Design, Synthesis and Biological Evaluation of Novel Thienylpyridyl- and Thioether-Containing Acetamides and Their Derivatives as Pesticidal Agents
Abstract
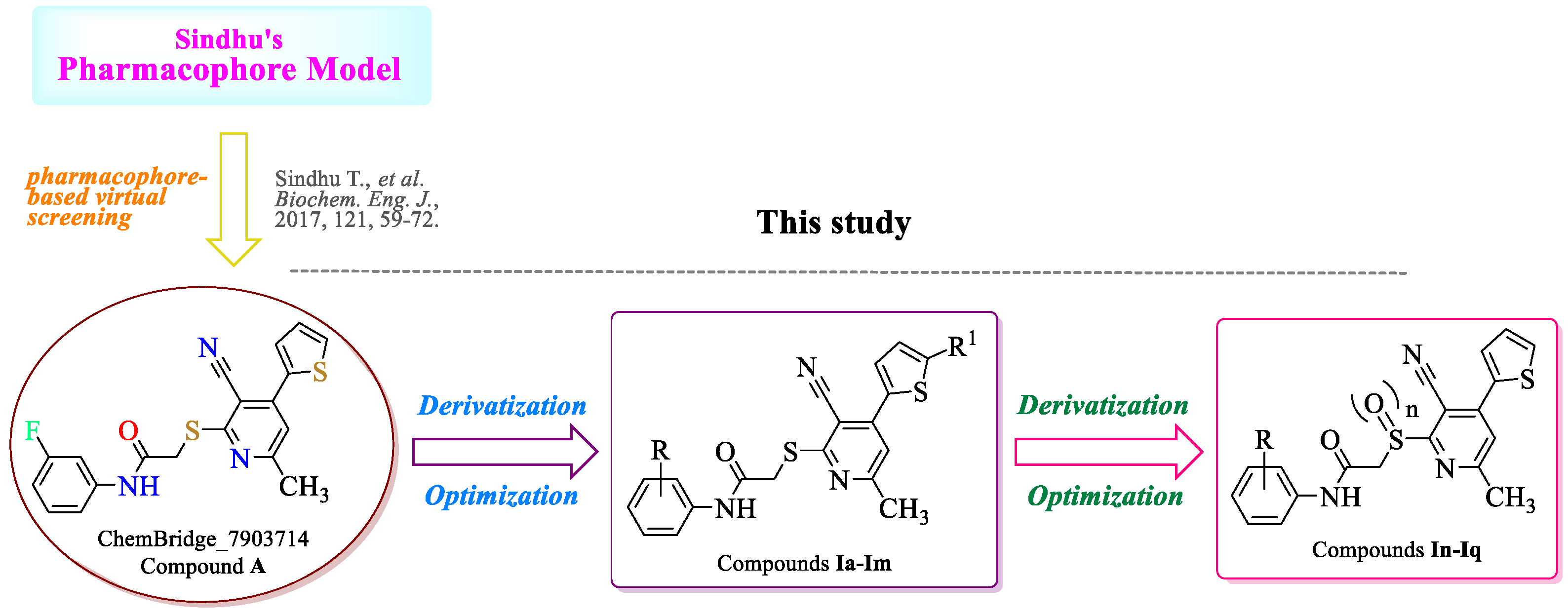
:1. Introduction
2. Results and Discussion
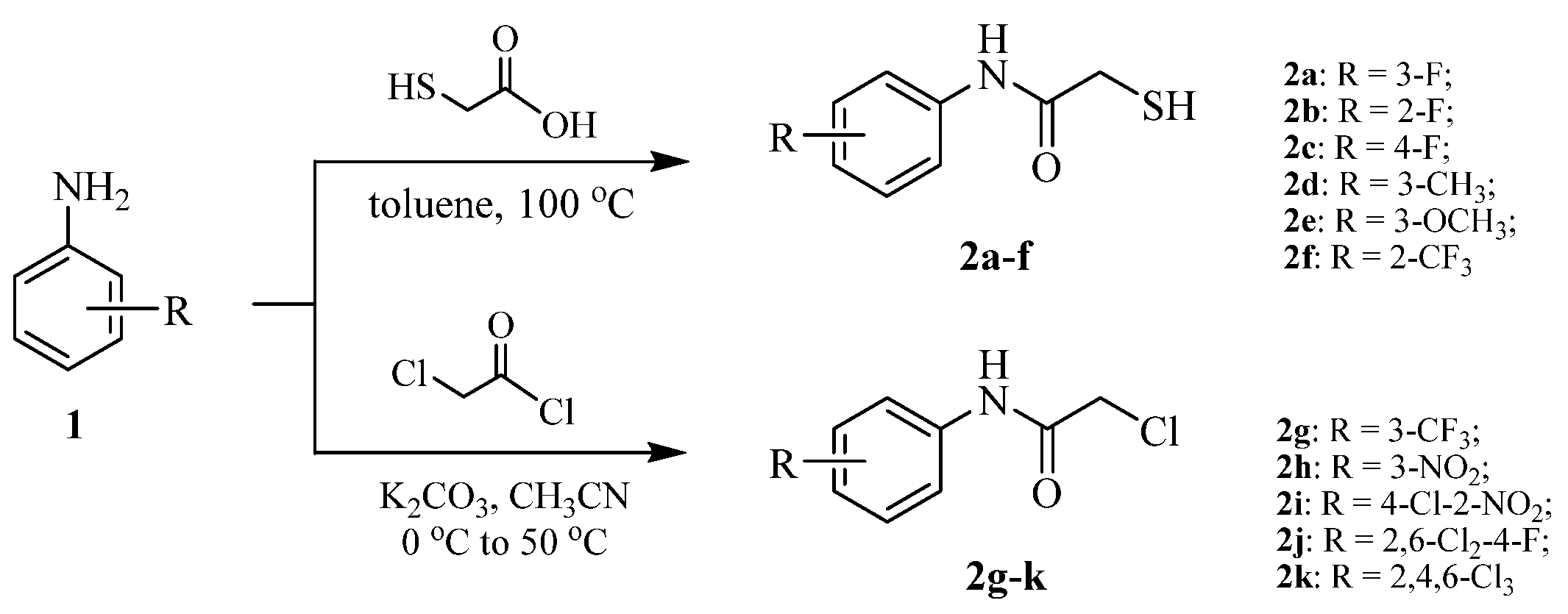
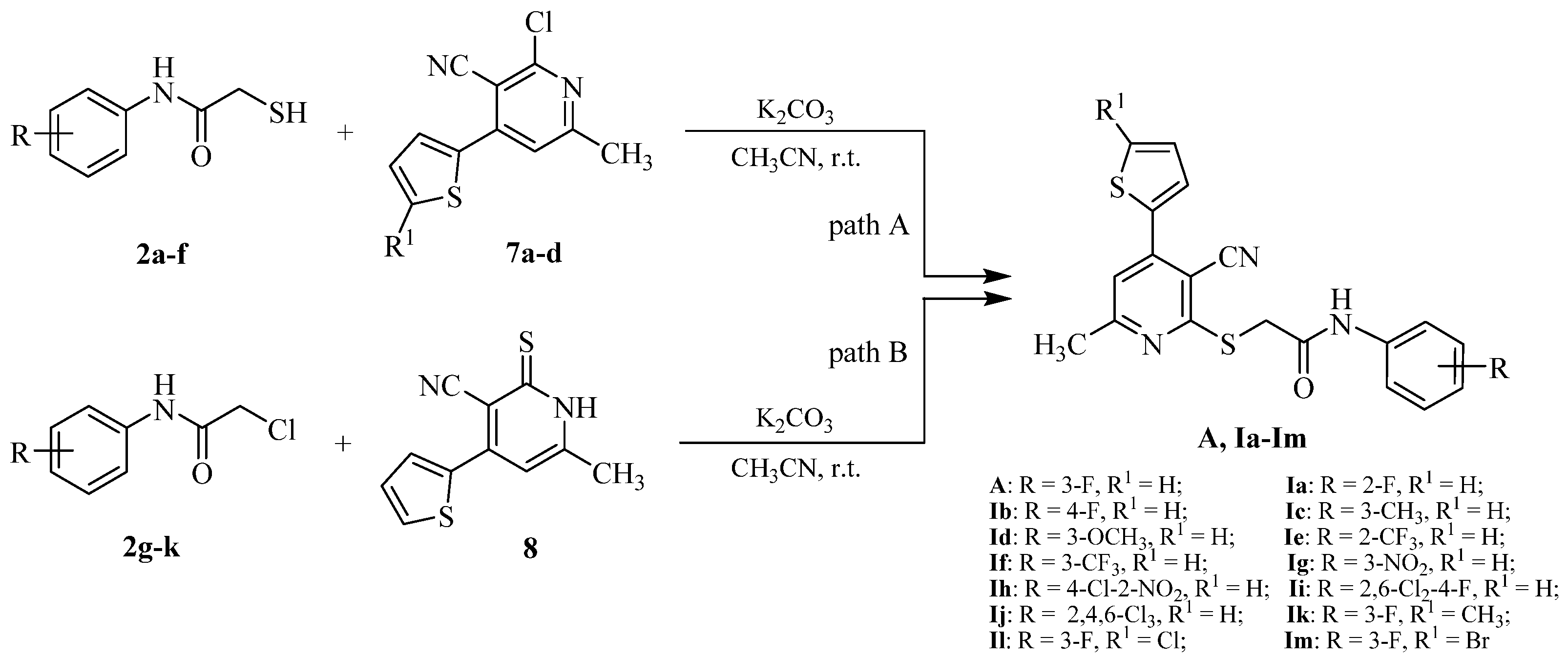
