Purification and Characterization of Antibacterial Activity against Phytopathogenic Bacteria in Culture Fluids from Ganoderma lucidum
Abstract
:1. Introduction
2. Results
2.1. Determination of Bioactive Culture Fluid Stability
2.2. Solubility Determination of Bioactive Compounds
2.3. Proteinase K Does Not Affect Antibacterial Activity
2.4. Size Determination of the Bioactive Compounds
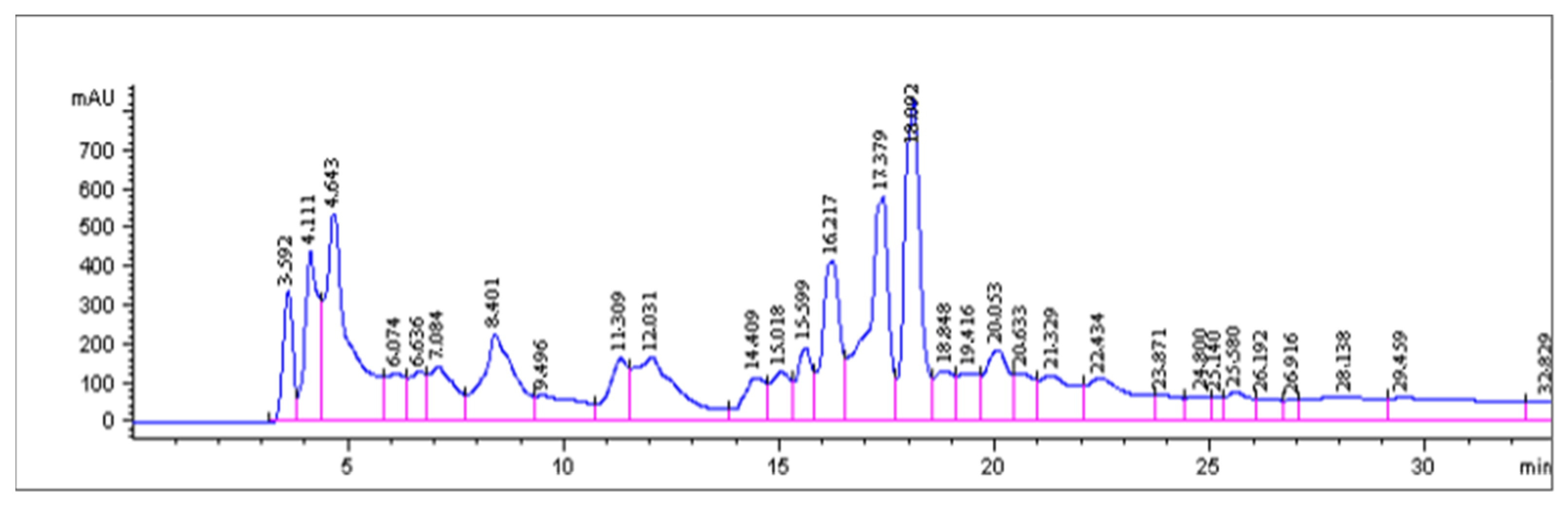
