Cold Atmospheric Plasma Jet as a Possible Adjuvant Therapy for Periodontal Disease
Abstract
:1. Introduction
2. Results
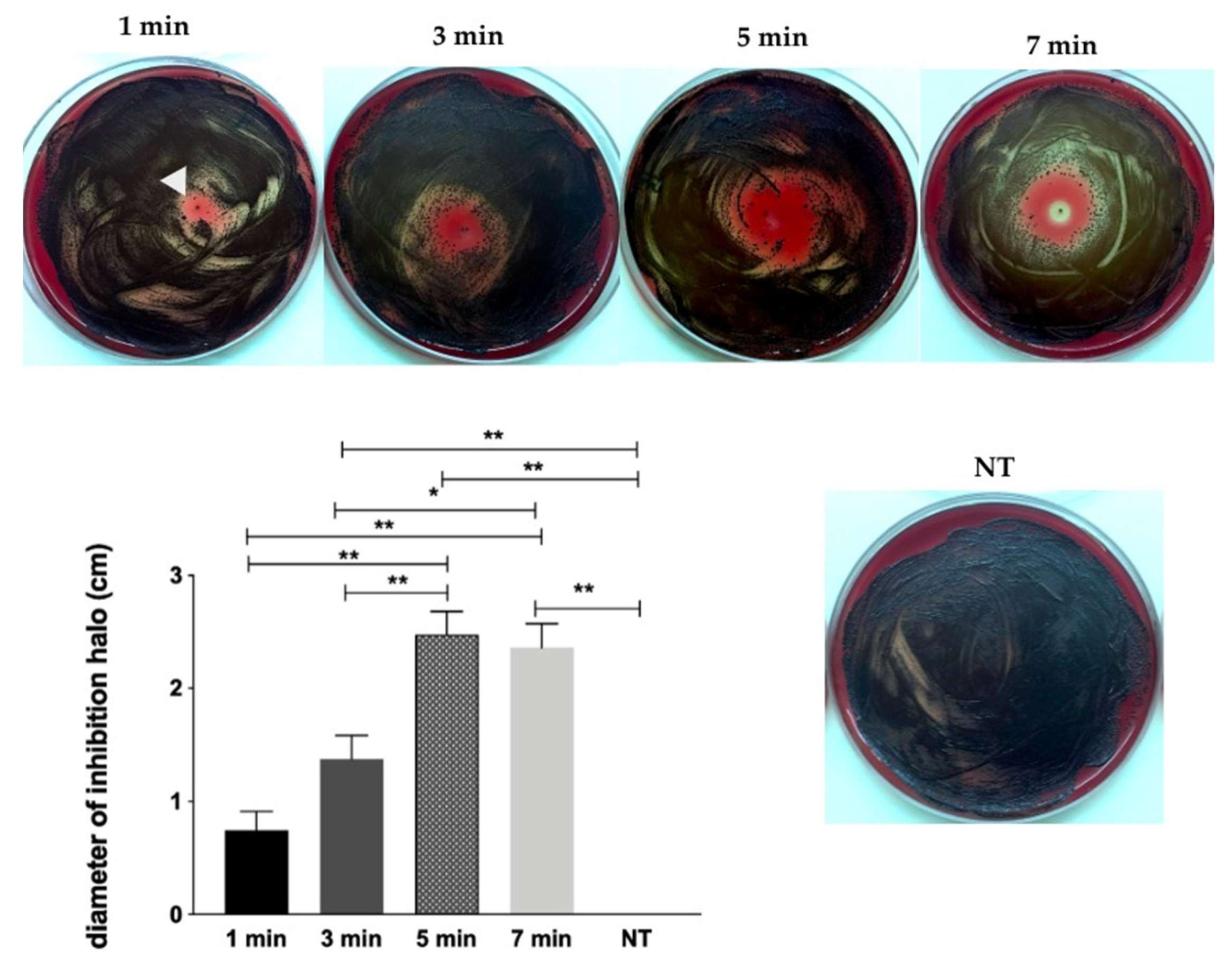
2.1. Screening of CAP Effect on P. gingivalis by Inhibition Halo Methodology
2.2. Effect of CAP on P. gingivalis and Streptococcus gordonii Dual Species Biofilms
Colony Forming Units (CFU) Counting
2.3. Cytotoxicity Evaluation
2.4. In Vivo Experiments
2.4.1. Histomorphometry
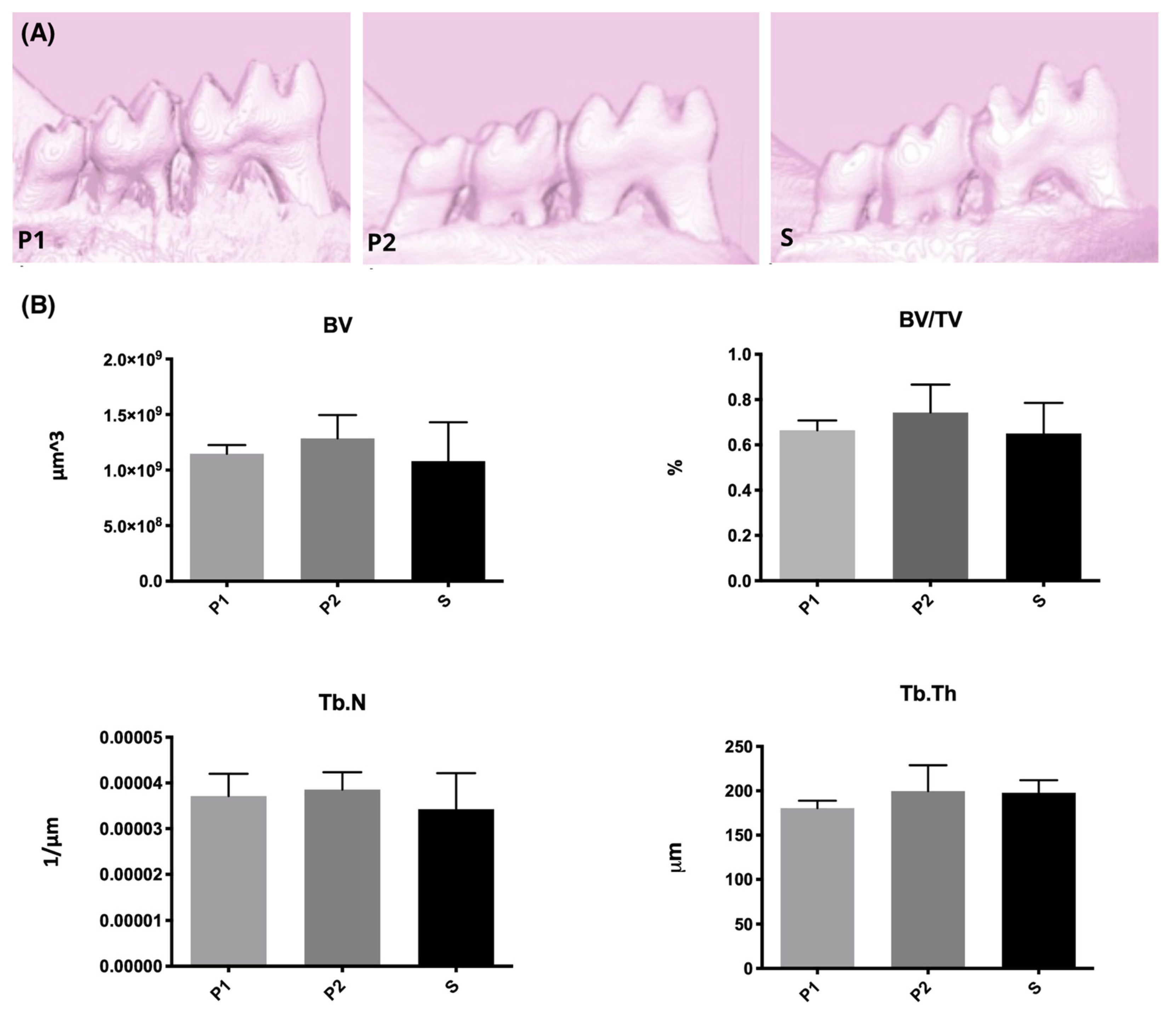
2.4.2. Microcomputed Tomography (Micro-CT)
