Pyrethroid and Etofenprox Resistance in Anopheles gambiae and Anopheles coluzzii from Vegetable Farms in Yaoundé, Cameroon: Dynamics, Intensity and Molecular Basis
Abstract
:1. Background
2. Results
2.1. Species Composition
2.2. Trends of Insecticide Resistance in Anopheles gambiae s.l. Populations
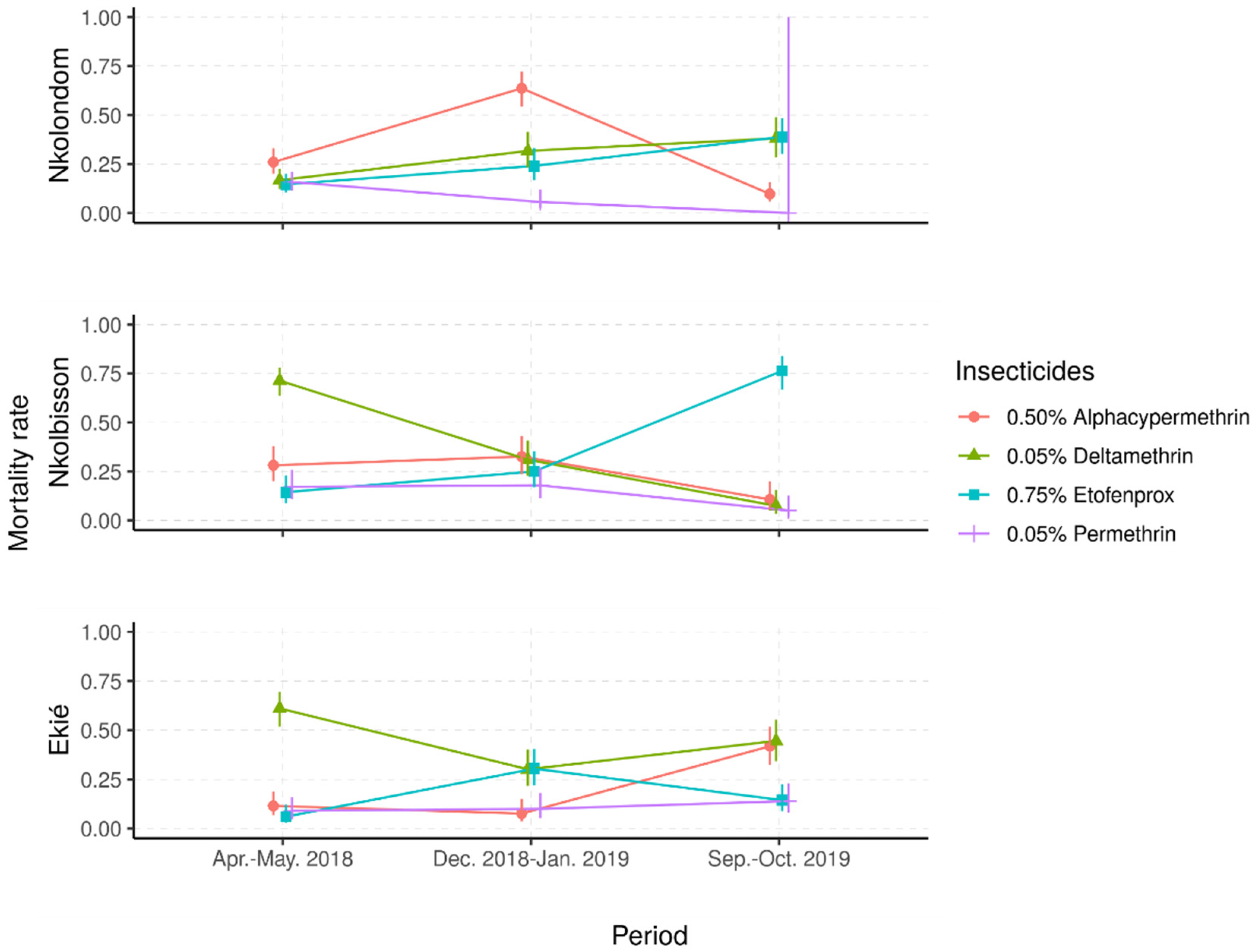
2.2.1. Resistance Frequencies
2.2.2. Status and Dynamics of Resistance Intensity
2.2.3. Relationship between the Frequency and the Intensity of Insecticide Resistance
2.3. Molecular Markers of Insecticide Resistance
3. Discussion
4. Material and Methods
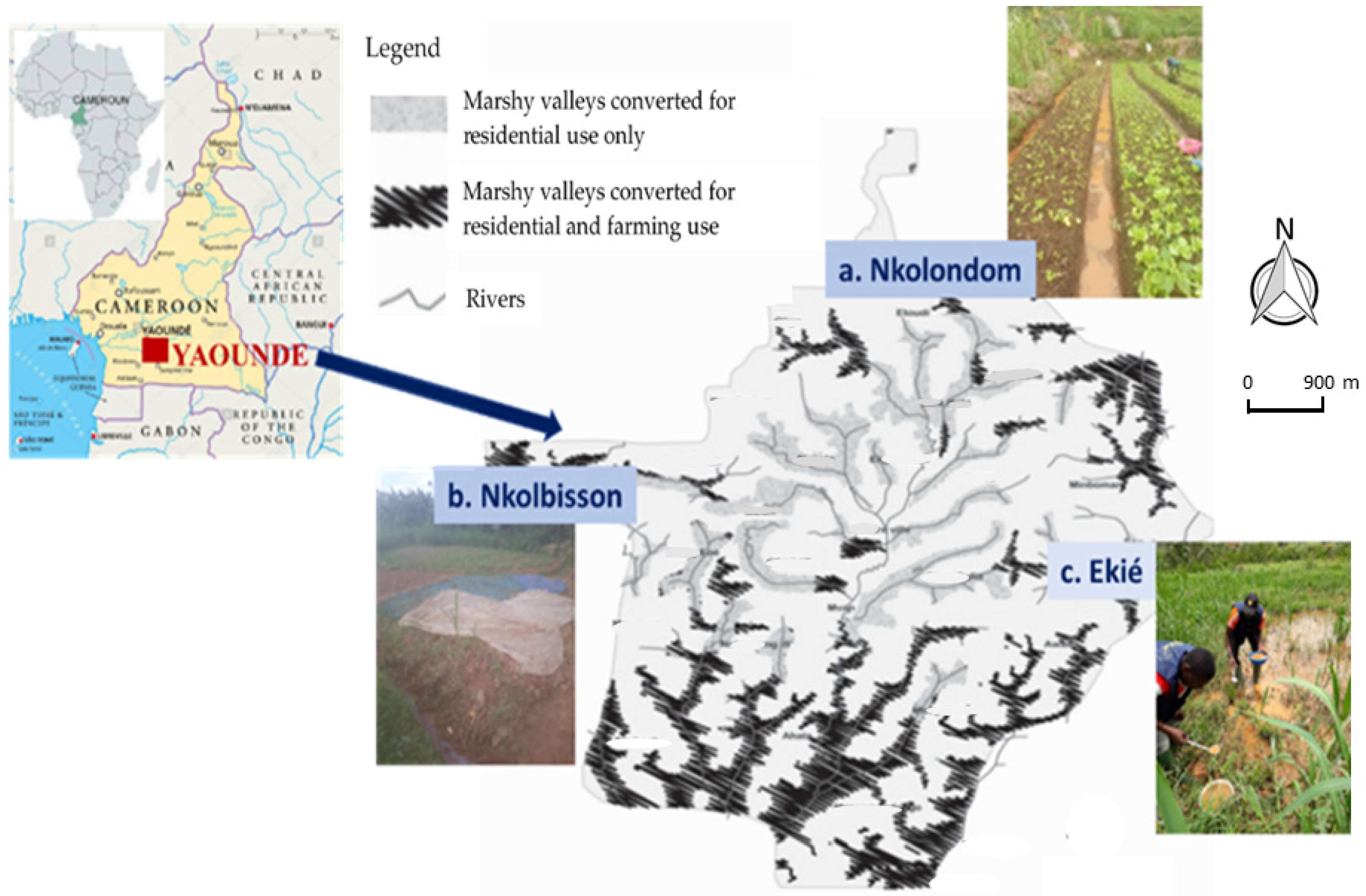
4.1. Study Sites
4.2. Mosquito Collection, Rearing and Processing
4.3. Insecticide Susceptibility Assays
4.4. Resistance Intensity Assays
4.5. Molecular Assays
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| ACTs | Artemisinin combination therapies |
| CSRS | Centre Suisse de Recherches Scientifique en Côte d’Ivoire |
| DC | Diagnostic concentrations |
| HL1 | High level 1 |
| HL2 | High level 2 |
| HL3 | High level 3 |
| IRS | Indoor residual spraying |
| Kdr | Knockdown resistance |
| LLINs | Long-lasting insecticidal nets |
| MR | Mortality rates |
| RR | Resistant homozygote |
| RS | Heterozygotes |
| RT-qPCR | Reverse-transcription quantitative polymerase chain reaction |
| SNP | Single-nucleotide polymorphism |
| SS | Susceptible homozygote |
| VCRU | Vector Control Research Unit |
| WHO | World Health Organization |
References
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.; Moyes, C.; Henry, A.; Eckhoff, P.A.; et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nat. Cell Biol. 2015, 526, 207–211. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. World Malaria Report 2020: 20 Years of Global Progress and Challenges. Available online: https://www.who.int/publications/i/item/9789240015791 (accessed on 29 July 2021).
- World Health Organization. Pre-Qualification Team: Pre-Qualified Vector Control Products. 2020. Available online: https://www.who.int/pq-vector-control/prequalified-lists/VCP_PQ-List_26August2020.pdf?ua=1 (accessed on 12 August 2021).