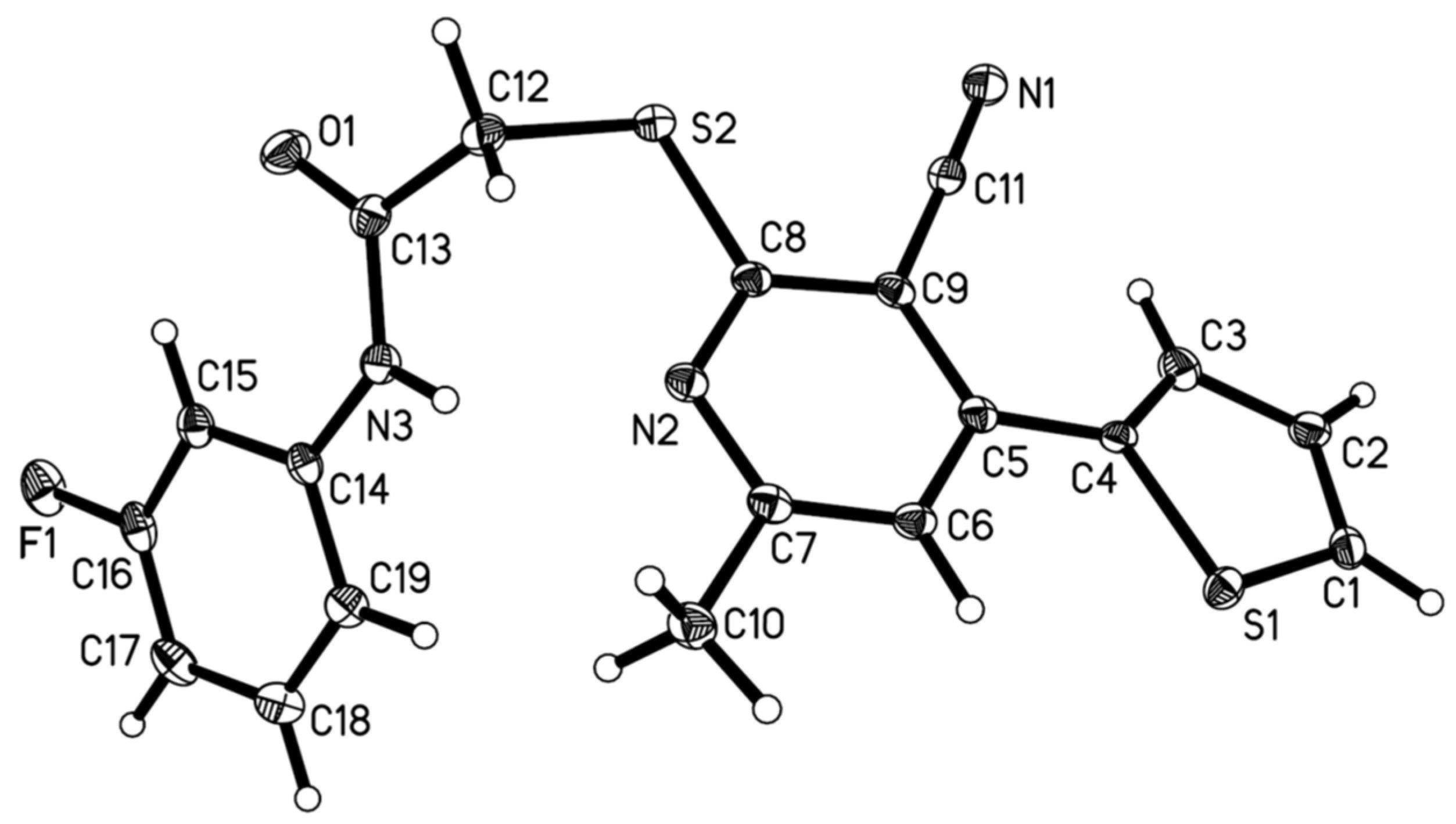
2.1. Chemistry
2.2. Biological Activities and Structure-Activity Relationship
2.2.1. Insecticidal Activity
2.2.2. Fungicidal Activity
3. Experimental Section
3.1. General Information
3.2. Chemistry
3.2.1. Synthetic Procedure of the Intermediates 2-Mercapto-N-arylacetamide (2a–f)
3.2.2. Synthetic Procedure of the Intermediates 2-Chloro-N-arylacetamide (2g–k)
3.2.3. Representative Synthetic Procedure of the Intermediates 4-(5-Substitutedthiophen-2-yl)-6-methyl-2-oxo-1,2-dihydropyridine-3-carbonitrile (6a–d)
3.2.4. Representative Synthetic Procedure of the Intermediates 2-Chloro-4-(5-chlorothiophen-2-yl)-6-methylnicotinonitrile (7a–d)
