Recent Advances in the Synthesis and Biomedical Applications of Heterocyclic NO-Donors
Abstract
:1. Introduction
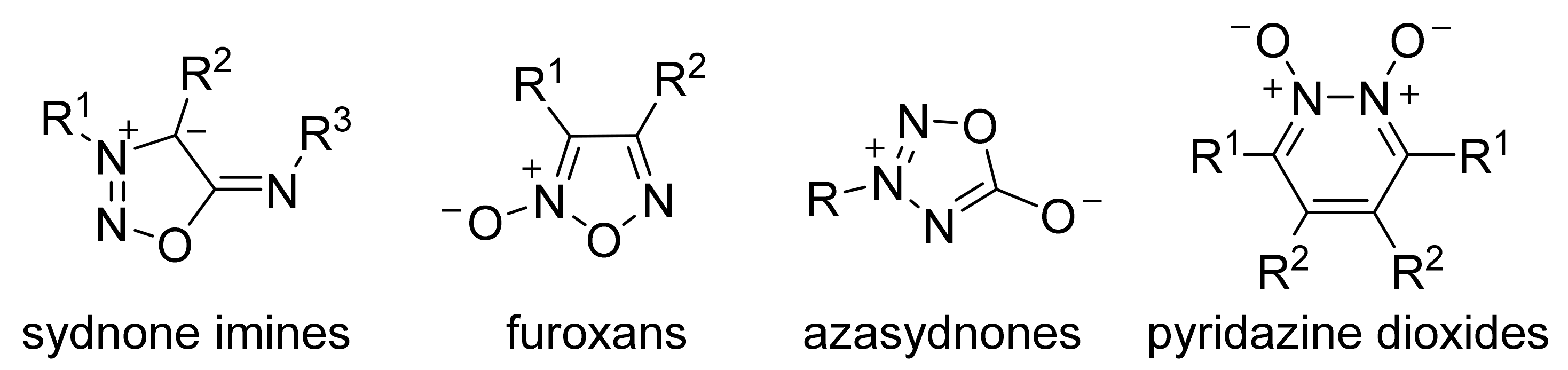
