Early-Age Hydration Reaction and Strength Formation Mechanism of Solid Waste Silica Fume Modified Concrete
Abstract
:1. Introduction
2. Experimental Program
2.1. Raw Materials and Mix Proportions of Concrete
2.2. Sample Preparation and Experimental Methods
3. Results
3.1. Working Performance of Fresh Concrete
3.2. Compressive Strength of Early-Age Concrete Cubes
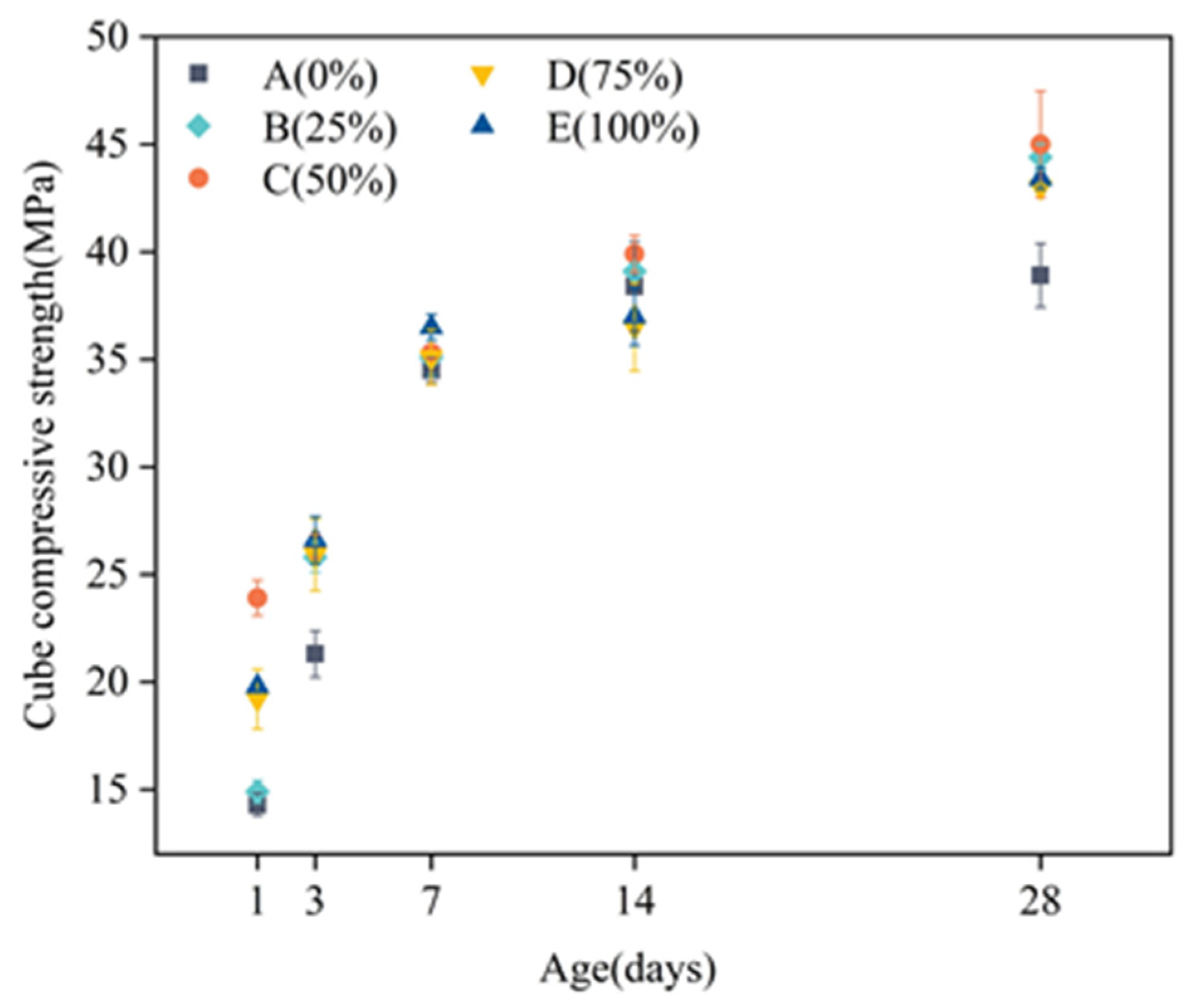
3.2.1. Influence of Age on the Compressive Strength of Concrete Cubes
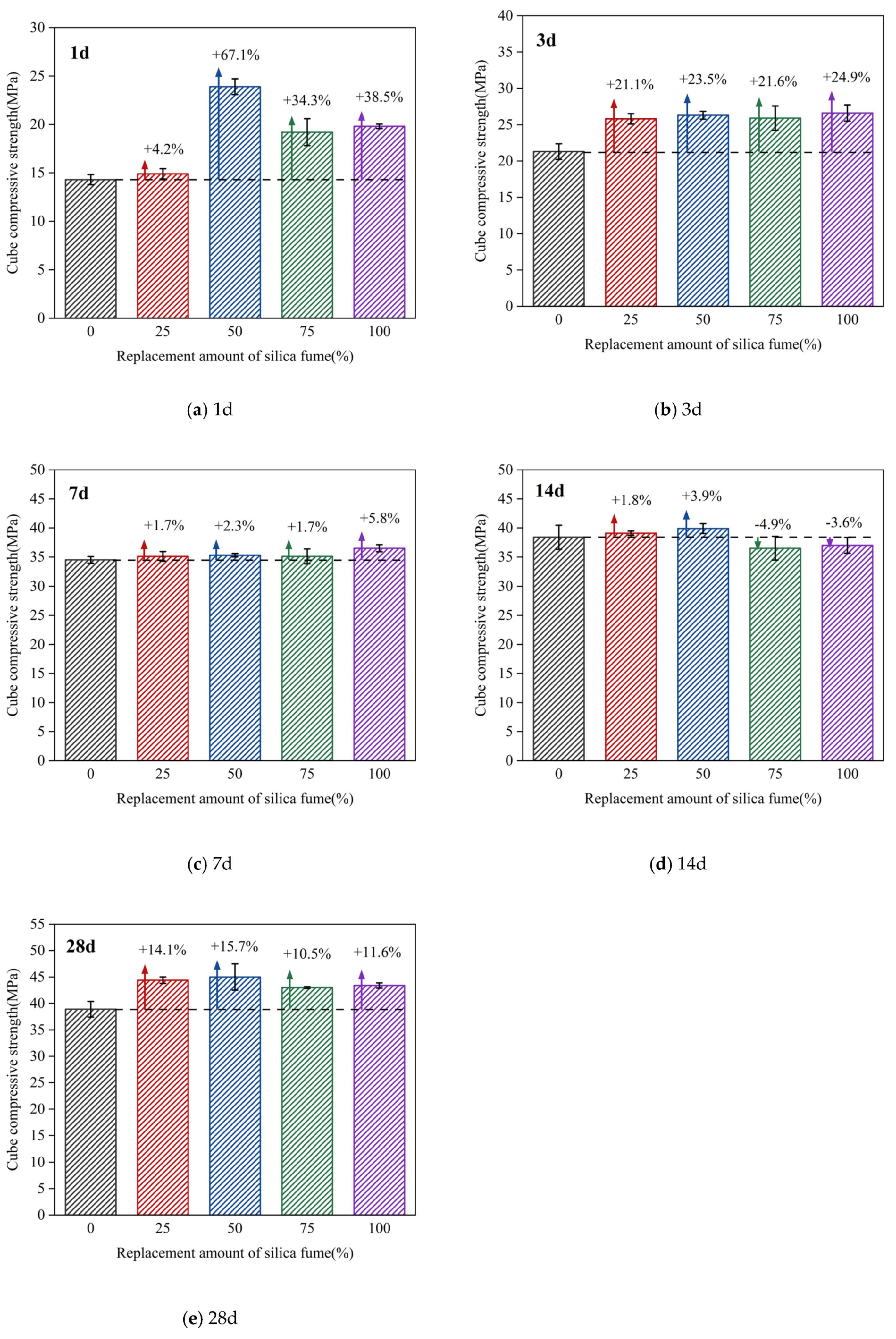
3.2.2. Effect of the Replacement Amount of Silica Fume on the Compressive Strength of Concrete Cubes
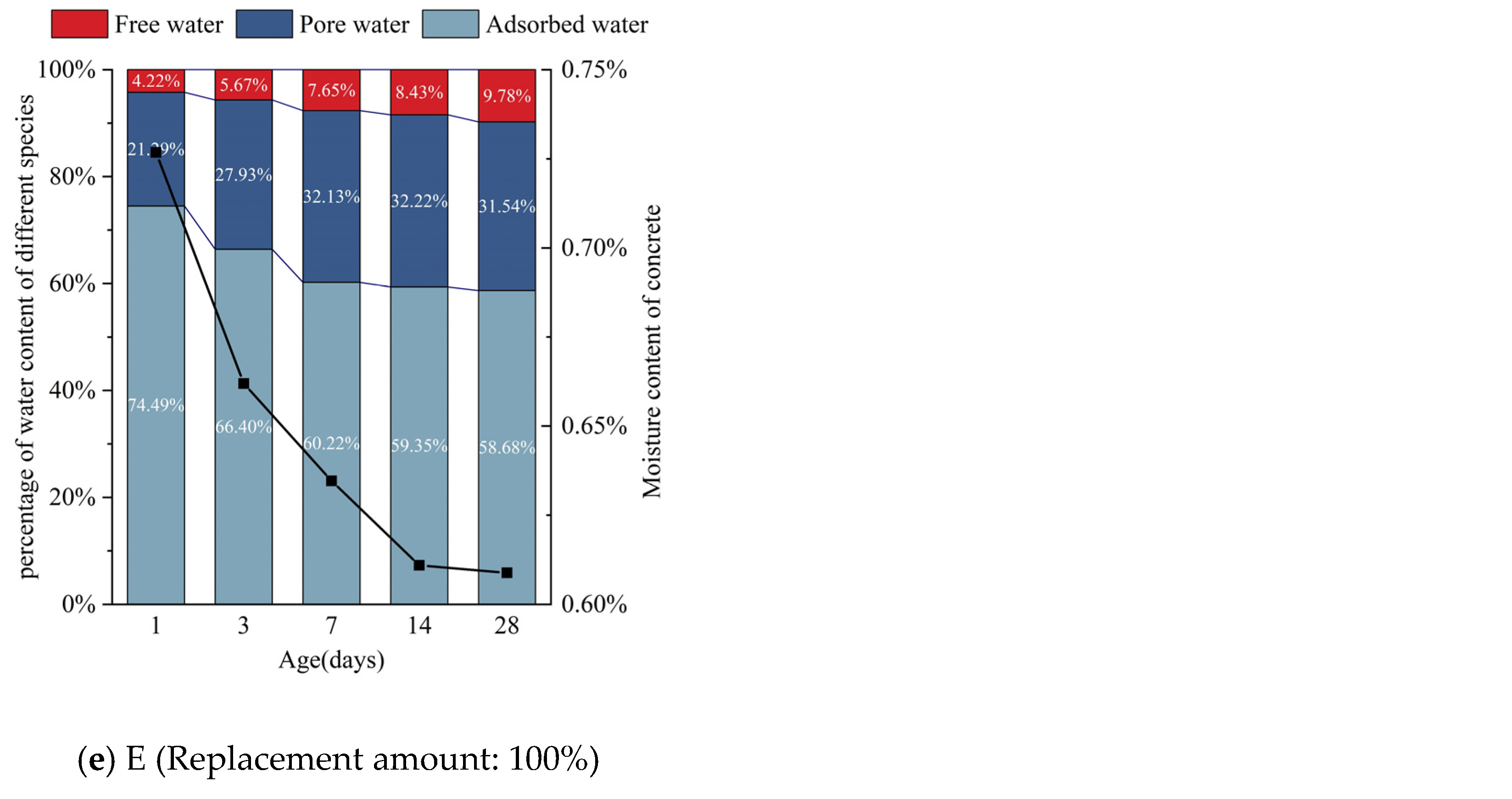
3.3. Hydration Reaction of Early-Age Concrete
3.3.1. Effect of Silica Fume Replacement Amount on the Hydration Reaction
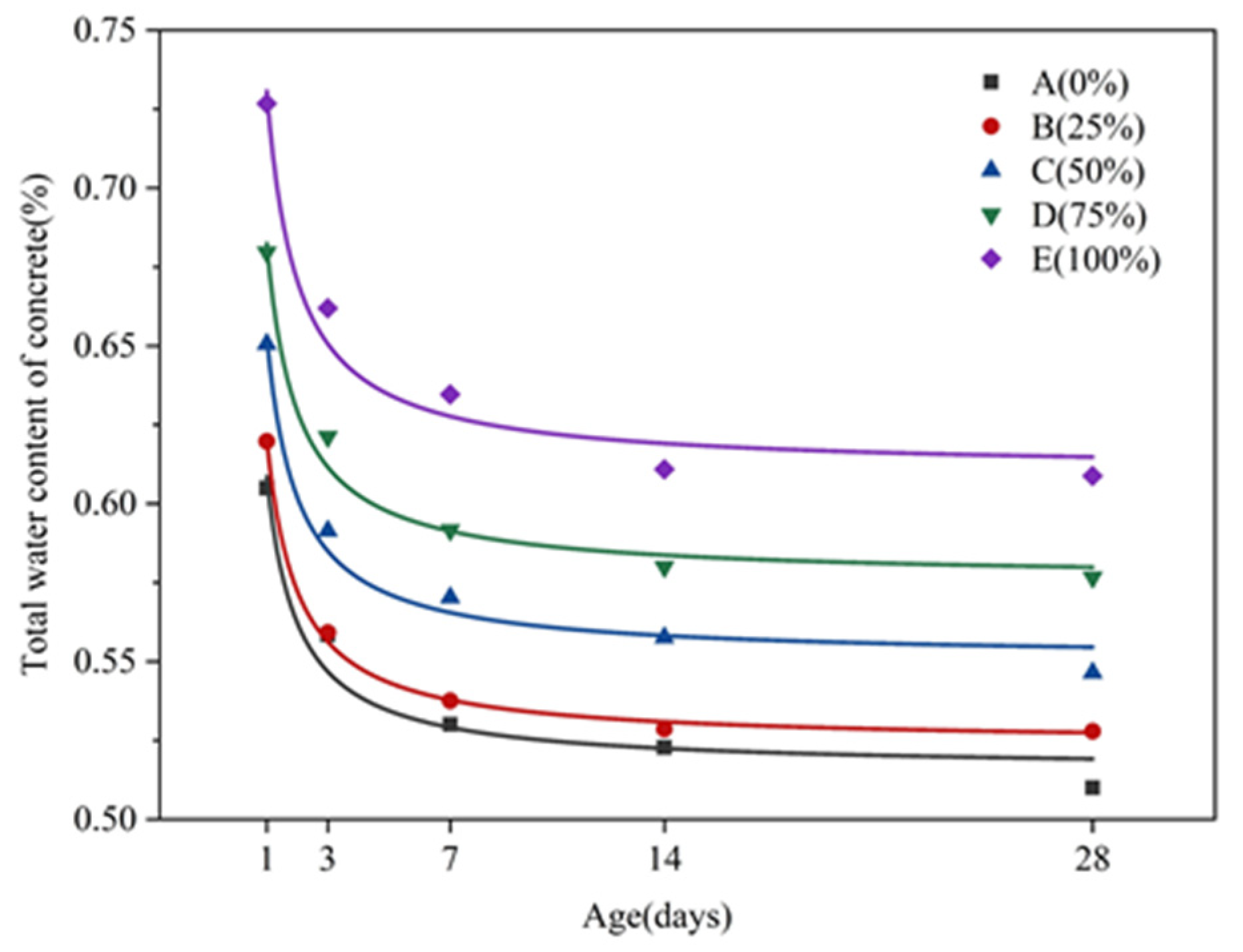
3.3.2. Influence of Age on Hydration Reaction
4. Discussion
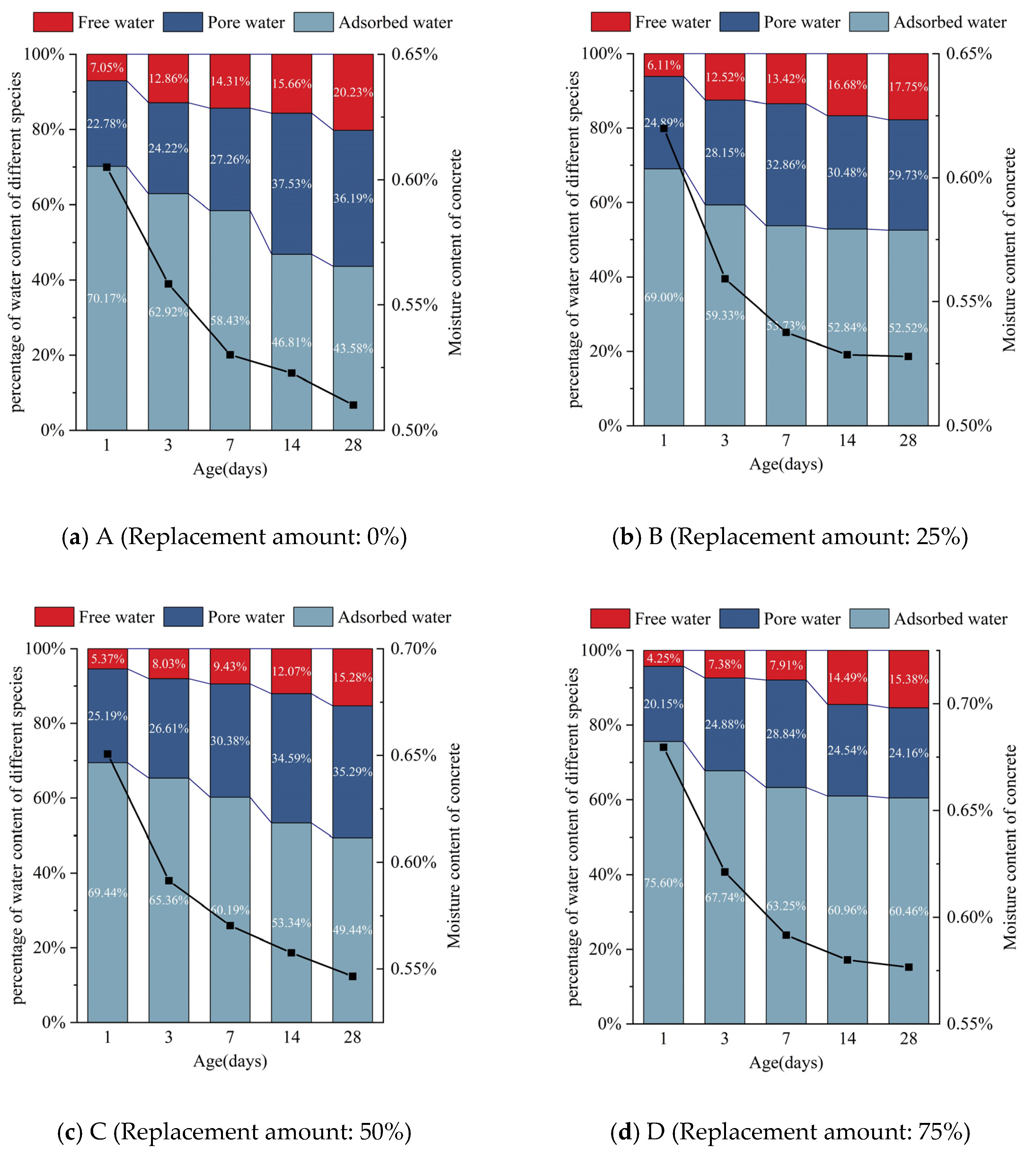
4.1. Relationship between Changes of Cube Compressive Strength and Water Content
4.2. Relationship between the Formation of Cube Compressive Strength, Microstructure and Hydration Products
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Khan, M.I.; Siddique, R. Utilization of silica fume in concrete: Review of durability properties. Resour. Conserv. Recycl. 2011, 57, 30–35. [Google Scholar] [CrossRef]