3.2.5. Synthetic Procedure of Intermediate 6-Methyl-4-(thiophen-2-yl)-2-thioxo-1,2-dihydropyridine-3-carbonitrile (8)
3.2.6. Synthetic Procedure of the Target Compounds A and Ia–Im
3.2.7. Synthetic Procedure of the Target Compounds In–Iq
3.3. Biological Assay
3.3.1. Insecticidal Assay against Oriental Armyworm (Mythimna separata Walker)
3.3.2. Insecticidal Assay against Diamondback Moth (Plutella xylostella L.)
3.3.3. Fungicidal Assay In Vitro
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sparks, T.C.; Lorsbach, B.A. Perspectives on the agrochemical industry and agrochemical discovery. Pest. Manag. Sci. 2017, 73, 672–677. [Google Scholar] [CrossRef]
- Jeanmart, S.; Edmunds, A.J.F.; Lamberth, C.; Pouliot, M. Synthetic approaches to the 2010-2014 new agrochemicals. Bioorg. Med. Chem. 2016, 24, 317–341. [Google Scholar] [CrossRef]
- Casida, J.E. Pesticide detox by design. J. Agric. Food Chem. 2018, 66, 9379–9383. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Yu, W.; Min, L.J.; Wedge, D.E.; Tan, C.X.; Weng, J.Q.; Wu, H.K.; Cantrell, C.L.; Bajsa-Hischel, J.; Hua, X.W.; et al. Synthesis and pesticidal activities of new quinoxalines. J. Agric. Food Chem. 2020, 68, 7324–7332. [Google Scholar] [CrossRef]
- Mahmoud, N.F.H.; El-Sewedy, A. Facile synthesis of novel heterocyclic compounds based on pyridine moiety with pharmaceutical activities. J. Heterocycl. Chem. 2020, 57, 1559–1572. [Google Scholar] [CrossRef]
- Morita, M.; Ueda, T.; Yoneda, T.; Koyanagi, T.; Haga, T. Flonicamid, a novel insecticide with a rapid inhibitory effect on aphid feeding. Pest. Manag. Sci. 2007, 63, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Guan, A.-Y.; Liu, C.-L.; Sun, X.-F.; Xie, Y.; Wang, M.-A. Discovery of pyridine-based agrochemicals by using Intermediate Derivatization Methods. Bioorg. Med. Chem. 2016, 24, 342–353. [Google Scholar] [CrossRef]
- Yu, C.-S.; Wang, Q.; Bajsa-Hirschel, J.; Cantrell, C.; Duke, S.O.; Liu, X.-H. Synthesis, crystal structure, herbicidal activity and SAR study of novel N-(arylmethoxy)-2-chloronicotinamides derived from nicotinic acid. J. Agric. Food Chem. 2021, 69, 6423–6430. [Google Scholar] [CrossRef]
- Plosker, G.L.; Robinson, D.M. Nilotinib. Drugs 2008, 68, 449–459. [Google Scholar] [CrossRef]
- Lahm, G.P.; Stevenson, T.M.; Selby, T.P.; Freudenberger, J.H.; Cordova, D.; Flexner, L.; Bellin, C.A.; Dubas, C.M.; Smith, B.K.; Hughes, K.A.; et al. RynaxypyrTM: A new insecticidal anthranilic diamide that acts as a potent and selective ryanodine receptor activator. Bioorg. Med. Chem. Lett. 2007, 17, 6274–6279. [Google Scholar] [CrossRef]