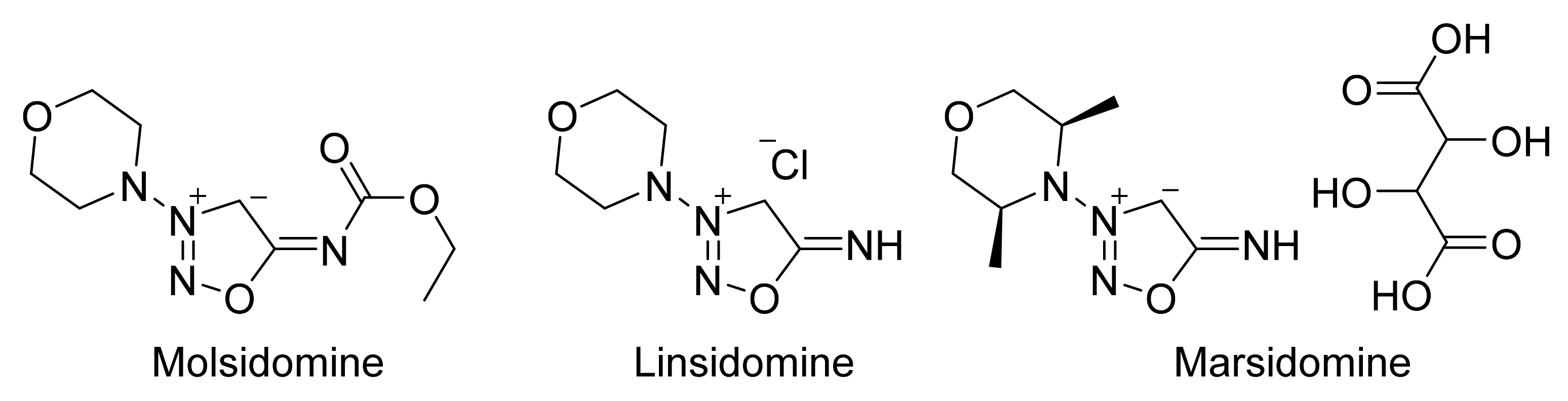
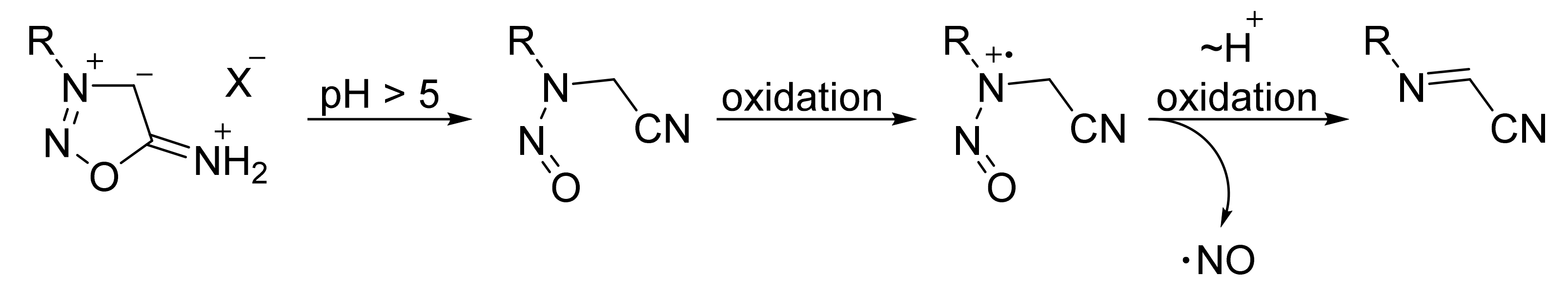
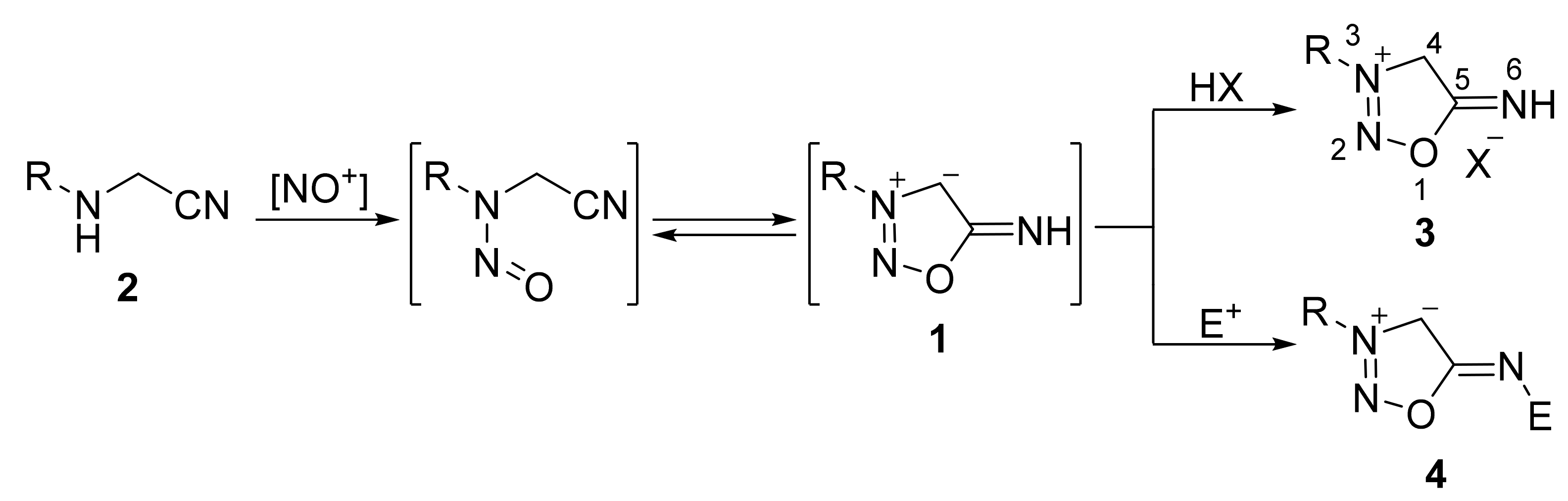
