Xanthone Glucosides: Isolation, Bioactivity and Synthesis
Abstract
:1. Introduction


2. Structure, Isolation and Bioactivity of Xanthone Glucoside
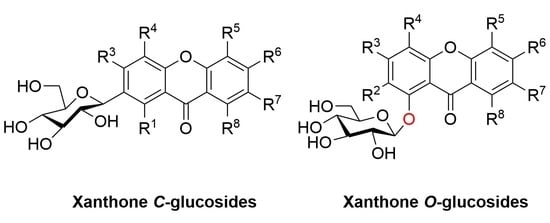
2.1. Xanthone C-Glucoside
2.1.1. Xanthone C-Glucoside from Liliaceae


2.1.2. Xanthone C-Glucoside from Iridaceae

2.1.3. Xanthone C-Glucoside from Arrabidaea

2.1.4. Xanthone C-Glucoside from Polygalaceae


2.1.5. Xanthone C-Glucoside from Gentianaceae


2.1.6. Xanthone C-Glucoside from Bombacaceae

2.1.7. Others

2.2. Xanthone O-Glucoside
2.2.1. Xanthone O-Glucoside from Gentianaceae


























2.2.2. Xanthone O-Glucoside from Clusiaceae

2.2.3. Xanthone O-Glucoside from Hypericaceae




2.2.4. Xanthone O-Glucoside from Iridaceae

2.2.5. Xanthone O-Glucoside from Polygalaceae

2.2.6. Xanthone O-Glucoside from Polygonaceae




2.2.7. Xanthone O-Glucoside from Polypodiaceae

2.2.8. Others




3. NMR Difference of Xanthone Glucosides
4. Synthesis of Xanthone Glucosides or Derivatives

5. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fiesel, T.; Gaid, M.; Muller, A.; Bartels, J.; El-Awaad, I.; Beuerle, T.; Ernst, L.; Behrends, S.; Beerhues, L. Molecular Cloning and Characterization of a Xanthone Prenyltransferase from Hypericum calycinum Cell Cultures. Molecules 2015, 20, 15616–15630. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Das, P.C.; Joshi, P.C. Naturally-occurring xanthones from terrestrial flora. J. Indian Chem. Soc. 1992, 69, 611–636. [Google Scholar]
- Peres, V.; Nagem, T.J. Naturally occurring, pentaoxygenated, hexaoxygenated and dimeric xanthones: A literature survey. Quim. Nova 1997, 20, 388–397. [Google Scholar] [CrossRef] [Green Version]
- Nhan, N.T.; Nguyen, P.H.; Tran, M.H.; Nguyen, P.D.; Tran, D.T.; To, D.C. Anti-inflammatory xanthone derivatives from Garcinia delpyana. J. Asian Nat. Prod. Res. 2021, 23, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Banik, K.; Harsha, C.; Bordoloi, D.; Lalduhsaki Sailo, B.; Sethi, G.; Leong, H.C.; Arfuso, F.; Mishra, S.; Wang, L.; Kumar, A.P.; et al. Therapeutic potential of gambogic acid, a caged xanthone, to target cancer. Cancer Lett. 2018, 416, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.M.; Kim, C.Y.; Ma, J.Y.; Lee, B. Xanthone-related compounds as an anti-browning and antioxidant food additive. Food Chem. 2019, 274, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Akao, Y.; Nakagawa, Y.; Iinuma, M.; Nozawa, Y. Anti-Cancer Effects of Xanthones from Pericarps of Mangosteen. Int. J. Mol. Sci. 2008, 9, 355–370. [Google Scholar] [CrossRef]
- Rukachaisirikul, V.; Kamkaew, M.; Sukavisit, D.; Phongpaichit, S.; Sawangchote, P.; Taylor, W.C. Antibacterial xanthones from the leaves of Garcinia nigrolineata. J. Nat. Prod. 2003, 66, 1531–1535. [Google Scholar] [CrossRef]
- He, L.; Zhu, C.F.; Yuan, Y.; Xu, Z.F.; Qiu, S.X. Specific glycosylated metabolites of α-mangostin by Cunninghamella blakesleana. Phytochem. Lett. 2014, 9, 175–178. [Google Scholar] [CrossRef]
- Gales, L.; Damas, A.M. Xanthones–A Structural Perspective. Curr. Med. Chem. 2005, 12, 2499–2515. [Google Scholar] [CrossRef]
- Klein, L.C.; Campos, A.; Niero, R.; Correa, R.; Vander Heyden, Y.; Cechinel, V. Xanthones and Cancer: From Natural Sources to Mechanisms of Action. Chem. Biodivers. 2020, 17, 30. [Google Scholar]
- Shan, T.; Ma, Q.; Guo, K.; Liu, J.; Li, W.; Wang, F.; Wu, E. Xanthones from Mangosteen Extracts as Natural Chemopreventive Agents: Potential Anticancer Drugs. Curr. Mol. Med. 2011, 11, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.B.; Xu, H.X. Caged Garcinia Xanthones: Development since 1937. Curr. Med. Chem. 2009, 16, 3775–3796. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; El-Ghorab, D.M.H.; El-Barbary, M.A.; Zayed, M.F.; Goransson, U.; Larsson, S.; Verpoorte, R. Naturally Occurring Xanthones; Latest Investigations: Isolation, Structure Elucidation and Chemosystematic Significance. Curr. Med. Chem. 2009, 16, 2581–2626. [Google Scholar] [CrossRef] [PubMed]
- Na, Y. Recent cancer drug development with xanthone structures. J. Pharm. Pharmacol. 2009, 61, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.; Fernandes, C.; Pinto, M.; Tiritan, M.E. Chiral Derivatives of Xanthones with Antimicrobial Activity. Molecules 2019, 24, 314. [Google Scholar] [CrossRef] [Green Version]
- Vieira, L.M.M.; Kijjoa, A. Naturally-Occurring Xanthones: Recent Developments. Curr. Med. Chem. 2005, 12, 2413–2446. [Google Scholar] [CrossRef]