2.4.3. Histological Effects of Plasma on Health Tissues
3. Discussion
4. Materials and Methods
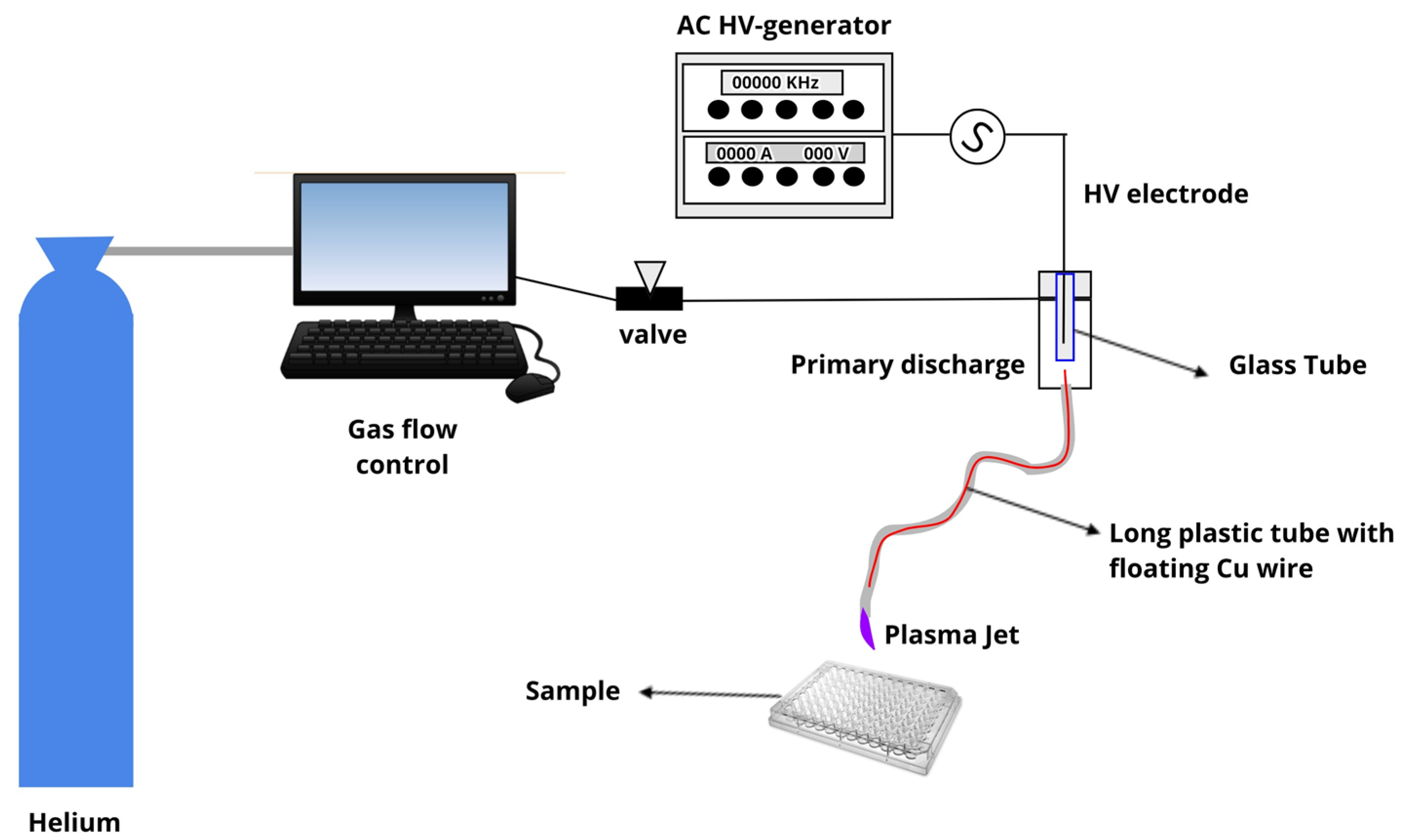
4.1. Plasma Jet
4.2. Screening of CAP Effect on P. gingivalis by Inhibition Halo Methodology
4.3. Effect of CAP on Dual Species Biofilm of P. gingivalis and Streptococcus gordonii
4.3.1. Colony Forming Units (CFU) Counting
4.3.2. Evaluation of Dual Species Biofilm Metabolic Activity by XTT Assay
4.4. Cytotoxicity Evaluation
4.5. In Vivo Experiments
4.5.1. CAP Effects on Experimental Periodontitis Induced in c57bl/6 Mice
- RP Group (root planning): Ligature was kept for 11 days, and root planning was carried out on day 11;
- P1 Group (plasma 1×): Ligature was kept for 11 days. On day 11, root planning was performed and, after the region was exposed to CAP for 5 min;
- P2 Group (plasma 2×) Ligature was kept for 11 days, and root planning was performed. The region was exposed to CAP for 5 min after ligature removal and 2 days after (Day 13) the plasma treatment was repeated.