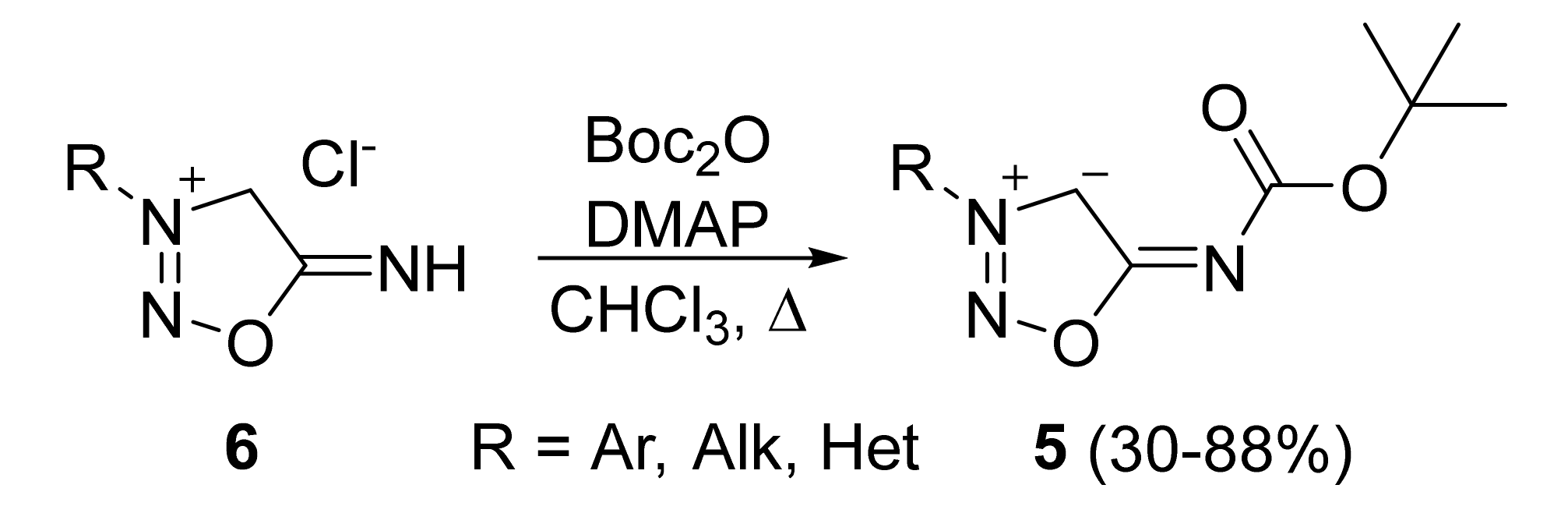
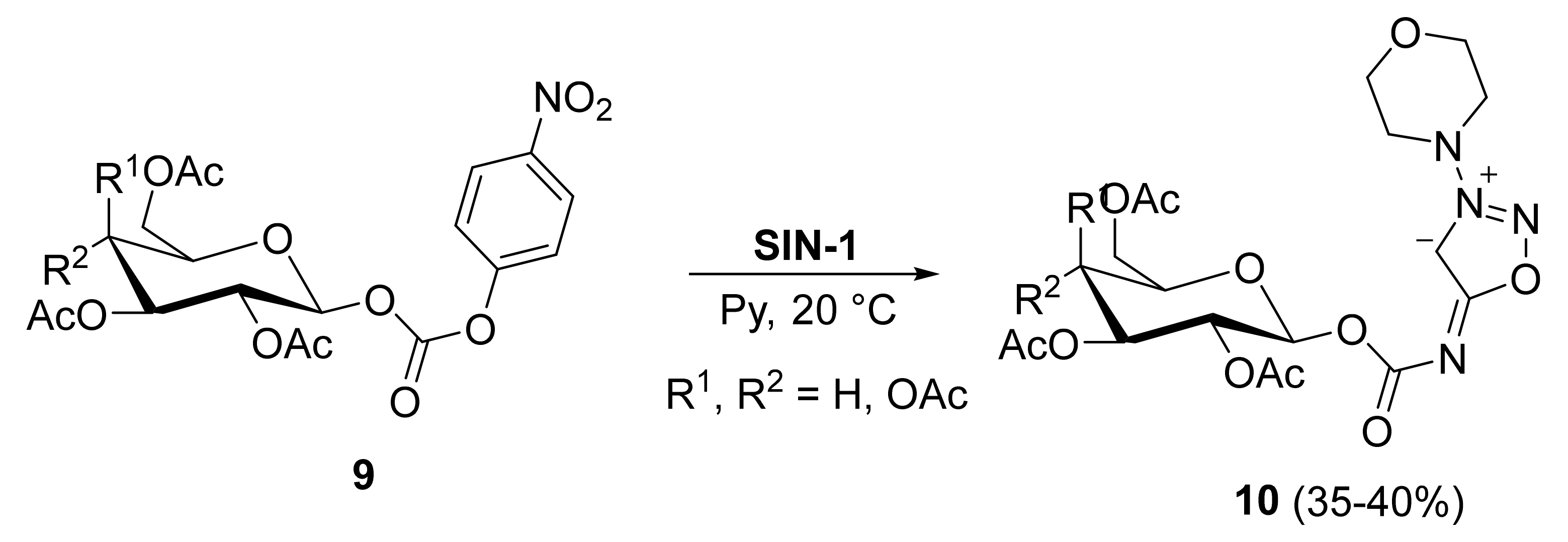
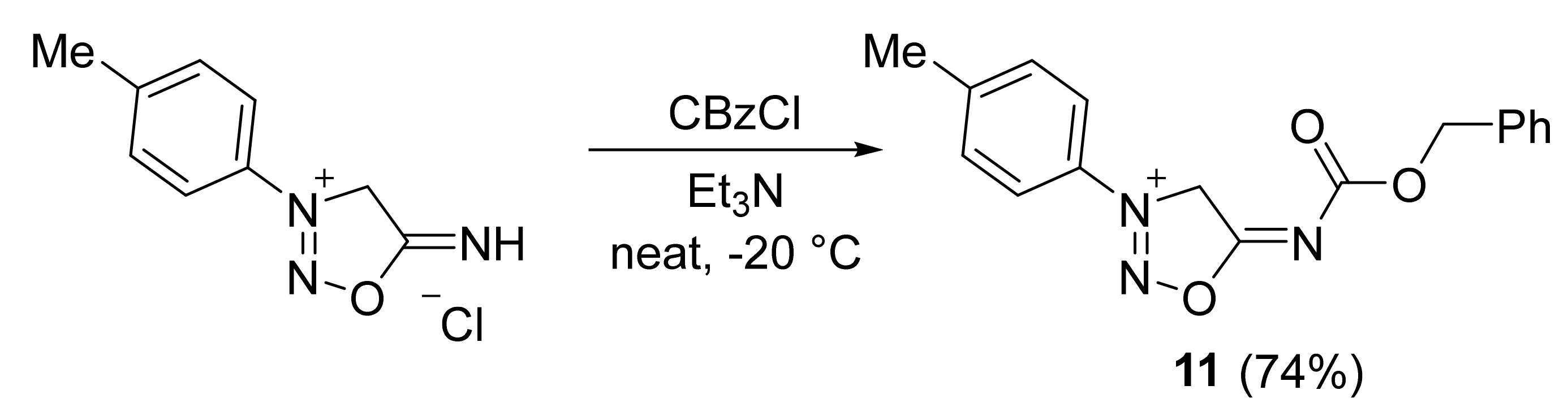
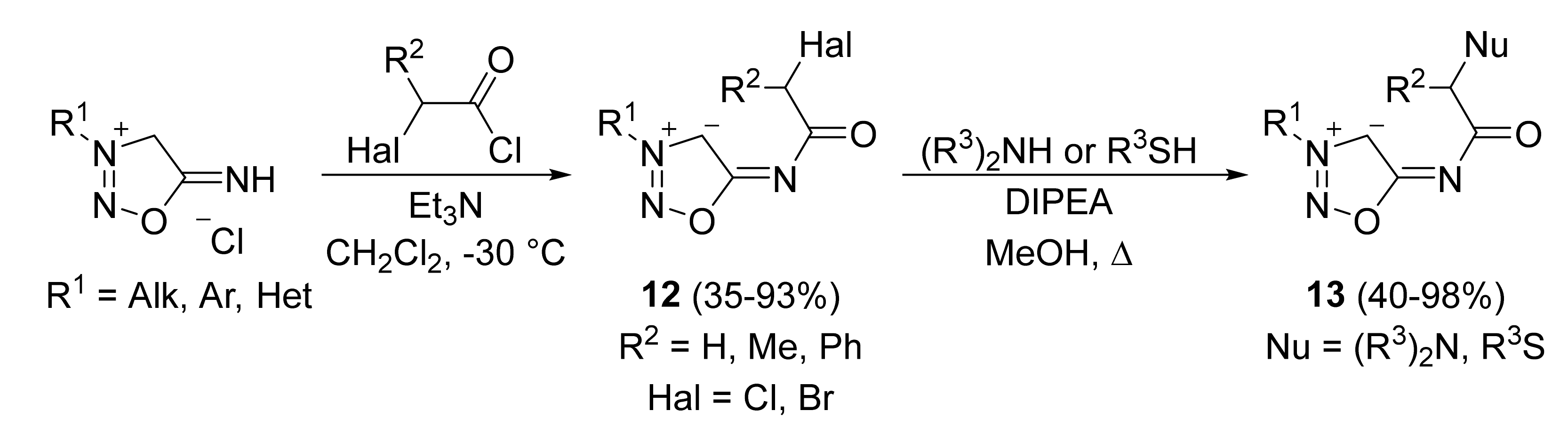