- Wu, Q.L.; Wang, S.P.; Du, L.J.; Yang, J.S.; Xiao, P.G. Xanthones from Hypericum japonicum and H-Henryi. Phytochemistry 1998, 49, 1395–1402. [Google Scholar] [CrossRef]
- Perest, V.; Nagem, T.J. Trioxygenated naturally occurring xanthones. Phywchemistry 1997, 44, 191–214. [Google Scholar] [CrossRef]
- Valdir, P.; Nagem, T.J.; de Oliveira, F.F. Tetraoxygenated naturally occurring xanthones. Phytochemistry 2000, 55, 683–710. [Google Scholar]
- Mangangcha, I.R.; Singh, R.K.B.; Lebeche, D.; Ali, S. Xanthone glucoside 2-beta-d-glucopyranosyl-1,3,6,7-tetrahydroxy-9H-xanthen-9-one binds to the ATP-binding pocket of glycogen synthase kinase 3 beta and inhibits its activity: Implications in prostate cancer and associated cardiovascular disease risk. J. Biomol. Struct. Dyn. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, S.; Sharma, P.V.; Chaudhuri, R.K. Chemical constituents of gentianaceae. X. Xanthone-O-glucosides of Swertia purpurascens Wall. J. Pharm. Sci. 1974, 63, 1286–1290. [Google Scholar] [CrossRef] [PubMed]
- Kren, V.; Martinkova, L. Glycosides in medicine: “The role of glycosidic residue in biological activity”. Curr. Med. Chem. 2001, 8, 1303–1328. [Google Scholar] [CrossRef]
- Septiana, I.; Nguyen, T.T.H.; Lim, S.; Lee, S.; Park, B.; Kwak, S.; Park, S.; Kim, S.B.; Kim, D. Enzymatic synthesis and biological characterization of a novel mangiferin glucoside. Enzym. Microb. Technol. 2020, 134, 109479. [Google Scholar] [CrossRef]
- Feng, S.T.; Wang, Z.Z.; Yuan, Y.H.; Sun, H.M.; Chen, N.H.; Zhang, Y. Mangiferin: A multipotent natural product preventing neurodegeneration in Alzheimer’s and Parkinson’s disease models. Pharmacol. Res. 2019, 146, 12. [Google Scholar] [CrossRef]
- Jo, C.; Yoon, K.Y.; Jang, E.J.; Kim, T.H. Degradation products of mangiferin by gamma irradiation with inhibitory effects on NO production. Biosci. Biotechnol. Biochem. 2016, 80, 2022–2024. [Google Scholar] [CrossRef] [Green Version]
- Yoshimi, N.; Matsunaga, K.; Katayama, M.; Yamada, Y.; Kuno, T.; Qiao, Z.; Hara, A.; Yamahara, J.; Mori, H. The inhibitory effects of mangiferin, a naturally occurring glucosylxanthone, in bowel carcinogenesis of male F344 rats. Cancer Lett. 2001, 163, 163–170. [Google Scholar] [CrossRef]
- Kim, G.E.; Kang, H.K.; Seo, E.S.; Jung, S.H.; Park, J.S.; Kim, D.H.; Kim, D.W.; Ahn, S.A.; Sunwoo, C.; Kim, D. Glucosylation of the flavonoid, astragalin by Leuconostoc mesenteroides B-512FMCM dextransucrase acceptor reactions and characterization of the products. Enzym. Microb. Technol. 2012, 50, 50–56. [Google Scholar] [CrossRef]
- Faizi, S.; Zikr-ur-Rehman, S.; Naz, A.; Versiani, M.A.; Dar, A.; Naqvi, S. Bioassay-guided studies on Bombax ceiba leaf extract: Isolation of shamimoside, a new antioxidant xanthone C-glucoside. Chem. Nat. Compd. 2012, 48, 774–779. [Google Scholar] [CrossRef]
- Finnegan, R.A.; Stephani, R.A.; Ganguli, G.; Ganguly, S.N.; Bhattacharya, A.K. Occurrence of mangiferin in Hiptage madablota Geartn. J. Pharm. Sci. 1968, 57, 1039–1040. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.F.; Han, G.Y.; Guo, X.M. Isolation and structure determination of xanthone glycosides of Anemarrhena asphodeloides. Acta Pharm. Sin. 1997, 32, 473–475. [Google Scholar]
- Iwashina, T.; Kitajima, J.; Shiuchi, T.; Itou, Y. Chalcones and other flavonoids from Asarum sensu lato (Aristolochiaceae). Biochem. Syst. Ecol. 2005, 33, 571–584. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Xu, X.J.; Liu, H.M. Chemical constituents from Mahkota dewa. J. Asian Nat. Prod. Res. 2006, 8, 119–123. [Google Scholar] [CrossRef]
- Talamond, P.; Mondolot, L.; Gargadennec, A.; de Kochko, A.; Hamon, S.; Fruchier, A.; Campa, C. First report on mangiferin (C-glucosyl-xanthone) isolated from leaves of a wild coffee plant, Coffea pseudozanguebariae (Rubiaceae). Acta Bot. Gallica 2008, 155, 513–519. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Li, M.H.; Zhang, N.; Huang, L.Q. Chemical constituents from Lomatogonium carinthiacum (Gentianaceae). Biochem. Syst. Ecol. 2011, 39, 766–768. [Google Scholar] [CrossRef]
- Khare, P.; Shanker, K. Mangiferin: A review of sources and interventions for biological activities. Biofactors 2016, 42, 504–514. [Google Scholar]
