Influence of the Use of an Ionic Liquid as Pre-Hydrodistillation Maceration Medium on the Composition and Yield of Cannabis sativa L. Essential Oil
Abstract
:1. Introduction
2. Results
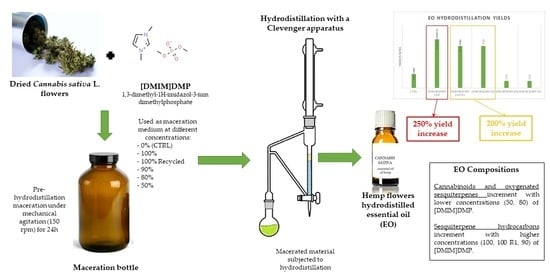
2.1. Essential Oil (EO) Compositions
2.2. Hydrodistillation Yields
3. Discussion
3.1. Essential Oil (EO) Compositions
3.2. Hydrodistillation Yields
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. NMR Analyses
4.3. 1,3-Dimethyl-1H-imidazol-3-ium Dimethylphosphate ([DMIM]DMP) Synthesis and Purification
4.4. Essential Oils (EOs) Maceration and Hydrodistillation
- Control (CTRL): 250 g of hydrodistilled water;
- Pure 1,3-dimethyl-1H-imidazol-3-ium dimethylphosphate ([DMIM]DMP 100): 250 g of [DMIM]DMP;
- Pure [DMIM]DMP recycled and purified after the previous hydrodistillation ([DMIM]DMP 100 R1): 250 of recycled and purified [DMIM]DMP;
- 50% water, 50% [DMIM]DMP ([DMIM]DMP 50): 125 g of hydrodistilled water and 125 g of [DMIM]DMP;
- 20% water, 80% [DMIM]DMP ([DMIM]DMP 80): 50 g hydrodistilled water and 200 g of [DMIM]DMP;
- 10% water, 90% [DMIM]DMP ([DMIM]DMP 90): 25 g of hydrodistilled water and 225 g of [DMIM]DMP.
4.5. Ionic Liquid Recycling
4.6. Gas Chromatography-Electron Impact Mass Spectrometry (GC–EIMS) Analyses and Peak Identifications
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hazekamp, A.; Fischedick, J.T. Cannabis—From cultivar to chemovar. Drug Test. Anal. 2012, 4, 660–667. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Ceccarini, L.; Tavarini, S.; Flamini, G.; Angelini, L.G. Valorisation of hemp inflorescence after seed harvest: Cultivation site and harvest time influence agronomic characteristics and essential oil yield and composition. Ind. Crops Prod. 2019, 139, 111541. [Google Scholar] [CrossRef]
- Stonehouse, G.C.; McCarron, B.J.; Guignardi, Z.S.; El Mehdawi, A.F.; Lima, L.W.; Fakra, S.C.; Pilon-Smits, E.A.H. Selenium Metabolism in Hemp (Cannabis sativa L.)—Potential for Phytoremediation and Biofortification. Environ. Sci. Technol. 2020, 54, 4221–4230. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Tehsin, Z.; Malik, S.T.; Asad, S.A.; Shahzad, M.; Bilal, M.; Shah, M.M.; Khan, S.A. Phytoremediation Potential of Hemp (Cannabis sativa L.): Identification and Characterization of Heavy Metals Responsive Genes. CLEAN–Soil Air Water 2016, 44, 195–201. [Google Scholar] [CrossRef]
- Linger, P.; Müssig, J.; Fischer, H.; Kobert, J. Industrial hemp (Cannabis sativa L.) growing on heavy metal contaminated soil: Fibre quality and phytoremediation potential. Ind. Crops Prod. 2002, 16, 33–42. [Google Scholar] [CrossRef]
- Mirizzi, F.; Troyano, V. Hemp Cultivation & Production in Europe in 2018; EIHA: Brussels, Belgium, 2018. [Google Scholar]
- Ascrizzi, R.; Iannone, M.; Cinque, G.; Marianelli, A.; Pistelli, L.; Flamini, G. “Hemping” the drinks: Aromatizing alcoholic beverages with a blend of Cannabis sativa L. flowers. Food Chem. 2020, 325, 126909. [Google Scholar] [CrossRef]
- Bedini, S.; Flamini, G.; Cosci, F.; Ascrizzi, R.; Benelli, G.; Conti, B. Cannabis sativa and Humulus lupulus essential oils as novel control tools against the invasive mosquito Aedes albopictus and fresh water snail Physella acuta. Ind. Crops Prod. 2016, 85, 318–323. [Google Scholar] [CrossRef] [Green Version]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Santini, G.; Fiorini, D.; Sut, S.; Dall’Acqua, S.; Canale, A.; Maggi, F. The essential oil from industrial hemp (Cannabis sativa L.) by-products as an effective tool for insect pest management in organic crops. Ind. Crops Prod. 2018, 122, 308–315. [Google Scholar] [CrossRef]