4.5.2. Histomorphometry
4.5.3. Microcomputed Tomography (Micro-CT)
4.5.4. Histological Effects of CAP on Health Tissues
4.6. Statistics
5. Conclusions
- -
- He-CAP jet was effective against planktonic growth and, most important, against dual-species biofilm (P. gingivalis + S. gordonii) when applied within 1 to 7 min time interval at 1.5 cm.
- -
- The twice repeated 5 min He plasma jet treatment revealed a tendency of mineral tissue improvement (micro-CT).
- -
- A slight, not significant, amelioration in Type I collagen percentage can be noted after two intercalated 5-min plasma applications.
- -
- CAP generated with Helium had no cytotoxic effects, in all tested time periods, using the adopted experimental parameters (e.g., plasma jet power, voltage signal and frequency, amplitude, duty cycle, gas flow, and plasma plume distance to the treated surface), both immediately after exposure and 24 h after treatment.
- -
- No physiological and histological alterations were induced by the in vivo treatments.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bainbridge, B.W.; Coats, S.R.; Darveau, R.P. Porphyromonas gingivalis lipopolysaccharide displays functionally diverse interactions with the innate host defense system. Ann. Periodontol. 2002, 7, 29–37. [Google Scholar] [CrossRef]
- Bartold, P.M.; Van Dyke, T.E. Periodontitis: A host-mediated disruption of microbial homeostasis. Unlearning learned concepts. Periodontology 2000 2013, 62, 203–217. [Google Scholar] [CrossRef] [Green Version]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef] [PubMed]
- Weltmann, K.D.; von Woedtke, T. Basic requirements for plasma sources in medicine. Eur. Phys. J. Appl. Phys. 2011, 55, 13807. [Google Scholar] [CrossRef]
- Kostov, K.G.; Rocha, V.; Koga-Ito, C.Y.; Matos, B.M.; Algatti, M.A.; Honda, R.Y.; Kayama, M.E.; Mota, R.P. Bacterial sterilization by a dielectric barrier discharge (DBD) in air. Surf. Coat. Technol. 2010, 204, 2954–2959. [Google Scholar] [CrossRef]
- Eom, C.S.; Lee, H.K.; Ye, S.; Park, S.M.; Cho, K.H. Use of selective serotonin reuptake inhibitors and risk of fracture: A systematic review and meta-analysis. J. Bone Miner. Res. 2012, 27, 1186–1195. [Google Scholar] [CrossRef]
- Küçük, D.; Savran, L.; Ercan, U.K.; Yarali, Z.B.; Karaman, O.; Kantarci, A.; Sağlam, M.; Köseoğlu, S. Evaluation of efficacy of non-thermal atmospheric pressure plasma in treatment of periodontitis: A randomized controlled clinical trial. Clin. Oral Investig. 2020, 24, 3133–3145. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, X.; Lu, Z.; Li, Y.; Lopes-Virella, M.F.; Yu, H.; Haycraft, C.J.; Li, Q.; Kirkwood, K.L.; Huang, Y. Simvastatin inhibits lipopolysaccharide-induced osteoclastogenesis and reduces alveolar bone loss in experimental periodontal disease. J. Periodontal. Res. 2014, 49, 518–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, G.M.; Corazza, B.J.; Moraes, R.M.; de Oliveira, F.E.; de Oliveira, L.D.; Franco, G.C.; Perrien, D.S.; Elefteriou, F.; Anbinder, A.L. The effect of an inhibitor of gut serotonin (LP533401) during the induction of periodontal disease. J. Periodontal. Res. 2016, 51, 661–668. [Google Scholar] [CrossRef] [Green Version]
- Van Dyke, T.E.; Sima, C. Understanding resolution of inflammation in periodontal diseases: Is chronic inflammatory periodontitis a failure to resolve? Periodontology 2000 2020, 82, 205–213. [Google Scholar] [CrossRef]
- Sczepanik, F.S.C.; Grossi, M.L.; Casati, M.; Goldberg, M.; Glogauer, M.; Fine, N.; Tenenbaum, H.C. Periodontitis is an inflammatory disease of oxidative stress: We should treat it that way. Periodontology 2000 2020, 84, 45–68. [Google Scholar] [CrossRef]
- McCombs, G.B.; Darby, M.L. New discoveries and directions for medical, dental and dental hygiene research: Low temperature atmospheric pressure plasma. Int. J. Dent. Hyg. 2010, 8, 10–15. [Google Scholar] [CrossRef]
- Laroussi, M.; Leipold, F. Evaluation of the roles of reactive species, heat and UV radiation in the inactivation of bacterial cells by air plasma at atmospheric pressure. Int. J. Mass Spectrom. 2004, 233, 81–86. [Google Scholar] [CrossRef]
- Kolb, J.F.; Mohamed, A.A.H.; Price, R.O.; Swanson, R.J.; Bowman, A.; Chiavarini, R.L.; Stacey, M.; Schoenbach, K.H. Cold atmospheric pressure air plasma jet for medical applications. Appl. Phys. Lett. 2008, 92, 241501. [Google Scholar] [CrossRef] [Green Version]
- Alkawareek, M.Y.; Algwari, Q.T.; Gorman, S.P.; Graham, W.G.; O’Connell, D.; Gilmore, B.F. Application of atmospheric pressure nonthermal plasma for the in vitro eradication of bacterial biofilms. FEMS Immunol. Med. Microbiol. 2012, 65, 381–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero-Preston, R.; Ogawa, T.; Uemura, M.; Shumulinsky, G.; Valle, B.L.; Pirini, F.; Ravi, R.; Sidransky, D.; Keidar, M.; Trink, B. Cold atmospheric plasma treatment selectively targets head and neck squamous cell carcinoma cells. Int. J. Mol. Med. 2014, 34, 941–946. [Google Scholar] [CrossRef] [Green Version]
- Eggers, B.; Marciniak, J.; Memmert, S.; Kramer, F.J.; Deschner, J.; Nokhbehsaim, M. The beneficial effect of cold atmospheric plasma on parameters of molecules and cell function involved in wound healing in human osteoblast-like cells in vitro. Odontology 2020, 108, 607–616. [Google Scholar] [CrossRef] [Green Version]