2.5. HPLC Analysis
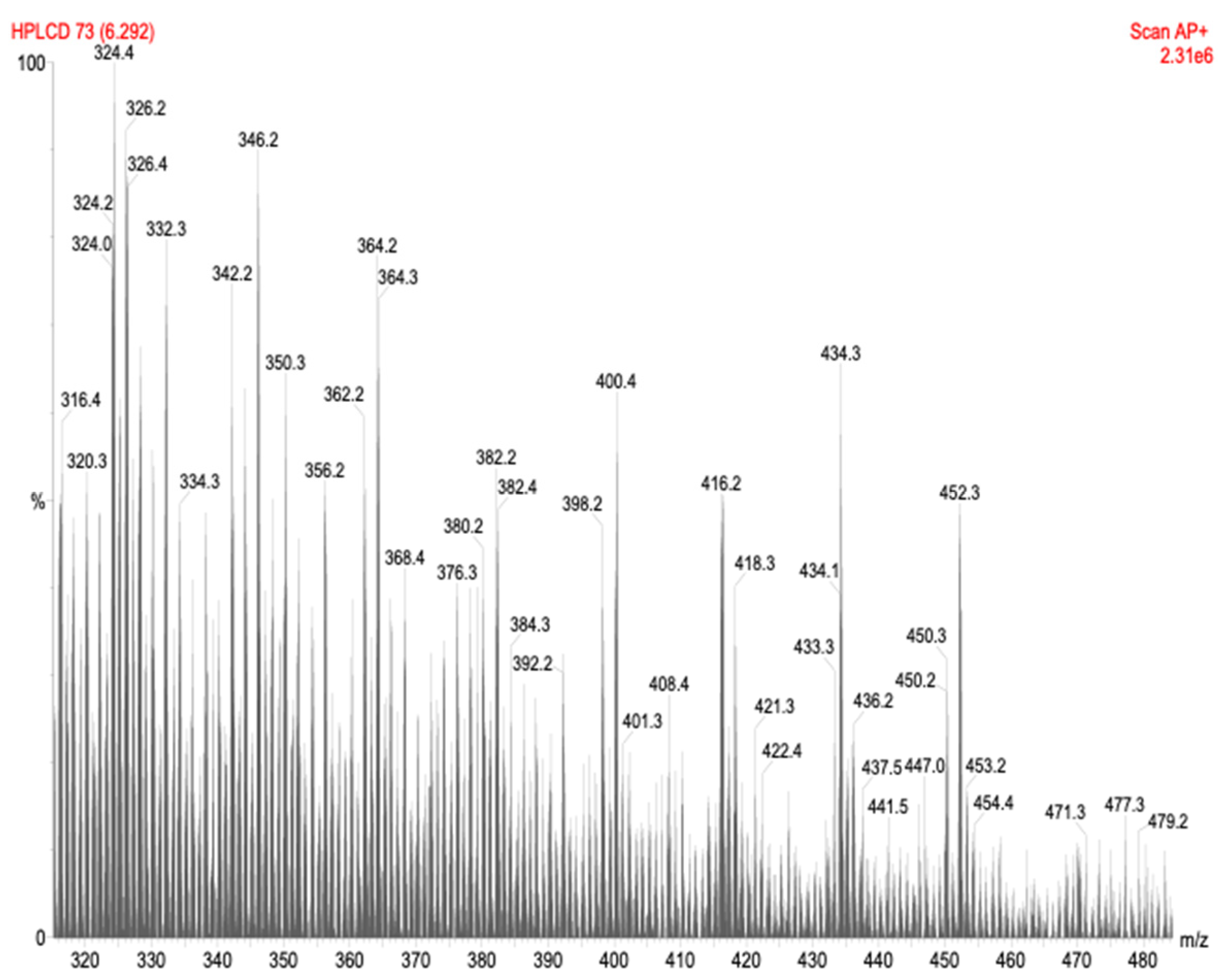
HPLC-APCI-MS Analysis
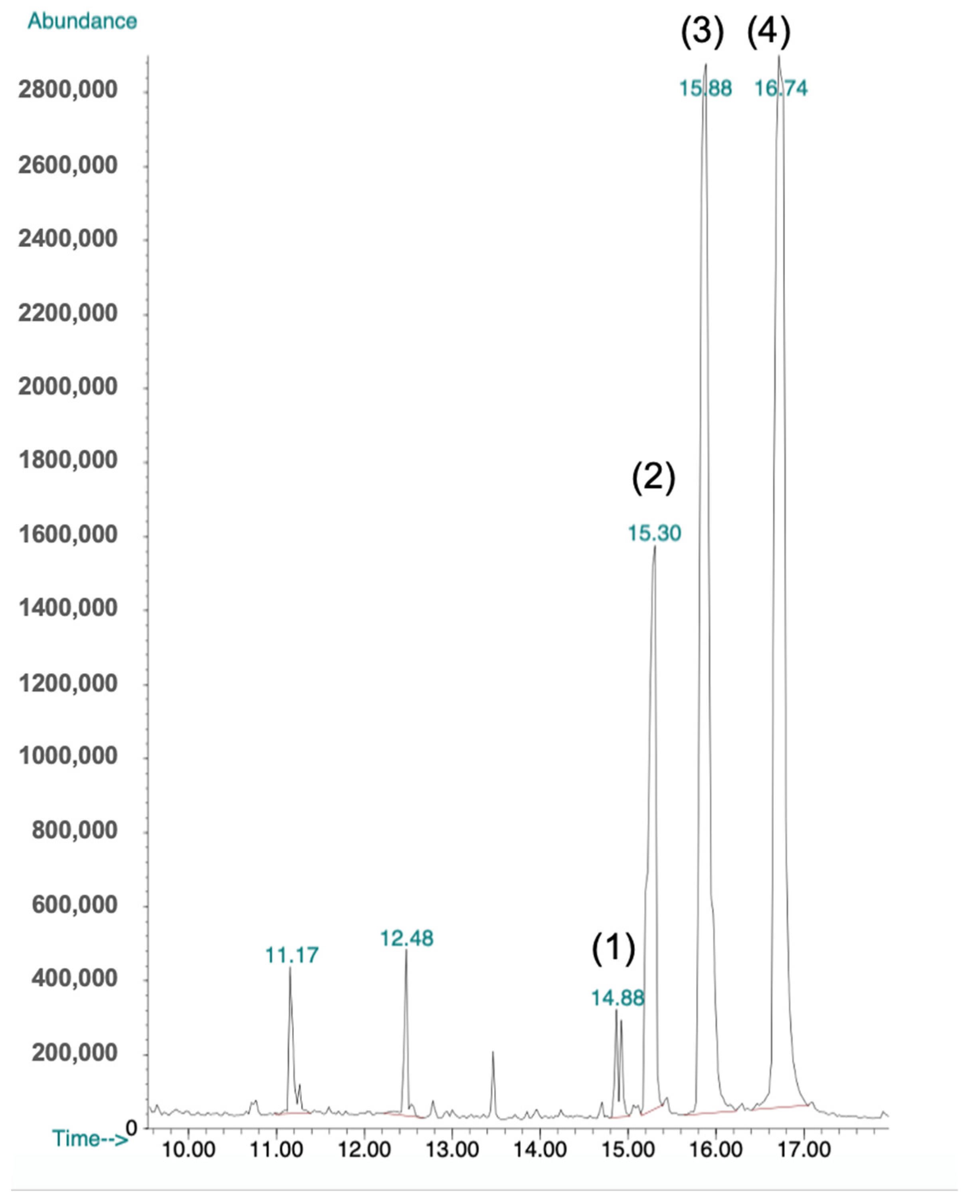
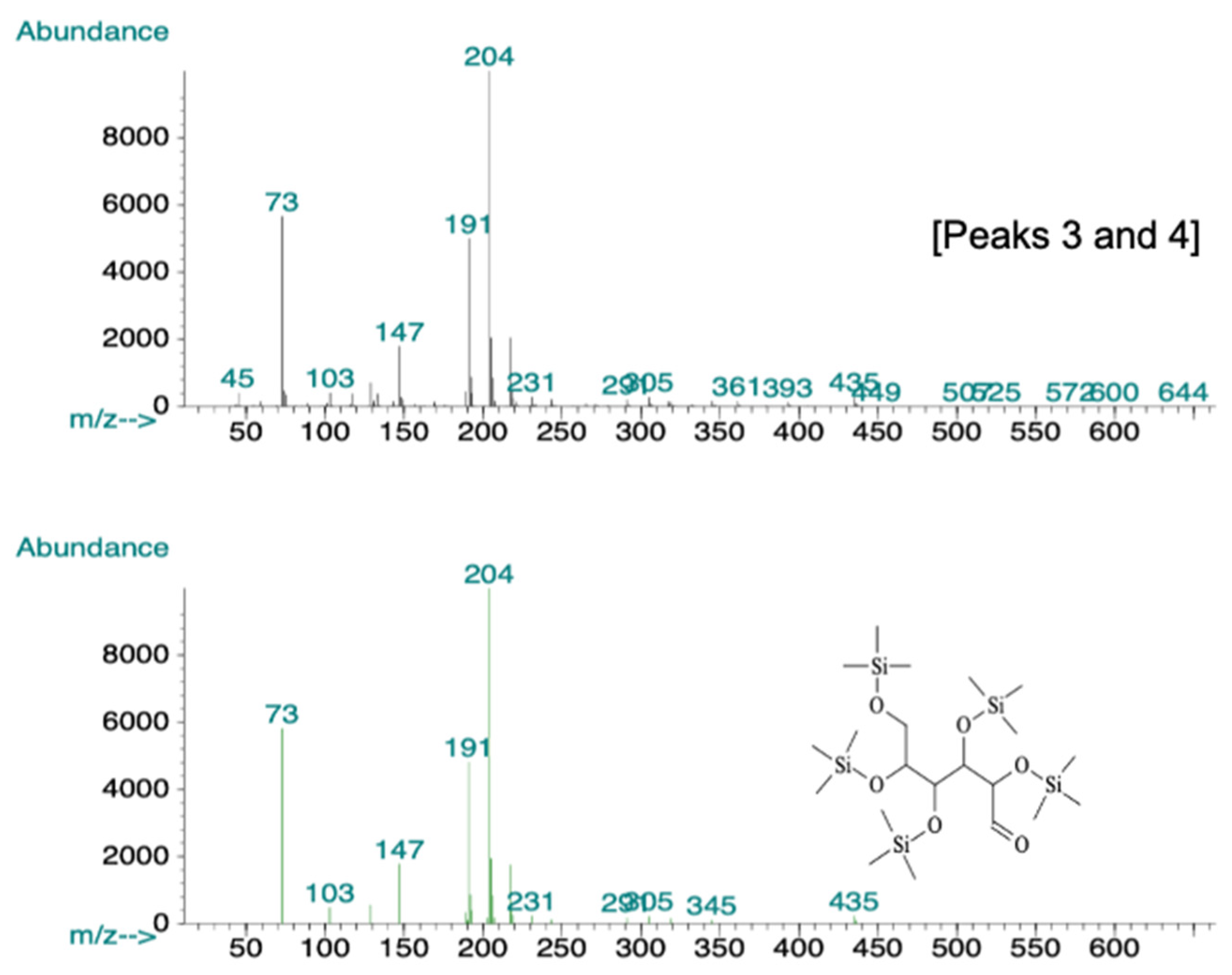
2.6. GC-MS Analysis
3. Discussion
4. Materials and Methods
4.1. Microbial Strains and Culture Conditions
4.2. Production of Bioactive Culture Fluids
4.3. Antibacterial Activity
- (1)
- The agar dilution method (poisoned agar) [25,26] was used to evaluate the antibacterial activity in two assays: culture fluid stability and Proteinase K sensitivity. The method consisted of incorporating in a 1:1 ratio the treatment (treated culture fluids) into a molten twofold concentrated NA medium and poured into 90 × 15 mm petri plates. The inoculation (15 μL) of the bacterial test at a concentration of 107 colony forming units (CFU)/mL was spotted onto the agar plate surface. Plates were incubated at 28 °C for 24 h. A mix of potato dextrose broth and twofold concentrated NA medium in a 1:1 ratio was poured into petri dishes to confirm the growth of the bacteria (control treatment). Plates were incubated at 28 °C for 24 h. Results were recorded as (+) = growth inhibition, (±) = partial growth inhibition, and (−) = no growth inhibition. Each experiment was repeated three times.