- Tabari, M.A.; Khodashenas, A.; Jafari, M.; Petrelli, R.; Cappellacci, L.; Nabissi, M.; Maggi, F.; Pavela, R.; Youssefi, M.R. Acaricidal properties of hemp (Cannabis sativa L.) essential oil against Dermanyssus gallinae and Hyalomma dromedarii. Ind. Crops Prod. 2020, 147, 112238. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Sikora, V.; Semerdjieva, I.B.; Kačániová, M.; Astatkie, T.; Dincheva, I. Grinding and Fractionation during Distillation Alter Hemp Essential Oil Profile and Its Antimicrobial Activity. Molecules 2020, 25, 3943. [Google Scholar] [CrossRef]
- Bertoli, A.; Tozzi, S.; Pistelli, L.; Angelini, L.G. Fibre hemp inflorescences: From crop-residues to essential oil production. Ind. Crops Prod. 2010, 32, 329–337. [Google Scholar] [CrossRef]
- Nissen, L.; Zatta, A.; Stefanini, I.; Grandi, S.; Sgorbati, B.; Biavati, B.; Monti, A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 2010, 81, 413–419. [Google Scholar] [CrossRef]
- Pieracci, Y.; Ascrizzi, R.; Terreni, V.; Pistelli, L.; Flamini, G.; Bassolino, L.; Fulvio, F.; Montanari, M.; Paris, R. Essential Oil of Cannabis sativa L: Comparison of Yield and Chemical Composition of 11 Hemp Genotypes. Molecules 2021, 26, 4080. [Google Scholar] [CrossRef]
- Vuerich, M.; Ferfuia, C.; Zuliani, F.; Piani, B.; Sepulcri, A.; Baldini, M. Yield and Quality of Essential Oils in Hemp Varieties in Different Environments. Agronomy 2019, 9, 356. [Google Scholar] [CrossRef] [Green Version]
- Campiglia, E.; Radicetti, E.; Mancinelli, R. Plant density and nitrogen fertilization affect agronomic performance of industrial hemp (Cannabis sativa L.) in Mediterranean environment. Ind. Crops Prod. 2017, 100, 246–254. [Google Scholar] [CrossRef]
- García-Tejero, I.F.; Durán Zuazo, V.H.; Pérez-Álvarez, R.; Hernández, A.; Casano, S.; Morón, M.; Muriel, J.L. Impact of Plant Density and Irrigation on Yield of Hemp (Cannabis sativa L.) in a Mediterranean Semi-arid Environment. J. Agric. Sci. Technol. 2014, 16, 887–895. [Google Scholar]
- Meier, C.; Mediavilla, V. Factors influencing the yield and the quality of hemp (Cannabis sativa L.) essential oil. J. Int. Hemp Assoc. 1998, 5, 16–20. [Google Scholar]
- Abdollahi, M.; Sefidkon, F.; Calagari, M.; Mousavi, A.; Mahomoodally, M.F. Impact of four hemp (Cannabis sativa L.) varieties and stage of plant growth on yield and composition of essential oils. Ind. Crops Prod. 2020, 155, 112793. [Google Scholar] [CrossRef]
- Fiorini, D.; Scortichini, S.; Bonacucina, G.; Greco, N.G.; Mazzara, E.; Petrelli, R.; Torresi, J.; Maggi, F.; Cespi, M. Cannabidiol-enriched hemp essential oil obtained by an optimized microwave-assisted extraction using a central composite design. Ind. Crops Prod. 2020, 154, 112688. [Google Scholar] [CrossRef]
- Naz, S.; Hanif, M.A.; Ansari, T.M.; Al-Sabahi, J.N. A Comparative Study on Hemp (Cannabis sativa) Essential Oil Extraction Using Traditional and Advanced Techniques. Guang Pu Xue Yu Guang Pu Fen Xi 2017, 37, 306–311. [Google Scholar] [PubMed]