- Wei, Z.; Yan, L.; Chen, Y.; Bao, C.; Deng, J.; Deng, J. Mangiferin inhibits macrophage classical activation via downregulating interferon regulatory factor 5 expression. Mol. Med. Rep. 2016, 14, 1091–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Z.L.; Lu, X.Q.; Gan, L.S.; Zhang, Q.W.; Lin, L.G. Xanthones, A Promising Anti-Inflammatory Scaffold: Structure, Activity, and Drug Likeness Analysis. Molecules 2020, 25, 598. [Google Scholar] [CrossRef] [Green Version]
- Sha, H.; Zeng, H.R.; Zhao, J.; Jin, H.Y. Mangiferin ameliorates gestational diabetes mellitus-induced placental oxidative stress, inflammation and endoplasmic reticulum stress and improves fetal outcomes in mice. Eur. J. Pharmacol. 2019, 859, 7. [Google Scholar] [CrossRef]
- Dar, A.; Faizi, S.; Naqvi, S.; Roome, T.; Zikr-ur-Rehman, S.; Ali, M.; Firdous, S.; Moin, S.T. Analgesic and antioxidant activity of mangiferin and its derivatives: The structure activity relationship. Biol. Pharm. Bull. 2005, 28, 596–600. [Google Scholar] [CrossRef] [Green Version]
- He, L.Y.; Peng, X.F.; Zhu, J.F.; Chen, X.; Liu, H.; Tang, C.Y.; Dong, Z.; Liu, F.Y.; Peng, Y.M. Mangiferin Attenuate Sepsis-Induced Acute Kidney Injury via Antioxidant and Anti-Inflammatory Effects. Am. J. Nephrol. 2014, 40, 441–450. [Google Scholar] [CrossRef]
- PardO-Andreu, G.L.; Barrios, M.F.; Curti, C.; Hernandez, I.; Merino, N.; Lemus, Y.; Martinez, L.; Riano, A.; Delgado, R. Protective effects of Mangifera indica L. extract (Vimang), and its major component mangiferin, on iron-induced oxidative damage to rat serum and liver. Pharmacol. Res. 2008, 57, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.H.; Luo, C.T.; Chen, H.R.; Lin, J.N.; Ye, C.L.; Mao, S.S.; Li, Y.L. Xanthones from Swertia mussotii as Multitarget-Directed Antidiabetic Agents. Chemmedchem 2014, 9, 1374–1377. [Google Scholar] [CrossRef] [PubMed]
- Muruganandan, S.; Srinivasan, K.; Gupta, S.; Gupta, P.K.; Lal, J. Effect of mangiferin on hyperglycemia and atherogenicity in streptozotocin diabetic rats. J. Ethnopharmacol. 2005, 97, 497–501. [Google Scholar] [CrossRef]
- Miura, T.; Ichiki, H.; Hashimoto, I.; Iwamoto, N.; Kato, M.; Kubo, M.; Ishihara, E.; Komatsu, Y.; Okada, M.; Ishida, T.; et al. Antidiabetic activity of a xanthone compound, mangiferin. Phytomedicine 2001, 8, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Wu, G.S.; Yu, Y.; Liu, H.; Yang, B.Y.; Kuang, H.X.; Wang, Q.H. Xanthones isolated from Gentianella acuta and their protective effects against H2O2-induced myocardial cell injury. Nat. Prod. Res. 2018, 32, 2171–2177. [Google Scholar] [CrossRef]
- Muruganandan, S.; Gupta, S.; Kataria, M.; Lal, J.; Gupta, P.K. Mangiferin protects the streptozotocin-induced oxidative damage to cardiac and renal tissues in rats. Toxicology 2002, 176, 165–173. [Google Scholar] [CrossRef]
- Prabhu, S.; Jainu, M.; Sabitha, K.E.; Devi, C.S.S. Effect of mangiferin on mitochondrial energy production in experimentally induced myocardial infarcted rats. Vasc. Pharmacol. 2006, 44, 519–525. [Google Scholar] [CrossRef]
- Zeng, Z.; Lin, C.J.; Wang, S.W.; Wang, P.F.; Xu, W.W.; Ma, W.Q.; Wang, J.L.; Xiang, Q.; Liu, Y.T.; Yang, J.M.; et al. Suppressive activities of mangiferin on human epithelial ovarian cancer. Phytomedicine 2020, 76, 9. [Google Scholar] [CrossRef]
- Deng, Q.; Tian, Y.X.; Liang, J.J. Mangiferin inhibits cell migration and invasion through Rac1/WAVE2 signalling in breast cancer. Cytotechnology 2018, 70, 593–601. [Google Scholar] [CrossRef]
- Lv, J.Z.; Wang, Z.J.; Zhang, L.; Wang, H.L.; Liu, Y.D.; Li, C.Y.; Deng, J.G.; Yi, W.; Bao, J.K. Mangiferin Induces Apoptosis and Cell Cycle Arrest in MCF-7 Cells Both in vitro and in vivo. J. Anim. Vet. Adv. 2013, 12, 352–359. [Google Scholar]
- Pinto, M.M.M.; Sousa, M.E.; Nascimento, M.S.J. Xanthone derivatives: New insights in biological activities. Curr. Med. Chem. 2005, 12, 2517–2538. [Google Scholar] [CrossRef]
- Sato, T.; Kawamoto, A.; Tamura, A.; Tatsumi, Y.; Fujii, T. Mechanism of antioxidant action of pueraria glycoside (PG)-1 (an isoflavonoid) and mangiferin (a xanthonoid). Chem. Pharm. Bull. 1992, 40, 721–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyas, A.; Syeda, K.; Ahmad, A.; Padhye, S.; Sarkar, F.H. Perspectives on Medicinal Properties of Mangiferin. Mini-Rev. Med. Chem. 2012, 12, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Andreu, G.P.; Delgado, R.; Velho, J.A.; Curti, C.; Vercesi, A.E. Iron complexing activity of mangiferin, a naturally occurring glucosylxanthone, inhibits mitochondrial lipid peroxidation induced by Fe2+-citrate. Eur. J. Pharmacol. 2005, 513, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Aritomi, M.; Kawasaki, T. A new xanthone C-glucoside, position isomer of mangiferin, from Anemarrhena asphodeloides Bunge. Chem. Pharm. Bull. 1970, 18, 2327–2333. [Google Scholar] [CrossRef] [Green Version]