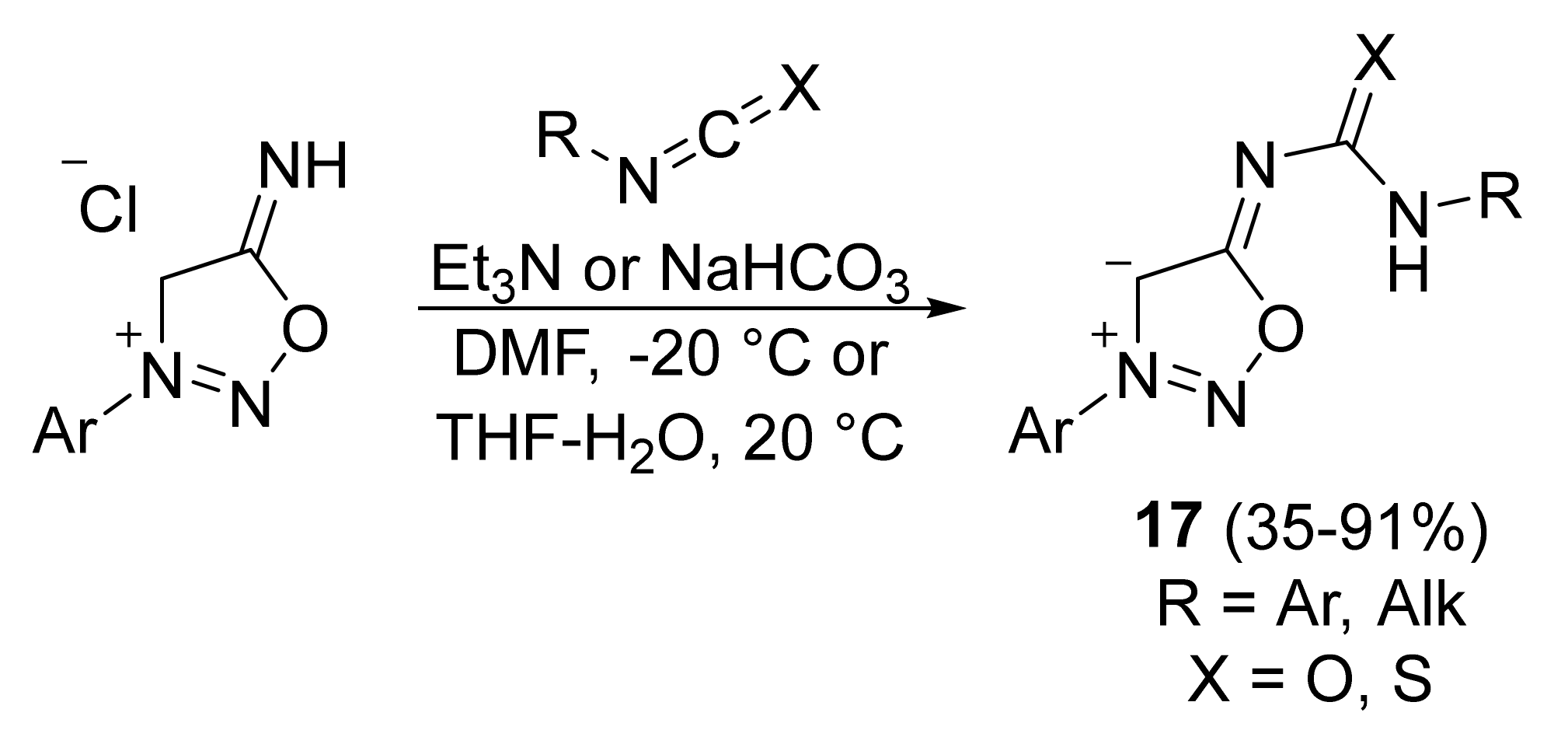
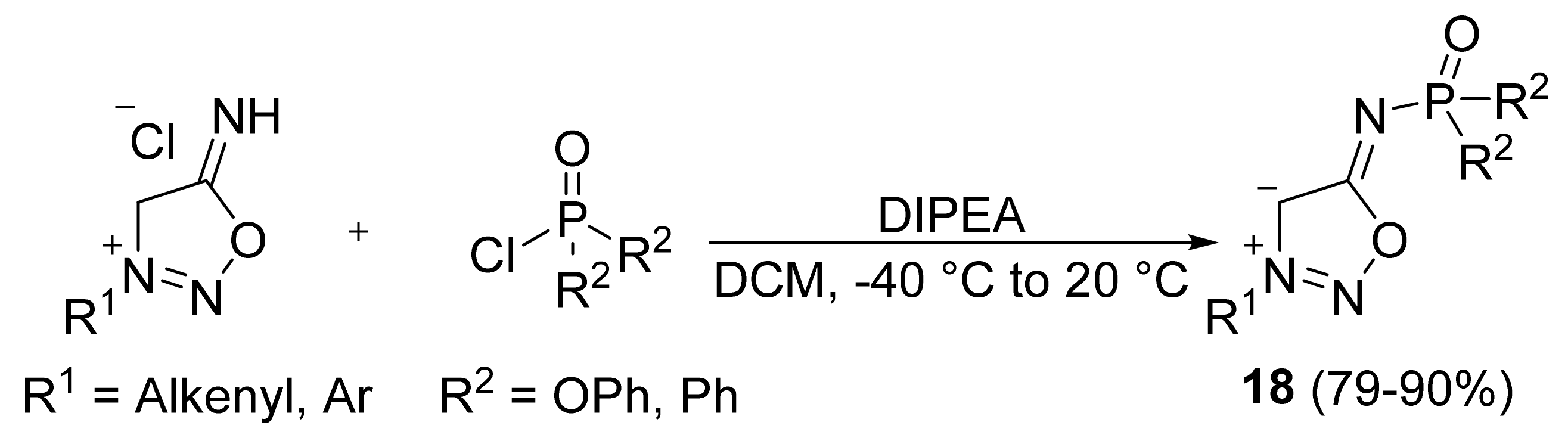
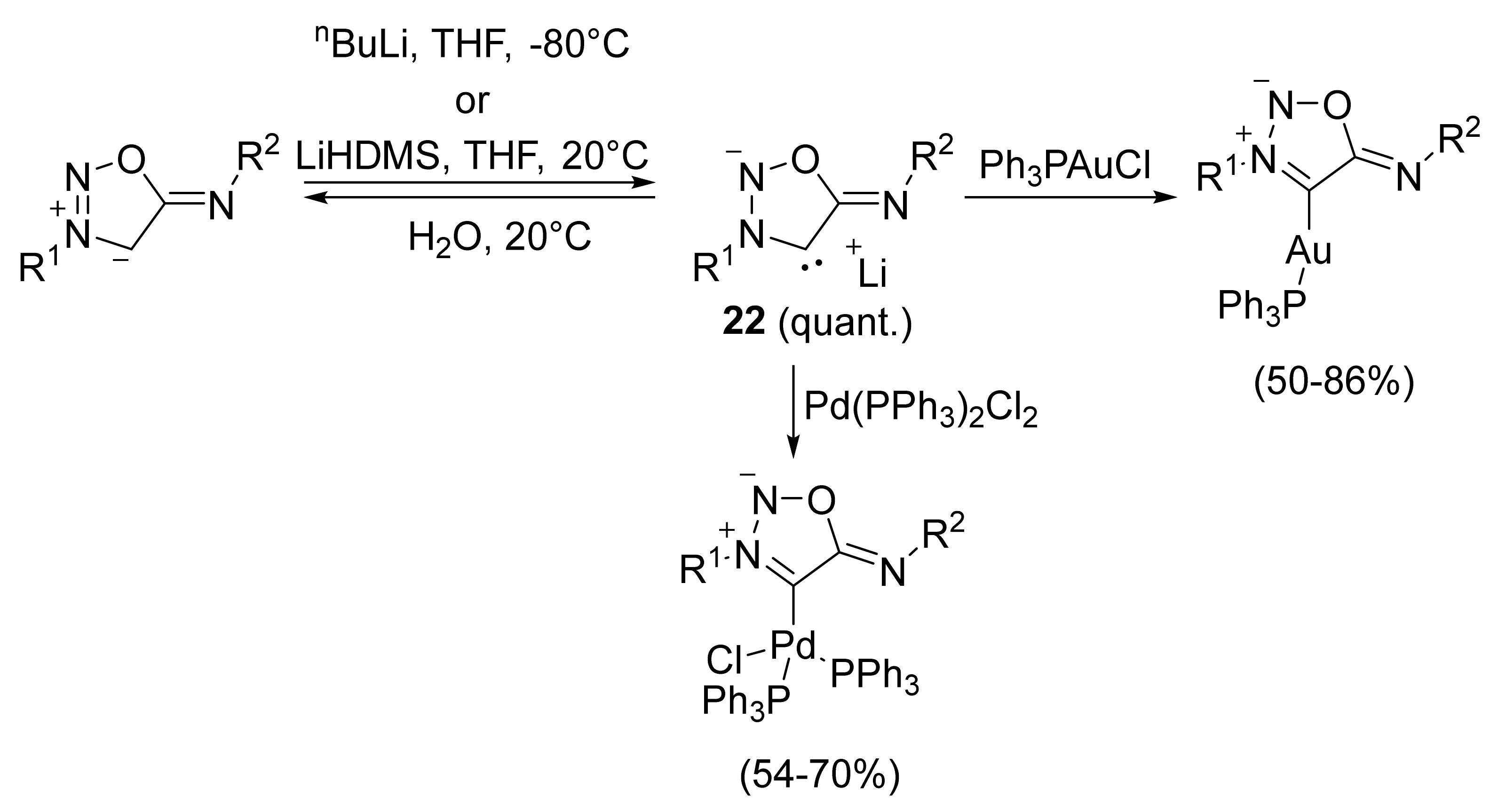