- Karmakar, A.; Mukundan, R.; Yang, P.; Batista, E.R. Solubility model of metal complex in ionic liquids from first principle calculations. RSC Adv. 2019, 9, 18506–18526. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-S.; Wang, X.-X.; Li, Y.; Jiang, K.; Shao, X.-Z.; Du, C.-J. Ionic liquids: Solubility parameters and selectivities for organic solutes. AIChE J. 2013, 59, 3034–3041. [Google Scholar] [CrossRef]
- Mezzetta, A.; Becherini, S.; Pretti, C.; Monni, G.; Casu, V.; Chiappe, C.; Guazzelli, L. Insights into the levulinate-based ionic liquid class: Synthesis, cellulose dissolution evaluation and ecotoxicity assessment. New J. Chem. 2019, 43, 13010–13019. [Google Scholar] [CrossRef]
- Morais, E.S.; Lopes, A.M.D.C.; Freire, M.G.; Freire, C.S.R.; Coutinho, J.A.P.; Silvestre, A.J.D. Use of Ionic Liquids and Deep Eutectic Solvents in Polysaccharides Dissolution and Extraction Processes towards Sustainable Biomass Valorization. Molecules 2020, 25, 3652. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhen, Y.; Jelle, B.P.; Boström, T. Measurements of ionic liquids thermal conductivity and thermal diffusivity. J. Therm. Anal. Calorim. 2017, 128, 279–288. [Google Scholar] [CrossRef]
- Yuan, W.-L.; Yang, X.; He, L.; Xue, Y.; Qin, S.; Tao, G.-H. Viscosity, Conductivity, and Electrochemical Property of Dicyanamide Ionic Liquids. Front. Chem. 2018, 6, 59. [Google Scholar] [CrossRef] [Green Version]
- Guglielmero, L.; Mezzetta, A.; Pomelli, C.S.; Chiappe, C.; Guazzelli, L. Evaluation of the effect of the dicationic ionic liquid structure on the cycloaddition of CO2 to epoxides. J. CO2 Util. 2019, 34, 437–445. [Google Scholar] [CrossRef]
- Martins, V.L.; Torresi, R.M. Ionic liquids in electrochemical energy storage. Curr. Opin. Electrochem. 2018, 9, 26–32. [Google Scholar] [CrossRef]
- Trujillo-Rodríguez, M.J.; Nan, H.; Varona, M.; Emaus, M.N.; Souza, I.D.; Anderson, J.L. Advances of Ionic Liquids in Analytical Chemistry. Anal. Chem. 2019, 91, 505–531. [Google Scholar] [CrossRef]
- Santos, M.M.; Alves, C.; Silva, J.; Florindo, C.; Costa, A.; Petrovski, Ž.; Marrucho, I.M.; Pedrosa, R.; Branco, L.C. Antimicrobial Activities of Highly Bioavailable Organic Salts and Ionic Liquids from Fluoroquinolones. Pharmaceutics 2020, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Grecchi, S.; Ferdeghini, C.; Longhi, M.; Mezzetta, A.; Guazzelli, L.; Khawthong, S.; Arduini, F.; Chiappe, C.; Iuliano, A.; Mussini, P.R. Chiral Biobased Ionic Liquids with Cations or Anions including Bile Acid Building Blocks as Chiral Selectors in Voltammetry. ChemElectroChem 2021, 8, 1377–1387. [Google Scholar] [CrossRef]
- Arnaboldi, S.; Mezzetta, A.; Grecchi, S.; Longhi, M.; Emanuele, E.; Rizzo, S.; Arduini, F.; Micheli, L.; Guazzelli, L.; Mussini, P.R. Natural-based chiral task-specific deep eutectic solvents: A novel, effective tool for enantiodiscrimination in electroanalysis. Electrochim. Acta 2021, 380, 138189. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Yao, S.; Song, H. Aqueous solubilization and extraction of curcumin enhanced by imidazolium, quaternary ammonium, and tropine ionic liquids, and insight of ionic liquids-curcumin interaction. J. Mol. Liq. 2020, 317, 113906. [Google Scholar] [CrossRef]