- Kokotkiewicz, A.; Luczkiewicz, M.; Pawlowska, J.; Luczkiewicz, P.; Sowinski, P.; Witkowski, J.; Bryl, E.; Bucinski, A. Isolation of xanthone and benzophenone derivatives from Cyclopia genistoides (L.) Vent. (honeybush) and their pro-apoptotic activity on synoviocytes from patients with rheumatoid arthritis. Fitoterapia 2013, 90, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Kang, G.D.; Jeong, J.J.; Choi, H.S.; Kim, D.H. Neomangiferin modulates the Th17/Treg balance and ameliorates colitis in mice. Phytomedicine 2016, 23, 131–140. [Google Scholar] [CrossRef]
- Arisawa, M.; Morita, N.; Kondo, Y.; Takemoto, T. The constiuents of Iris florentina L. (3). structure of irisxanthone, a know C-glucosylxanthone. Chem. Pharm. Bull. 1973, 21, 2562–2565. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Mageed, W.M.; Al-Wahaibi, L.H.; Al-Saleem, M.S.M.; Gouda, Y.G.; Abdel-Kader, M.S.; Ibraheim, Z.Z. Phytochemical and chemotaxonomic study on Iris albicans Lange leaves. Biochem. Syst. Ecol. 2018, 76, 32–34. [Google Scholar] [CrossRef]
- Bukvicki, D.; Novakovic, M.; Ab Ghani, N.; Marin, P.D.; Asakawa, Y. Secondary metabolites from endemic species Iris adriatica Trinajstic ex Mitic (Iridaceae). Nat. Prod. Res. 2018, 32, 1849–1852. [Google Scholar] [CrossRef]
- Xie, G.Y.; Chen, Y.J.; Wen, R.; Xu, J.Y.; Wu, S.S.; Qin, M.J. Chemical constituents from rhizomes of Iris germanica. China J. Chin. Mater. Med. 2014, 39, 846–850. [Google Scholar]
- Alkhalil, S.; Tosa, H.; Iinuma, M. A xanthone C-glycoside from Iris Nigricans. Phytochemistry 1995, 38, 729–731. [Google Scholar] [CrossRef]
- Pauletti, P.M.; Castro-Gamboa, I.; Silva, D.H.S.; Young, M.C.M.; Tomazela, D.M.; Eberlin, M.N.; Bolzani, V.D. New antioxidant C-glucosylxanthones from the stems of Arrabidaea samydoides. J. Nat. Prod. 2003, 66, 1384–1387. [Google Scholar] [CrossRef]
- Martin, F.; Hay, A.E.; Cressend, D.; Reist, M.; Vivas, L.; Gupta, M.P.; Carrupt, P.A.; Hostettmann, K. Antioxidant C-Glucosylxanthones from the Leaves of Arrabidaea patellifera. J. Nat. Prod. 2008, 71, 1887–1890. [Google Scholar] [CrossRef] [PubMed]
- Miyase, T.; Noguchi, H.; Chen, X.M. Sucrose Esters and Xanthone C-Glycosides from the Roots of Polygala sibirica. J. Nat. Prod. 1999, 62, 993–996. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, W.; Tu, P.F.; Xu, X.J. Xanthone Glycosides from Polygala tenuifolia and Their Conformational Analyses. J. Nat. Prod. 2005, 68, 875–879. [Google Scholar] [CrossRef]
- Chang, H.T.; Tu, P.F. New oligosaccharide esters and xanthone C-glucosides from Polygala telephioides. Helv. Chim. Acta 2007, 90, 944–950. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Nishihara, M.; Osumi, Y.; Hakamatsuka, T.; Goda, Y.; Uchiyama, N.; Ozeki, Y. Structural Analysis of Polygalaxanthones, C-Glucosyl Xanthones of Polygala tenuifolia Roots. Chem. Pharm. Bull. 2019, 67, 1242–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.F.; Chen, S.B.; Gao, J.C.; Song, H.L.; Wu, L.J.; Chen, S.L.; Tu, P.F. Xanthone glycosides from herbs of Polygala hongkongensis Hemsl and their antioxidant activities. J. Asian Nat. Prod. Res. 2008, 10, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, T.; KrstiC-Milosevic, D.; Aljancic, I.; Savikin, K.; Menkovic, N.; Radanovic, D.; Milosavljevic, S. Phytochemical re-investigation of Gentiana utriculosa. Nat. Prod. Res. 2009, 23, 466–469. [Google Scholar] [CrossRef]
- Schaufelberger, D.; Hostettmann, K. Chemistry and pharmacology of Gentiana lactea. Planta Med. 1988, 54, 219–221. [Google Scholar] [CrossRef]
- Fujita, M.; Inoue, T. Studies on the Constituents of Iris florentina L. II. C-Glucosides of Xanthones and Flavones from the Leaves. Chem. Pharm. Bull. 1982, 30, 2342–2348. [Google Scholar] [CrossRef] [Green Version]
- Shi, T.X.; Wang, S.; Zeng, K.W.; Tu, P.F.; Jiang, Y. Inhibitory constituents from the aerial parts of Polygala tenuifolia on LPS-induced NO production in BV2 microglia cells. Bioorg. Med. Chem. Lett. 2013, 23, 5904–5908. [Google Scholar] [CrossRef]