- Ahmad, O.A. Production of high-performance silica fume concrete. Am. J. Appl. Sci. 2017, 14, 1031–1038. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Wen, J.; Wu, J.; Ma, W.; Xu, M.; Wei, K.; Zhang, Z.; Zhang, L.; Xu, J. Recovery and purification of metallic silicon from waste silicon slag in electromagnetic induction furnace by slag refining method. J. Clean. Prod. 2019, 229, 1335–1341. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Q.; Chen, X.; Liu, H.; Zhang, J.; Zhang, J.; Yang, Y.; Wei, J. The role of MgO in the thermal behavior of MgO-silica fume pastes. J. Therm. Anal. Calorim. 2017, 127, 1897–1909. [Google Scholar] [CrossRef]
- Goodarzi, A.R.; Goodarzi, S.H.; Akbari, H.R. Assessing geo-mechanical and micro-structural performance of modified expansive clayey soil by silica fume as industrial waste. Iran. J. Sci. Technol. Trans. Civ. Eng. 2015, 39, 333–350. [Google Scholar]
- Janotka, I.; Nürnbergerová, T. Effect of temperature on structural quality of the cement paste and high-strength concrete with silica fume. Nucl. Eng. Des. 2005, 235, 2019–2032. [Google Scholar] [CrossRef]
- Sezer, G.I. Compressive strength and sulfate resistance of limestone and/or silica fume mortars. Constr. Build. Mater. 2012, 26, 613–618. [Google Scholar] [CrossRef]
- Bhanja, S.; Sengupta, B. Influence of silica fume on the tensile strength of concrete. Cem. Concr. Res. 2005, 35, 743–747. [Google Scholar] [CrossRef]
- Poon, C.S.; Kou, S.C.; Lam, L. Compressive strength, chloride diffusivity and pore structure of high performance metakaolin and silica fume concrete. Constr. Build. Mater. 2006, 20, 858–865. [Google Scholar] [CrossRef]