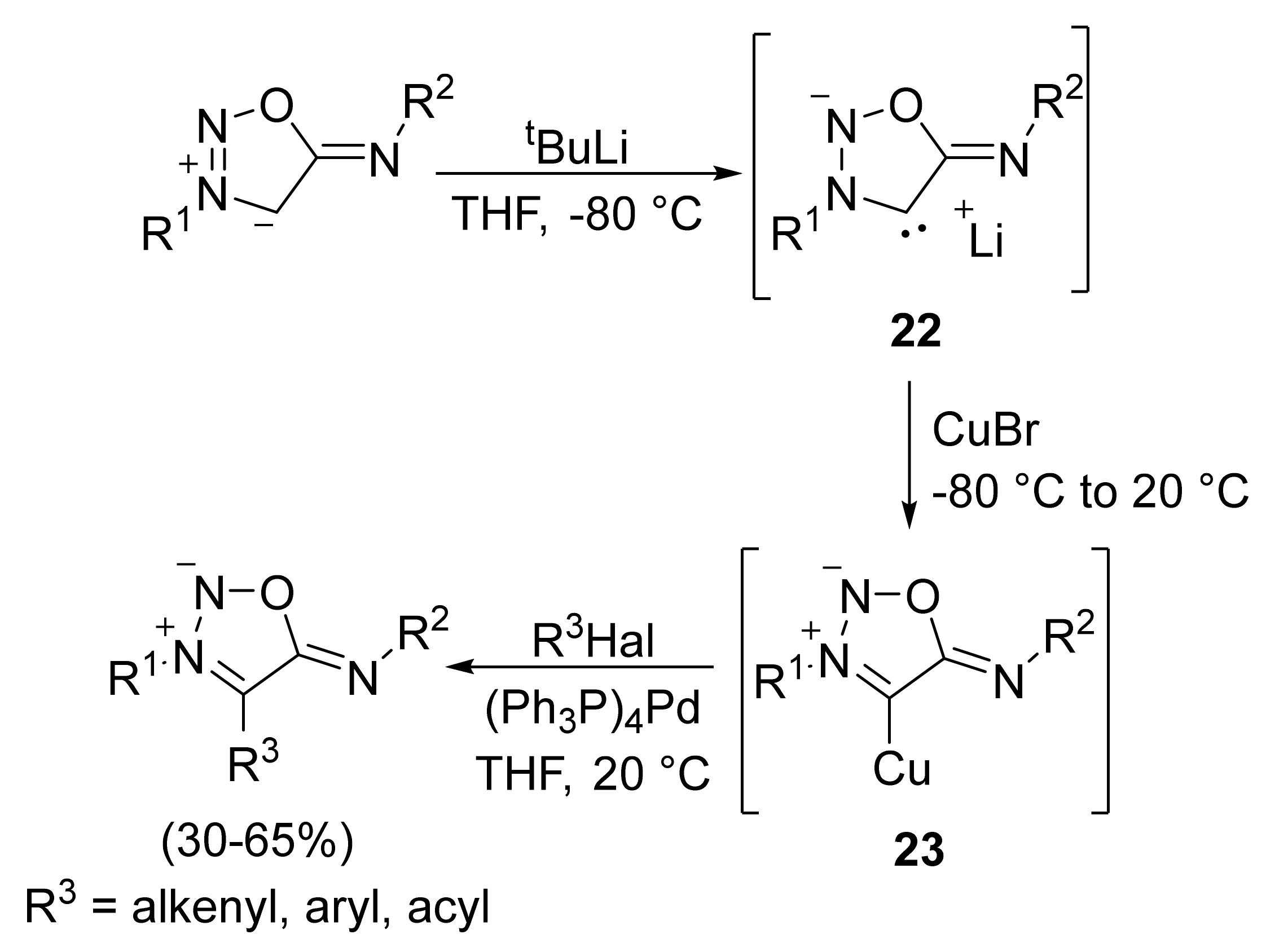
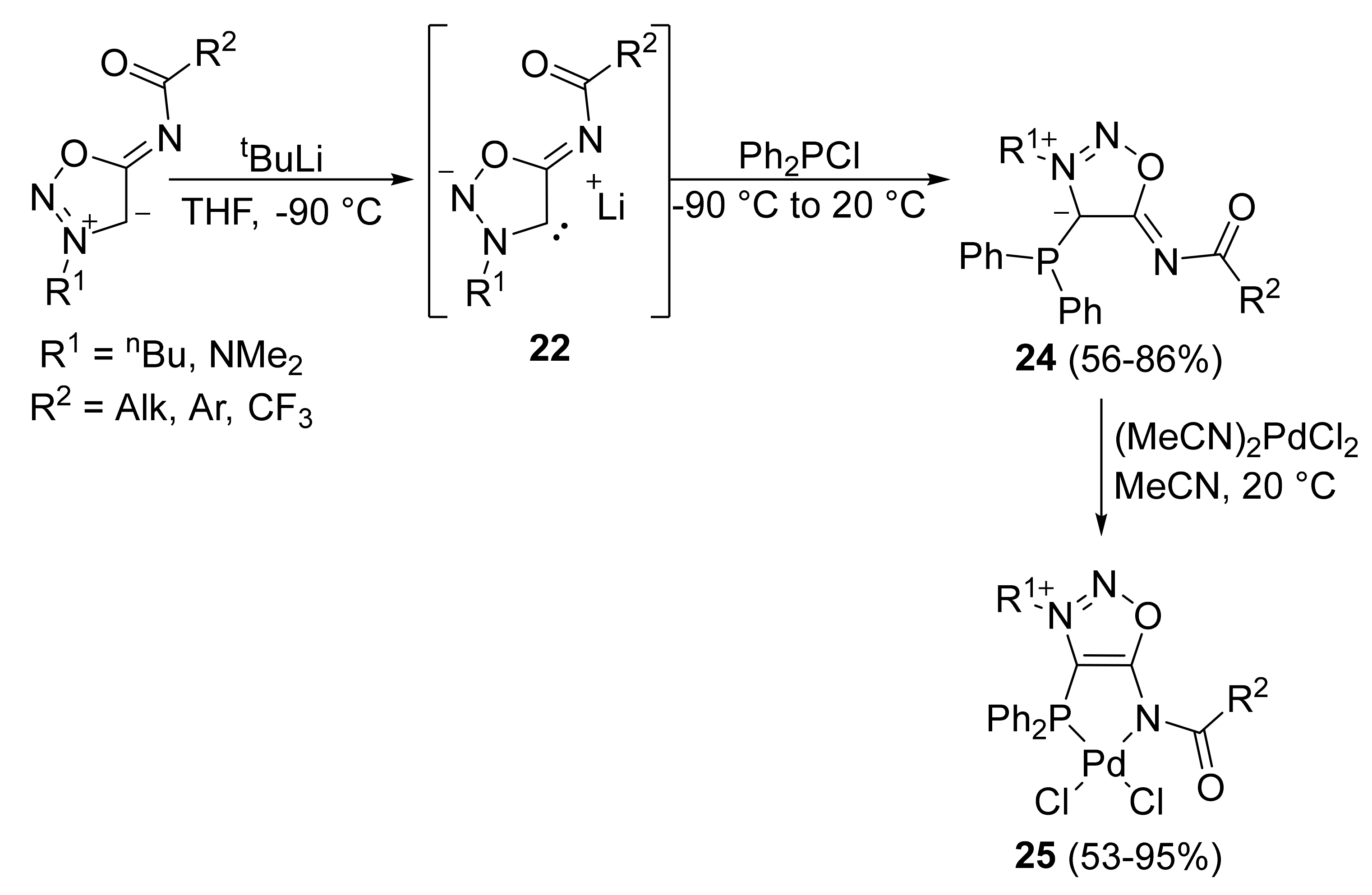
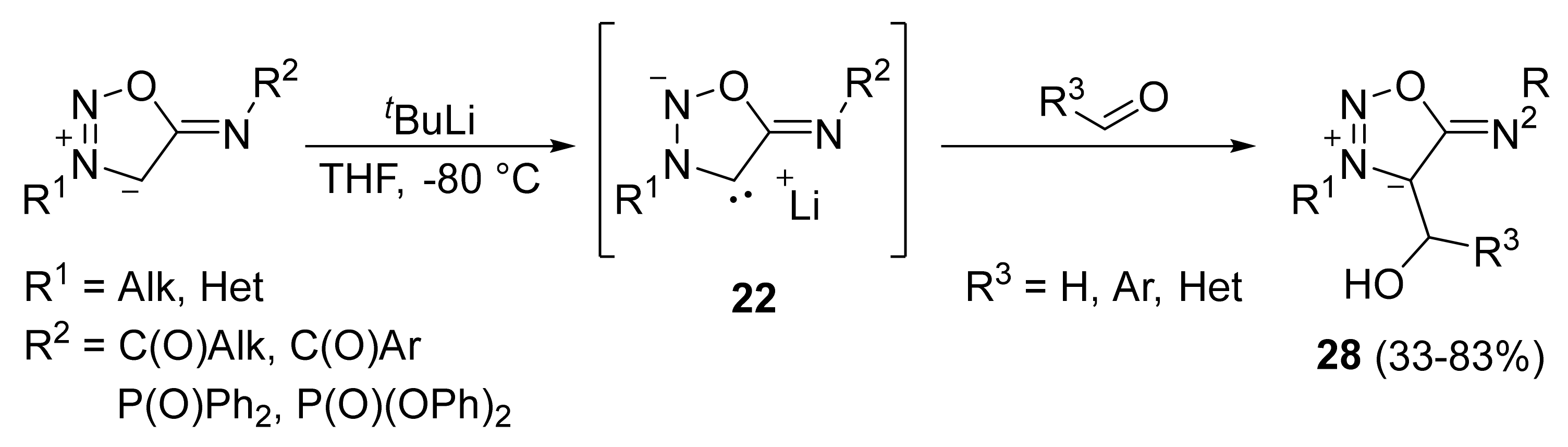
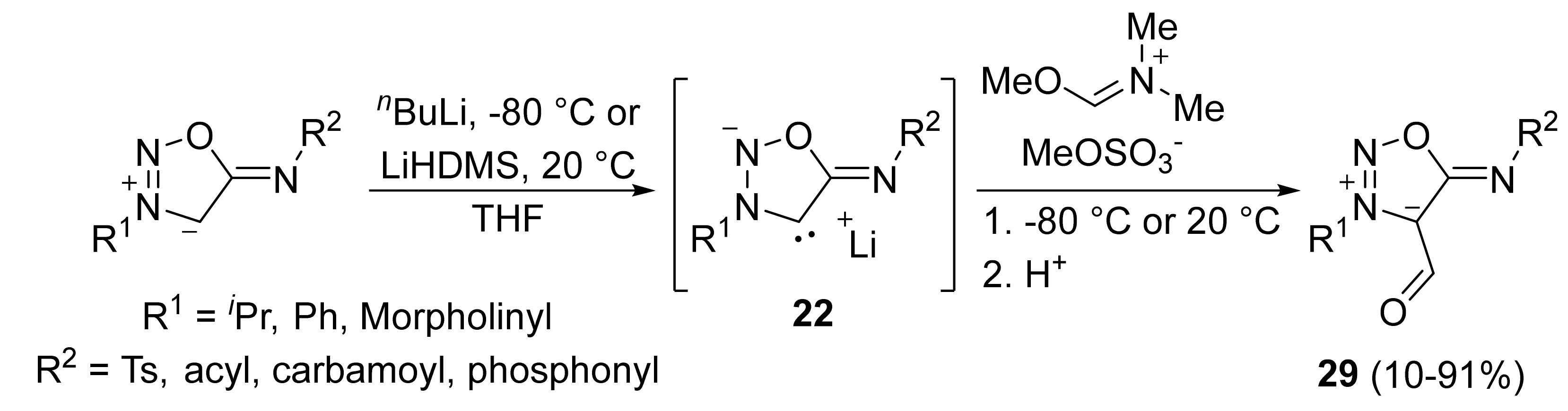