- Tan, P.; Hou, C.Y.; Liu, Y.L.; Lin, L.J.; Cordell, G.A. Swertipunicoside. The First Bisxanthone C-Glycoside. J. Org. Chem. 1991, 56, 7130–7133. [Google Scholar] [CrossRef]
- Tan, P.; Hou, C.Y.; Liu, Y.L.; Lin, L.J.; Cordell, G.A. 3-O-demethylswertipunicoside from Swertia punicea. Phytochemistry 1992, 31, 4313–4315. [Google Scholar] [CrossRef]
- Du, X.G.; Wang, W.; Zhang, S.P.; Pu, X.P.; Zhang, Q.Y.; Ye, M.; Zhao, Y.Y.; Wang, B.R.; Khan, I.A.; Guo, D.A. Neuroprotective Xanthone Glycosides from Swertia punicea. J. Nat. Prod. 2010, 73, 1422–1426. [Google Scholar] [CrossRef]
- Luo, C.T.; Mao, S.S.; Liu, F.L.; Yang, M.X.; Chen, H.; Kurihara, H.; Li, Y. Antioxidant xanthones from Swertia mussotii, a high altitude plant. Fitoterapia 2013, 91, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zou, D.Z.; Bai, S.; Li, Z.H.; Zhang, C.H.; Li, M.H. Chemical constituents from Gentianella turkestanorum (Gentianaceae). Biochem. Syst. Ecol. 2016, 65, 89–92. [Google Scholar] [CrossRef]
- Abdel-Mageed, W.M.; Bayoumi, S.A.H.; Chen, C.; Vavricka, C.J.; Li, L.; Malik, A.; Dai, H.; Song, F.; Wang, L.; Zhang, J.; et al. Benzophenone C-glucosides and gallotannins from mango tree stem bark with broad-spectrum anti-viral activity. Bioorg. Med. Chem. 2014, 22, 2236–2243. [Google Scholar] [CrossRef] [PubMed]
- Stout, G.H.; Balkenhol, W.J. Xanthones of the Gentianaceae-I Frasera caroliniensis Walt. Tetrahedron 1969, 25, 1947–1960. [Google Scholar] [CrossRef]
- Tomimori, T.; Komatsu, M. Studies on the Constituents of Swertia japonica. VI. on the Flavonoid and Xanthone Constituents of Swertia randaiensis. HAYATA amd S. swertopsis MAKINO. Yakugaku Zasshi 1969, 89, 1276–1282. [Google Scholar] [CrossRef] [Green Version]
- Tomimori, T.; Yoshizaki, M.; Nanba, T. Studies on the Nepalese Crude Drugs.I. On the Flavonoid and Xanthone Constituents of the Plants of Swertia spp. Yakugaku Zasshi 1973, 93, 442–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hostettmann, K.; Tabacchi, R.; Jacot-Guillarmod, A. Contribution à la phytochimie du genre Gentiana, VI. Etude des xanthones dans les feuilles de Gentiana bavarica L. Helv. Chim. Acta 1974, 57, 294–301. [Google Scholar] [CrossRef]
- Hostettmann, K.; Miura, I. A New Xanthone Diglucoside from Swertia perennis L. Helv. Chim. Acta 1977, 60, 262–264. [Google Scholar] [CrossRef]
- Ghosal, S.; Sharma, P.V.; Jaiswal, D.K. Chemical constituents of Gentianaceae XXIII: Tetraoxygenated and pentaoxygenated xanthones and xanthone O-glucosides of Swertia angustifolia Buch.-Ham. J. Pharm. Sci. 1978, 67, 55–60. [Google Scholar] [CrossRef]
- Dhasmana, H.; Garg, H.S. Two xanthone glucosides from Halenia elliptica. Phytochemrstry 1989, 28, 2819–2821. [Google Scholar] [CrossRef]
- Sun, H.F.; Hu, B.L.; Ding, J.Y.; Fan, S.F. The glucosides from Swertia mussotii Franch. Acta Bot. Sin. 1991, 33, 31–37. [Google Scholar]
- Recio-Iglesias, M.C.; Marston, A.; Hosteyitman, K. Xanthones and secoiridoid glucosides of Halenia campanulata. Phytochemistry 1992, 31, 1387–1389. [Google Scholar] [CrossRef]
- Rodriguez, S.; Wolfender, J.L.; Odontuya, G.; Purev, O.; Hostettmann, K. Xanthones, secoiridoids and flavonoids from Halenia corniculata. Phytochemistry 1995, 40, 1265–1272. [Google Scholar] [CrossRef]
- Otsuka, H.; Triptexanthosides, A.-E. Xanthone Glycosides from Aerial Parts of Tripterospermum japonicum. Chem. Pharm. Bull. 1999, 47, 962–965. [Google Scholar] [CrossRef] [Green Version]
- Menkovic, N.; Savikin-Fodulovic, K.; Bulatovic, V.; Aljancic, I.; Juranic, N.; Macura, S.; Vajs, V.; Milosavljevic, S. Xanthones from Swertia punctata. Phytochemistry 2002, 61, 415–420. [Google Scholar] [CrossRef]
- Zeng, G.Y.; Tan, G.S.; Xu, K.P.; Xu, X.P.; Li, F.S.; Tan, J.B.; Hu, G.Y. Water-soluble chemical constituents of Swertia davidi Franch. Acta Pharm. Sin. 2004, 39, 351–353. [Google Scholar]
- Kaldas, M.; Hostettmann, K.; Jacot-Guillarmod, A. Contribution à la phytochimie du genre Gentiana IX. Etude de composés flavoniques et xanthoniques dans les feuilles de gentiana CampestrisL. 1ère communication. Helv. Chim. Acta 1974, 57, 2557–2561. [Google Scholar] [CrossRef]
- Rana, V.S.; Rawat, M.S.M. A New Xanthone Glycoside and Antioxidant Constituents from the Rhizomes of Swertia speciosa. Chem. Biodivers. 2005, 2, 1310–1315. [Google Scholar] [CrossRef]
- Hajimehdipoor, H.; Dijoux-Franca, M.G.; Mariotte, A.M.; Amanzadeh, Y.; Sadat-Ebrahimi, S.E.; Ghazi-Khansari, M. Two new xanthone diglycosides from Swertia longifolia Boiss. Nat. Prod. Res. 2006, 20, 1251–1257. [Google Scholar] [CrossRef]
