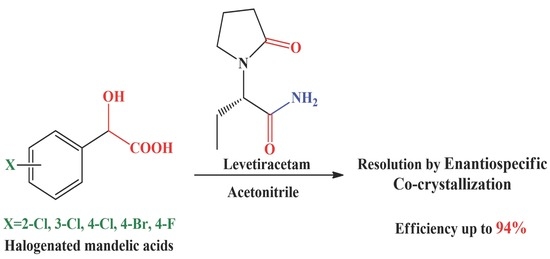
Resolution of Halogenated Mandelic Acids through Enantiospecific Co-Crystallization with Levetiracetam
Abstract
:1. Introduction
2. Results and Discussion
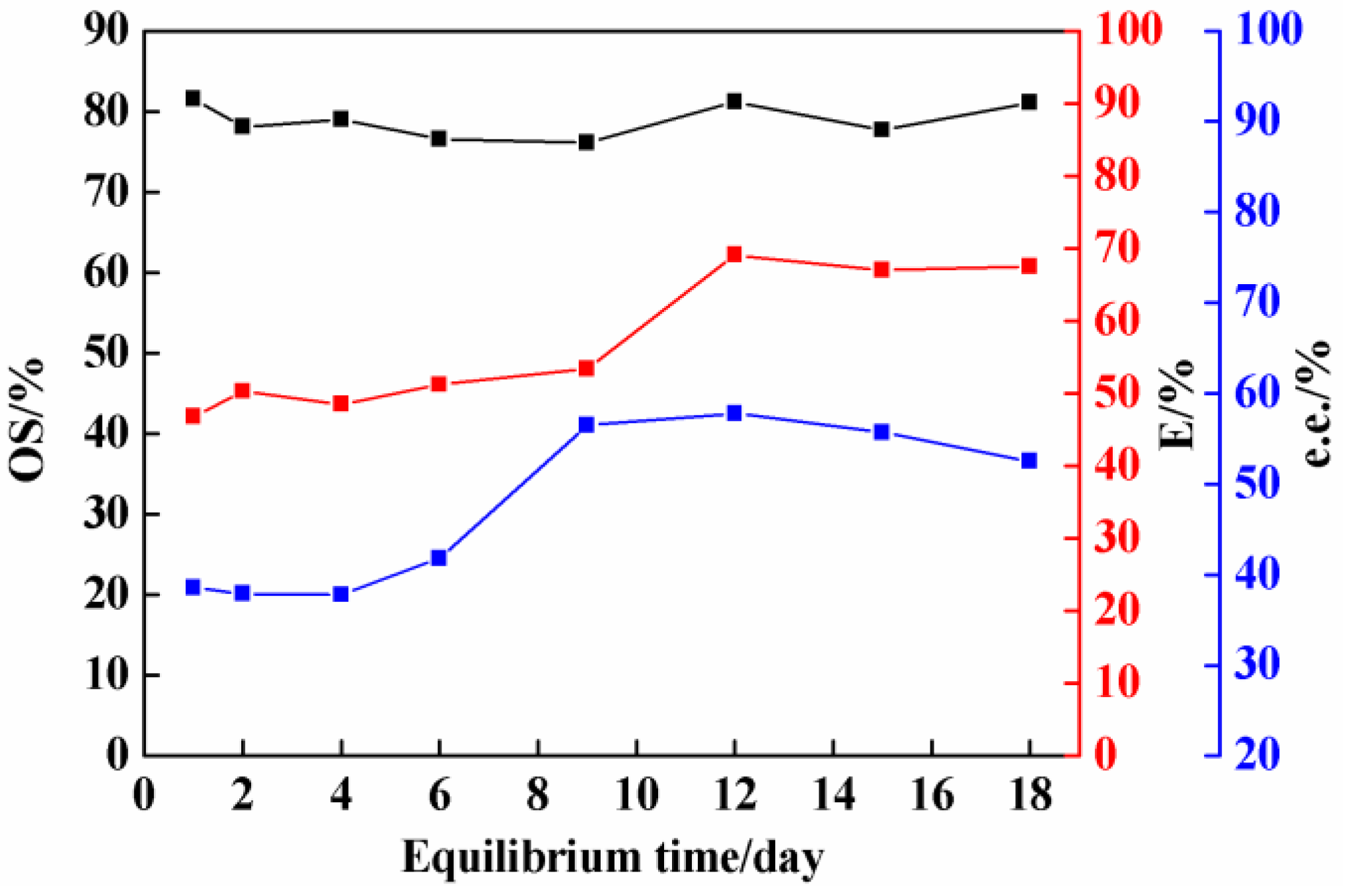
2.1. Effect of Equilibrium Time on Co-Crystal Resolution
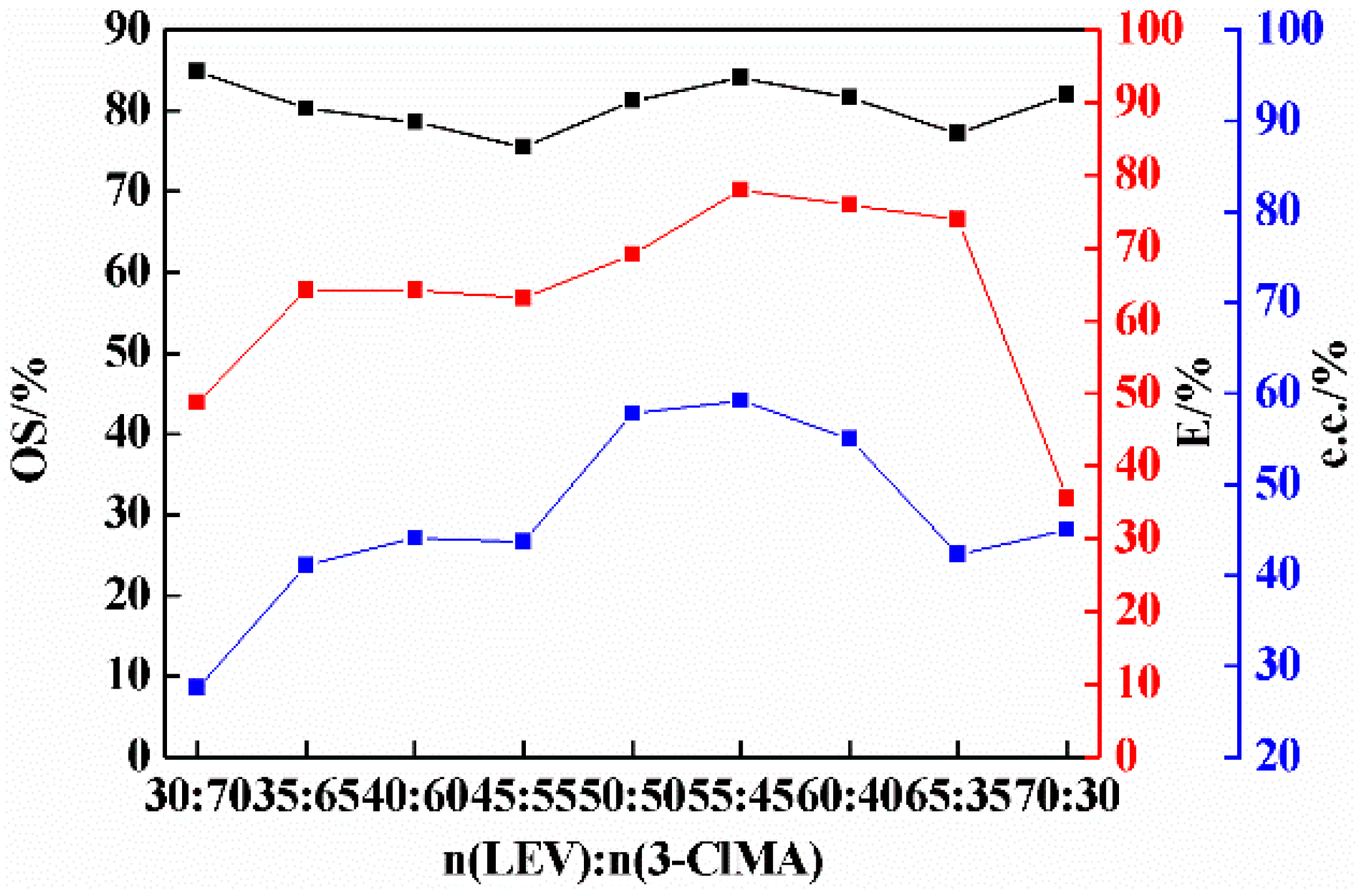
2.2. Effect of Molar Ratio on Co-Crystal Resolution
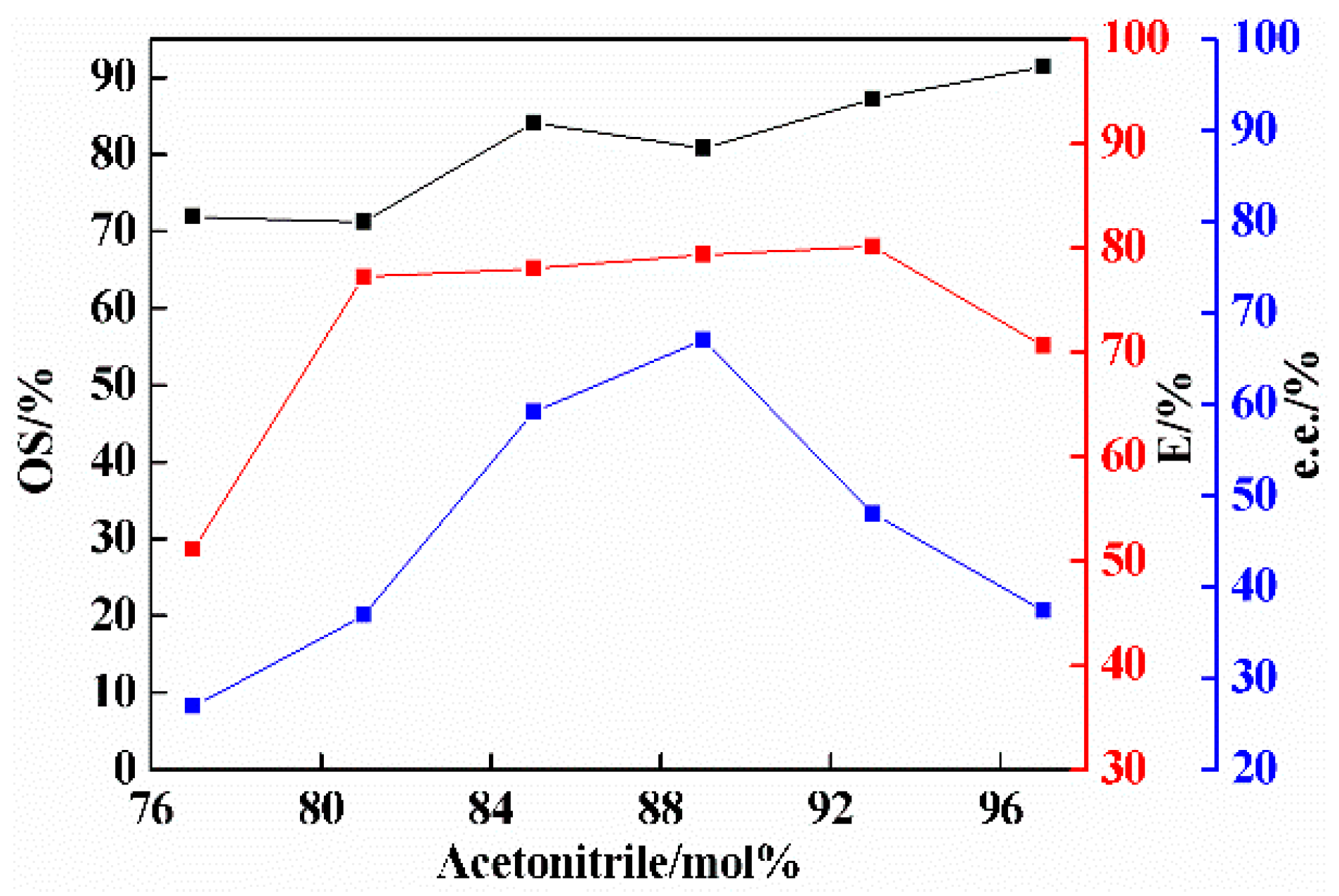
2.3. Effect of Amount of Solvent on Co-Crystal Resolution
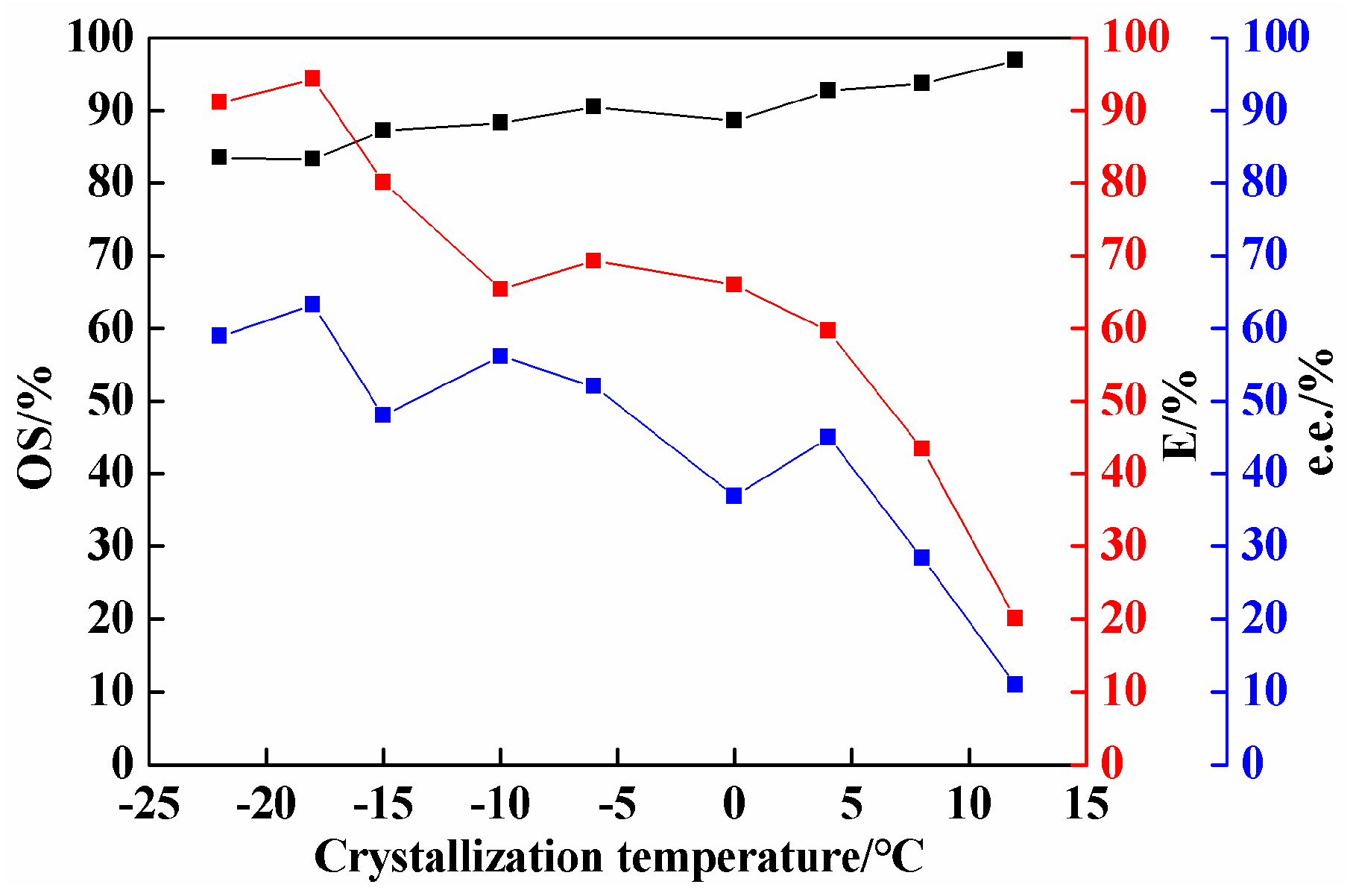
2.4. Effect of Crystallization Temperature on Co-Crystal Resolution
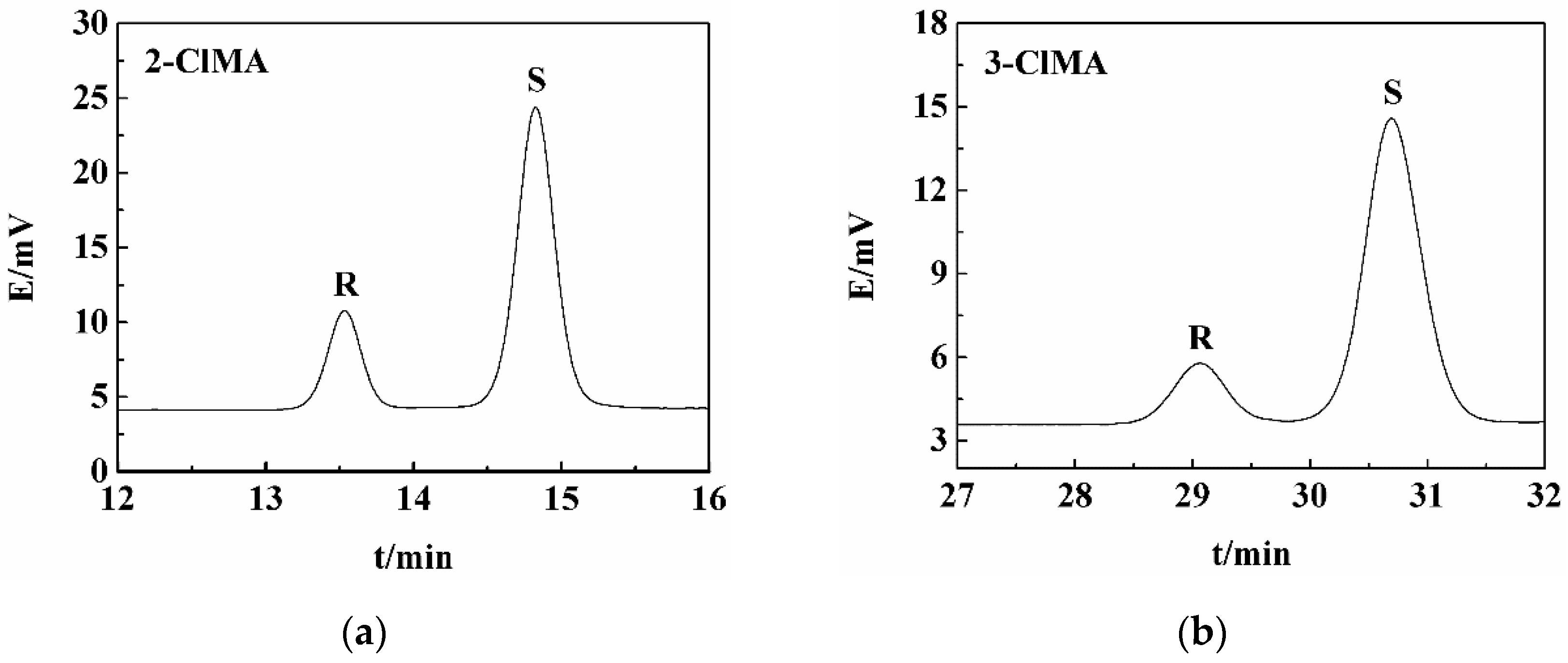
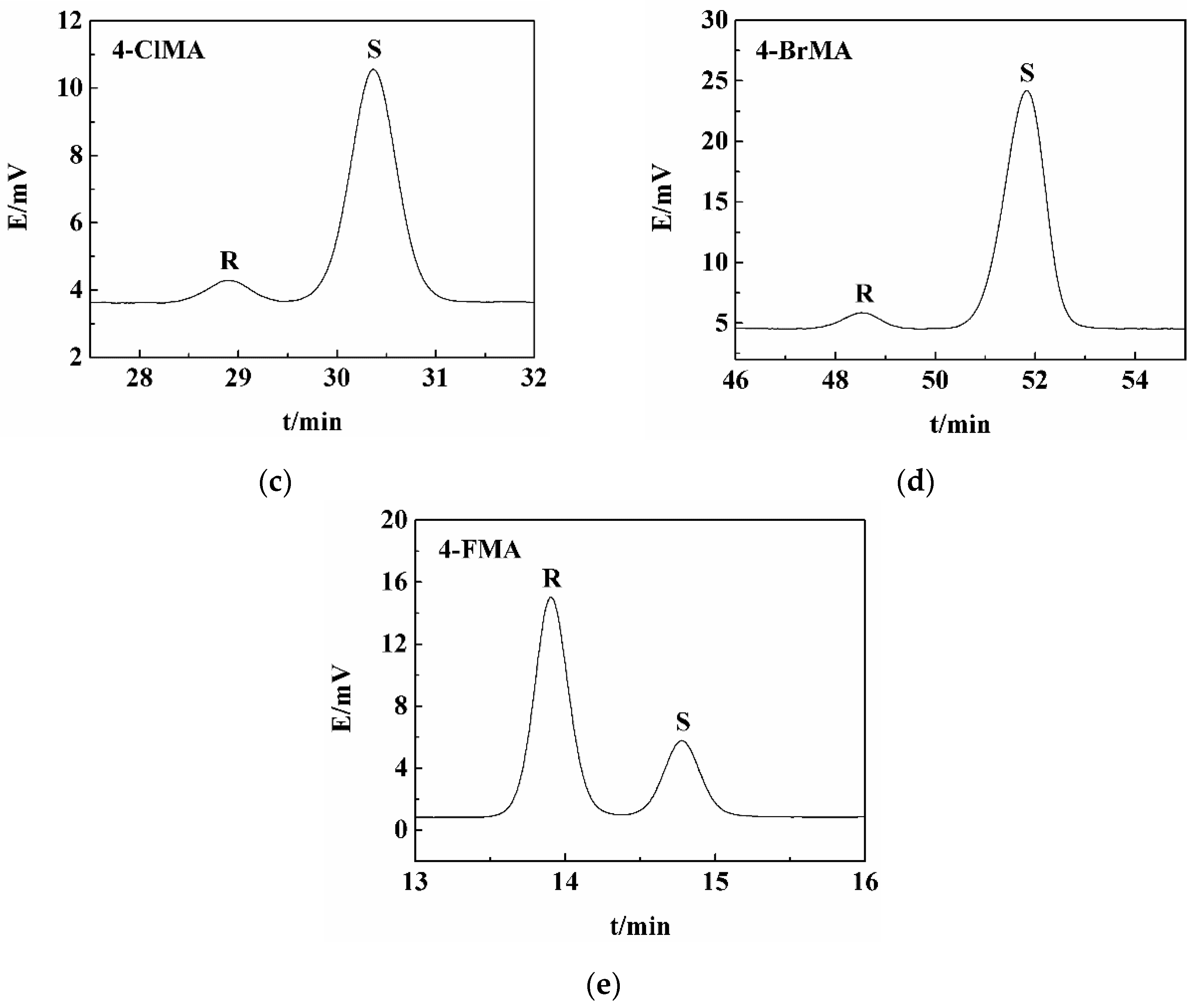
2.5. Resolution of Other Halogenated Mandelic Acids by LEV
3. Materials and Methods
3.1. Materials
3.2. Sample Analysis
3.3. The Procedure of LEV Resolving 3-ClMA
3.4. The Procedure of LEV Resolving 2-ClMA
3.5. The Procedure of LEV Resolving 4-ClMA
3.6. The Procedure of LEV Resolving 4-BrMA
3.7. The Procedure of LEV Resolving 4-FMA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Green, J.B.; Bethel, M.A.; Armstrong, P.W.; Buse, J.B.; Engel, S.S.; Garg, J.; Josse, R.; Kaufman, K.D.; Koglin, J.; Korn, S.; et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2015, 373, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, X. Current situation of chiral agrochemical products. World Pestic. 2019, 41, 3–14. (In Chinese) [Google Scholar]
- Ajikumar, P.K.; Tyo, K.; Carlsen, S.; Mucha, O.; Phon, T.H.; Stephanopoulos, G. Opportunities for biosynthesis of natural product drugs using engineered microorganisms. Mol. Pharm. 2008, 5, 167–190. [Google Scholar] [CrossRef]