- Díaz, A.E.; Paradela, L.S.; Sánchez-Gálvez, V. The effect of silica fume and metakaolin on glass-fibre reinforced concrete (GRC) ageing. Mater. Construcción 2010, 60, 67–82. [Google Scholar]
- Memon, F.A.; Nuruddin, M.F.; Shafiq, N. Effect of silica fume on the fresh and hardened properties of fly ash-based self-compacting geopolymer concrete. Int. J. Miner. Metall. Mater. 2013, 20, 205–213. [Google Scholar] [CrossRef]
- Bhalla, N.; Sharma, S.; Sharma, S.; Siddique, R. Monitoring early-age setting of silica fume concrete using wave propagation techniques. Constr. Build. Mater. 2018, 162, 802–815. [Google Scholar] [CrossRef]
- Negi, P.; Chakraborty, T.; Kaur, N.; Bhalla, S. Investigations on effectiveness of embedded PZT patches at varying orientations for monitoring concrete hydration using EMI technique. Constr. Build. Mater. 2018, 169, 489–498. [Google Scholar] [CrossRef]
- Kachanov, V.K.; Sokolov, I.V.; Fedorov, M.B.; Kontsov, R.V. The use of complex-modulated signals to increase the accuracy of measurements of the velocity of ultrasound in concrete. Meas. Tech. 2015, 58, 817–822. [Google Scholar] [CrossRef]
- Makul, N. Dielectric permittivity of various cement-based materials during the first 24 h hydration. Open J. Inorg. Non-Met. Mater. 2013, 3, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Soutsos, M.N.; Bungey, J.H.; Millard, S.G.; Shaw, M.R.; Patterson, A. Dielectric properties of concrete and their influence on radar testing. NDT E Int. 2001, 34, 419–425. [Google Scholar] [CrossRef]