- Murray, A.P.; Faraoni, M.B.; Castro, M.J.; Alza, N.P.; Cavallaro, V. Natural AChE Inhibitors from Plants and their Contribution to Alzheimer’s Disease Therapy. Curr. Neuropharmacol. 2013, 11, 388–413. [Google Scholar] [CrossRef] [Green Version]
- Nalivaeva, N.N.; Turner, A.J. AChE and the amyloid precursor protein (APP)—Cross-talk in Alzheimer’s disease. Chem.-Biol. Interact. 2016, 259, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Sherif, F.; Gottfries, C.G.; Alafuzoff, I.; Oreland, L. Brain gamma-aminobutyrate aminotransferase (GABA-T) and monoamine-oxidase (MAO) in patients with alzheimers-disease. J. Neural Transm.-Park. Dis. Dement. Sect. 1992, 4, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Urbain, A.; Marston, A.; Grilo, L.S.; Bravo, J.; Purev, O.; Purevsuren, B.; Batsuren, D.; Reist, M.; Carrupt, P.A.; Hostettmann, K. Xanthones from Gentianella amarella ssp acuta with acetylcholinesterase and monoamine oxidase inhibitory activities. J. Nat. Prod. 2008, 71, 895–897. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Xu, X.M.; Rinderspacher, K.A.; Cai, C.Q.; Ma, Y.; Long, C.L.; Feng, J.C. Two new compounds from Comastoma pedunlulatum. J. Asian Nat. Prod. Res. 2011, 13, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Liu, B.; Zhang, S.D.; Hou, Q.; Qi, L.L.; Zhou, Q.Y. Cytotoxicity, apoptosis-inducing effects and structure-activity relationships of four natural xanthones from Gentianopsis paludosa Ma in HepG2 and HL-60 cells. Nat. Prod. Res. 2011, 25, 669–683. [Google Scholar] [CrossRef]
- Gao, L.; Zhou, Y.; Yan, H.; Huang, F.; Wen, R.; Li, G. Two new xanthone glucosides from Swertia mussotii Franch. Heterocycles 2011, 83, 1897–1902. [Google Scholar]
- Wan, L.S.; Min, Q.X.; Wang, Y.L.; Yue, Y.D.; Chen, J.C. Xanthone glycoside constituents of Swertia kouitchensis with α-glucosidase inhibitory activity. J. Nat. Prod. 2013, 76, 1248–1253. [Google Scholar] [CrossRef]
- Yue, Y.D.; Zhang, Y.T.; Liu, Z.X.; Min, Q.X.; Wan, L.S.; Wang, Y.L.; Xiao, Z.Q.; Chen, J.C. Xanthone Glycosides from Swertia bimaculata with α-Glucosidase Inhibitory Activity. Planta Med. 2014, 80, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Tong, Y.F.; Wang, Q.H. Two new xanthones from Lomatogonium carinthiacum. Chin. J. Nat. Med. 2014, 12, 693–696. [Google Scholar]
- Mahendran, G.; Manoj, M.; Murugesh, E.; Sathish Kumar, R.; Shanmughavel, P.; Rajendra Prasad, K.J.; Narmatha Bai, V. In vivo anti-diabetic, antioxidant and molecular docking studies of 1,2,8-trihydroxy-6-methoxy xanthone and 1,2-dihydroxy-6-methoxyxanthone-8-O-β-d-xylopyranosyl isolated from Swertia corymbosa. Phytomedicine 2014, 21, 1237–1248. [Google Scholar] [CrossRef]
- Lu, S.; Tanaka, N.; Kawazoe, K.; Murakami, K.; Damdinjav, D.; Dorjbal, E.; Kashiwada, Y. Tetrahydroxanthones from Mongolian medicinal plant Gentianella amarella ssp. acuta. J. Nat. Med. 2016, 70, 780–788. [Google Scholar] [CrossRef]
- Shi, K.L.; Wang, Y.Q.; Jiang, Q.; Liao, Z.X. Chemical Constituents of Gentianella azurea. Chin. J. Nat. Med. 2010, 8, 425–428. [Google Scholar]
- Xu, K.P.; Li, F.S.; Liu, J.F.; Tan, J.B.; Zhang, L.H.; Zeng, G.Y.; Tan, G.S. Studies on Chemical Constituents of Swertia nervosa (G.Don) Wall. Chin. Pharm. J. 2008, 43, 1612–1615. [Google Scholar]
- Tan, G.S.; Xu, P.S.; Tian, H.Y.; Xu, K.P.; Dai, Z.Y. Studies on the chemical constituents of Swertia davidi. Chin. Pharm. J. 2000, 35, 441–443. [Google Scholar]
- Tian, C.W.; Zhang, T.J.; Wang, L.L.; Shan, Q.; Jiang, L.H. The hepatoprotective effect and chemical constituents of total iridoids and xanthones extracted from Swertia mussotii Franch. J. Ethnopharmacol. 2014, 154, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, G.; Liu, H.; Xing, N.; Sun, Y.; Zhai, Y.; Yang, B.; Kong, A.T.; Kuang, H.; Wang, Q. Cardioprotective effect of the xanthones from Gentianella acuta against myocardial ischemia/reperfusion injury in isolated rat heart. Biomed. Pharmacother. 2017, 93, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Tosa, H.; Iinuma, M.; Murakami, K.; Ito, T.; Tanaka, T.; Chelladurai, V.; Riswan, S. Three xanthones from Poeciloneuron pauciflorum and Mammea acuminata. Phytochemistry 1997, 45, 133–136. [Google Scholar] [CrossRef]