- Diabate, A.; Baldet, T.; Chandre, F.; Akoobeto, M.; Guiguemde, T.R.; Darriet, F.; Brengues, C.; Guillet, P.; Hemingway, J.; Small, G.J.; et al. The role of agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae s.l. in Burkina Faso. Am. J. Trop. Med. Hyg. 2002, 67, 617–622. [Google Scholar] [CrossRef] [Green Version]
- Yadouleton, A.; Martin, T.; Padonou, G.; Chandre, F.; Asidi, A.; Djogbenou, L.; Dabire, R.; Aikpon, R.; Boko, M.; Glitho, I.; et al. Cotton pest management practices and the selection of pyrethroid resistance in Anopheles gambiae populations in northern Benin. Parasites Vectors 2011, 4, 60. [Google Scholar] [CrossRef] [Green Version]
- Talom, A.D.; Tchuinkam, T.; Zeukeng, F.; Demano, M.L.; Kuate, A.F.; Lehman, G.L.; Djouaka, R. Susceptibility of Anopheles gambiae s.l. to pyrethroid insecticides in vegetable farms in the city of Yaoundé, Cameroon. J. Entomol. Zool Stud. 2020, 8, 1851–1858. [Google Scholar]
- Mouhamadou, C.S.; de Souza, S.S.; Fodjo, B.K.; Zoh, M.G.; Bli, N.K.; Koudou, B.G. Evidence of insecticide resistance selection in wild Anopheles coluzzii mosquitoes due to agricultural pesticide use. Infect. Dis. Poverty 2019, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Brogdon, W.G.; McAllister, J.C. Insecticide resistance and vector control. Emerg. Infect. Dis. 1998, 4, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Corbel, V.; N’Guessan, R. Distribution, Mechanisms, Impact and Management of Insecticide Resistance in Malaria Vectors: A Pragmatic Review. In Anopheles Mosquitoes―New Insights into Malaria Vectors; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef] [Green Version]
- Antonio-Nkondjio, C.; Tene Fossog, B.; Ndo, C.; Menze Djantio, B.; Zebaze Togouet, S.; Awono-Ambene, P.; Costantini, C.; Wondji, C.S.; Ranson, H. Anopheles gambiae distribution and insecticide resistance in the cities of Douala and Yaoundé (Cameroon): Influence of urban agriculture and pollution. Malar. J. 2011, 10, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministère de la Santé. Programme National de Lutte Contre le Paludisme au Cameroun. Plan Stratégique National de Lutte Contre le Paludisme, 2020–2024. Available online: file:///C:/Users/josye/AppData/Local/Temp/PlanstrategiquenationalPaludisme2020–2024.pdf (accessed on 29 July 2021).
- Antonio-Nkondjio, C.; Ndo, C.; Njiokou, F.; Bigoga, J.; Awono-Ambene, P.; Etang, J.; Ekobo, A.S.; Wondji, C.S. Review of malaria situation in Cameroon: Technical viewpoint on challenges and prospects for disease elimination. Parasites Vectors 2019, 12, 501. [Google Scholar] [CrossRef] [Green Version]
- Etang, J.; Manga, L.; Chandre, F.; Guillet, P.; Fondjo, E.; Mimpfoundi, R.; Toto, J.C.; Fontenille, D. Insecticide susceptibility status of Anopheles gambiae s.l. (Diptera: Culicidae) in the Republic of Cameroon. J. Med. Entomol. 2003, 40, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Etang, J.; Chouaibou, M.; Ndjemai, H.; Chandre, F.; Morlais, I.; Fondjo, E.; Simard, F.; Brengues, C.; Nwane, P. First report of knockdown mutations in the malaria Anopheles gambiae from Cameroon. Am. J. Trop. Med. Hyg. 2006, 74, 795–797. [Google Scholar] [CrossRef]
- Antonio-Nkondjio, C.; Sonhafouo-Chiana, N.; Ngadjeu, C.S.; Doumbe-Belisse, P.; Talipouo, A.; Djamouko-Djonkam, L.; Kopya, E.; Bamou, R.; Awono-Ambene, P.; Wondji, C.S. Review of the evolution of insecticide resistance in main malaria vectors in Cameroon from 1990 to 2017. Parasites Vectors 2017, 10, 472. [Google Scholar] [CrossRef]
- Nwane, P.; Etang, J.; Chouaïbou, M.; Toto, J.C.; Mimpfoundi, R.; Simard, F. Kdr-based insecticide resistance in Anopheles gambiae s.s. populations in Cameroon: Spread of the L1014F and L1014S mutations. BMC Res. Notes 2011, 4, 463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandeng, S.E.; Awono-Ambene, H.P.; Bigoga, J.D.; Ekoko, W.E.; Binyang, J.; Piameu, M.; Mbakop, L.R.; Fesuh, B.N.; Mvondo, N.; Tabue, N.; et al. Spatial and temporal development of deltamethrin resistance in malaria vectors of the Anopheles gambiae complex from North Cameroon. PLoS ONE 2019, 14, e0212024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bamou, R.; Sonhafouo-Chiana, N.; Mavridis, K.; Tchuinkam, T.; Wondji, C.S.; Vontas, J.; Antonio-Nkondjio, C. Status of Insecticide Resistance and Its Mechanisms in Anopheles gambiae and Anopheles coluzzii Populations from Forest Settings in South Cameroon. Genes 2019, 10, 741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etang, J.; Manga, L.; Toto, J.C.; Guillet, P.; Fondjo, E.; Chandre, F. Spectrum of metabolic-based resistance to DDT and pyrethroids in Anopheles gambiae s.l. populations from Cameroon. J. Vector Ecol. 2007, 32, 123–133. [Google Scholar] [CrossRef]