- He, Q.; Zhu, J.; Gomaa, H.; Jennings, M.; Rohani, S. Identification and characterization of solid-state nature of 2-chloromandelic acid. J. Pharm. Sci. 2009, 98, 1835–1844. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Pappo, D.; Tsang, K.Y.; Gibe, R.; Chen, D.Y.K. A chiral pool based synthesis of platensimycin. Angew. Chem. Int. Ed. 2008, 47, 944–946. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Lu, J.M.; Wang, X.R.; Shao, L.X. Palladium (II)-N-heterocyclic carbene complexes derived from proline: Synthesis and characterization. Tetrahedron 2010, 66, 7970–7974. [Google Scholar] [CrossRef]
- Knowles, W.S.; Sabacky, M.J.; Vineyard, B.D. Catalytic asymmetric hydrogenation. Ann. N. Y. Acad. Sci. 1973, 214, 119–124. [Google Scholar] [CrossRef]
- Gong, X.M.; Qin, Z.; Li, F.L.; Zeng, B.B.; Zheng, G.W.; Xu, J.H. Development of an engineered ketoreductase with simultaneously improved thermostability and activity for making a bulky atorvastatin precursor. ACS Catal. 2019, 9, 147–153. [Google Scholar] [CrossRef]
- Savile, C.K.; Janey, J.M.; Mundorff, E.C.; Moore, J.C.; Tam, S.; Jarvis, W.R.; Colbeck, J.C.; Krebber, A.; Fleitz, F.J.; Brands, J.; et al. Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture. Science 2010, 329, 305–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaser, H.U. The chiral pool as a source of enantioselective catalysts and auxiliaries. Chem. Rev 1992, 92, 935–952. [Google Scholar] [CrossRef]