- Selby, T.P.; Lahm, G.P.; Stevenson, T.M. A retrospective look at anthranilic diamide insecticides: Discovery and lead optimization to chlorantraniliprole and cyantraniliprole. Pest. Manag. Sci. 2017, 73, 658–665. [Google Scholar] [CrossRef]
- Ando, W. Photooxidation of organosulfur compounds. J. Sulfur Chem. 1981, 1, 147–207. [Google Scholar] [CrossRef]
- Timmons, P.; Outcalt, R.; Cramp, S.; Kwiatkowski, P.; Lopes, A.; Sinodis, D.; Cain, P. Pyrrole Insecticides. EP372982 A2, 13 June 1990. application date 8 December 1989. [Google Scholar]
- Tohnishi, M.; Nakao, H.; Furuya, T.; Seo, A.; Kodama, H.; Tsubata, K.; Fujioka, S.; Kodama, H.; Hirooka, T.; Nishimatsu, T. Flubendiamide, a novel insecticide highly active against lepidopterous insect pests. J. Pestic. Sci. 2005, 30, 354–360. [Google Scholar] [CrossRef] [Green Version]
- Sindhu, T.; Venkatesan, T.; Gracy, G.R.; Jalali, S.K.; Rai, A. Exploring the resistance-developing mutations on Ryanodine receptor in diamondback moth and binding mechanism of its activators using computational study. Biochem. Eng. J. 2017, 121, 59–72. [Google Scholar] [CrossRef]
- Trist, I.M.L.; Nannetti, G.; Tintori, C.; Fallacara, A.L.; Deodato, D.; Mercorelli, B.; Palù, G.; Wijtmans, M.; Gospodova, T.; Edink, E.; et al. 4,6-Diphenylpyridines as promising novel anti-influenza agents targeting the PA-PB1 protein-protein interaction: Structure-activity relationships exploration with the aid of molecular modeling. J. Med. Chem. 2016, 59, 2688–2703. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Qin, Y.; Yan, H.; Song, X.; Zhong, R. Synthesis, biological evaluation and molecular modeling studies of N-aryl-2-arylthioacetamides as non-nucleoside HIV-1 reverse transcriptase inhibitors. Chem. Biol. Drug Des. 2010, 76, 330–339. [Google Scholar]
- Wang, W.; Xu, W. Preparation of 2-(2-pyridinylthio)-Acetamide Derivatives as Antibacterial Agents. CN101704780 A, 12 May 2010. application date 27 October 2009. [Google Scholar]
- Bakhite, E.A.; Abdel-Rahman, A.E.; Mohamed, O.S.; Thabet, E.A. Synthesis and reactions of new thienopyridines, pyridothienopyrimidines and pyridothienotriazines. Bull. Korean Chem. Soc. 2002, 23, 1709–1714. [Google Scholar] [CrossRef]