- Lai, W.L.; Kind, T.; Wiggenhauser, H. Frequency-dependent dispersion of high-frequency ground penetrating radar wave in concrete. NDT E Int. 2011, 44, 267–273. [Google Scholar] [CrossRef]
- Lai, W.L.; Kind, T.; Wiggenhauser, H. Using ground penetrating radar and time–frequency analysis to characterize construction materials. NDT E Int. 2011, 44, 111–120. [Google Scholar] [CrossRef]
- Lai, W.L.; Kind, T.; Kruschwitz, S.; Wöstmann, J.; Wiggenhauser, H. Spectral absorption of spatial and temporal ground penetrating radar signals by water in construction materials. NDT E Int. 2014, 67, 55–63. [Google Scholar]
- Lai, W.L.; Kind, T.; Wiggenhauser, H. A study of concrete hydration and dielectric relaxation mechanism using ground penetrating radar and short-time fourier transform. Eurasip J. Adv. Signal Process. 2010, 1, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Barbhuiya, S.A.; Gbagbo, J.K.; Russell, M.I.; Basheer, P.A.M. Properties of fly ash concrete modified with hydrated lime and silica fume. Constr. Build. Mater. 2009, 23, 3233–3239. [Google Scholar] [CrossRef]
- Langan, B.W.; Weng, K.; Ward, M.A. Effect of silica fume and fly ash on heatof hydration of Portland cement. Cem. Concr. Res. 2002, 32, 1045–1051. [Google Scholar] [CrossRef]
- Sun, Z.; Voigt, T.; Shah, S.P. Rheometric and ultrasonic investigations of viscoelastic properties of fresh Portland cement pastes. Cem. Concr. Res. 2006, 36, 278–287. [Google Scholar] [CrossRef]