- Davoren, J.E.; Martin, S.F. Enantioselective synthesis and structure revision of solandelactone E. J. Am. Chem. Soc. 2007, 129, 510–511. [Google Scholar] [CrossRef] [Green Version]
- Knowles, W.S. Asymmetric hydrogenations (Nobel Lecture). Hüthig Wepf Verl. 2002, 41, 1998–2007. [Google Scholar]
- Wang, Y.N.; Chai, J.L.; You, C.; Zhang, J.; Mi, X.L.; Zhang, L.; Luo, S.Z. π-Coordinating chiral primary amine/palladium synergistic catalysis for asymmetric allylic alkylation. J. Am. Chem. Soc. 2020, 142, 3184–3195. [Google Scholar] [CrossRef] [PubMed]
- Hayama, N.; Kuramoto, R.; Foldes, T.; Nishibayashi, K.; Kobayashi, Y.; Papai, I.; Takemoto, Y. Mechanistic insight into asymmetric hetero-michael addition of α, β-unsaturated carboxylic acids catalyzed by multifunctional thioureas. J. Am. Chem. Soc. 2018, 140, 12216–12225. [Google Scholar] [CrossRef] [PubMed]
- Che, W.; Li, Y.Z.; Liu, J.C.; Zhu, S.F.; Xie, J.H.; Zhou, Q.L. Stereodiverse iterative synthesis of 1,3-polyol arrays through asymmetric catalytic hydrogenation. Formal total synthesis of (-)-cyanolide A. Org. Lett. 2019, 21, 2369–2373. [Google Scholar] [CrossRef]
- Guo, Z.M.; Jin, Y.; Liang, T.; Liu, Y.F.; Xu, Q.; Liang, X.M.; Lei, A.W. Synthesis, chromatographic evaluation and hydrophilic interaction/reversed-phase mixed-mode behavior of a “Click β-cyclodextrin” stationary phase. J. Chromatogr. A 2009, 1216, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.Q.; Zeng, Y.; Hua, Y.T.; Cheng, Y.Q.; Guo, Z.M. Enantioselective liquid-liquid extraction of racemic ibuprofen by L-tartaric acid derivatives. J. Chem. Eng. Data 2014, 59, 2517–2522. [Google Scholar] [CrossRef]