- Elsaman, T.; Fares, M.; Abdel-Aziz, H.A.; Attia, M.I.; Ghabbour, H.A.; Dawood, K.M. A facile synthesis of pyrido[2’,3’:3,4]pyrazolo[1,5-a]pyrimidine and pyrido[2’,3’:3,4]pyrazolo[5,1-c][1,2,4]triazine bearing a thiophene moiety. J. Chem. 2013, 2013, 463515. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.-L.; Li, Q.-N.; Zhan, Y.-Z.; Xiong, L.-X.; Yu, S.-J.; Li, Z.-M. The synthesis and biological activities of [5-(4-substituted phenylsulfinyl/sulfonyl)-1,3-dimethyl-1H-pyrazol-4-yl]-arylmethanones. Phosphorus Sulfur Silicon Relat. Elem. 2014, 189, 483–491. [Google Scholar] [CrossRef]
- Swain, R.C.; Misra, R.N.; Sircar, S.S.G. The use of some thioglycolanilides in inorganic analysis. II. J. Indian Chem. Soc. 1956, 33, 329–334. [Google Scholar]
- Hernández-Núñez, E.; Tlahuext, H.; Moo-Puc, R.; Torres-Gómez, H.; Reyes-Martínez, R.; Cedillo-Rivera, R.; Nava-Zuazo, C.; Navarrete-Vazquez, G. Synthesis and in vitro trichomonicidal, giardicidal and amebicidal activity of N-acetamide(sulfonamide)-2-methyl-4-nitro-1H-imidazoles. Eur. J. Med. Chem. 2009, 44, 2975–2984. [Google Scholar] [CrossRef]
- Desai, A.D.; Chikhalia, K.H. Synthesis and studies of 1-[2-(aryl amino-2-oxo ethyl)amino]-4-(N-methyl piperazino)-benzene derivatives. E-J. Chem. 2005, 2, 15–20. [Google Scholar] [CrossRef]
- Hall, D.M.; Turner, E.E. Structure and antimalarial activity. III. Some benzimidazoles. J. Chem. Soc. 1948, 70, 1909–1911. [Google Scholar] [CrossRef]
- Gowda, B.T.; Usha, K.M.; Jyothi, K. Infrared, 1H and 13C NMR spectral studies on di- and tri-substituted N-aryl amides, 2,6-X2C6H3NHCOCH3-iXi and 2,4,6-X3C6H2NHCOCH3-iXi (X = Cl or CH3 and i = 0, 1, 2 or 3). Z. FuerNat. A Phys. Sci. 2004, 59, 69–76. [Google Scholar] [CrossRef]
- Shatsauskas, A.L.; Abramov, A.A.; Chernenko, S.A.; Kostyuchenko, A.S.; Fisyuk, A.S. Synthesis and photophysical properties of 3-amino-4-arylpyridin-2(1H)-ones. Synthesis 2020, 52, 227–238. [Google Scholar] [CrossRef]
- Wang, B.-L.; Zhu, H.-W.; Ma, Y.; Xiong, L.-X.; Li, Y.-Q.; Zhao, Y.; Zhang, J.-F.; Chen, Y.-W.; Zhou, S.; Li, Z.-M. Synthesis, insecticidal activities, and SAR studies of novel pyridylpyrazole acid derivatives based on amide bridge modification of anthranilic diamide insecticides. J. Agric. Food Chem. 2013, 61, 5483–5493. [Google Scholar] [CrossRef]
- Wang, B.; Wang, H.; Liu, H.; Xiong, L.; Yang, N.; Zhang, Y.; Li, Z. Synthesis and structure-insecticidal activity relationship of novel phenylpyrazole carboxylic acid derivatives containing fluorine moiety. Chin. Chem. Lett. 2020, 31, 739–745. [Google Scholar] [CrossRef]