- Kadri, E.-H.; Duval, R. Hydration heat kinetics of concrete with silica fume. Constr. Build. Mater. 2009, 23, 3388–3392. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Ai, K.; Zhu, L. An NMR-based experimental study on the pore structure of the hydration process of mine filling slurry. Adv. Civ. Eng. 2018, 6, 4720356. [Google Scholar] [CrossRef] [Green Version]
- Holthausen, R.S.; Raupach, M. Monitoring the internal swelling in cementitious mortars with single-sided 1H nuclear magnetic resonance. Cem. Concr. Res. 2018, 111, 138–146. [Google Scholar] [CrossRef]
- Jehng, J.Y.; Sprague, D.T.; Halperin, W.P. Pore structure of hydrating cement paste by magnetic resonance relaxation analysis and freezing. Magn. Reson. Imaging 1996, 14, 785–791. [Google Scholar] [CrossRef]
- Hou, P.; Muzenda, T.R.; Li, Q.; Chen, H.; Kawashima, S.; Sui, T.; Yong, H.; Xie, N.; Cheng, X. Mechanisms dominating thixotropy in limestone calcined clay cement (LC3). Cem. Concr. Res. 2021, 140, 106316. [Google Scholar] [CrossRef]
- Zhou, K.; Ai, K.; Zhang, J.; Li, J. Nuclear magnetic resonance characteristics in fresh filling slurry. Sci. Technol. Rev. 2013, 31, 50–53. [Google Scholar]
- Liu, L.; He, Z.; Cai, X.; Fu, S. Application of low-field NMR to the pore structure of concrete. Appl. Magn. Reson. 2021, 52, 15–31. [Google Scholar] [CrossRef]
- Lura, P.; Winnefeld, F.; Fang, X. A simple method for determining the total amount of physically and chemically bound water of different cements. J. Therm. Anal. Calorim. 2017, 130, 653–660. [Google Scholar] [CrossRef]