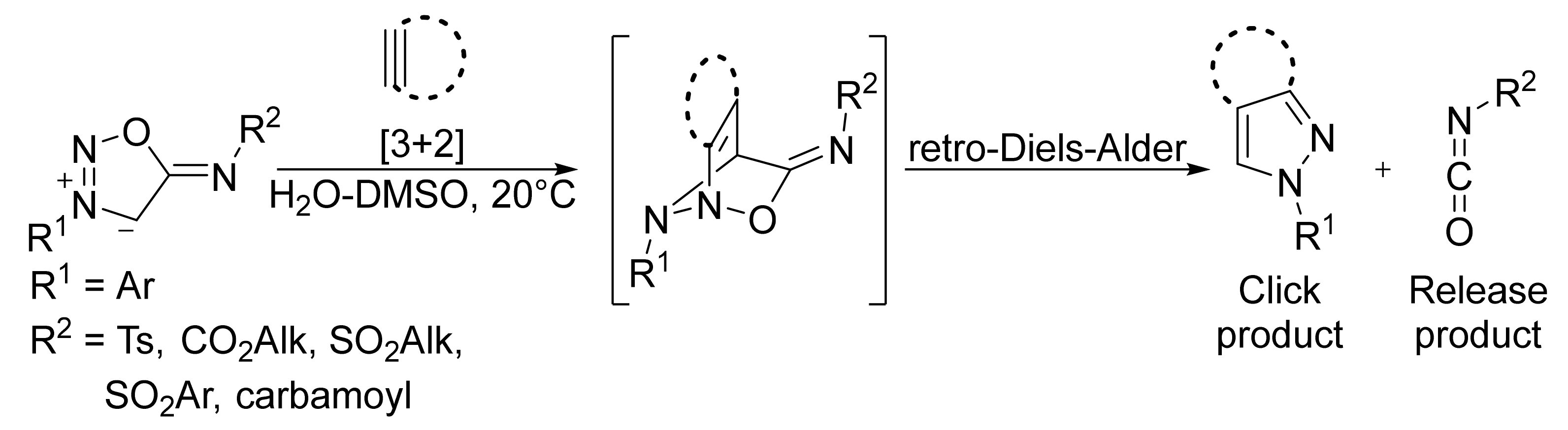
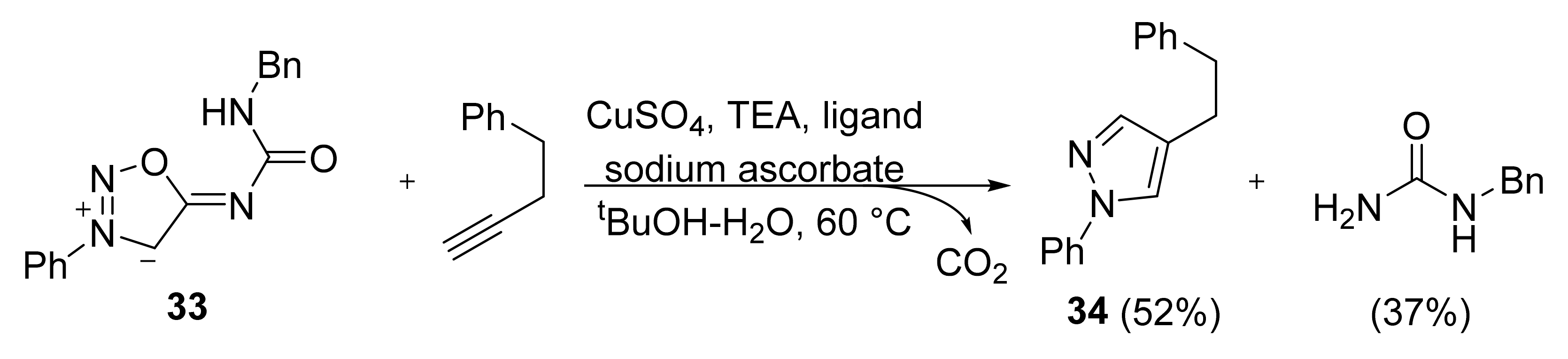
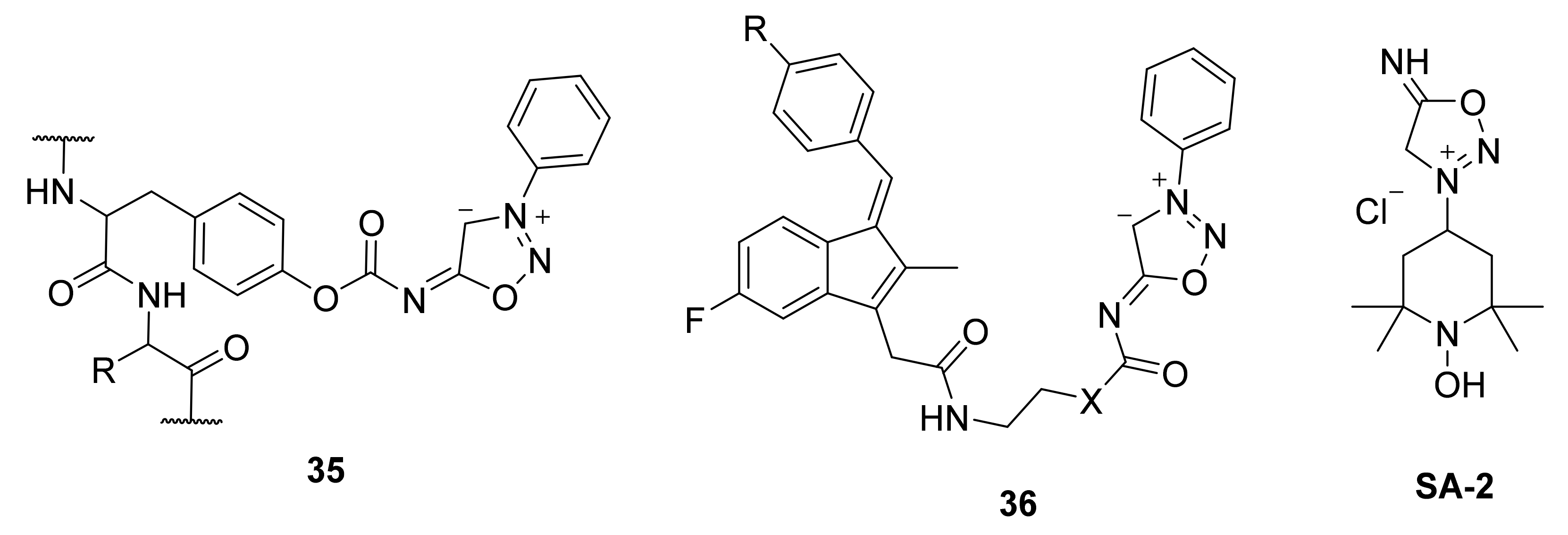