- (2)
- The disk diffusion method [25,27] was used to evaluate antibacterial activity using the following assays: solubility of the bioactive compounds (testing different solvents), dialisis tubing, and bioactive fractions in the gel exclusion chromatography and HPLC. The method consisted of loading onto the paper disks (9 mm diameter) separately four times with 100 μL of the culture fluid samples and drying them between each application under aseptic conditions. Loaded disks were then evenly distributed on the bacterial lawn of NA plates. Bacterial lawns were made by spreading 100 μL of bacterial suspensions at 107 colony forming units (CFU)/mL. Plates were sealed with parafilm and incubated at 28 °C. After 24 h, inhibition zone diameters were recorded. Each experiment was repeated three times.
4.4. Determination of Bioactive Culture Fluid Stability
4.5. Solubility of Antibacterial Compounds
4.6. Proteinase-K Sensitivity
4.7. Size Determination
4.7.1. Dialysis Tubing
4.7.2. Gel Exclusion Chromatography
4.8. Reverse Phase HPLC Separation of Methanol Soluble Bioactive Fraction
HPLC-APCI-MS Analysis of the Bioactive Fraction
4.9. GC-MS Analysis of the Antibacterial Fraction
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bao, X.-F.; Wang, X.-S.; Dong, Q.; Fang, J.-N.; Li, X.-Y. Structural features of immunologically active polysaccharides from Ganoderma lucidum. Phytochemistry 2002, 59, 175–181. [Google Scholar] [CrossRef]
- Su, C.; Sun, C.; Juan, S.; Hu, C.; Ke, W.; Sheu, M. Fungal mycelia as the source of chitin and polysaccharides and their applications as skin substitutes. Biomaterials 1997, 18, 1169–1174. [Google Scholar] [CrossRef]
- Battle, J.; Ha, T.; Li, C.; Della Beffa, V.; Rice, P.; Kalbfleisch, J.; Browder, W.; Williams, D. Ligand binding to the (1-->3) beta-D-glucan receptor stimulates NFkappaB activation, but not apoptosis in U937 cells. Biochem. Biophysic. Res. Commun. 1998, 249, 499–504. [Google Scholar] [CrossRef]
- Diamond, M.; Garcia-Aguilar, J.; Bickford, J.; Corbi, A.; Springer, T. The I domain is a major recognition site on the leukocyte integrin Mac- 1 (CD11b/CD18) for four distinct adhesion ligands. J. Cell Biol. 1993, 120, 1031–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müeller, A.; Raptis, J.; Rice, P.; Kalbfleisch, J.; Stout, R.; Ensley, H.; Browder, W.; Williams, D. The influence of glucan polymer structure and solution conformation on binding to (1-->3)-beta-D-glucan receptors in a human monocyte-like cell line. Glycobiology 2000, 10, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.; Rice, P.; Ensley, H.; Coogan, P.; Kalbfleish, J.; Kelley, J.; Love, E.; Portera, C.; Ha, T.; Browder, I.; et al. Receptor binding and internalization of a water-soluble (1-->3)-beta-D- glucan biologic response modifier in two monocyte/macrophage cell lines. J. Immunol. 1996, 156, 3418–3425. [Google Scholar] [PubMed]