- Yang, L.; Gao, D.; Zhang, Y.; She, W. Study on water and chloride transport in cracked mortar using X-ray CT, gravimetric method and natural immersion method. Constr. Build. Mater. 2018, 176, 652–664. [Google Scholar] [CrossRef]
- Krishnan, J.M.; Rao, C.L. Permeability and bleeding of asphalt concrete using mixture theory. Int. J. Eng. Sci. 2001, 39, 611–627. [Google Scholar] [CrossRef]
- Rong, Z.; Sun, W.; Xiao, H.; Jiang, G. Effects of nano-SiO2 particles on the mechanical and microstructural properties of ultra-high performance cementitious composites. Cem. Concr. Compos. 2015, 56, 25–31. [Google Scholar] [CrossRef]
- Rong, Z.D.; Sun, W.; Xiao, H.J.; Wang, W. Effect of silica fume and fly ash on hydration and microstructure evolution of cement based composites at low water–binder ratios. Constr. Build. Mater. 2014, 51, 446–450. [Google Scholar] [CrossRef]
- Zhao, S.; Sun, W. Effect of silica fume and fly ash on pore structures of blended pastes at low water to binder ratios. Adv. Cem. Res. 2015, 27, 506–514. [Google Scholar] [CrossRef]
- She, A.M.; Yao, W. Probing the hydration of composite cement pastes containing fly ash and silica fume by proton NMR spin-lattice relaxation. Sci. China-Technol. Sci. 2010, 53, 1471–1476. [Google Scholar] [CrossRef]
- Dutta, D.; Thokchom, S.; Ghosh, P.; Ghosh, S. Effect of silica fume additions on porosity of fly ash geopolymers. J. Eng. Appl. Sci. 2010, 5, 74–79. [Google Scholar]
- Suarez, L.; Abu-Lebdeh, T.M.; Picornell, M.; Hamoush, S.A. Investigating the role of fly ash and silica fume in the cement hydration process. Am. J. Eng. Appl. Sci. 2016, 9, 134–145. [Google Scholar] [CrossRef]