- Elanga-Ndille, E.; Nouage, L.; Ndo, C.; Binyang, A.; Assatse, T.; Nguiffo-Nguete, D.; Djonabaye, D.; Irwing, H.; Tene-Fossog, B.; Wondji, C.S. The G119S Acetylcholinesterase (Ace-1) Target Site Mutation Confers Carbamate Resistance in the Major Malaria Vector Anopheles gambiae from Cameroon: A Challenge for the Coming IRS Implementation. Genes 2019, 10, 790. [Google Scholar] [CrossRef] [Green Version]
- Etang, J.; Pennetier, C.; Piameu, M.; Bouraima, A.; Chandre, F.; Awono-Ambene, P.; Coosemans, M.; Corbel, V. When intensity of deltamethrin resistance in Anopheles gambiae s.l. leads to loss of Long-Lasting Insecticidal Nets bio-efficacy: A case study in north Cameroon. Parasites Vectors 2016, 9, 132. [Google Scholar] [CrossRef] [Green Version]
- Nguendo Yongsi, H.B.; Ntetu Lutumba, A.; Bryant, R.C.; Ojuku, T.; Thora, H.M. Uncontrolled Draining of Rainwater and Health. Consequences in Yaoundé―Cameroon. Acta Univ. 2009, 19, 20–30. [Google Scholar]
- Nwane, P.; Etang, J.; Chouaïbou, M.; Toto, J.C.; Koffi, A.; Mimpfoundi, R.; Simard, F. Multiple insecticide resistance mechanisms in Anopheles gambiae s.l. populations from Cameroon, Central Africa. Parasites Vectors 2013, 6, 41. [Google Scholar] [CrossRef] [Green Version]
- Fouet, C.; Ashu, A.F.; Ambadiang, M.M.; Tchapga, W.; Wondji, C.S.; Kamdem, C. Resistance of Anopheles gambiae to the new insecticide clothianidin associated with unrestricted use of agricultural neonicotinoids in Yaoundé, Cameroon. BioRxiv 2020. [Google Scholar] [CrossRef]
- Bamou, R.; Mbakop, L.R.; Kopya, E.; Ndo, C.; Awono-Ambene, P.; Tchuinkam, T.; Rono, M.K.; Mwangangi, J.; Antonio-Nkondjio, C. Changes in malaria vector bionomics and transmission patterns in the equatorial forest region of Cameroon between 2000 and 2017. Parasites Vectors 2018, 11, 464. [Google Scholar] [CrossRef]
- Djamouko-Djonkam, L.; Mounchili-Ndam, S.; Kala-Chouakeu, N.; Nana-Ndjangwo, S.; Kopya, E.; Sonhafouo-Chiana, N.; Talipouo, A.; Ngadjeu, C.S.; Doumbe-Belisse, P.; Bamou, R.; et al. Spatial distribution of Anopheles gambiae sensu lato larvae in the urban environment of Yaoundé, Cameroon. Infect. Dis. Poverty 2019, 8, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diabaté, A.; Dabire, R.K.; Millogo, N.; Lehmann, T. Evaluating the Effect of Postmating Isolation Between Molecular Forms of Anopheles gambiae (Diptera: Culicidae). J. Med. Entomol. 2007, 44, 60–64. [Google Scholar] [CrossRef]
- Gimonneau, G.; Bouyer, J.; Morand, S.; Besansky, N.J.; Diabate, A.; Simard, F. A behavioral mechanism underlying ecological divergence in the malaria mosquito Anopheles gambiae. Behav. Ecol. 2010, 21, 1087–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nwane, P.; Etang, J.; Chouaibou, M.; Toto, J.C.; Kerah-Hinzoumbé, C.; Mimpfoundi, R.; Awono-Ambene, H.P.; Simard, F. Trends in DDT and pyrethroid resistance in Anopheles gambiae s.s. populations from urban and agro-industrial settings in southern Cameroon. BMC Infect. Dis. 2009, 9, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. World Health Organization Recommended Insecticides for Indoor Residual Spraying against Malaria Vectors. 2015. Available online: https://www.who.int/neglected_diseases/vector_ecology/vector-control/Insecticides (accessed on 28 July 2021).
- Chanda, E.; Alister Kandyata, A.; Javan Chanda, J.; Phiri, F.N.; Muzia, L.; Kamuliwo, M. The Efficacy of Vectron 20 WP, Etofenprox, for Indoor Residual Spraying in Areas of High Vector Resistance to Pyrethroids and Organochlorines in Zambia. ISRN Prev. Med. 2013, 2013, 371934. [Google Scholar] [CrossRef] [Green Version]
- Mfopou, Y.C.M.; Traore, M.; Kenmogne, P.P.N.; Aboubakar, A.; Manguele, G.S.F.; Maboune, S.A.T.; Ndam, J.R.N.; Gnankambary, Z.; Nacro, H.B. Structure of Vegetables Farming and Farmer’s Perception of Soil and Water Degradation in Two Peri urban Areas in Yaounde Cameroon. Open J. Soil Sci. 2017, 7, 333–346. [Google Scholar] [CrossRef] [Green Version]
- Talipouo, A.; Ngadjeu, C.S.; Patricia Doumbe-Belisse, P.; Landre Djamouko-Djonkam, L.; Sonhafouo-Chiana, N.; Edmond Kopya, E.; Bamou, R.; Awono-Ambene, P.; Woromogo, S.; Kekeunou, S.; et al. Malaria prevention in the city of Yaoundé: Knowledge and practices of urban dwellers. Malar. J. 2019, 18, 167. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Test Procedures: For Insecticide Resistance Monitoring in Malaria Vector Mosquitoes; World Health Organization: Geneva, Switzerland, 2018; 56p, Available online: https://apps.who.int/iris/bitstream/handle/10665/250677/9789241511575-eng.pdf (accessed on 8 September 2021).