- Wang, B.-L.; Zhu, H.-W.; Li, Z.-M.; Zhang, X.; Yu, S.-J.; Ma, Y.; Song, H.-B. One-pot synthesis, structure and structure-activity relationship of novel bioactive diphenyl/diethyl (3-bromo-1-(3-chloropyridin-2-yl)-1H-pyrazol-5-yl)(arylamino) methylphosphonates. Pest. Manag. Sci. 2019, 75, 3273–3281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shang, J.; Li, H.; Liu, H.; Song, H.; Wang, B.; Li, Z. Synthesis of novel N-pyridylpyrazole derivatives containing 1,2,4-oxadiazole moiety via 1,3-dipolar cycloaddition and their structures and biological activities. Chin. Chem. Lett. 2020, 31, 1276–1280. [Google Scholar] [CrossRef]


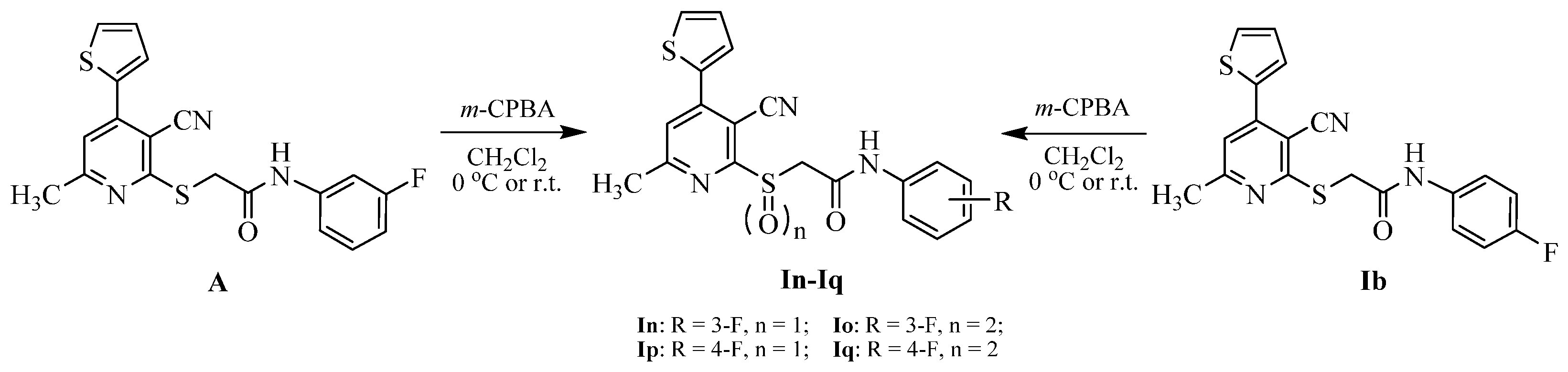
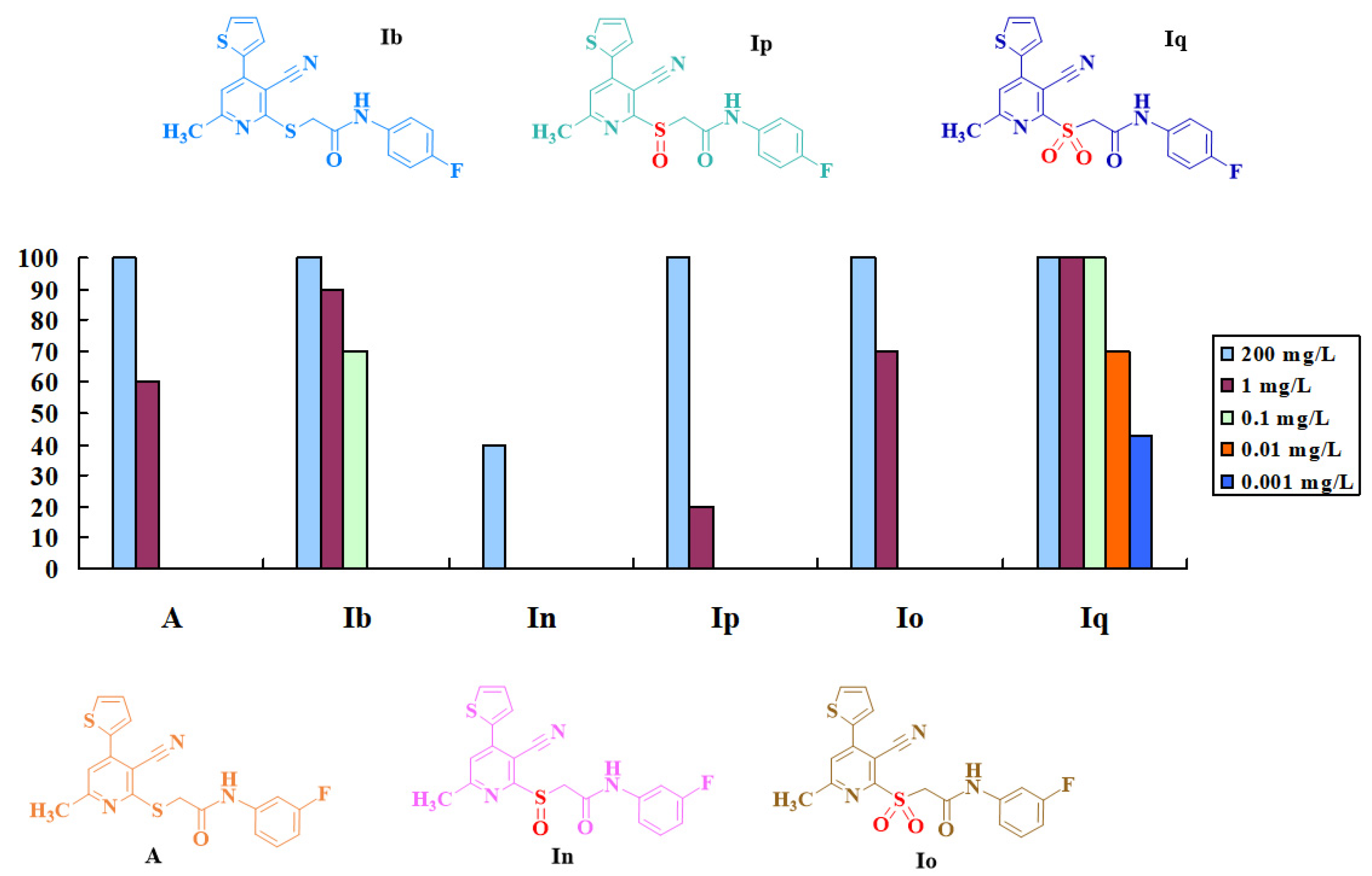
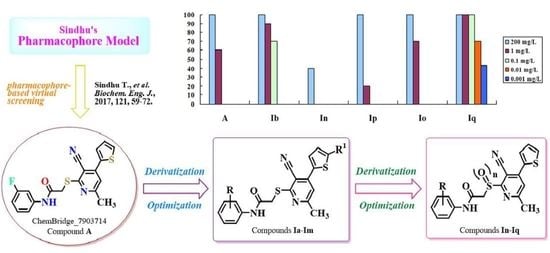
| Compd. | Lethality Rate (%) against Mythimna Separata Walker at 200 mg/L | Lethality Rate (%) against Plutella xylostella L. at Conc. (mg/L) | ||||||
|---|---|---|---|---|---|---|---|---|
| 200 | 100 | 10 | 1 | 0.1 | 0.01 | 0.001 | ||
| A | 60 | 100 | 100 | 90 | 60 | n.t. a | n.t. | n.t. |
| Ia | 20 | 100 | 90 | 75 | n.t. | n.t. | n.t. | n.t. |
| Ib | 30 | 100 | 100 | 100 | 90 | 70 | n.t. | n.t. |
| Ic | 20 | 85 | 60 | 37 | n.t. | n.t. | n.t. | n.t. |
| Id | 40 | 100 | 93 | 80 | n.t. | n.t. | n.t. | n.t. |
| Ie | 30 | 93 | 71 | 55 | n.t. | n.t. | n.t. | n.t. |
| If | 20 | 92 | 77 | 47 | n.t. | n.t. | n.t. | n.t. |
| Ig | 0 | 100 | 100 | 85 | 50 | n.t. | n.t. | n.t. |
| Ih | 35 | 100 | 88 | 63 | 20 | n.t. | n.t. | n.t. |
| Ii | 40 | 90 | 73 | 40 | n.t. | n.t. | n.t. | n.t. |
| Ij | 30 | 80 | 47 | n.t. | n.t. | n.t. | n.t. | n.t. |
| Ik | 70 | 50 | n.t. | n.t. | n.t. | n.t. | n.t. | n.t. |
| Il | 50 | 74 | 55 | n.t. | n.t. | n.t. | n.t. | n.t. |
| Im | 30 | 65 | n.t. | n.t. | n.t. | n.t. | n.t. | n.t. |
| In | 50 | 40 | n.t. | n.t. | n.t. | n.t. | n.t. | n.t. |
| Io | 30 | 100 | 100 | 88 | 70 | n.t. | n.t. | n.t. |
| Ip | 50 | 100 | 90 | 57 | 20 | n.t. | n.t. | n.t. |
| Iq | 30 | 100 | 100 | 100 | 100 | 100 | 70 | 43 |
| chlorantraniliprole | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 83 (37 b) |
| cartap | n.t. | 93 | 67 | 40 | n.t. | n.t. | n.t. | n.t. |
| triflumuron | n.t. | 100 | 100 | 77 | 50 | n.t. | n.t. | n.t. |
| Compd. | y = a + bx | LC50 (mg/L) | R |
|---|---|---|---|
| Iq | y = 8.47 + 1.24x | 0.0016 | 0.9394 |
| triflumuron | y = 5.31 + 0.71x | 0.3627 | 0.9697 |