- Thornton, B.; Vetvicka, V.; Pitman, M.; Goldman, R.; Ross, G. Analysis of the sugar specificity and molecular location of the beta- glucan-binding lectin site of complement receptor type 3 (CD11b/CD18). J. Immunol. 1996, 156, 1235–1246. [Google Scholar] [PubMed]
- Xia, Y.; Vetvicka, V.; Yan, J.; Hanikyrova, M.; Mayadas, T.; Ross, G. The beta-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. J. Immunol. 1999, 162, 2281–2290. [Google Scholar] [PubMed]
- Wasser, S.; Weis, A. Therapeutic effects of medicinal properties of substances occurring in higher basidiomycete mushrooms: A current perspective. Crit. Rev. Immunol. 1999, 19, 65–96. [Google Scholar]
- Tanaka, S.; Ko, K.; Kino, K.; Tsuchiya, K.; Yamashita, A.; Murasugi, A.; Sakuma, S.; Tsunoo, H. Complete amino acid sequence of an immunomodulatory protein, Ling-Zhi-8 (LZ-8). An immunomodulator from a fungus, Ganoderma lucidum, having similarity to immunoglobulin variable regions. J. Biol. Chem. 1989, 264, 16372–16377. [Google Scholar] [CrossRef]
- Wang, S.; Hsu, M.; Hsu, H.; Tzeng, C.; Lee, S.; Shiao, M.; Ho, C. The anti-tumor effect of Ganoderma lucidum is mediated by cytokines released from activated macrophages and T lymphocytes. Int. J. Cancer 1997, 70, 699–705. [Google Scholar] [CrossRef]
- Suay, I.; Arenal, F.; Asensio, F.; Basilio, A.; Cabello, M.; Díez, M.; García, J.; González del Val, A.; Gorrochategui, J.; Hernández, P.; et al. Screening of basidiomycetes for antimicrobial activities. Antonie Leeuwenhoek 2000, 78, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Mothana, R.; Jansen, R.; Julich, W.; Lindequist, U. Ganomycins A and B, new antimicrobial farnesyl hydroquinones from basidiomycete Ganoderma pfeifferi. J. Nat. Prod. 2000, 63, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Smania, A.; Monache, F.D.; Loguericio-Leite, C.; Smania, E.F.A.; Gerber, A.L. Antimicrobial activity of basidiomycetes. Int. J. Med. Mushr. 2001, 3, 87. [Google Scholar] [CrossRef]
- Zjawiony, J. Biologically active compounds from Aphyllophorales (Polypore) fungi. J. Nat. Prod. 2004, 67, 300–310. [Google Scholar] [CrossRef]
- Brian, D. Antibiotics produced by fungi. Bat Rev. 1951, 17, 357–430. [Google Scholar] [CrossRef]
- Robles-Hernández, L.; Ojeda-Barrios, D.L.; González-Franco, A.C.; Hernández-Huerta, J.; Salas-Salazar, N.A.; Hernández-Rodríguez, O.A. Susceptibilidad de aislados de Xanthomonas campestris pv. vesicatoria a Streptomyces y extractos bioactivos de Ganoderma. Acta Univ. 2017, 27, 30–39. [Google Scholar] [CrossRef]