- Gao, J.; Fang, C.; Lin, Y.; Nie, F.; Ji, H.; Liu, S. Enhanced extraction of astaxanthin using aqueous biphasic systems composed of ionic liquids and potassium phosphate. Food Chem. 2020, 309, 125672. [Google Scholar] [CrossRef]
- Ran, L.; Yang, C.; Xu, M.; Yi, Z.; Ren, D.; Yi, L. Enhanced aqueous two-phase extraction of proanthocyanidins from grape seeds by using ionic liquids as adjuvants. Sep. Purif. Technol. 2019, 226, 154–161. [Google Scholar] [CrossRef]
- Sales, I.; Abranches, D.O.; Costa, P.; Sintra, T.E.; Ventura, S.P.M.; Mattedi, S.; Coutinho, J.A.P.; Freire, M.G.; Pinho, S.P. Enhancing Artemisinin Solubility in Aqueous Solutions: Searching for Hydrotropes based on Ionic Liquids. Fluid Phase Equilib. 2021, 534, 112961. [Google Scholar] [CrossRef]
- Gao, J.; You, J.; Kang, J.; Nie, F.; Ji, H.; Liu, S. Recovery of astaxanthin from shrimp (Penaeus vannamei) waste by ultrasonic-assisted extraction using ionic liquid-in-water microemulsions. Food Chem. 2020, 325, 126850. [Google Scholar] [CrossRef]
- Neto, R.T.; Santos, S.A.O.; Oliveira, J.; Silvestre, A.J.D. Biorefinery of high polymerization degree proanthocyanidins in the context of circular economy. Ind. Crops Prod. 2020, 151, 112450. [Google Scholar] [CrossRef]
- De Faria, E.L.P.; Ferreira, A.M.; Cláudio, A.F.M.; Coutinho, J.A.P.; Silvestre, A.J.D.; Freire, M.G. Recovery of Syringic Acid from Industrial Food Waste with Aqueous Solutions of Ionic Liquids. ACS Sustain. Chem. Eng. 2019, 7, 14143–14152. [Google Scholar] [CrossRef]
- Pistelli, L.; Giovanelli, S.; Margari, P.; Chiappe, C. Considerable effect of dimethylimidazolium dimethylphosphate in cinnamon essential oil extraction by hydrodistillation. RSC Adv. 2016, 6, 52421–52426. [Google Scholar] [CrossRef]
- Ascrizzi, R.; González-Rivera, J.; Pomelli, C.S.; Chiappe, C.; Margari, P.; Costagli, F.; Longo, I.; Tiné, M.R.; Flamini, G.; Duce, C. Ionic liquids, ultra-sounds and microwaves: An effective combination for a sustainable extraction with higher yields. The cumin essential oil case. React. Chem. Eng. 2017, 2, 577–589. [Google Scholar] [CrossRef]
- Pacifico, D.; Miselli, F.; Micheler, M.; Carboni, A.; Ranalli, P.; Mandolino, G. Genetics and Marker-assisted Selection of the Chemotype in Cannabis sativa L. Mol. Breed. 2006, 17, 257–268. [Google Scholar] [CrossRef]
- Wahlström, R.M.; Suurnäkki, A. Enzymatic hydrolysis of lignocellulosic polysaccharides in the presence of ionic liquids. Green Chem. 2015, 17, 694–714. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, X.; Yang, M.; Singh, S.; Cheng, G. Transforming lignocellulosic biomass into biofuels enabled by ionic liquid pretreatment. Bioresour. Technol. 2021, 322, 124522. [Google Scholar] [CrossRef]
- Kuhlmann, E.; Himmler, S.; Giebelhaus, H.; Wasserscheid, P. Imidazolium dialkylphosphates—A class of versatile, halogen-free and hydrolytically stable ionic liquids. Green Chem. 2007, 9, 233–242. [Google Scholar] [CrossRef]
- Chiappe, C.; Margari, P.; Mezzetta, A.; Pomelli, C.S.; Koutsoumpos, S.; Papamichael, M.; Giannios, P.; Moutzouris, K. Temperature effects on the viscosity and the wavelength-dependent refractive index of imidazolium-based ionic liquids with a phosphorus-containing anion. Phys. Chem. Chem. Phys. 2017, 19, 8201–8209. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology. NIST/EPA/NIH Mass Spectral Library; The NIST Mass Spectrometry Data Center: Gaithersburg, MD, USA, 2014.