- Ahadji-Dabla, K.M.; Chabi, J.; Apetogbo, G.Y.; Hadi, M.P.; Ketoh, G.K. Pyrethroid Resistance Intensity of Anopheles gambiae sensu lato (Diptera: Culicidae) from Phase II Hut Trial Station in Kolokope, Eastern Plateau Togo: A Potential Site to Assess the Next Generation of Long-Lasting Insecticidal Nets. BioRxiv 2020. [Google Scholar] [CrossRef]
- Sovi, A.; Keita, C.; Sinaba, Y.; Dicko, A.; Traore, I.; Cisse, M.B.; Koita, O.; Dengela, D.O.; Flatley, C.; Bankineza, E.; et al. Anopheles gambiae (s.l.) exhibit high intensity pyrethroid resistance throughout Southern and Central Mali (2016–2018): PBO or next generation LLINs may provide greater control. Parasites Vectors 2020, 13, 1–16. [Google Scholar] [CrossRef]
- Awolola, T.S.; Adeogun, A.; Olakiigbe, A.K.; Oyeniyi, T.; Olukosi, Y.A.; Okoh, H.; Arowolo, T.; Akila, J.; Oduola, A.; Amajoh, C.N. Pyrethroids resistance intensity and resistance mechanisms in Anopheles gambiae from malaria vector surveillance sites in Nigeria. PLoS ONE 2018, 13, e0205230. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global Report on Insecticide Resistance in Malaria Vectors: 2010–2016; World Health Organization: Geneva, Switzerland, 2018; Available online: https://creativecommons.org/licenses/by-nc-sa/3.0/igo (accessed on 23 June 2021).
- Elanga-Ndille, E.; Binyang, A.; Ndo, C.; Assatse, T.; Nouage, L.; Tchouakui, M.; Tene-Fossog, B.; Kekeunou, S.; Wondji, C.S. Entomological indicators of malaria transmission and insecticide resistance profile of Anopheles gambiae at the early phase of irrigated rice farming in the forest area of central Cameroon. Wellcome Open Res. 2020, 5, 190. [Google Scholar] [CrossRef]
- Stevenson, B.J.; Bibby, J.; Pignatelli, P.; Muangnoicharoen, S.; O’Neill, P.M.; Lian, L.Y.; Muller, P.; Nikou, D.; Steven, A.; Hemingway, J.; et al. Cytochrome P450 6M2 from the malaria vector Anopheles gambiae metabolizes pyrethroids: Sequential metabolism of deltamethrin revealed. Insect Biochem. Mol. Biol. 2011, 41, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Vontas, J.; Katsavou, E.; Mavridis, K. Cytochrome P450-based metabolic insecticide resistance in Anopheles and Aedes mosquito vectors: Muddying the waters. Pestic. Biochem. Physiol. 2020, 170, 104666. [Google Scholar] [CrossRef] [PubMed]
- Ranson, H.; Jensen, B.; Wang, X.; Prapanthadara, L.; Hemingway, J.; Collins, F.H. Genetic mapping of two loci affecting DDT resistance in the malaria vector Anopheles gambiae. Insect Mol. Biol. 2000, 9, 499–507. [Google Scholar] [CrossRef]
- Yaounde, Climate Yaounde, Temperatures Yaounde, Weather Averages. Available online: http://www.yaounde.climatemps.com/ (accessed on 23 June 2021).
- Nguegang, P.A. L’agriculture Urbaine et périurbaine à Yaoundé: Analyse Multifonctionnelle d’une activité Montante en économie de survie. Ph.D. Thesis, Sciences Agronomiques et Ingénierie Biologique, Université libre de Bruxelles, Brussels, Belgium, 2008. Available online: file:///C:/Users/josye/AppData/Local/Temp/TheseNguegangProsper.pdf (accessed on 23 June 2021).