- Porpiglia, N.; Musile, G.; Bortolotti, F.; Palo, E.F.D.; Tagliaro, F. Chiral separation and determination of ketamine and nor-ketamine in hair by capillary electrophoresis. Forensic Sci. Int. 2016, 266, 304–310. [Google Scholar] [CrossRef]
- Kosjek, B.; Tellers, D.M.; Biba, M.; Farr, R.; Moore, J.C. Biocatalytic and chemocatalytic approaches to the highly stereoselective 1, 2-reduction of an α, β-unsaturated ketone. Tetrahedron Asymmetry 2006, 17, 2798–2803. [Google Scholar] [CrossRef]
- Singh, K.; Ingole, P.G.; Bajaj, H.C.; Gupta, H. Preparation, characterization and application of β-cyclodextrin-glutaraldehyde crosslinked membrane for the enantiomeric separation of amino acids. Desalination 2012, 298, 13–21. [Google Scholar] [CrossRef]
- Onozawa, T.; Kogure, N.; Takayama, H.; Kitajima, M. Elucidation of absolute configuration of ophiorrhiside A by comparison of ECD spectra with that of model chiral compound having a 1,2,3,4-tetrahydro-β-carbolin-3-one skeleton. Heterocycles 2021, 102, 35. [Google Scholar]
- Sun, J.Y.; Xing, Y.P.; Song, J.X.; Guo, X.J. Chiral separation of 4 drugs by capillary electrophoresis. J. Shenyang Pharm. Univ. 2015, 32, 199–203. [Google Scholar]
- He, Q.; Peng, Y.F.; Rohani, S. Diastereomeric resolution of p-chloromandelic acid with (R)-phenylethylamine. Chirality 2010, 22, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Bánhegyi, D.F.; Pálovics, E. The Stoichiometry, structure and possible formation of crystalline diastereomeric salts. Symmetry 2021, 13, 667. [Google Scholar] [CrossRef]
