Ipertrofan Revisited—The Proposal of the Complete Stereochemistry of Mepartricin A and B
Abstract
:1. Introduction
2. Results
2.1. A Brief Introduction to the Procedure
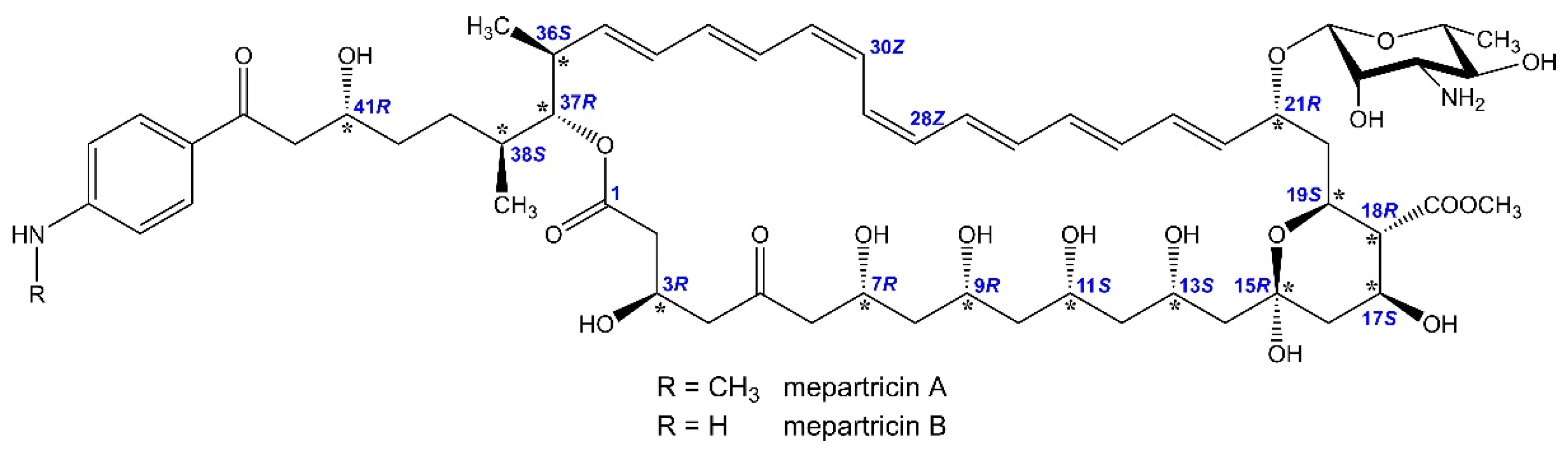
2.2. Assignment of the Stereochemistry of Mepartricin A and B
3. Discussion
4. Materials and Methods
4.1. NMR Spectroscopy
4.2. Molecular Modeling Studies
4.3. Haasnoot–de Leeuw–Altona (HLA) Equation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A
| Mepartricin A and B | |||||
|---|---|---|---|---|---|
| Position | δC, Type | δH | JH,H (Hz) | ROE Contacts | |
| Aglycone | |||||
| 1 | 170.88, | C | – | – | – |
| 2a | 43.67, | CH2 | 2.664 | 15.4 (2b), 9.2 (3) | 2b, 3, 4a |
| 2b | 2.948 | 15.4 (2a), 3.4 (3) | 2a, 3, 4b | ||
| 3 | 63.82, | CH | 4.963 | 9.2 (2a), 3.4 (2b), 8.5 (4a), 5.0 (4b) | 2a, 2b, 4a, 4b, 34, Me38 |
| 4a | 51.34, | CH2 | 2.836 | 8.5 (3), 17.2 (4b) | 2a, 3, 4b |
| 4b | 3.099 | 5.0 (3), 17.2 (4b) | 2b, 3, 4a | ||
| 5 | 208.49, | C | – | – | – |
| 6a | 51.30, | CH2 | 2.558 | 16.5 (6b), 1.9 (7) | 4a, 6b, 7 |
| 6b | 2.821 | 16.5 (6a), 9.6 (7) | 4b, 6a, 7 | ||
| 7 | 67.40, | CH | 4.698 | 1.9 (6a), 9.6 (6b), 2.0 (8a), 9.4 (8b) | 6a, 6b, 8a, 8b, 9, 28, 29 |
| 8a | 43.86, | CH2 | 1.334 | 2.0 (7), 13.5 (8b), 1.9 (9) | 6a, 7, 8b, 9, 10a |
| 8b | 1.773 | 9.4 (7), 13.5 (8b), 9.8 (9) | 6b, 7, 8a, 10b | ||
| 9 | 73.04, | CH | 4.217 | 1.9 (8a), 9.8 (8b), 2.0 (10a), 10.0 (10b) | 7, 8a, 10a, 11, 26, 28 |
| 10a | 44.34, | CH2 | 1.406 | 2.0 (9), 14.0 (10b), 1.8 (11) | 8a, 9, 10b, 11, 12a |
| 10b | 1.668 | 10.0 (9), 14.0 (10a), 10.3 (11) | 8b, 10a | ||
| 11 | 73.82, | CH | 4.269 | 1.8 (10a), 10.3 (10b), 2.1 (12a), 9.4 (12b) | 9, 10a, 12a, 13, 24, 26 |
| 12a | 44.09, | CH2 | 1.357 | 2.1 (11), 13.3 (12b), 1.9 (13) | 10a, 11, 12b, 13, 14a |
| 12b | 1.677 | 9.4 (11), 13.3 (12a), 9.9 (13) | 12a, 14b | ||
| 13 | 69.36, | CH | 4.751 | 1.9 (12a), 9.9 (12b), 2.1 (14a), 9.5 (14b) | 11, 12a, 14a, 22, 24 |
| 14a | 46.68, | CH2 | 1.730 | 2.1 (13), 14.5 (14b) | 12a, 13, 14b |
| 14b | 1.945 | 9.5 (13), 14.5 (14a) | 12b, 14a, 16b | ||
| 15 | 98.01, | C | – | – | – |
| 16a | 45.28, | CH2 | 1.734 | 12.4 (16b), 10.4 (17) | 16b, 18 |
| 16b | 2.536 | 12.4 (16a), 4.3 (17) | 14b, 16a, 17 | ||
| 17 | 66.34, | CH | 5.006 | 10.4 (16a), 4.3 (16b), 10.1 (18) | 16b, 18, 19 |
| 18 | 58.59, | CH | 2.809 | 10.1 (17), 10.2 (19) | 16a, 17, 19, 20a |