- Doumbe-Belisse, P.; Ngadjeu, C.S.; Sonhafouo-Chiana, N.; Talipouo, A.; Djamouko-Djonkam, L.; Kopya, E.; Bamou, R.; Toto, J.C.; Mounchili, S.; Tabue, R.; et al. High malaria transmission sustained by Anopheles gambiae s.l. occurring both indoors and outdoors in the city of Yaoundé, Cameroon. Wellcome Open Res. 2018, 3, 164. [Google Scholar] [CrossRef] [PubMed]
- Van der Kolk, M.; Etti Tebo, A.; Nimpaye, H.; Ngo Ndombol, D.; Sauerwein, R.; Eling, W. Transmission of Plasmodium falciparum in urban Yaoundé Cameroon is seasonal and age-dependent. Trans. R. Soc. Trop. Med. Hyg. 2003, 97, 375–379. [Google Scholar] [CrossRef]
- Lumsden, W.H.R.; Service, M.W. Mosquito Ecology. Field Sampling Methods. J. Appl. Ecol. 1977, 14, 651. [Google Scholar] [CrossRef]
- Gillies, M.T.; Coetzee, M. A supplement to the Anophelinae of Africa South of the Sahara. Publ. Afr. Inst. Med. Res. 1987, 55, 141–143. [Google Scholar]
- Gillies, M.T.; De Meillon, B. The Anophelinae of Africa South of the Sahara (Ethiopian Zoogeographical Region.); Publications of the South African Institute for Medical Research: Johannesburg, South Africa, 1968; pp. 1–343. [Google Scholar]
- Mavridis, K.; Wipf, N.; Müller, P.; Traoré, M.M.; Muller, G.; Vontas, J. Detection and Monitoring of Insecticide Resistance Mutations in Anopheles gambiae: Individual vs. Pooled Specimens. Genes 2018, 9, 479. [Google Scholar] [CrossRef] [Green Version]
- Bass, C.; Williamson, M.S.; Field, L.M. Development of a multiplex real-time PCR assay for identification of members of the Anopheles gambiae species complex. Acta Trop. 2008, 107, 50–53. [Google Scholar] [CrossRef]
- Christopher, M.J.; Liyanapathirana, M.; Agossa, F.R.; Weetman, D.; Ranson, H.; Donnelly, M.J.; Wilding, C.S. Footprints of positive selection associated with a mutation (N1575Y) in the voltage-gated sodium channel of Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2012, 109, 6614–6619. [Google Scholar]
- Bass, C.; Nikou, D.; Donnelly, M.J.; Williamson, M.S.; Ranson, H.; Ball, A.; Vontas, J.; Field, L.M. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: A comparison of two new high-throughput assays with existing methods. Malar. J. 2007, 6, 111. [Google Scholar] [CrossRef] [Green Version]
- Bass, C.; Nikou, D.; Vontas, J.; Williamson, M.S.; Field, L.M. Development of high-throughput real-time PCR assays for the identification of insensitive acetylcholinesterase (ace-1R) in Anopheles gambiae. Pestic. Biochem. Physiol. 2010, 96, 80–85. [Google Scholar] [CrossRef]
- Mavridis, K.; Wipf, N.; Medves, S.; Erquiaga, I.; Müller, P.; Vontas, J. Rapid multiplex gene expression assays for monitoring metabolic resistance in the major malaria vector Anopheles gambiae. Parasites Vectors 2019, 12, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vontas, J.; Mavridis, K. Vector population monitoring tools for insecticide resistance management: Myth or fact? Pestic. Biochem. Physiol. 2019, 161, 54–60. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative Expression Software Tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H.; Navarro, D.; Pedersen, T.L. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]

| Colony | n | Species | (%) | Genotype | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| kdr L995F/S | kdr N1570Y | Ace-1 G280S | |||||||||||||||||
| n | SS | RwS | RwRw | RwRe | Freq | n | SS | SR | RR | Freq | n | SS | SR | RR | Freq | ||||
| Nkolondom | 50 | Anopheles gambiae s.s. | 100 | 50 | 1 | 26 | 22 | 1 | 0.72 | 32 | 22 | 10 | 0 | 0.16 | 50 | 37 | 13 | 0 | 0.13 |
| Nkolbisson | 50 | Anopheles gambiae s.s. | 4 | 2 | 0 | 2 | 0 | 0 | 0.50 | − | − | − | − | − | 2 | 2 | 0 | 0 | 0 |
| Anopheles coluzzii | 96 | 48 | 0 | 31 | 17 | 0 | 0.65 | 30 | 30 | 0 | 0 | 0 | 48 | 48 | 0 | 0 | 0 | ||