- Jeon, T.H.; Kang, H.J.; Svirid, A.; Lyakhov, A.; Kovalenko, V.; Cho, C.G. Chiral resolution of racemic 2-pyrone diels-alder cycloadduct by diastereomeric salt formation. Bull. Korean Chem. Soc. 2019, 40, 910–913. [Google Scholar] [CrossRef]
- Wong, L.W.Y.; Vashchenko, E.V.; Zhao, Y.; Sung, H.H.Y.; Vashchenko, V.V.; Mikhailenko, V.; Krivoshey, A.I.; Williams, I.D. Tandem crystallization strategies for resolution of 3,3,3-trifluorolactic acid [CF3CH(OH)COOH] by chiral benzylamines. Chirality 2019, 31, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Tumanova, N.; Seiler, V.; Tumanov, N.; Robeyns, K.; Filinchuk, Y.; Wouters, J.; Leyssens, T. Structural analysis of D-phenylglycinamide salts uncovers potential pitfalls in chiral resolution via diastereomeric salt formation. Cryst. Growth Des. 2019, 19, 3652–3659. [Google Scholar] [CrossRef]
- Ferretti, R.; Zanitti, L.; Casulli, A.; Cirilli, R. Green high-performance liquid chromatography enantioseparation of lansoprazole using a cellulose-based chiral stationary phase under ethanol/water mode. J. Sep. Sci. 2016, 39, 1418–1424. [Google Scholar] [CrossRef]
- Sonika, B.; Ravi, B. Enantioresolution of (R, S)-baclofen by liquid chromatography: A review. Biomed. Chromatogr. 2017, 31, 3833–3847. [Google Scholar]
- La, Z.; Charton, J.; Etienne, L.; Bourey, J.; Lipka, E. Supercritical fluid chromatography and liquid chromatography for isomeric separation of a multiple chiral centers analyte. J. Chromatogr. A 2021, 1651, 462270. [Google Scholar] [CrossRef]