| 19 | 66.79, | CH | 5.082 | 10.2 (18), 10.5 (20a) | 17, 18, 20b, 22, 1′, 2′ |
| 20a | 37.72, | CH2 | 1.999 | 10.5 (19), 15.8 (20b) | 18, 20b, 21 |
| 20b | 2.447 | 15.8 (20a), 5.6 (21) | 19, 20a, 21, 1′ | ||
| 21 | 75.70, | CH | 4.936 | 5.6 (20b), 9.1 (22) | 20a, 20b, 22, 23, 1′ |
| 22 | 137.00, | CH | 6.507 | 9.1 (21), 15.4 (23) | 13, 19, 21, 24 |
| 23 | 129.99, | CH | 6.392 | 15.4 (22), 10.9 (24) | 21, 25 |
| 24 | 134.31, | CH | 6.753 | 10.9 (23), 15.5 (25) | 11, 13, 22, 26 |
| 25 | 132.85, | CH | 6.544 | 15.5 (24). 11.2 (26) | 23, 27 |
| 26 | 135.21, | CH | 6.789 | 11.2 (25), 15.2 (27) | 9, 11, 24, 28 |
| 27 | 125.11, | CH | 7.048 | 15.2 (26), 11.0 (28) | 25, 30 |
| 28 | 130.49, | CH | 6.616 | 11.0 (27), 11.5 (29) | 7, 9, 26, 29 |
| 29 | 128.25, | CH | 7.052 | 11.5 (28), 10.8 (30) | 7, 28, 32 |
| 30 | 124.88, | CH | 6.736 | 10.8 (29). 11.3 (31) | 27, 31 |
| 31 | 130.47, | CH | 6.275 | 11.3 (30), 10.9 (32) | 30, 33 |
| 32 | 128.11, | CH | 7.261 | 10.9 (31), 15.5 (33) | 29, 34 |
| 33 | 134.10, | CH | 6.370 | 15.5 (32), 11.2 (34) | 31, 35 |
| 34 | 133.17, | CH | 6.489 | 11.2 (33), 15.5 (35) | 3, 32, 36 |
| 35 | 137.63, | CH | 5.610 | 15.5 (34), 9.3 (36) | 33, 36, 37, Me36 |
| 36 | 40.05, | CH | 2.592 | 9.3 (35), 9.9 (37) | 34, 35, 37, 39ab, Me36, Me38 |
| 37 | 78.29, | CH | 5.109 | 9.9 (36), 2.3 (38) | 35, 36, 38, 39ab, Me36, Me38 |
| 38 | 33.88, | CH | 1.948 | 2.3 (37), ? (39ab)* | 37, 39ab, 40a, 40b, 41, Me36, Me38 |
| 39ab* | 30.77, | CH2 | A: 1.801 B: 1.799 | ? (38, 40a, 40b)* | 36, 37, 38, 40a, 40b, 41, Me36, Me38 |
| 40a | A: 35.65, B: 35.67, | CH | A: 1.918 B: 1.902 | ? (39ab)*, 15.9 (40b), 4.5 (41) | 38, 39ab, 40b, 41, 42a, 42b, Me38 |
| 40b | A: 1.853 B: 1.854 | ? (39ab)*, 15.9 (40a), 8.5 (41) | 38, 39ab, 40a, 42a, 42b | ||
| 41 | A: 68.35, B: 68.30, | CH | A: 4.589 B: 4.572 | 4.5 (40a), 8.5 (40b), 3.2 (42a), 9.3 (42b) | 38, 39ab, 40a, 42a, 45/45′ |
| 42a | A: 46.00, B: 45.95, | CH2 | A: 3.204 B: 3.183 | 3.2 (41), 15.4 (42b) | 40a, 40b, 41, 42b, 45/45′ |
| 42b | A: 3.390 B: 3.366 | 9.3 (41), 15.4 (42a) | 40a, 40b, 42a, 45/45′ | ||
| 43 | A: 197.72, B: 197.64, | C | – | – | – |
| Me36 | 16.26, | CH3 | 0.971 | 6.7 (36) | 35, 36, 37, 38, 39ab |
| Me38 | 13.01, | CH3 | 1.053 | 6.5 (38) | 3, 36, 37, 38, 39ab, 40a |
| COOMe | 174.05, | C | – | – | – |
