a Th-H Bond via Single or Double Hydroboration of Inactivated Imines or Nitriles. ACS Catal. 2019, 9, 5947–5956. [Google Scholar] [CrossRef]
- Yan, D.; Wu, X.; Xiao, J.; Zhu, Z.; Xu, X.; Bao, X.; Yao, Y.; Shen, Q.; Xue, M. n-Butyllithium catalyzed hydroboration of imines and alkynes. Org. Chem. Front. 2019, 6, 648–653. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.T.; Lee, J.H.; Hwang, H.; An, D.K. Lithium bromide: An inexpensive and efficient catalyst for imine hydroboration with pinacolborane at room temperature. RSC Adv. 2020, 10, 34421–34427. [Google Scholar] [CrossRef]
- Ould, D.M.C.; Carden, J.L.; Page, R.; Melen, R.L. Synthesis and Reactivity of Fluorinated Triaryl Aluminum Complexes. Inorg. Chem. 2020, 59, 14891–14898. [Google Scholar] [CrossRef]
- Panda, T.K.; Banerjee, I.; Sagar, S. Alkali Metal-Promoted Facile Synthesis of Secondary Amines from Imines and Carbodiimides. Appl. Organomet. Chem. 2020, 34, e5765. [Google Scholar] [CrossRef]
- Bazkiaei, A.R.; Wiseman, M.; Findlater, M. Iron-catalysed hydroboration of non-activated imines and nitriles: Kinetic and mechanistic studies. RSC Adv. 2021, 11, 15284–15289. [Google Scholar] [CrossRef]
- Query, I.P.; Squier, P.A.; Larson, E.M.; Isley, N.A.; Clark, T.B. Alkoxide-Catalyzed Reduction of Ketones with Pinacolborane. J. Org. Chem. 2011, 76, 6452–6456. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, M.; Kucinski, K.; Hreczycho, G.; Hoffmann, M. Catalytic and non-catalytic hydroboration of carbonyls: Quantum-chemical studies. Org. Biomol. Chem. 2021, 19, 3004–3015. [Google Scholar] [CrossRef]
- Blackwell, J.M.; Piers, W.E.; Parvez, M.; McDonald, R. Solution and Solid-State Characteristics of Imine Adducts with Tris(pentafluorophenyl)borane. Organometallics 2002, 21, 1400–1407. [Google Scholar] [CrossRef]
- Euzenat, L.; Horhant, D.; Ribourdouille, Y.; Duriez, C.; Alcaraz, G.; Vaultier, M. Monomeric (dialkylamino)boranes: A new and efficient boron source in palladium catalyzed C-B bond formation with aryl halides. Chem. Commun. 2003, 18, 2280–2281. [Google Scholar] [CrossRef] [PubMed]
- Bellham, P.; Hill, M.S.; Kociok-Koehn, G.; Liptrot, D.J. Bespoke synthesis of unsymmetrical diaminoboranes by alkaline earth catalysis. Chem. Commun. 2013, 49, 1960–1962. [Google Scholar] [CrossRef]
- Rosello-Merino, M.; Rama, R.J.; Diez, J.; Conejero, S. Catalytic dehydrocoupling of amine-boranes and amines into diaminoboranes: Isolation of a Pt(ii), Shimoi-type, η1-BH complex. Chem. Commun. 2016, 52, 8389–8392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, S.; Guijarro, F.G.; Garcia Ruano, J.L.; Cid, M.B. A General Aminocatalytic Method for the Synthesis of Aldimines. J. Am. Chem. Soc. 2014, 136, 1082–1089. [Google Scholar] [CrossRef]
- Rauch, M.; Kar, S.; Kumar, A.; Avram, L.; Shimon, L.J.W.; Milstein, D. Metal-Ligand Cooperation Facilitates Bond Activation and Catalytic Hydrogenation with Zinc Pincer Complexes. J. Am. Chem. Soc. 2020, 142, 14513–14521. [Google Scholar] [CrossRef] [PubMed]
- Corre, Y.; Iali, W.; Hamdaoui, M.; Trivelli, X.; Djukic, J.P.; Agbossou-Niedercorn, F.; Michon, C. Efficient hydrosilylation of imines using catalysts based on iridium(III) metallacycles. Catal. Sci. Technol. 2015, 5, 1452–1458. [Google Scholar] [CrossRef]
- Collados, J.F.; Toledano, E.; Guijarro, D.; Yus, M. Microwave-assisted solvent-free synthesis of enantiomerically pure N-(tert-butylsulfinyl)imines. J. Org. Chem. 2012, 77, 5744–5750. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Xu, G.; Tang, W. Enantioselective palladium-catalyzed C(sp2)-H carbamoylation. Tetrahedron 2019, 75, 3239–3247. [Google Scholar] [CrossRef]
- Rahman, O.; Kihlberg, T.; Langstroem, B. Synthesis of [11C]/(13C)amines via carbonylation followed by reductive amination. Org. Biomol. Chem. 2004, 2, 1612–1616. [Google Scholar] [CrossRef]
- Reeves, J.T.; Visco, M.D.; Marsini, M.A.; Grinberg, N.; Busacca, C.A.; Mattson, A.E.; Senanayake, C.H. A General Method for Imine Formation Using B(OCH2CF3)3. Org. Lett. 2015, 17, 2442–2445. [Google Scholar] [CrossRef]
- Ciotonea, C.; Hammi, N.; Dhainaut, J.; Marinova, M.; Ungureanu, A.; El Kadib, A.; Michon, C.; Royer, S. Phyllosilicate-derived nickel-cobalt bimetallic nanoparticles for the catalytic hydrogenation of imines, oximes and N-heteroarenes. ChemCatChem 2020, 12, 4652–4663. [Google Scholar] [CrossRef]
- Landge, V.G.; Maxwell, J.M.; Chand-Thakuri, P.; Kapoor, M.; Diemler, E.T.; Young, M.C. Palladium-Catalyzed Regioselective Arylation of Unprotected Allylamines. JACS Au 2021, 1, 13–22. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).