- Shuang, Y.Z.; Zhang, T.C.; Zhong, H.; Li, L.S. Simultaneous enantiomeric determination of multiple triazole fungicides in fruits and vegetables by chiral liquid chromatography/tandem mass spectrometry on a bridged bis (β-cyclodextrin)-bonded chiral stationary phase. Food Chem. 2020, 345, 128842. [Google Scholar] [CrossRef]
- Guy, L.; Crassous, J.; Andraud, C. CRC Handbook of optical resolutions via diastereomeric salt formation. Chirality 2002, 24, 21. [Google Scholar] [CrossRef]
- Brittain, H.G. Vibrational spectroscopic studies of cocrystals and salts. 4. cocrystal products formed by benzylamine, alpha-methylbenzylamine, and their chloride salts. Cryst. Growth Des. 2011, 11, 2500–2509. [Google Scholar] [CrossRef]
- Schultheiss, N.; Bethune, S.; Henck, J.O. Nutraceutical cocrystals: Utilizing pterostilbene as a cocrystal former. Crystengcomm 2010, 12, 2436–2442. [Google Scholar] [CrossRef]
- Kavanagh, O.N.; Croker, D.M.; Walker, G.M.; Zaworotko, M.J. Pharmaceutical cocrystals: From serendipity to design to application. Drug Discov. Today 2019, 24, 796–804. [Google Scholar] [CrossRef] [Green Version]
- Wouters, J.; Grepioni, F.; Braga, D.; Kaminski, R.M.; Rome, S.; Aerts, L.; Quere, L. Novel pharmaceutical compositions through co-crystallization of racetams and Li+ salts. Crystengcomm 2013, 15, 8898–8902. [Google Scholar] [CrossRef]
- Springuel, G.; Leyssens, T. Innovative chiral resolution using enantiospecific cocrystallization in solution. Cryst. Growth Des. 2012, 12, 3374–3378. [Google Scholar] [CrossRef]