| COOMe | 51.38, | CH3 | 3.696 | – | 2′, 3′ |
| NHMe | A: 29.27, B: –, | CH3 | A: 2.791 B: – | – | 46/46′ |
| Aromatic Moiety | |||||
| 45/45′ | A: 131.04, B: 131.21, | CH | 8.148 | 8.7 (46/46′) | 41, 42a, 42b, 46/46′ |
| 46/46′ | A: 110.91, B: 113.23, | CH | 6.783 | 8.7 (45/45′) | 45/45′, NHMe |
| C*CO | A: 154.39, B: 154.21, | C | – | – | – |
| C*NH | A: 126.16, B: 126.69, | C | – | – | – |
| Mycosamine Moiety | |||||
| 1’ | 98.34, | CH | 4.906 | 1.8 (2′) | 2′, 3′, 5′, 19, 20b, 21 |
| 2’ | 71.19, | CH | 4.439 | 1.8 (1′), 3.4 (3′) | 1′, 3′, 19, COOMe |
| 3’ | 57.91, | CH | 3.120 | 3.4 (2′), 9.6 (4′) | 1′, 2′, 5′, COOMe |
| 4’ | 74.19, | CH | 3.806 | 9.6 (3′), 9.8 (5′) | 6′ |
| 5’ | 74.36, | CH | 3.669 | 9.8 (4)’, 6.1 (6′) | 1′, 3′, 6′ |
| 6’ | 18.37, | CH3 | 1.589 | 6.1 (5′) | 4′, 5′ |
References
- Bruzzese, T.; Binda, I.; di Nardo, A.; Ghielmetti, G.; Riva, M. Partricin Methyl Ester, A Semisynthetic Polyene Antibiotic. Experientia 1972, 28, 1515–1516. [Google Scholar] [CrossRef]
- Cirillo Marucco, E.; Pagliarulo, A.; Piccinno, A.; di Rienzo, U. Mepartricin in the Treatment of Benign Prostatic Hyperplasia. Minerva Urol. Nefrol. 1988, 40, 101–104. [Google Scholar]
- Boehm, S.; Nirnberger, G.; Ferrari, P. Estrogen Suppression as a Pharmacotherapeutic Strategy in the Medical Treatment of Benign Prostatic Hyperplasia: Evidence for Its Efficacy from Studies with Mepartricin. Wien. Klin. Wochenschr. 1998, 110, 817–823. [Google Scholar] [PubMed]
- Petrone, U.; Gaspari, G.; Magnocavallo, N.; Petrone, D.; Tucci, C.; Marascia, G. Use of Mepartricin in the Treatment of Benign Prostatic Hypertrophy. Evaluation of Clinical and Functional Parameters. Minerva Urol. Nefrol. 1988, 40, 89–91. [Google Scholar] [PubMed]
- de Rose, A.F.; Gallo, F.; Giglio, M.; Carmignani, G. Role of Mepartricin in Category III Chronic Nonbacterial Prostatitis/Chronic Pelvic Pain Syndrome: A Randomized Prospective Placebo-Controlled Trial. Urology 2004, 63, 13–16. [Google Scholar] [CrossRef] [PubMed]
- del Vecchio, S.; Ulissi, A.; Monache, M.D.; Tavanti, A.; Rapocci, M.; Ruozi, P.; de Bernardi, M.; Ricci, G.L. Faecal Elimination of Steroids in Rats after Oral Administration of Mepartricin. J. Int. Med. Res. 1990, 18, 468–472. [Google Scholar] [CrossRef]