- Fu, P.; Zhang, W.D.; Liu, R.H.; Li, T.Z.; Shen, Y.H.; Li, H.L.; Zhang, W.; Chen, H.S. Two new xanthones from Hypericum japonicum. Nat. Prod. Res. 2006, 20, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, K.; Yamamota, R.; Oku, H. Patulosides A and B, novel xanthone glycosides from cell suspension cultures of Hypericum patulum. J. Nat. Prod. 1999, 62, 906–908. [Google Scholar] [CrossRef]
- Kitanov, G.M.; Nedialkov, P.T. Xanthohypericoside, a new xanthone-O-glucoside from Hypericum annulatum. Pharmazie 2000, 55, 397–398. [Google Scholar]
- Yan, X.T.; An, Z.; Huangfu, Y.; Zhang, Y.T.; Li, C.H.; Chen, X.; Liu, P.L.; Gao, J.M. Polycyclic polyprenylated acylphloroglucinol and phenolic metabolites from the aerial parts of Hypericum elatoides and their neuroprotective and anti-neuroinflammatory activities. Phytochemistry 2019, 159, 65–74. [Google Scholar] [CrossRef]
- Woo, K.W.; Lee, K.H.; Jang, J.H.; Kim, M.S.; Cho, H.W.; Cho, J.H.; An, B. Anti-inflammatory Constituents from the Aerial Parts of Iris minutiaurea. Nat. Prod. Commun. 2016, 11, 817–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.K.; Chan, C.L.; Leung, H.W.; Yeung, H.W.; Xiao, P.G. Xanthones from Polygala caudata. Phytochemistry 1999, 51, 953–958. [Google Scholar] [CrossRef]
- Abd El-Kader, A.M.; Ahmed, A.S.; Nafady, A.M.; Ibraheim, Z.Z. Xanthone and lignan glycosides from the aerial parts of Polygonum bellardii all growing in Egypt. Pharmacogn. Mag. 2013, 9, 135–143. [Google Scholar] [PubMed]
- Li, J.; Jiang, Y.; Tu, P.F. Xanthone O-Glycosides and Benzophenone O-Glycosides from the Roots of Polygala tricornis. J. Nat. Prod. 2005, 68, 1802–1804. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Tu, P.F. Xanthone O-glycosides from Polygala tenuifolia. Phytochemistry 2002, 60, 813–816. [Google Scholar] [CrossRef]
- He, K.; Fan, L.L.; Wu, T.T.; Du, J. A new xanthone glycoside from Pyrrosia sheareri. Nat. Prod. Res. 2019, 33, 2982–2987. [Google Scholar] [CrossRef]
- Rezanka, T.; Dembitsky, V.M. Identification of acylated xanthone glycosides by liquid chromatography-atmospheric pressure chemical ionization mass spectrometry in positive and negative modes from the lichen Umbilicaria proboscidea. J. Chromatogr. A 2003, 995, 109–118. [Google Scholar] [CrossRef]
- Rezanka, T.; Jachymova, J.; Dembitsky, V.M. Prenylated xanthone glucosides from Ural’s lichen Umbilicaria proboscidea. Phytochemistry 2003, 62, 607–612. [Google Scholar] [CrossRef]
- Eltamany, E.E.; Abdelmohsen, U.R.; Ibrahim, A.K.; Hassanean, H.A.; Hentschel, U.; Ahmed, S.A. New antibacterial xanthone from the marine sponge-derived Micrococcus sp. EG45. Bioorg. Med. Chem. Lett. 2014, 24, 4939–4942. [Google Scholar] [CrossRef]
- Yang, B.J.; Chen, G.D.; Li, Y.J.; Hu, D.; Guo, L.D.; Xiong, P.; Gao, H. A New Xanthone Glycoside from the Endolichenic Fungus Sporormiella irregularis. Molecules 2016, 21, 764. [Google Scholar] [CrossRef]
- Yoneyama, T.; Iguchi, M.; Yoshii, K.; Elshamy, A.I.; Ban, S.; Noji, M.; Umeyama, A. Xanthone glucoside from an insect pathogenic fungus Conoideocrella luteorostrata NBRC106950. Nat. Prod. Res. 2021, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Vermes, B.; Seligmann, O.; Wagner, H. Synthesis of xanthone O-glycosides. III. synthesis of 1-O and 8-O-β-d-glycosides of 5-O-methylbellidifolin and de-O-methylbellidifolin. Helv. Chim. Acta 1985, 68, 2359–2366. [Google Scholar] [CrossRef]
- Hu, H.G.; Wang, M.J.; Zhao, Q.J.; Liao, H.L.; Cai, L.Z.; Song, Y.; Zhang, J.; Yu, S.C.; Chen, W.S.; Liu, C.M.; et al. Synthesis of mangiferin derivatives as protein tyrosine phosphatase 1B inhibitors. Chem. Nat. Compd. 2007, 43, 663–666. [Google Scholar] [CrossRef]
- Wei, X.; Liang, D.; Ning, M.; Wang, Q.; Meng, X.; Li, Z. Semi-synthesis of neomangiferin from mangiferin. Tetrahedron Lett. 2014, 55, 3083–3086. [Google Scholar] [CrossRef]
- Wei, X.; Liang, D.; Wang, Q.; Meng, X.; Li, Z. Total synthesis of mangiferin, homomangiferin, and neomangiferin. Org. Biomol. Chem. 2016, 14, 8821–8831. [Google Scholar] [CrossRef]
- Zarena, A.S.; Sankar, K.U. Synthesis of α-mangostin-d-glucoside in supercritical carbon dioxide media. J. Food Sci. Technol. 2015, 52, 6547–6555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuoi, T.L.; Pandey, R.P.; Gurung, R.B.; Dhakal, D.; Sohng, J.K. Efficient enzymatic systems for synthesis of novel α-mangostin glycosides exhibiting antibacterial activity against Gram-positive bacteria. Appl. Microbiol. Biotechnol. 2014, 98, 8527–8538. [Google Scholar]