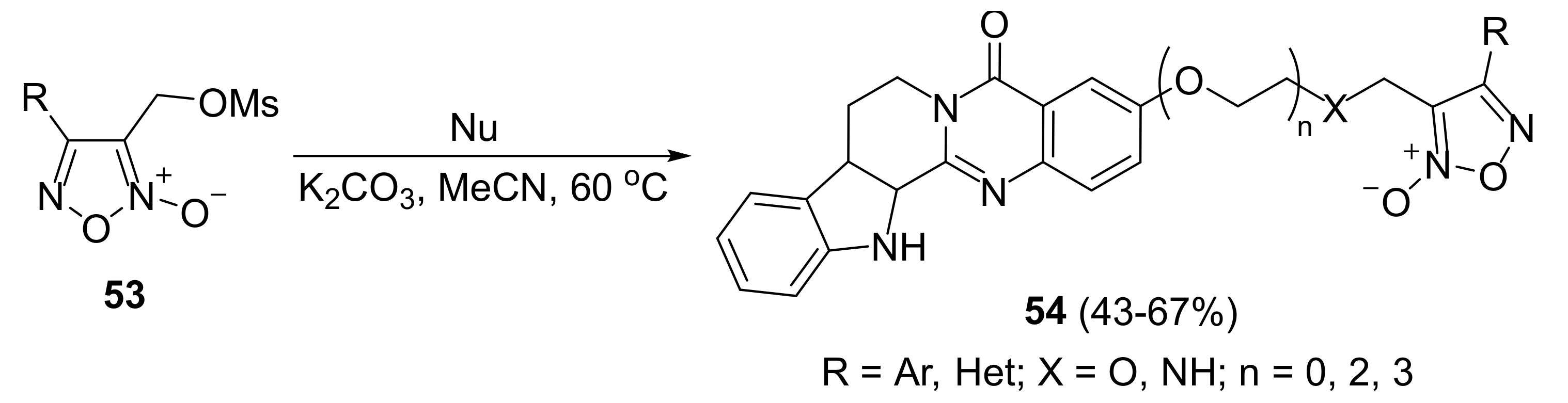
2. Sydnone Imines
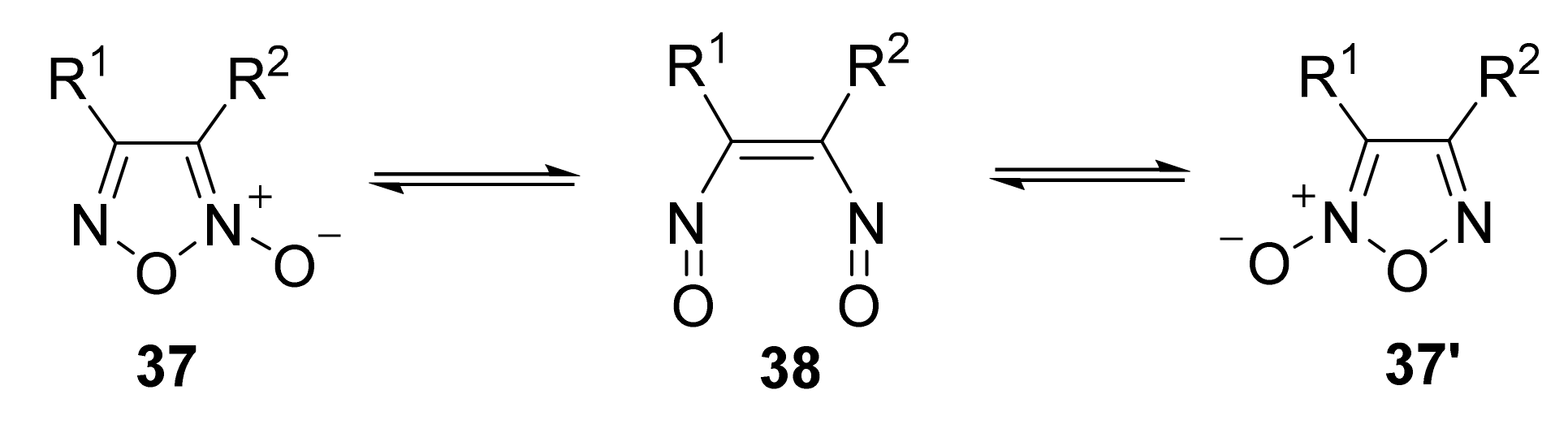
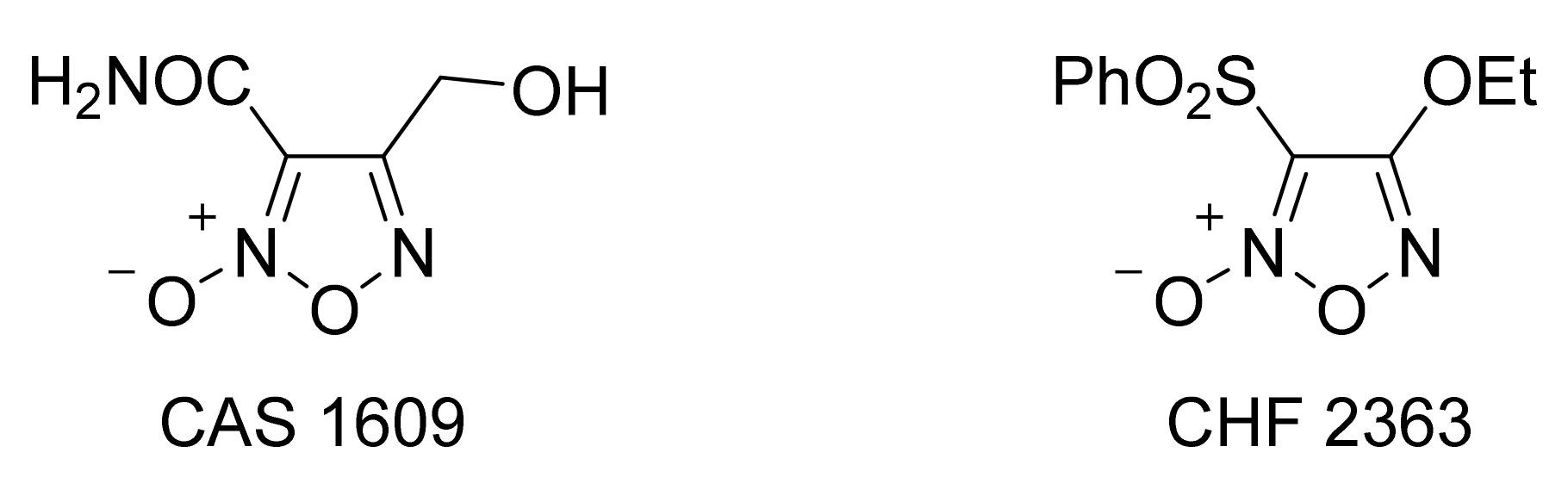