| Ekié | 50 | Anopheles coluzzii | 100 | 50 | 0 | 40 | 10 | 0 | 0.6 | 30 | 30 | 0 | 0 | 0 | 50 | 50 | 0 | 0 | 0 |
| Kisumu | 50 | Anopheles gambiae s.s. | 100 | 50 | 50 | 0 | 0 | 0 | 0.0 | 30 | 30 | 0 | 0 | 0 | 50 | 50 | 0 | 0 | 0 |
| Insecticide | Colony | Insecticide Concentration (%) | Resistance Variable | Mortality Rate (%) per Survey Period | ||
|---|---|---|---|---|---|---|
| Apr.–May 2018 | Dec. 2018–Jan. 2019 | Sept.–Oct. 2019 | ||||
| Deltamethrin | Kisumu | 0.05dc | F | 100 | 99 | 100 |
| Nkolondom | 0.05dc | F | 16.0 | 31.7 | 45.2 | |
| 0.510× | I | |||||
| Nkolbisson | 0.05dc | F | 56.7 | 31.0 | 4.9 | |
| 0.510× | I | |||||
| Ekié | 0.05dc | F | 61.1 | 30.1 | 44.6 | |
| 0.510× | I | |||||
| Permethrin | Kisumu | 0.75dc | F | 98.7 | 100 | 99 |
| Nkolondom | 0.75dc | F | 8.7 | 5.6 | 0.0 | |
| 7.510× | I | |||||
| Nkolbisson | 0.75dc | F | 16.3 | 17.9 | 5.1 | |
| 7.510× | I | |||||
| Ekié | 0.75dc | F | 7.2 | 10.0 | 13.9 | |
| 7.510× | I | |||||
| Alpha-cypermethrin | Kisumu | 0.05dc | F | 100 | 100 | 100 |
| Nkolondom | 0.05dc | F | 17.8 | 63.7 | 3.2 | |
| 0.510× | I | |||||
| Nkolbisson | 0.05dc | F | 17.8 | 32.6 | 10.7 | |
| 0.510× | I | |||||
| Ekié | 0.05dc | F | 11.5 | 7.5 | 41.8 | |
| 0.510× | I | |||||
| Etofenprox | Kisumu | 0.5dc | F | 100 | 100 | 99 |
| Nkolondom | 0.5dc | F | 9.4 | 24.1 | 38.9 | |
| 5.010× | I | |||||
| Nkolbisson | 0.5dc | F | 14.3 | 25.0 | 76.3 | |
| 5.010× | I | |||||
| Ekié | 0.5dc | F | 6.0 | 30.4 | 14.4 | |
| 5.010× | I | |||||
| Resistance intensity | Moderate | High Level I | High Level II | High Level III | ||
| Mortality rate to 10× | >98% | 76–98% | 50–75% | <50% | ||
| Gene | Mechanism | Nkolondom | Nkolbisson |
|---|---|---|---|
| Cyp6p3 | Metabolic Resistance | 0.4 (0.2–1.1) | 1.5 (0.8–3.8) |
| Cyp6m2 | 17.1 (5.9–47.5) * | 18.9 (5.7–56.6) * | |
| Cyp9k1 | 6.0 (2.94–13.5) * | 6.8 (3.6–15.3) * | |
| Cyp6p4 | 2.9 (1.8–4.4) * | 6.2 (4.7–9.3) * | |
| Cyp6z1 | 2.6 (1.8–3.6) * | 3.4 (2.3–4.5) * | |
| Gste2 | 12.8 (7.9–23.2) * | 52.0 (29.2–93.8) * | |
| Cyp6p1 | 0.8 (0.4–1.6) | 2.1 (0.9–5.1) | |
| Cyp4g16 | Cuticular Resistance | 2.4 (2.1–2.7) * | 2.5 (1.9–3.2) * |
| Insecticides | Collection Period | 1× DC | 5× DC | 10× DC | N | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ekié | Nkolondom | Nkolbisson | Ekié | Nkolondom | Nkolbisson | Ekié | Nkolondom | Nkolbisson | |||
| Delta | Apr.–May 2018 | 113 | 106 | 106 | 124 | 91 | 84 | MD | MD | MD | 624 |
| Dec. 2018–Jan. 2019 | 93 | 108 | 100 | 102 | 101 | 91 | MD | MD | MD | 595 | |
| Sept.–Oct. 2019 | 83 | 90 | 81 | 80 | 84 | 142 | 101 | 88 | 81 | 830 | |
| Perm | Apr.–May 2018 | 111 | 103 | 104 | 112 | 81 | 85 | 118 | 83 | 114 | 911 |
| Dec. 2018–Jan. 2019 | 90 | 107 | 95 | 103 | 104 | 86 | 101 | 104 | 97 | 887 | |
| Sept.–Oct. 2019 | 86 | 99 | 87 | 90 | 92 | 89 | MD | 91 | 88 | 722 | |
| Alpha | Apr.–May 2018 | 113 | 101 | 83 | 115 | 106 | 116 | 110 | 84 | 89 | 917 |
| Dec. 2018–Jan. 2019 | 93 | 110 | 89 | 102 | 103 | 99 | 101 | 85 | 97 | 879 | |
| Sept.–Oct. 2019 | 98 | 85 | 80 | 94 | 87 | 85 | MD | 94 | 94 | 717 | |
| Etofenprox | Apr.–May 2018 | 116 | 96 | 98 | 116 | 83 | 123 | 122 | 80 | 87 | 921 |
| Dec. 2018–Jan. 2019 | 92 | 104 | 84 | 99 | 93 | 89 | MD | 89 | 90 | 740 | |
| Sept.–Oct. 2019 | 104 | 91 | 93 | 81 | 108 | 94 | 93 | 80 | 95 | 839 | |
| N | 1192 | 1200 | 1100 | 1218 | 1133 | 1183 | 746 | 878 | 932 | 9582 | |
| Assayed Marker | Oligonucleotides Name | Sequence | Assay Name |
|---|---|---|---|
| Species Identification | S200-6.1 F | TCGCCTTAGACCTTGCGTTA | Molecular Forms |
| S200-6.1 R | CGCTTCAAGAATTCGAGATAC | Molecular Forms | |
| AgM-P | ACCGCGCCGCCATACGTAGGA | An. coluzzii | |
| AgS-P | ATGTCTAATAGTCTCAATAGT | An. gambiae | |
| Kdr L995 mutation | Kdr-F | CATTTTTCTTGGCCACTGTAGTGAT | kdr |
| Kdr-R | CGATCTTGGTCCATGTTAATTTGCA | kdr | |