| R1 | R2 | |
|---|---|---|
| 10 |  | H |
| 11 |  | H |
| 12 | H |  |
| 13 |  | H |
| 14 |  | H |
| 15 |  | H |
| R1 | R2 | R3 | R4 | R5 | R6 | |
|---|---|---|---|---|---|---|
| 56 | H | OMe | Gentiobiosyl | OMe | OMe | H |
| 57 | H | OMe | Primeverosyl | OMe | OMe | H |
| 58 | OMe | H | Gentiobiosyl | OMe | OMe | OMe |
| 59 | H | OMe | Primeverosyl | OMe | OMe | OMe |
| 60 | OMe | OH | Gentiobiosyl | OMe | OMe | OMe |
| 61 | OMe | OH | Primeverosyl | OMe | OMe | OMe |
| 62 | OMe | OMe | Gentiobiosyl | OMe | OMe | OMe |
| 63 | OMe | OMe | Primeverosyl | OMe | OMe | OMe |
| R1 | R2 | R3 | R4 | R5 | R6 | |
|---|---|---|---|---|---|---|
| 90 | O-glc(6–1)-xyl | OMe | OMe | H | OH | OMe |
| 91 | O-glc(6–1)-xyl | OMe | OMe | H | OMe | OMe |
| 92 | O-glc | OMe | OH | H | OH | OMe |
| 93 | O-glc(6–1)-xyl | OMe | H | OMe | H | OMe |
| 94 | O-glc(6–1)-xyl | OMe | H | OMe | OMe | OMe |
| 95 | OH | O-glc(6–1)-xyl | OMe | OMe | H | OH |
| 96 | O-glc(6–1)-glc | OMe | H | OMe | H | H |
| 97 | O-glc(6–1)-glc | OMe | H | OMe | H | OH |
| 98 | OH | OMe | H | H | O-glc(2–1)-rha | OH |
| 99 | OH | OMe | H | H | O-rha | O-glc |
| R1 | R2 | R3 | R4 | R5 | R6 | R7 | |
|---|---|---|---|---|---|---|---|
| 100 | O-glc(6–1)-xyl | OMe | OMe | OMe | OMe | H | OH |
| 101 | OH | OMe | OMe | OMe | OMe | H | O-glc(6–1)-xyl |
| 102 | O-glc(6–1)-xyl | H | OMe | OMe | OMe | H | OH |
| 103 | OH | H | OMe | OMe | OMe | H | O-glc(6–1)-xyl |
| 104 | O-glc(6–1)-xyl | H | OH | OMe | OMe | H | OH |
| 105 | O-glc(6–1)-glc | H | OH | OMe | OMe | H | OH |
| 106 | O-glc(6–1)-glc | H | OH | OMe | OMe | H | OH |
| Category | Compound [Ref.] | C-1′ | C-3′ | C-5′ |
|---|---|---|---|---|
| Xanthone C-glucoside | 1 [34] | 73.6 | 79.5 | 82.1 |
| 10 [62] | 75.4 | 81.6 | 82.7 | |
| 22 [69] | 74.04 | 79.60 | 80.55 | |
| 29 [77] | 74.1 | 77.9 | 81.3 | |
| 31 [78] | 74.4 | 80.3 | 82.9 | |
| Xanthone O-glucoside | 69 [92] | 102 | 76.4 | 76.3 |
| 79 [77] | 103.6 | 76.0 | 77.5 | |
| 87 [78] | 103.7 | 76.2 | 77.8 | |
| 119 [115] | 105.4 | 77.2 | 76.0 | |
| 123 [118] | 100.4 | 76.4 | 77.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Wang, Y.; Wu, H.; Yuan, M.; Zheng, C.; Xu, H. Xanthone Glucosides: Isolation, Bioactivity and Synthesis. Molecules 2021, 26, 5575. https://doi.org/10.3390/molecules26185575
Huang Q, Wang Y, Wu H, Yuan M, Zheng C, Xu H. Xanthone Glucosides: Isolation, Bioactivity and Synthesis. Molecules. 2021; 26(18):5575. https://doi.org/10.3390/molecules26185575
Chicago/Turabian StyleHuang, Qing, Youyi Wang, Huaimo Wu, Man Yuan, Changwu Zheng, and Hongxi Xu. 2021. "Xanthone Glucosides: Isolation, Bioactivity and Synthesis" Molecules 26, no. 18: 5575. https://doi.org/10.3390/molecules26185575
APA StyleHuang, Q., Wang, Y., Wu, H., Yuan, M., Zheng, C., & Xu, H. (2021). Xanthone Glucosides: Isolation, Bioactivity and Synthesis. Molecules, 26(18), 5575. https://doi.org/10.3390/molecules26185575