- Adams, R.P.R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 193-263-3-219. [Google Scholar]
- Choi, Y.H.; Kim, H.K.; Hazekamp, A.; Erkelens, C.; Lefeber, A.W.M.; Verpoorte, R. Metabolomic Differentiation of Cannabis sativa Cultivars Using 1H NMR Spectroscopy and Principal Component Analysis. J. Nat. Prod. 2004, 67, 953–957. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Flamini, G.; Giusiani, M.; Stefanelli, F.; Deriu, V.; Chericoni, S. VOCs as fingerprints for the chemical profiling of hashish samples analyzed by HS-SPME/GC–MS and multivariate statistical tools. Forensic Toxicol. 2018, 36, 243–260. [Google Scholar] [CrossRef]



| Compounds | l.r.i. 1 | Relative Abundance (AVG ± SD) | |||||
|---|---|---|---|---|---|---|---|
| CTRL | [DMIM]DMP 100 | [DMIM]DMP 100 R1 | [DMIM]DMP 90 | [DMIM]DMP 80 | [DMIM]DMP 50 | ||
| hexanal | 805 | - 2 | - | 0.1 ± 0.03 | 0.2 ± 0.02 | - | - |
| α-pinene 3 | 939 | 2.1 ± 0.71 BC | 2.8 ± 0.03 B | 5.4 ± 1.10 A | 5.4 ± 0.89 A | 0.9 ± 0.30 C | 4.9 ± 0.90 A |
| camphene | 954 | - | - | 0.1 ± 0.03 | 0.1 ± 0.02 | - | 0.2 ± 0.05 |
| β-pinene | 980 | 0.8 ± 0.28 B | 0.8 ± 0.00 B | 2.1 ± 0.39 A | 2.2 ± 0.34 A | 0.7 ± 0.20 B | 2.4 ± 0.38 A |
| myrcene | 991 | 2.1 ± 0.58 B | 2.2 ± 0.05 B | 7.6 ± 1.08 A | 6.7 ± 0.87 A | 1.9 ± 0.60 B | 7.5 ± 1.27 A |
| δ-3-carene | 1012 | - | - | 0.1 ± 0.02 | 0.2 ± 0.03 | - | 0.2 ± 0.03 |
| p-cymene | 1024 | 0.2 ± 0.04 | 0.2 ± 0.00 | 0.3 ± 0.04 | 0.4 ± 0.06 | 0.2 ± 0.06 | 0.5 ± 0.06 |
| limonene | 1031 | 2.2 ± 0.64 B | 2.2 ± 0.03B | 2.9 ± 0.37 A,B | 2.7 ± 0.33 A,B | 1.2 ± 0.35 C | 3.0 ± 0.36 A |
| 1,8-cineole | 1035 | 0.6 ± 0.18 | 0.8 ± 0.02 | 0.5 ± 0.07 | 0.5 ± 0.06 | 0.3 ± 0.07 | 0.3 ± 0.06 |
| (E)-β-ocimene | 1050 | 0.2 ± 0.04 | 0.2 ± 0.01 | 0.4 ± 0.04 | 0.2 ± 0.03 | - | 0.3 ± 0.02 |
| γ-terpinene | 1062 | 0.1 ± 0.01 | 0.1 ± 0.08 | - | - | - | - |
| cis-sabinene hydrate | 1070 | 0.1 ± 0.08 | - | - | - | - | - |
| terpinolene | 1089 | 1.2 ± 0.28 BC | 1.0 ± 0.03C | 2.0 ± 0.18 A | 1.4 ± 0.14 B | 0.5 ± 0.11 D | 1.9 ± 0.13 A |
| methyl benzoate | 1091 | - | 0.2 ± 0.01 | - | - | - | - |
| linalool | 1100 | 0.3 ± 0.06 | 0.2 ± 0.03 | 0.3 ± 0.00 | 0.4 ± 0.06 | 0.4 ± 0.08 | 0.4 ± 0.03 |
| perillene | 1101 | - | - | 0.1 ± 0.07 | 0.1 ± 0.01 | 0.1 ± 0.07 | 0.2 ± 0.03 |
| nonanal | 1103 | - | - | 0.1 ± 0.01 | 0.1 ± 0.03 | - | 0.2 ± 0.02 |
| fenchol | 1115 | 0.3 ± 0.09 | 0.2 ± 0.01 | 0.3 ± 0.01 | 0.3 ± 0.04 | 0.3 ± 0.05 | 0.4 ± 0.02 |