- Mahasneh, A.; Darby, M.; Tolle, S.L.; Hynes, W.; Laroussi, M.; Karakas, E. Inactivation of Porphyromonas gingivalis by Low-Temperature Atmospheric Pressure Plasma. Plasma Med. 2011, 1, 191–204. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Du, X.; Hu, L.; Chen, L. The expression of hBDs in the gingival tissue and keratinocytes from healthy subjects and periodontitis patients. Arch. Oral Biol. 2014, 59, 193–198. [Google Scholar] [CrossRef]
- Xiong, Z.; Du, T.; Lu, X.; Cao, Y.; Pan, Y. How deep can plasma penetrate into a biofilm? Appl. Phys. Lett. 2011, 98, 221503. [Google Scholar] [CrossRef]
- Kleineidam, B.; Nokhbehsaim, M.; Deschner, J.; Wahl, G. Effect of cold plasma on periodontal wound healing-an in vitro study. Clin. Oral Investig. 2019, 23, 1941–1950. [Google Scholar] [CrossRef]
- Arndt, S.; Unger, P.; Wacker, E.; Shimizu, T.; Heinlin, J.; Li, Y.F.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L.; Bosserhoff, A.K.; et al. Cold atmospheric plasma (CAP) changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo. PLoS ONE 2013, 8, e79325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arndt, S.; Landthaler, M.; Zimmermann, J.L.; Unger, P.; Wacker, E.; Shimizu, T.; Li, Y.F.; Morfill, G.E.; Bosserhoff, A.K.; Karrer, S. Effects of cold atmospheric plasma (CAP) on ß-defensins, inflammatory cytokines, and apoptosis-related molecules in keratinocytes in vitro and in vivo. PLoS ONE 2015, 10, e0120041. [Google Scholar] [CrossRef] [Green Version]
- Brun, P.; Pathak, S.; Castagliuolo, I.; Palù, G.; Zuin, M.; Cavazzana, R.; Martines, E. Helium generated cold plasma finely regulates activation of human fibroblast-like primary cells. PLoS ONE 2014, 9, e104397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezaeinezhad, A.; Eslami, P.; Mirmiranpour, H.; Ghomi, H. The effect of cold atmospheric plasma on diabetes-induced enzyme glycation, oxidative stress, and inflammation; in vitro and in vivo. Sci. Rep. 2019, 9, 19958. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xiong, Z.; Du, T.; Zhou, X.; Cao, Y.; Lu, X. Bacterial-killing effect of atmospheric pressure non-equilibrium plasma jet and oral mucosa response. J. Huazhong Univ. Sci. Technol. Med. Sci. 2011, 31, 852–856. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, K.H.; Park, S.Y.; Yoon, S.Y.; Kim, G.H.; Lee, Y.M.; Rhyu, I.C.; Seol, Y.J. The bactericidal effect of an atmospheric-pressure plasma jet on. J. Periodontal Implant Sci. 2019, 49, 319–329. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, Y.; Xie, P.; Ao, X.; Zheng, Z.; Dong, X.; Li, H.; Yu, Q.; Zhu, Z.; Chen, M.; et al. Non-thermal plasma reduces periodontitis-induced alveolar bone loss in rats. Biochem. Biophys. Res. Commun. 2018, 503, 2040–2046. [Google Scholar] [CrossRef]