- Lotti, T.; Mirone, V.; Prezioso, D.; de Bernardi, M.; Rapocci, M.P.; Ruozi, P. Observations on Some Hormone Fractions in Patients with Benign Prostatic Hyperplasia Treated with Mepartricin. Curr. Ther. Res. 1988, 44, 402–409. [Google Scholar]
- Shakutou, S.; Bandoh, K.; Yoshinaka, Y.; Kobayashi, H.; Yamanaka, H. Effects of Mepartricin, a Polyene Macrolide Agent, on Fecal Excretion and Serum Concentration of Estrogen and Number of Prostatic Estrogen Receptors in Immature Rats. Prostate 1999, 38, 17–27. [Google Scholar] [CrossRef]
- Omura, S.; Tanaka, H. Macrolide Antibiotics. Chemistry, Biology and Practice, 1st ed.; Academic Press Inc.: London, UK, 1984. [Google Scholar]
- Bruzzese, T.; Ferrari, R. Partricin. Patent US3773925 A, 20 November 1973. [Google Scholar]
- Bruzzese, T.; Rimaroli, C.; Bonabello, A.; Ferrari, E.; Signorini, M. Amide Derivatives of Partricin A with Potent Antifungal Activity. Eur. J. Med. Chem. 1996, 31, 965–972. [Google Scholar] [CrossRef]
- Tweit, R.C.; Pandey, R.C.; Rinehart Jr., K. L. Characterization of the Antifungal and Antiprotozoal Antibiotic Partricin and Structural Studies on Partricins A and B. J. Antibiot. 1982, 35, 997–1012. [Google Scholar] [CrossRef] [Green Version]
- Golik, J.; Zieliński, J.; Borowski, E. The Structure of Mepartricin A and Mepartricin, B. J. Antibiot. 1980, 33, 904–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowiński, P.; Gariboldi, P.; Czerwiński, A.; Borowski, E. The Structure of Vacidin A, an Aromatic Heptaene Macrolide Antibiotic. I. Complete Assignment of the 1H NMR Spectrum and Geometry of the Polyene Chromophore. J. Antibiot. 1989, 42, 1631–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowiński, P.; Gariboldi, P.; Pawlak, J.K.; Borowski, E. The Structure of Vacidin A, an Aromatic Heptaene Macrolide Antibiotic. II. Stereochemistry of the Antibiotic. J. Antibiot. 1989, 42, 1639–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowiński, P.; Pawlak, J.; Borowski, E.; Gariboldi, P. Stereostructure of Gedamycin. Pol. J. Chem. 1995, 69, 213–217. [Google Scholar]
- Borzyszkowska-Bukowska, J.; Szczeblewski, P.; Konkol, A.; Grynda, J.; Szwarc-Karabyka, K.; Laskowski, T. The Complete Stereochemistry of the Antibiotic Candicidin A3 (Syn. Ascosin A3, Levorin A3). Nat. Prod. Res. 2020, 34. [Google Scholar] [CrossRef] [PubMed]