| cis-p-menth-2-en-1-ol | 1123 | 0.2 ± 0.06 | 0.1 ± 0.11 | 0.2 ± 0.01 | 0.2 ± 0.04 | 0.2 ± 0.04 | 0.2 ± 0.00 |
| trans-p-mentha-2,8-dien-1-ol | 1125 | - | - | - | 0.2 ± 0.02 | - | - |
| cis-p-mentha-2,8-dien-1-ol | 1138 | - | - | 0.2 ± 0.00 | 0.4 ± 0.04 | 0.9 ± 0.18 | 0.4 ± 0.01 |
| pinocarveol | 1140 | - | - | - | - | 0.1 ± 0.07 | - |
| trans-verbenol | 1145 | - | - | 0.1 ± 0.08 | - | 0.1 ± 0.03 | 0.1 ± 0.01 |
| (E)-tagetone | 1146 | 0.1 ± 0.09 | - | - | - | - | - |
| borneol | 1167 | 0.1 ± 0.01 | - | - | 0.1 ± 0.10 | 0.1 ± 0.01 | 0.1 ± 0.09 |
| 4-terpineol | 1178 | 0.4 ± 0.07 | 0.4 ± 0.05 | 0.2 ± 0.01 | 0.5 ± 0.03 | 0.2 ± 0.04 | 0.3 ± 0.00 |
| p-cymen-8-ol | 1185 | 0.2 ± 0.01 | - | - | 0.4 ± 0.01 | 0.3 ± 0.05 | 0.2 ± 0.02 |
| α-terpineol | 1190 | 0.4 ± 0.04 | 0.2 ± 0.02 | 0.3 ± 0.02 | 0.5 ± 0.03 | 0.4 ± 0.07 | 0.4 ± 0.00 |
| carveol | 1228 | - | - | - | 0.1 ± 0.01 | - | - |
| α-ylangene | 1372 | - | - | 0.1 ± 0.01 | - | - | - |
| isocaryophyllene | 1405 | 0.2 ± 0.01 | 0.3 ± 0.05 | 0.4 ± 0.06 | 0.3 ± 0.01 | 0.3 ± 0.00 | 0.1 ± 0.09 |
| β-caryophyllene | 1418 | 32.1 ± 0.68 B | 40.3 ± 1.42 A | 30.3 ± 1.00 B | 22.5 ± 0.54 C | 15.6 ± 1.27 D | 10.2 ± 0.06 E |
| trans-α-bergamotene | 1438 | 0.2 ± 0.06 | 0.5 ± 0.04 | 0.5 ± 0.03 | 0.3 ± 0.01 | 0.3 ± 0.00 | - |
| α-guaiene | 1440 | 0.3 ± 0.06 | 0.6 ± 0.02 | 0.4 ± 0.01 | 0.3 ± 0.01 | 0.2 ± 0.00 | - |
| α-himachalene | 1450 | - | 0.1 ± 0.13 | - | 0.1 ± 0.09 | 0.1 ± 0.00 | - |
| α-humulene | 1455 | 13.1 ± 0.13 B | 15.3 ± 0.47 A | 12.4 ± 0.43 C | 10.0 ± 0.22 D | 7.8 ± 0.45 E | 5.3 ± 0.04 F |
| (E)-beta-farnesene | 1459 | - | 0.2 ± 0.23 | 0.4 ± 0.06 | 0.3 ± 0.06 | 0.2 ± 0.02 | - |
| alloaromadendrene | 1461 | 0.7 ± 0.04 C | 1.1 ± 0.05 A | 0.9 ± 0.04 B | 0.7 ± 0.01 C | 0.6 ± 0.02 D | 0.3 ± 0.00 E |
| γ-gurjunene | 1469 | - | - | 0.3 ± 0.01 | 0.2 ± 0.01 | 0.5 ± 0.02 | 0.2 ± 0.02 |
| γ-muurolene | 1476 | - | - | 0.1 ± 0.08 | 0.1 ± 0.00 | 0.1 ± 0.00 | - |
| β-chamigrene | 1477 | - | - | - | 0.6 ± 0.01 | - | - |
| γ-selinene | 1482 | 0.6 ± 0.06 | 0.9 ± 0.04 | 0.7 ± 0.08 | - | 0.5 ± 0.01 | 0.1 ± 0.19 |
| β-selinene | 1485 | 2.4 ± 0.13 B | 3.8 ± 0.01 A | 2.6 ± 0.17 B,C | 2.1 ± 0.02 B | 1.8 ± 0.02 B,C | 1.0 ± 0.01C |
| valencene | 1492 | 1.9 ± 0.02 C | 3.4 ± 0.03 A | 2.3 ± 0.06 B | 1.7 ± 0.13 C | 1.4 ± 0.01D | 0.7 ± 0.07 E |
| α-bulnesene | 1505 | 0.4 ± 0.04 | 0.8 ± 0.02 | 0.4 ± 0.02 | 0.3 ± 0.01 | 0.2 ± 0.04 | 0.1 ± 0.09 |
| α-farnesene | 1507 | - | - | 0.5 ± 0.04 | 0.3 ± 0.03 | - | 0.1 ± 0.08 |