- Brun, P.; Bernabè, G.; Marchiori, C.; Scarpa, M.; Zuin, M.; Cavazzana, R.; Zaniol, B.; Martines, E. Antibacterial efficacy and mechanisms of action of low power atmospheric pressure cold plasma: Membrane permeability, biofilm penetration and antimicrobial sensitization. J. Appl. Microbiol. 2018, 125, 398–408. [Google Scholar] [CrossRef]
- Ranjan, R.; Krishnamraju, P.V.; Shankar, T.; Gowd, S. Nonthermal Plasma in Dentistry: An Update. J. Int. Soc. Prev. Community Dent. 2017, 7, 71–75. [Google Scholar] [CrossRef]
- Branco-de-Almeida, L.S.; Franco, G.C.; Castro, M.L.; Dos Santos, J.G.; Anbinder, A.L.; Cortelli, S.C.; Kajiya, M.; Kawai, T.; Rosalen, P.L. Fluoxetine Inhibits Inflammatory Response and Bone Loss in a Rat Model of Ligature-Induced Periodontitis. J. Periodontol. 2011, 83, 664–671. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, M.R.; de Aquino, S.G.; Coimbra, L.S.; Spolidorio, L.C.; Kirkwood, K.L.; Rossa, C. Curcumin modulates the immune response associated with LPS-induced periodontal disease in rats. Innate Immun. 2012, 18, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Gilmore, B.F.; Flynn, P.B.; O’Brien, S.; Hickok, N.; Freeman, T.; Bourke, P. Cold Plasmas for Biofilm Control: Opportunities and Challenges. Trends Biotechnol. 2018, 36, 627–638. [Google Scholar] [CrossRef]
- Borges, A.C.; Nishime, T.M.C.; Kostov, K.G.; Lima, G.d.M.G.; Gontijo, A.V.L.; Carvalho, J.N.M.M.; Honda, R.Y.; Koga-Ito, C.Y. Cold atmospheric pressure plasma jet modulates Candida albicans virulence traits. Clin. Plasma Med. 2017, 7, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Song, K.; Zhou, X.; Xiong, Z.; Du, T.; Lu, X.; Cao, Y. Effects of non-equilibrium plasma in the treatment of ligature-induced peri-implantitis. J. Clin. Periodontol. 2015, 42, 478–487. [Google Scholar] [CrossRef]
- Carreiro, A.F.P.; Delben, J.A.; Guedes, S.; Silveira, E.J.D.; Janal, M.N.; Vergani, C.E.; Pushalkar, S.; Duarte, S. Low-temperature plasma on peri-implant-related biofilm and gingival tissue. J. Periodontol. 2019, 90, 507–515. [Google Scholar] [CrossRef]
- Berglundh, T.; Zitzmann, N.U.; Donati, M. Are peri-implantitis lesions different from periodontitis lesions? J. Clin. Periodontol. 2011, 38 (Suppl. 11), 188–202. [Google Scholar] [CrossRef]
- Dionigi, C.; Larsson, L.; Carcuac, O.; Berglundh, T. Cellular expression of DNA damage/repair and reactive oxygen/nitrogen species in human periodontitis and peri-implantitis lesions. J. Clin. Periodontol. 2020, 47, 1466–1475. [Google Scholar] [CrossRef]
- Nishime, T.M.C.; Wagner, R.; Kostov, K.G. Study of Modified Area of Polymer Samples Exposed to a He Atmospheric Pressure Plasma Jet Using Different Treatment Conditions. Polymers 2020, 12, 1028. [Google Scholar] [CrossRef]