- Szczeblewski, P.; Laskowski, T.; Kubacki, B.; Dziergowska, M.; Liczmańska, M.; Grynda, J.; Kubica, P.; Kot-Wasik, A.; Borowski, E. Analytical Studies on Ascosin, Candicidin and Levorin Multicomponent Antifungal Antibiotic Complexes. the Stereostructure of Ascosin A2. Sci. Rep. 2017, 7, 40158. [Google Scholar] [CrossRef]
- Szwarc, K.; Szczeblewski, P.; Sowiński, P.; Borowski, E.; Pawlak, J. The Structure, Including Stereochemistry, of Levorin A1. Magn. Reson. Chem. 2015, 53, 479–484. [Google Scholar] [CrossRef]
- Szwarc, K.; Szczeblewski, P.; Sowiński, P.; Borowski, E.; Pawlak, J. The Stereostructure of Candicidin, D. J. Antibiot. 2015, 68, 504–510. [Google Scholar] [CrossRef]
- Sowiński, P.; Pawlak, J.; Borowski, E.; Gariboldi, P. Stereostructure of Rimocidin. J. Antibiot. 1995, 48, 1288–1291. [Google Scholar] [CrossRef] [Green Version]
- Pawlak, J.; Sowiński, P.; Borowski, E.; Gariboldi, P. Stereostructure and NMR Characterization of the Antibiotic Candidin. J. Antibiot. 1993, 46, 1598–1604. [Google Scholar] [CrossRef]
- Płosiński, M.; Laskowski, T.; Sowiński, P.; Pawlak, J. Stereostructure of Mycoheptin A2. Magn. Reson. Chem. 2012, 50, 818–822. [Google Scholar] [CrossRef]
- Sowiński, P.; Pawlak, J.; Borowski, E.; Gariboldi, P. 1H NMR Model Studies of Amphotericin B: Comparison of X-Ray and NMR Stereochemical Data. Magn. Reson. Chem. 1992, 30, 275–279. [Google Scholar] [CrossRef]
- Seroka, P.; Płosiński, M.; Czub, J.; Sowiński, P.; Pawlak, J. Monosaccharides as Internal Probes for the Determination of the Absolute Configuration of 2-Butanol. Magn. Reson. Chem. 2006, 44, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, T.; Szwarc, K.; Szczeblewski, P.; Sowiński, P.; Borowski, E.; Pawlak, J. Monosaccharides as Potential Chiral Probes for the Determination of the Absolute Configuration of Secondary Alcohols. J. Nat. Prod. 2016, 79. [Google Scholar] [CrossRef]
- Haasnoot, C.A.G.; de Leeuw, F.A.A.M.; Altona, C. The Relationship between Proton–proton NMR Coupling Constants and Substituent Electronegativities-I. An Empirical Generalization of the Karplus Equation. Tetrahedron 1980, 36, 2783–2792. [Google Scholar] [CrossRef]
- Sheehan, J.; Murphy, C.D.; Caffrey, P. New Insights into Polyene Macrolide Biosynthesis in Couchioplanes Caeruleus. Mol. Biosyst. 2017, 13, 866–873. [Google Scholar] [CrossRef] [Green Version]