- Sanchez-Guadarrama, O.; Mendoza-Navarro, F.; Cedillo-Cruz, A.; Jung-Cook, H.; Arenas-García, J.; Delgado-Díaz, A.; Herrera-Ruiz, D.; Morales-Rojas, H.; Hopfl, H. Chiral resolution of RS-praziquantel via diastereomeric co-crystal pair formation with L-malic acid. Cryst. Growth Des. 2015, 16, 307–314. [Google Scholar] [CrossRef]
- Vishweshwar, P.; Mcmahon, J.A.; Bis, J.A.; Zaworotko, M.J. Pharmaceutical co-crystal. J. Pharm. Sci. 2006, 95, 499–516. [Google Scholar] [CrossRef]
- Wang, J.; Ao, Q.; Peng, Y.F.; Zhao, H.L.; Tong, T.Z. Preparation and characterization of cocrystal of levetiracetam and halogenated mandelic acids. J. Chem. Eng. Chin. Univ. 2021. under review (In Chinese) [Google Scholar]
- He, Q.; Rohani, S.; Zhu, J.; Gomaa, H. Resolution of sertraline with (R)-mandelic acid: Chiral discrimination mechanism study. Chirality 2012, 24, 119–128. [Google Scholar] [CrossRef]
- He, Q.; Gomaa, H.; Rohani, S.; Zhu, J.; Jennings, M. Chiral discrimination in diastereomeric salts of chlorine-substituted mandelic acid and phenylethylamine. Chirality 2010, 22, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Cheney, M.L.; Choudhury, A.R.; Desiraju, G.R.; Dikundwar, A.G.; Dubey, R.; Duggirala, N.; et al. Polymorphs, salts, and cocrystals: What’s in a name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Stahly, G.P. Diversity in single- and multiple-component crystals. The search for and prevalence of polymorphs and cocrystals. Cryst. Growth Des. 2007, 7, 1007–1026. [Google Scholar] [CrossRef] [Green Version]
- Childs, S.L.; Stahly, G.P.; Park, A. The salt-cocrystal continuum: The influence of crystal structure on ionization state. Mol. Pharm. 2007, 4, 323–338. [Google Scholar] [CrossRef] [Green Version]