- Borges, A.C.; Nishime, T.M.C.; de Moura Rovetta, S.; Lima, G.M.G.; Kostov, K.G.; Thim, G.P.; de Menezes, B.R.C.; Machado, J.P.B.; Koga-Ito, C.Y. Cold Atmospheric Pressure Plasma Jet Reduces Trichophyton rubrum Adherence and Infection Capacity. Mycopathologia 2019, 184, 585–595. [Google Scholar] [CrossRef]
- Borges, A.C.; Lima, G.M.G.; Nishime, T.M.C.; Gontijo, A.V.L.; Kostov, K.G.; Koga-Ito, C.Y. Amplitude-modulated cold atmospheric pressure plasma jet for treatment of oral candidiasis: In vivo study. PLoS ONE 2018, 13, e0199832. [Google Scholar] [CrossRef] [PubMed]
- Kostov, K.G.; Nishime, T.M.C.; Machida, M.; Borges, A.C.; Prysiazhnyi, V.; Koga-Ito, C.Y. Study of cold atmospheric plasma jet at the end of flexible plastic tube for microbial decontamination. Plasma Process. Polym. 2015, 12, 9. [Google Scholar] [CrossRef]
- ISO 10993–5—Biological Evaluation of Medical Devices—Part 5—Tests for In Vitro Cytotoxicity. In Standardization ItIOf, Editor [Internet]. 2009. Available online: https://www.iso.org/obp/ui#iso:std:iso:10993:-5:ed-3:v1:en (accessed on 30 May 2021).


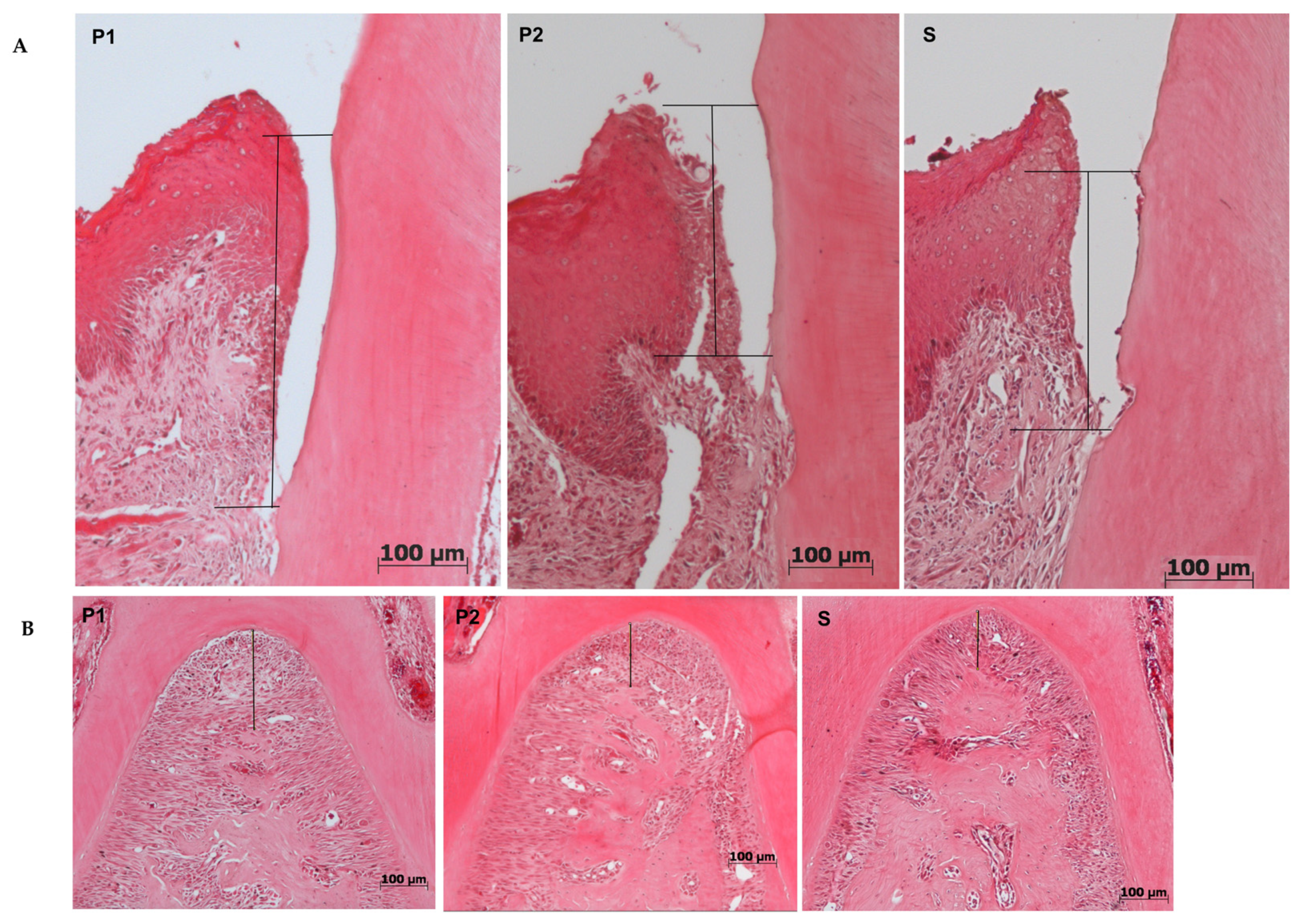
| P1 (Mean ± SD) | P2 (Mean ± SD) | S (Mean ± SD) | |
|---|---|---|---|
| Furcation (μm) | 142.0 ± 42.6 | 147.00 ± 42.3 | 148.00 ± 44.5 |
| JEC-JE (μm) | 242.1 ± 96.9 | 225.9 ± 61.4 | 235.6 ± 60.2 |
| % of collagen | 12.7 ± 5.9 | 13.3 ± 5.2 | 12.9 ± 5.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, G.d.M.G.; Borges, A.C.; Nishime, T.M.C.; Santana-Melo, G.d.F.; Kostov, K.G.; Mayer, M.P.A.; Koga-Ito, C.Y. Cold Atmospheric Plasma Jet as a Possible Adjuvant Therapy for Periodontal Disease. Molecules 2021, 26, 5590. https://doi.org/10.3390/molecules26185590
Lima GdMG, Borges AC, Nishime TMC, Santana-Melo GdF, Kostov KG, Mayer MPA, Koga-Ito CY. Cold Atmospheric Plasma Jet as a Possible Adjuvant Therapy for Periodontal Disease. Molecules. 2021; 26(18):5590. https://doi.org/10.3390/molecules26185590
Chicago/Turabian StyleLima, Gabriela de Morais Gouvêa, Aline Chiodi Borges, Thalita Mayumi Castaldelli Nishime, Gabriela de Fatima Santana-Melo, Konstantin Georgiev Kostov, Marcia Pinto Alves Mayer, and Cristiane Yumi Koga-Ito. 2021. "Cold Atmospheric Plasma Jet as a Possible Adjuvant Therapy for Periodontal Disease" Molecules 26, no. 18: 5590. https://doi.org/10.3390/molecules26185590
APA StyleLima, G. d. M. G., Borges, A. C., Nishime, T. M. C., Santana-Melo, G. d. F., Kostov, K. G., Mayer, M. P. A., & Koga-Ito, C. Y. (2021). Cold Atmospheric Plasma Jet as a Possible Adjuvant Therapy for Periodontal Disease. Molecules, 26(18), 5590. https://doi.org/10.3390/molecules26185590