- Szczeblewski, P.; Laskowski, T.; Balka, A.; Borowski, E.; Milewski, S. Light-Induced Transformation of the Aromatic Heptaene Antifungal Antibiotic Candicidin D into Its All-Trans Isomer. J. Nat. Prod. 2018, 81, 1540–1545. [Google Scholar] [CrossRef]
- Ishii, K.; Miyashiro, S. Process for Producing a Polyene Antibiotic. Patent US5244661 A, 14 September 1993. [Google Scholar]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM General Force Field: A Force Field for Drug-like Molecules Compatible with the CHARMM All-Atom Additive Biological Force Fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian Inc.: Wallingford, UK, 2013. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Vázquez, A.; Carlos, J.C.; Sardina, F.J.; Casanueva, J.; Díez, E. A Graphical Tool for the Prediction of Vicinal Proton–proton 3JHH Coupling Constants. J. Chem. Inf. Comput. Sci. 2004, 44, 1680–1685. [Google Scholar] [CrossRef] [PubMed]

| Coupling Protons (i,j) | Measured 3Ji/j (Hz) | Calculated 3Ĵi/j (Hz) | Absolute Difference between the Experimental and Calculated 3Ji/j (Hz) | ||
|---|---|---|---|---|---|
| 41R | 41S | 41R | 41S | ||
| H40a, H41 | 4.5 | 3.8 | 2.3 | 0.7 | 2.2 |
| H40b, H41 | 8.5 | 8.7 | 9.7 | 0.2 | 1.2 |
| H41, H42a | 3.2 | 3.1 | 4.5 | 0.1 | 1.3 |
| H41, H42b | 9.3 | 9.2 | 6.0 | 0.1 | 3.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczeblewski, P.; Andrałojć, W.; Polit, J.; Żabka, A.; Winnicki, K.; Laskowski, T. Ipertrofan Revisited—The Proposal of the Complete Stereochemistry of Mepartricin A and B. Molecules 2021, 26, 5533. https://doi.org/10.3390/molecules26185533
Szczeblewski P, Andrałojć W, Polit J, Żabka A, Winnicki K, Laskowski T. Ipertrofan Revisited—The Proposal of the Complete Stereochemistry of Mepartricin A and B. Molecules. 2021; 26(18):5533. https://doi.org/10.3390/molecules26185533
Chicago/Turabian StyleSzczeblewski, Paweł, Witold Andrałojć, Justyna Polit, Aneta Żabka, Konrad Winnicki, and Tomasz Laskowski. 2021. "Ipertrofan Revisited—The Proposal of the Complete Stereochemistry of Mepartricin A and B" Molecules 26, no. 18: 5533. https://doi.org/10.3390/molecules26185533
APA StyleSzczeblewski, P., Andrałojć, W., Polit, J., Żabka, A., Winnicki, K., & Laskowski, T. (2021). Ipertrofan Revisited—The Proposal of the Complete Stereochemistry of Mepartricin A and B. Molecules, 26(18), 5533. https://doi.org/10.3390/molecules26185533