| kdrWT-P | CTTACGACTAAATTTC | Wild type-kdr | |
| kdrRw-P | ACGACAAAATTTC | West-kdr | |
| kdrRe-P | ACGACTGAATTTC | East-kdr | |
| Kdr N1570Y mutation | 1575-F | TGGATCGCTAGAAATGTTCATGACA | Kdr+ |
| 1575-R | CGAGGAATTGCCTTTAGAGGTTTCT | Kdr+ | |
| N1575-P | ATTTTTTTCATTGCATTATAGTAC | Wild type-kdr+ | |
| Y1575-P | TTTTTCATTGCATAATAGTAC | Mutant-kdr+ | |
| Ace1 G280S mutation | ACE1-F | GGCCGTCATGCTGTGGAT | iAChe |
| ACE1-R | GCGGTGCCGGAGTAGA | iAChe | |
| Ace 1G_WT-P | TTCGGCGGCGGCT | Wild type-iAChe | |
| Ace 1G_MT-P | TTCGGCGGCAGCT | Mutant-iAChe | |
| Normalizer | Rps7F | CCACCATCGAACACAAAGTTGA | (A)-(D) [RG] |
| Rps7-R | TGCTGCAAACTTCGGCTATTC | (A)-(D) [RG] | |
| Rps7-P | FAM-CCGTGACGTTACGTTCGAATTCCCA-BHQ1 | (A)-(D) [RG] | |
| Detoxification Enzyme(metabolic resistance) | Cyp6p3-F | ACAATGTGATTGACGAAACCCT | (A) |
| Cyp6p3-R | GGATCACATGCTTTGTGCCG | (A) | |
| Cyp6p3-P | HEX-ACCCGCGTACCGTCTGTGGACT-BHQ1 | (A) | |
| Cyp6m2-F | CTGGCGTTGAATCCAGAGGT | (A) | |
| Cyp6m2-R | GATACTTGCGCAGTGATTCATTAAG | (A) | |
| Cyp6m2-P | ATTO647N-AGAGAAATCCTGCAAAAGCACAACGGAGA-BHQ3 | (A) | |
| Cyp9k1-F | CCGACACGTGGTGATGGATAC | (B) | |
| Cyp9k1-R | CGTCGTCGGTCCAGTCAAC | (B) | |
| Cyp9k1-P | HEX-CAATCTTCTGATGCAGGCCCGCAA-BHQ1 | (B) | |
| Cyp6p4-F | CTGGACAACGTTATCAATGAAACC | (B) | |
| Cyp6p4-R | GCACGGTGTAATCACGCATC | (B) | |
| Cyp6p4-P | ATTO647N-CCGATCGAGTCACTTTCGCGCG-BHQ3 | (B) | |
| Cyp6z1-F | CCCGCAACTGTATCGGTCTG | (C) | |
| Cyp6z1-R | TTCGGTGCCAGTGTGATTGA | (C) | |
| Cyp6z1-P | HEX-TGATGCTGTCCCGATTTAACTTTTCGGC-BHQ1 | (C) | |
| Gste2-F | CCGGAATTTGTGAAGCTAAACC | (C) | |
| Gste2-R | GCTTGACGGGGTCTTTCGG | (C) | |
| Gste2-P | ATTO647N-CGGTACGATCATCACCGAGAGCCAC-BHQ3 | (C) | |
| Cyp6p1-F | ACAGGTGGTGAACGAAACCC | (D) | |
| Cyp6p1-R | GGTGTAATCCTGTCCCGCAA | (D) | |
| Cyp6p1-P | HEX-CCGCTCGAAACGACGCTGCG-BHQ1 | (D) | |
| Cuticular hydrocarbon synthesis (cuticular resistance) | Cyp4g16-F | GTCCAAGAAGTTGCGTCGGAC | (D) |
| Cyp4g16-R | TCTTCGATTTGCGTTGACGTG | (D) | |
| Cyp4g16-P | ATTO647N-CTGCAGGCCGACATCATTTTGAAGC-BHQ3 | (D) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piameu, M.; Nwane, P.; Toussile, W.; Mavridis, K.; Wipf, N.C.; Kouadio, P.F.; Mbakop, L.R.; Mandeng, S.; Ekoko, W.E.; Toto, J.C.; et al. Pyrethroid and Etofenprox Resistance in Anopheles gambiae and Anopheles coluzzii from Vegetable Farms in Yaoundé, Cameroon: Dynamics, Intensity and Molecular Basis. Molecules 2021, 26, 5543. https://doi.org/10.3390/molecules26185543
Piameu M, Nwane P, Toussile W, Mavridis K, Wipf NC, Kouadio PF, Mbakop LR, Mandeng S, Ekoko WE, Toto JC, et al. Pyrethroid and Etofenprox Resistance in Anopheles gambiae and Anopheles coluzzii from Vegetable Farms in Yaoundé, Cameroon: Dynamics, Intensity and Molecular Basis. Molecules. 2021; 26(18):5543. https://doi.org/10.3390/molecules26185543
Chicago/Turabian StylePiameu, Michael, Philippe Nwane, Wilson Toussile, Konstantinos Mavridis, Nadja Christina Wipf, Paraudie France Kouadio, Lili Ranaise Mbakop, Stanislas Mandeng, Wolfgang Eyisap Ekoko, Jean Claude Toto, and et al. 2021. "Pyrethroid and Etofenprox Resistance in Anopheles gambiae and Anopheles coluzzii from Vegetable Farms in Yaoundé, Cameroon: Dynamics, Intensity and Molecular Basis" Molecules 26, no. 18: 5543. https://doi.org/10.3390/molecules26185543
APA StylePiameu, M., Nwane, P., Toussile, W., Mavridis, K., Wipf, N. C., Kouadio, P. F., Mbakop, L. R., Mandeng, S., Ekoko, W. E., Toto, J. C., Ngaffo, K. L., Ngo Etounde, P. K., Ngantchou, A. T., Chouaibou, M., Müller, P., Awono-Ambene, P., Vontas, J., & Etang, J. (2021). Pyrethroid and Etofenprox Resistance in Anopheles gambiae and Anopheles coluzzii from Vegetable Farms in Yaoundé, Cameroon: Dynamics, Intensity and Molecular Basis. Molecules, 26(18), 5543. https://doi.org/10.3390/molecules26185543