| β-bisabolene | 1509 | - | 0.3 ± 0.04 | - | - | 0.1 ± 0.06 | - |
| trans-γ-cadinene | 1514 | - | - | - | 0.1 ± 0.07 | 0.1 ± 0.11 | - |
| 7-epi-α-selinene | 1517 | 0.3 ± 0.01 | 0.5 ± 0.02 | 0.5 ± 0.13 | 0.4 ± 0.01 | 0.5 ± 0.04 | 0.1 ± 0.19 |
| β-cadinene | 1520 | - | 0.2 ± 0.00 | - | 0.2 ± 0.00 | - | - |
| δ-cadinene | 1523 | - | 0.2 ± 0.00 | 0.2 ± 0.21 | - | 0.1 ± 0.16 | - |
| selina-3,7(11)-diene | 1542 | 1.8 ± 0.35 D,E | 2.4 ± 0.04 D | 2.1 ± 0.07C | 1.8 ± 0.04 B | 1.6 ± 0.11 A | 1.2 ± 0.09 E |
| α-calacorene | 1546 | - | 0.2 ± 0.04 | - | 0.1 ± 0.13 | - | - |
| elemol | 1549 | 0.4 ± 0.04 | 0.3 ± 0.01 | 0.3 ± 0.03 | 0.6 ± 0.01 | 0.8 ± 0.07 | 0.8 ± 0.04 |
| (E)-nerolidol | 1565 | 0.3 ± 0.06 | - | 0.3 ± 0.08 | 0.7 ± 0.01 | 1.1 ± 0.09 | 1.0 ± 0.11 |
| palustrol | 1568 | 0.5 ± 0.09 | - | 0.4 ± 0.13 | 0.5 ± 0.01 | - | 0.8 ± 0.06 |
| caryophyllene alcohol | 1569 | - | - | - | - | 0.9 ± 0.09 | - |
| caryophyllene oxide | 1582 | 12.8 ± 0.78 D | 7.3 ± 0.44 F | 10.2 ± 0.50 E | 15.9 ± 0.37 C | 23.6 ± 0.35 A | 22.0 ± 0.25 B |
| viridiflorol | 1590 | - | - | - | 0.2 ± 0.25 | 0.5 ± 0.13 | 0.2 ± 0.31 |
| humulene oxide | 1606 | 4.3 ± 0.16 D | 2.5 ± 0.09 F | 3.2 ± 0.16 E | 4.9 ± 0.04 C | 7.8 ± 0.10 A | 7.2 ± 0.15 B |
| selin-6-en-4-ol | 1618 | 1.7 ± 0.18 B | 1.0 ± 0.14 D | 0.9 ± 0.05 D | 1.3 ± 0.02 C | 1.8 ± 0.14 B | 2.4 ± 0.18 A |
| 1-epi-cubenol | 1629 | 0.2 ± 0.22 | - | 0.2 ± 0.06 | 0.1 ± 0.11 | 0.7 ± 0.03 | - |
| caryophylla-4(14),8(15)-dien-5-ol | 1636 | 3.8 ± 0.37 B | 1.9 ± 0.37 C | 1.9 ± 0.21 D | 2.9 ± 0.07 C | 5.2 ± 0.25 A | 4.9 ± 0.35 A |
| β-eudesmol | 1649 | - | - | - | - | - | 0.2 ± 0.28 |
| α-eudesmol | 1653 | 0.4 ± 0.01 | - | - | - | - | - |
| neointermedeol | 1660 | 1.5 ± 0.09 A | 0.8 ± 0.21 B | 0.5 ± 0.05 B | 0.9 ± 0.02 B | 1.8 ± 0.16 A | 1.9 ± 0.47 A |
| 14-hydroxy-9-epi-(E)-caryophyllene | 1665 | 1.7 ± 0.24 C | 0.7 ± 0.14 D | 0.8 ± 0.08 D | 1.4 ± 0.03 C | 3.0 ± 0.29 A | 2.6 ± 0.29 B |
| α-bisabolol | 1685 | 0.7 ± 0.08 | 0.5 ± 0.05 | 0.4 ± 0.01 | 0.5 ± 0.03 | 0.9 ± 0.13 | 0.8 ± 0.11 |
| juniper camphor | 1695 | 0.5 ± 0.07 | 0.3 ± 0.05 | 0.2 ± 0.01 | 0.4 ± 0.01 | 0.6 ± 0.04 | 0.6 ± 0.06 |
| hexahydrofarnesyl acetone | 1845 | - | - | 0.8 ± 0.12 | 0.8 ± 0.05 | 0.9 ± 0.14 | 0.4 ± 0.00 |
| cannabidiol | 2431 | 3.4 ± 0.49 B | 0.9 ± 0.13 C | 0.6 ± 0.04 C | 1.1 ± 0.01 C | 6.0 ± 0.90 A | 6.7 ± 0.39 A |
| Δ9-tetrahydro-cannabinol | 2468 | 0.1 ± 0.04 B | - B | - B | - B | 0.1 ± 0.08 B | 1.3 ± 0.66 A |