- Robinson, J. Undergraduate Instrumental Analysis, 5th ed.; Marcel Dekker Inc.: New York, NY, USA, 1995; pp. 688–761. [Google Scholar]
- Voet, D.; Voet, G. Biochemistry, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1995; pp. 251–276. [Google Scholar]
- Mitscher, L. Plant-derived antibiotics. In Antibiotics Isolation, Separation and Purification; Weinstein, M., Wagman, M.G., Eds.; Elsevier North-Holland Inc.: New York, NY, USA, 1978; pp. 463–478. [Google Scholar]
- Prapulla, S.; Subhaprada, V.; Karanth, N. Microbial production of oligosaccharides: A review. In Advances in Applied Microbiology; Neidleman, S., Laskin, A., Bennett, J., Gadd, G., Eds.; Academic Press: San Diego, CA, USA, 2000; Volume 47, pp. 299–343. [Google Scholar] [CrossRef]
- Ahse, U.; Schroeder, R. Streptomycin inhibits splicing of group I introns by competition with the guanosine substrate. Nucleic Acid. Res. 1991, 19, 2261–2265. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.J.; Min, B.S.; Ahn, E.M.; Nakamura, N.; Lee, H.K.; Hattori, M. New triterpene aldehyde, lucialdehydes A-C, from Ganoderma lucidum and their cytotoxicity against murine and human tumor cells. Chem. Pharm. Bull. 2002, 50, 837–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, G.; Jolles, P. Immunostimulating agents: What next? A review of their present and potential medical applications. Eur. J. Biochem. 1996, 242, 1–19. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Baker, C.N.; Stocker, S.A.; Culver, D.H.; Thornsberry, C. Comparison of the E Test to agar dilution, broth microdilution, and agar diffusion susceptibility testing techniques by using a special challenge set of bacteria. J. Clin. Microbiol. 1991, 29, 533–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauli, A.; Schilcher, H. In vitro antimicrobial activities of essential oils monographed in the European Pharmacopoeia. In Handbook of Essential Oils, Science, Technology, and Applications, 6th ed.; Can Baser, K.H., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: New York, NY, USA, 2010; pp. 353–548. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Andrew, S.M.; Titus, J.A.; Zumstein, L. Dialysis and Concentration of Protein Solutions. Curr. Protoc. Toxicol. 2002. [Google Scholar] [CrossRef]
- Anonymous. Gel Filtration—Principles and Methods; Amersham Biosciences: Piscataway, NJ, USA, 2002; pp. 9–120. [Google Scholar]
- Kuga, S. Pore size distribution analysis of gel substances by size exclusion chromatography. J. Chromatogr. A 1981, 206, 449–461. [Google Scholar] [CrossRef]