- Springuel, G.; Collard, L.; Leyssens, T. Ternary and quaternary phase diagrams: Key tools for chiral resolution through solution cocrystallization. Crystengcomm 2013, 15, 7951–7958. [Google Scholar] [CrossRef]

| Solid Phase (HPLC Analysis) | OS(OR)/% | E/% | e.e./% in Liquid Phase | |
|---|---|---|---|---|
| 2-Chloromandelic acid (2-ClMA) | LEV-(S)-2-ClMA | 78 | 82 | 76 |
| 3-Chloromandelic acid (3-ClMA) | LEV-(S)-3-ClMA | 83 | 94 | 63 |
| 4-Chloromandelic acid (4-ClMA) | LEV-(S)-4-ClMA | 88 | 18 | 23 |
| 4-Bromomandelic acid (4-BrMA) | LEV-(S)-4-BrMA | 71 | 28 | 21 |
| 4-Fluoromandelic acid (4-FMA) | LEV-(R)-4-FMA | 90 | 54 | 29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Peng, Y. Resolution of Halogenated Mandelic Acids through Enantiospecific Co-Crystallization with Levetiracetam. Molecules 2021, 26, 5536. https://doi.org/10.3390/molecules26185536
Wang J, Peng Y. Resolution of Halogenated Mandelic Acids through Enantiospecific Co-Crystallization with Levetiracetam. Molecules. 2021; 26(18):5536. https://doi.org/10.3390/molecules26185536
Chicago/Turabian StyleWang, Jie, and Yangfeng Peng. 2021. "Resolution of Halogenated Mandelic Acids through Enantiospecific Co-Crystallization with Levetiracetam" Molecules 26, no. 18: 5536. https://doi.org/10.3390/molecules26185536
APA StyleWang, J., & Peng, Y. (2021). Resolution of Halogenated Mandelic Acids through Enantiospecific Co-Crystallization with Levetiracetam. Molecules, 26(18), 5536. https://doi.org/10.3390/molecules26185536