| Monoterpene hydrocarbons | 8.8 ± 2.59 B | 9.5 ± 0.16 B | 21.0 ± 3.26 A | 19.3 ± 2.70 A | 5.5 ± 1.62 B | 20.8 ± 3.18 A | |
| Oxygenated monoterpenes | 2.7 ± 0.67 A,B,C | 1.9 ± 0.20 C | 2.1 ± 0.06 B,C | 3.7 ± 0.45 A | 3.3 ± 0.76 A | 2.9 ± 0.09 A,B | |
| Sesquiterpene hydrocarbons | 54.0 ± 0.11B | 71.1 ± 1.55 A | 55.1 ± 2.11 B | 42.2 ± 1.16 C | 32.0 ± 1.23 D | 19.3 ± 0.81 E | |
| Oxygenated sesquiterpenes | 28.7 ± 2.39 C | 15.4 ± 1.23 E | 19.3 ± 1.27 D | 30.1 ± 0.83 C | 48.8 ± 1.16 A | 45.3 ± 2.17 B | |
| Apocarotenoids | - D | - D | 0.8 ± 0.12 B | 0.8 ± 0.05 A,B | 0.9 ± 0.14 A | 0.4 ± 0.00 C | |
| Cannabinoids | 3.5 ± 0.54 C | 0.9 ± 0.13 D | 0.6 ± 0.04 D | 1.1 ± 0.01 D | 6.1 ± 0.98 B | 8.0 ± 0.27 A | |
| Other non-terpene derivatives | - C | 0.2 ± 0.01 B | 0.2 ± 0.02 B | 0.3 ± 0.05 A | - C | 0.2 ± 0.02 B | |
| Total identified (%) | 97.8 ± 0.44 | 99.1 ± 0.18 | 99.0 ± 0.11 | 97.5 ± 1.26 | 96.6 ± 1.33 | 96.9 ± 0.58 | |
| Distillation yield (% w/w) | 0.03 ± 0.01 B | 0.06 ± 0.01 A | 0.07 ± 0.01 A | 0.06 ± 0.00 A | 0.01 ± 0.00 C | 0.01 ± 0.00 C | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezzetta, A.; Ascrizzi, R.; Martinelli, M.; Pelosi, F.; Chiappe, C.; Guazzelli, L.; Flamini, G. Influence of the Use of an Ionic Liquid as Pre-Hydrodistillation Maceration Medium on the Composition and Yield of Cannabis sativa L. Essential Oil. Molecules 2021, 26, 5654. https://doi.org/10.3390/molecules26185654
Mezzetta A, Ascrizzi R, Martinelli M, Pelosi F, Chiappe C, Guazzelli L, Flamini G. Influence of the Use of an Ionic Liquid as Pre-Hydrodistillation Maceration Medium on the Composition and Yield of Cannabis sativa L. Essential Oil. Molecules. 2021; 26(18):5654. https://doi.org/10.3390/molecules26185654
Chicago/Turabian StyleMezzetta, Andrea, Roberta Ascrizzi, Marco Martinelli, Filomena Pelosi, Cinzia Chiappe, Lorenzo Guazzelli, and Guido Flamini. 2021. "Influence of the Use of an Ionic Liquid as Pre-Hydrodistillation Maceration Medium on the Composition and Yield of Cannabis sativa L. Essential Oil" Molecules 26, no. 18: 5654. https://doi.org/10.3390/molecules26185654
APA StyleMezzetta, A., Ascrizzi, R., Martinelli, M., Pelosi, F., Chiappe, C., Guazzelli, L., & Flamini, G. (2021). Influence of the Use of an Ionic Liquid as Pre-Hydrodistillation Maceration Medium on the Composition and Yield of Cannabis sativa L. Essential Oil. Molecules, 26(18), 5654. https://doi.org/10.3390/molecules26185654