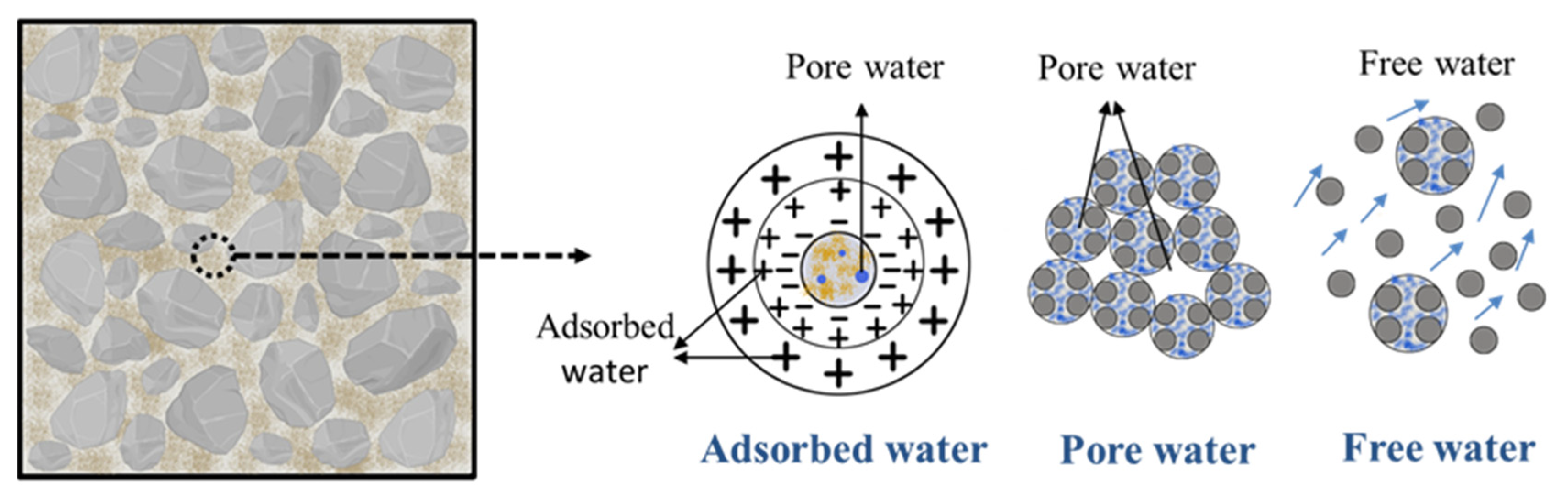




| Cement Type | Ignition Loss (%) | Stability | Setting Time (min) | Compressive Strength (Mpa) | Flexural Strength (Mpa) | |||
|---|---|---|---|---|---|---|---|---|
| Initial | Final | 3 d | 28 d | 3 d | 28 d | |||
| P·O 42.5 | 4.00 | Qualified | 175 | 245 | 28.0 | 48.8 | 5.1 | 7.7 |
| Material | Apparent Density (g/cm3) | Water Demand Ratio (%) | Moisture Content (%) | Specific Surface Area (m2/kg) | Ignition Loss (%) | Activity Index (%) | |
|---|---|---|---|---|---|---|---|
| 7 d | 28 d | ||||||
| Fly ash | 2.56 | 88 | 0.40 | 994 | 4.70 | / | 73 |
| Silica fume | 2.19 | 120 | 0.50 | 18,000 | 3.70 | 107 | 125 |
| Material | SiO2 | Al2O3 | CaO | Fe2O3 | MgO | SO3 |
|---|---|---|---|---|---|---|
| Cement | 22.81 | 5.62 | 61.43 | 3.36 | 1.35 | 2.17 |
| Fly ash | 49.02 | 31.56 | 4.88 | 6.97 | 0.83 | 1.2 |
| Silica fume | 86.30 | - | - | - | - | - |
| Code | Replacement Amount of Silica Fume | Silica Fume | Fly Ash | Cement | Coarse Aggregate | Sand | Water | Superplasticizer | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Small | Medium | Large | ||||||||
| A | 0% | 0 | 48 | 192 | 434 | 434 | 577 | 620 | 115 | 1.2 |
| B | 25% | 12 | 36 | 192 | 434 | 434 | 577 | 620 | 115 | 1.2 |
| C | 50% | 24 | 24 | 192 | 434 | 434 | 577 | 620 | 115 | 1.2 |
| D | 75% | 36 | 12 | 192 | 434 | 434 | 577 | 620 | 115 | 1.2 |
| E | 100% | 48 | 0 | 192 | 434 | 434 | 577 | 620 | 115 | 1.2 |
| Code | Slump (mm) | Air Content (%) |