- Paszczynski, A.; Crawford, R.; Funk, D.; Goodell, B. De novo synthesis of 4, 5-dimethoxycatechol and 2, 5-dimethoxyhydroquinone by the brown rot fungus Gloeophyllum trabeum. Appl. Environ. Microbiol. 1999, 65, 674–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
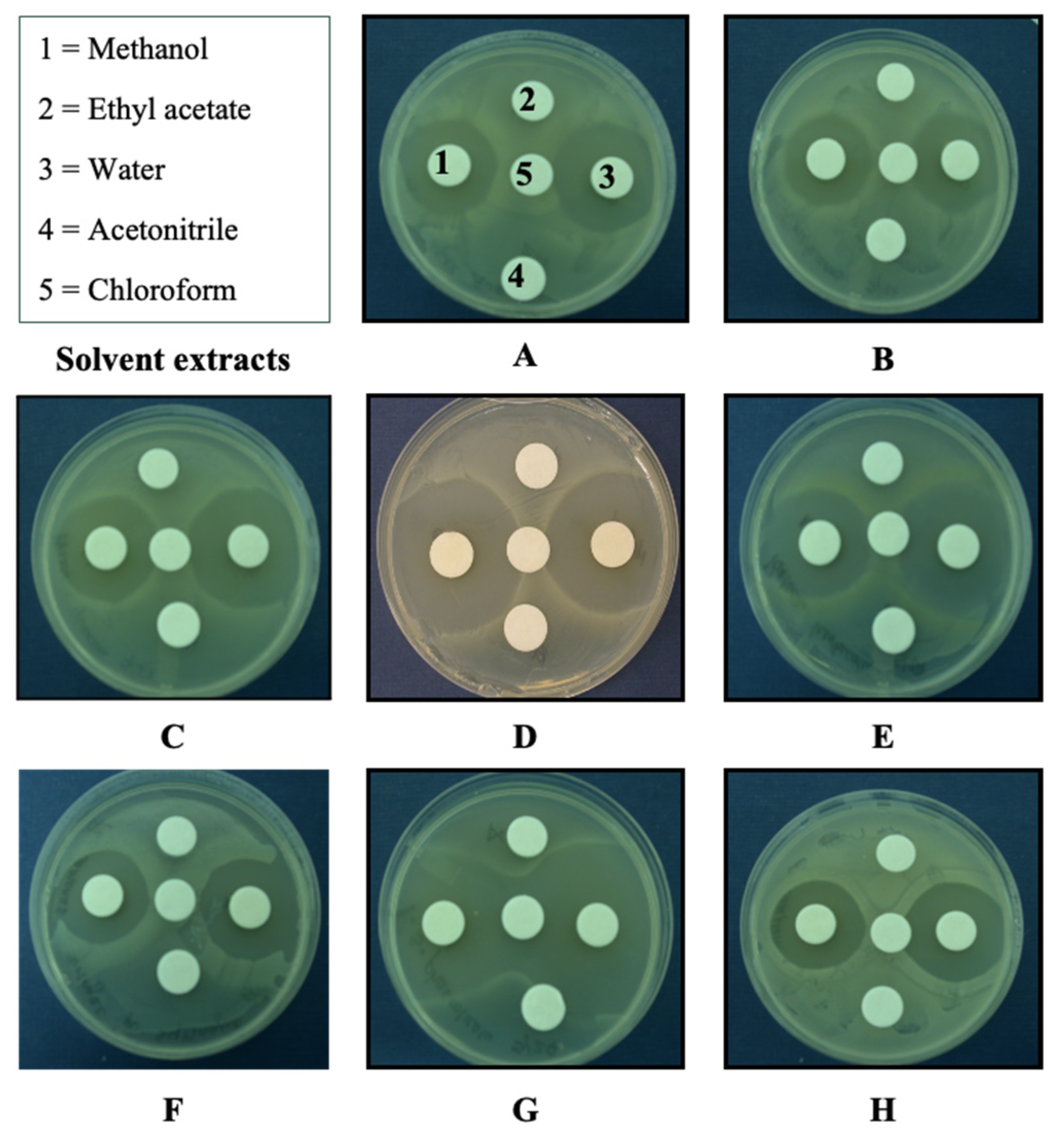


| Bacterial Species | Fresh Culture Fluids | 90-Day-Old Culture Fluids | Boiled Culture Fluids | PDB/NA Control ** |
|---|---|---|---|---|
| Acidovorax avenae | + * | + | + | − |
| Agrobacterium rhizogenes | + | + | + | − |
| Agrobacterium tumefaciens | + | + | + | − |
| Brenneria quercina | ± | ± | − | − |
| Burkholderia cepacia | − | − | − | − |
| Erwinia carotovora subsp. carotovora | + | + | + | − |
| Pseudomonas fluorescens | − | − | − | − |
| Pseudomonas syringae pv. syringae | + | + | + | − |
| Rathayibacter tritici | ± | ± | − | − |
| Xanthomonas campestris pv. campestris | + | + | + | − |
| Bacterial Species | Proteinase K Assay * | Dialysis Tubing Assay ** | ||
|---|---|---|---|---|
| Treated CF | Non-Treated CF | Tubing CF | Beaker Diffused CF | |
| Acidovorax avenae | + *** | + | - | + |
| Agrobacterium rhizogenes | + | + | - | + |
| Agrobacterium tumefaciens | + | + | - | + |
| Brenneria quercina | NT | NT | - | ± |
| Acidovorax avenae | + | + | - | + |
| Erwinia carotovora subsp. carotovora | + | + | - | + |
| Pseudomonas syringae pv. syringae | + | + | - | + |
| Pantoea herbicola | ± | ± | - | ± |
| Xanthomonas campestris pv. campestris | + | + | - | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robles-Hernández, L.; Salas-Salazar, N.A.; Gonzalez-Franco, A.C. Purification and Characterization of Antibacterial Activity against Phytopathogenic Bacteria in Culture Fluids from Ganoderma lucidum. Molecules 2021, 26, 5553. https://doi.org/10.3390/molecules26185553
Robles-Hernández L, Salas-Salazar NA, Gonzalez-Franco AC. Purification and Characterization of Antibacterial Activity against Phytopathogenic Bacteria in Culture Fluids from Ganoderma lucidum. Molecules. 2021; 26(18):5553. https://doi.org/10.3390/molecules26185553
Chicago/Turabian StyleRobles-Hernández, Loreto, Nora A. Salas-Salazar, and Ana C. Gonzalez-Franco. 2021. "Purification and Characterization of Antibacterial Activity against Phytopathogenic Bacteria in Culture Fluids from Ganoderma lucidum" Molecules 26, no. 18: 5553. https://doi.org/10.3390/molecules26185553
APA StyleRobles-Hernández, L., Salas-Salazar, N. A., & Gonzalez-Franco, A. C. (2021). Purification and Characterization of Antibacterial Activity against Phytopathogenic Bacteria in Culture Fluids from Ganoderma lucidum. Molecules, 26(18), 5553. https://doi.org/10.3390/molecules26185553