| chlorantraniliprole | y = 9.75 + 1.13x | 0.0001 | 0.9570 |
| Compd. | % Growth Inhibition | |||||
|---|---|---|---|---|---|---|
| Sclerotinia sclerotiorum | Botrytis cinerea | Pellicularia sasakii | Fusarium oxysporum | Physalospora piricola | Rhizoctonia cerealis | |
| A | 60.7 | 32.3 | 36.1 | 10.8 | 44.7 | 69.2 |
| Ia | 8.9 | 25.8 | 11.1 | 13.5 | 44.7 | 47.7 |
| Ib | 17.9 | 29.0 | 13.9 | 10.8 | 39.5 | 32.3 |
| Ic | 14.3 | 12.9 | 5.6 | 10.8 | 71.1 | 13.8 |
| Id | 53.6 | 6.5 | 27.8 | 13.5 | 81.6 | 33.8 |
| Ie | 17.9 | 25.8 | 13.9 | 10.8 | 55.3 | 52.3 |
| If | 17.9 | 16.1 | 5.6 | 13.5 | 44.7 | 36.9 |
| Ig | 25.0 | 22.6 | 13.9 | 8.1 | 7.9 | 29.2 |
| Ih | 8.9 | 25.8 | 27.8 | 16.2 | 44.7 | 67.7 |
| Ii | 17.9 | 6.5 | 25.0 | 10.8 | 44.7 | 38.5 |
| Ij | 17.9 | 16.1 | 27.8 | 8.1 | 21.1 | 29.2 |
| Ik | 7.1 | 9.7 | 5.6 | 2.7 | 44.7 | 38.5 |
| Il | 17.9 | 29.0 | 13.9 | 8.1 | 57.9 | 52.3 |
| Im | 14.3 | 16.1 | 11.1 | 8.1 | 23.7 | 21.5 |
| In | 44.6 | 16.1 | 19.4 | 8.1 | 31.6 | 43.1 |
| Io | 91.1 | 41.9 | 27.8 | 16.2 | 57.9 | 35.4 |
| Ip | 80.4 | 35.5 | 27.8 | 10.8 | 71.1 | 21.5 |
| Iq | 85.7 | 45.2 | 11.1 | 16.2 | 44.7 | 36.9 |
| chlorothalonil | 98.2 | 74.2 | 100.0 | 73.0 | 84.2 | 89.2 |
| azoxystrobin | 92.9 | 29.0 | 66.7 | 59.5 | 92.1 | 96.9 |
| triadimefon | 98.2 | 64.5 | 83.3 | 70.3 | 65.8 | 96.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Yang, N.; Xiong, L.; Wang, B. Design, Synthesis and Biological Evaluation of Novel Thienylpyridyl- and Thioether-Containing Acetamides and Their Derivatives as Pesticidal Agents. Molecules 2021, 26, 5649. https://doi.org/10.3390/molecules26185649
Li H, Yang N, Xiong L, Wang B. Design, Synthesis and Biological Evaluation of Novel Thienylpyridyl- and Thioether-Containing Acetamides and Their Derivatives as Pesticidal Agents. Molecules. 2021; 26(18):5649. https://doi.org/10.3390/molecules26185649
Chicago/Turabian StyleLi, Huan, Na Yang, Lixia Xiong, and Baolei Wang. 2021. "Design, Synthesis and Biological Evaluation of Novel Thienylpyridyl- and Thioether-Containing Acetamides and Their Derivatives as Pesticidal Agents" Molecules 26, no. 18: 5649. https://doi.org/10.3390/molecules26185649
APA StyleLi, H., Yang, N., Xiong, L., & Wang, B. (2021). Design, Synthesis and Biological Evaluation of Novel Thienylpyridyl- and Thioether-Containing Acetamides and Their Derivatives as Pesticidal Agents. Molecules, 26(18), 5649. https://doi.org/10.3390/molecules26185649