|---|---|---|
| A | 90 | 1.42 |
| B | 76 | 0.90 |
| C | 70 | 0.82 |
| D | 62 | 0.90 |
| E | 55 | 1.30 |
| Code | a | b | R2 | c | d | R2 |
|---|---|---|---|---|---|---|
| A | 18.851 | 14.717 | 0.9292 | 0.09273 | 0.51588 | 0.9590 |
| B | 20.485 | 15.818 | 0.9891 | 0.09656 | 0.52404 | 0.9975 |
| C | 15.421 | 22.004 | 0.9575 | 0.10216 | 0.55093 | 0.9800 |
| D | 16.378 | 19.114 | 0.9784 | 0.10634 | 0.57613 | 0.9828 |
| E | 16.261 | 19.926 | 0.9668 | 0.12022 | 0.61053 | 0.9690 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, T.; Hua, C.; Sun, Q.; Tang, L.; Yi, Y.; Pan, X. Early-Age Hydration Reaction and Strength Formation Mechanism of Solid Waste Silica Fume Modified Concrete. Molecules 2021, 26, 5663. https://doi.org/10.3390/molecules26185663
Luo T, Hua C, Sun Q, Tang L, Yi Y, Pan X. Early-Age Hydration Reaction and Strength Formation Mechanism of Solid Waste Silica Fume Modified Concrete. Molecules. 2021; 26(18):5663. https://doi.org/10.3390/molecules26185663
Chicago/Turabian StyleLuo, Tao, Cheng Hua, Qiang Sun, Liyun Tang, Yu Yi, and Xiaofeng Pan. 2021. "Early-Age Hydration Reaction and Strength Formation Mechanism of Solid Waste Silica Fume Modified Concrete" Molecules 26, no. 18: 5663. https://doi.org/10.3390/molecules26185663
APA StyleLuo, T., Hua, C., Sun, Q., Tang, L., Yi, Y., & Pan, X. (2021). Early-Age Hydration Reaction and Strength Formation Mechanism of Solid Waste Silica Fume Modified Concrete. Molecules, 26(18), 5663. https://doi.org/10.3390/molecules26185663