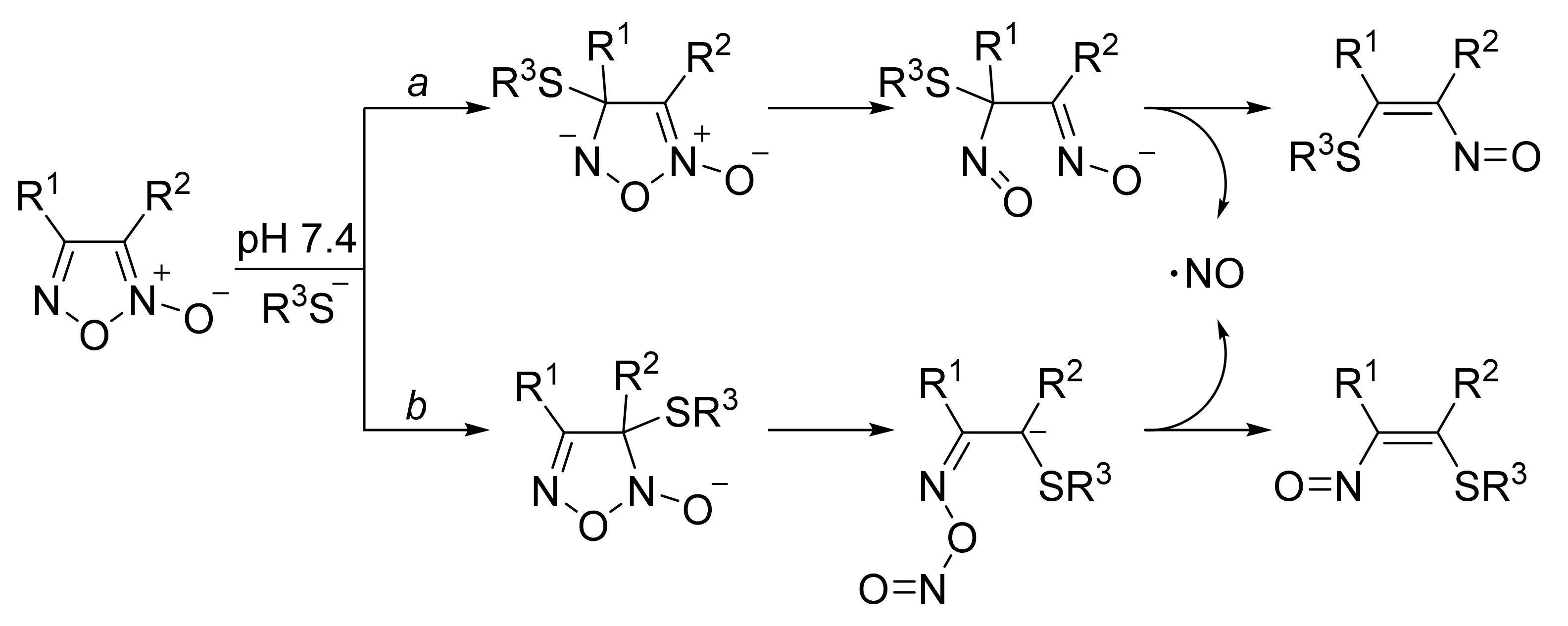
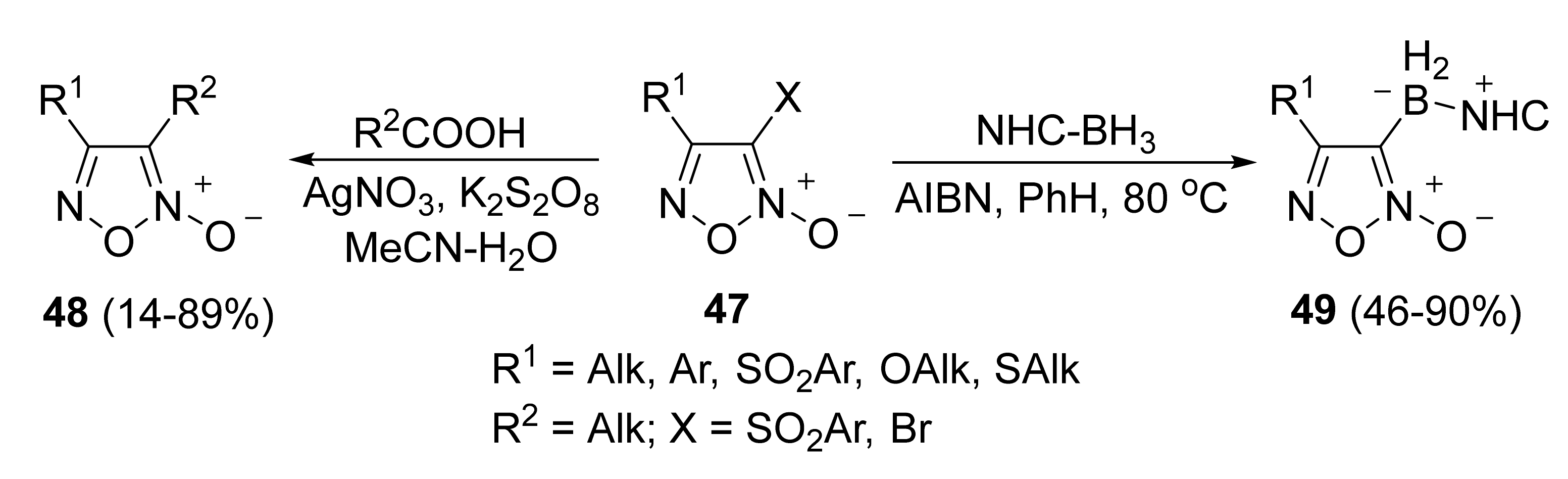
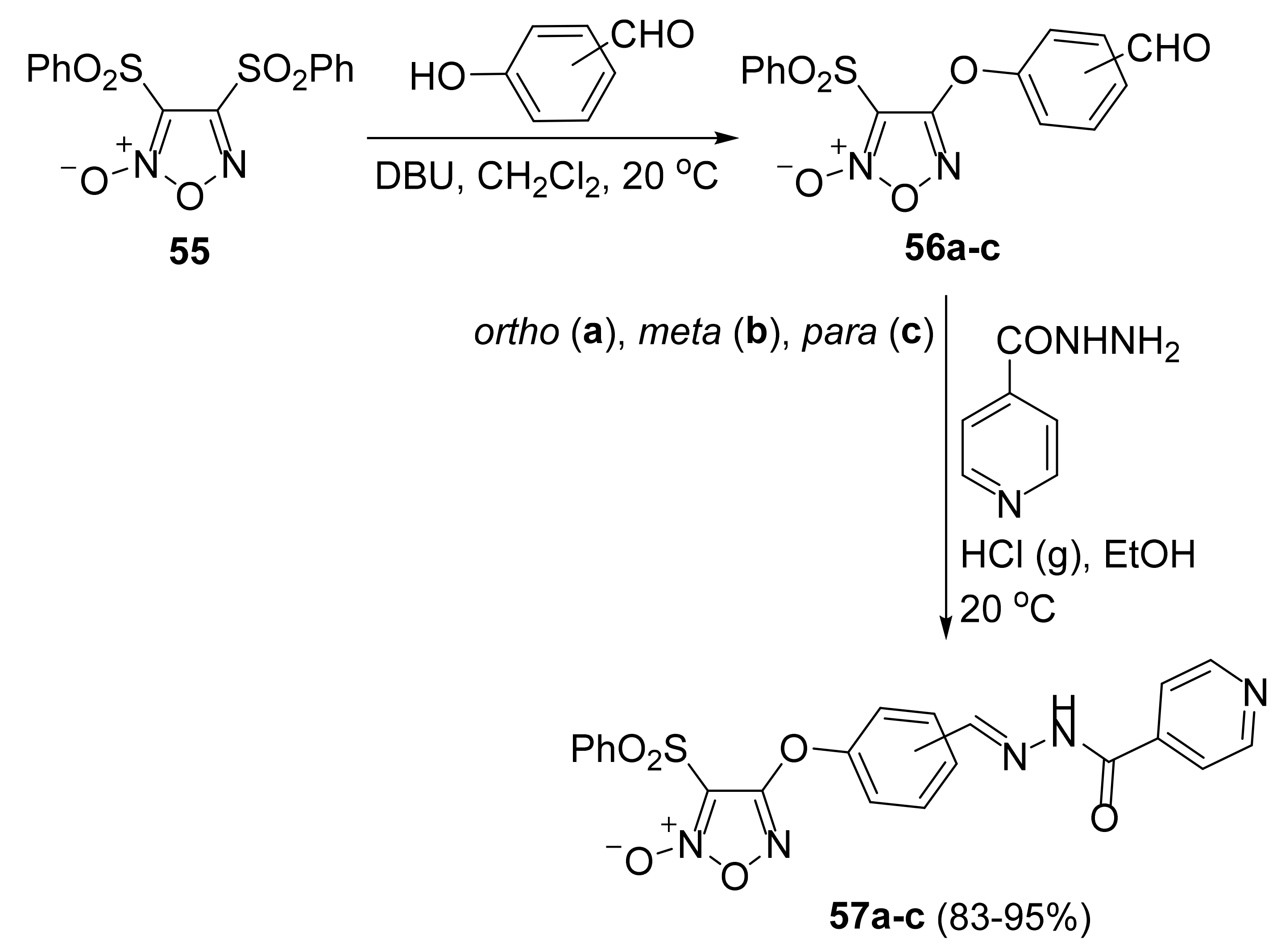
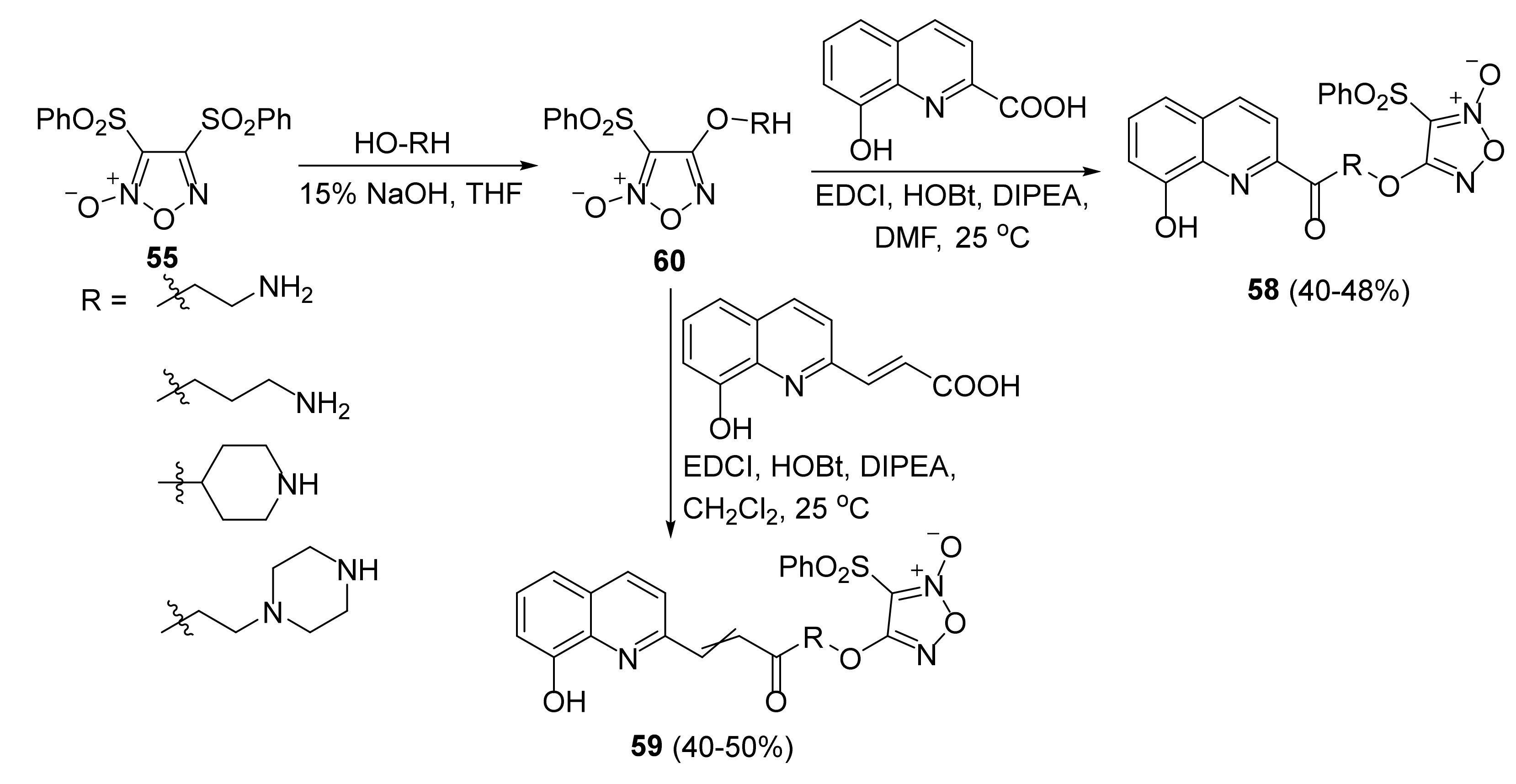
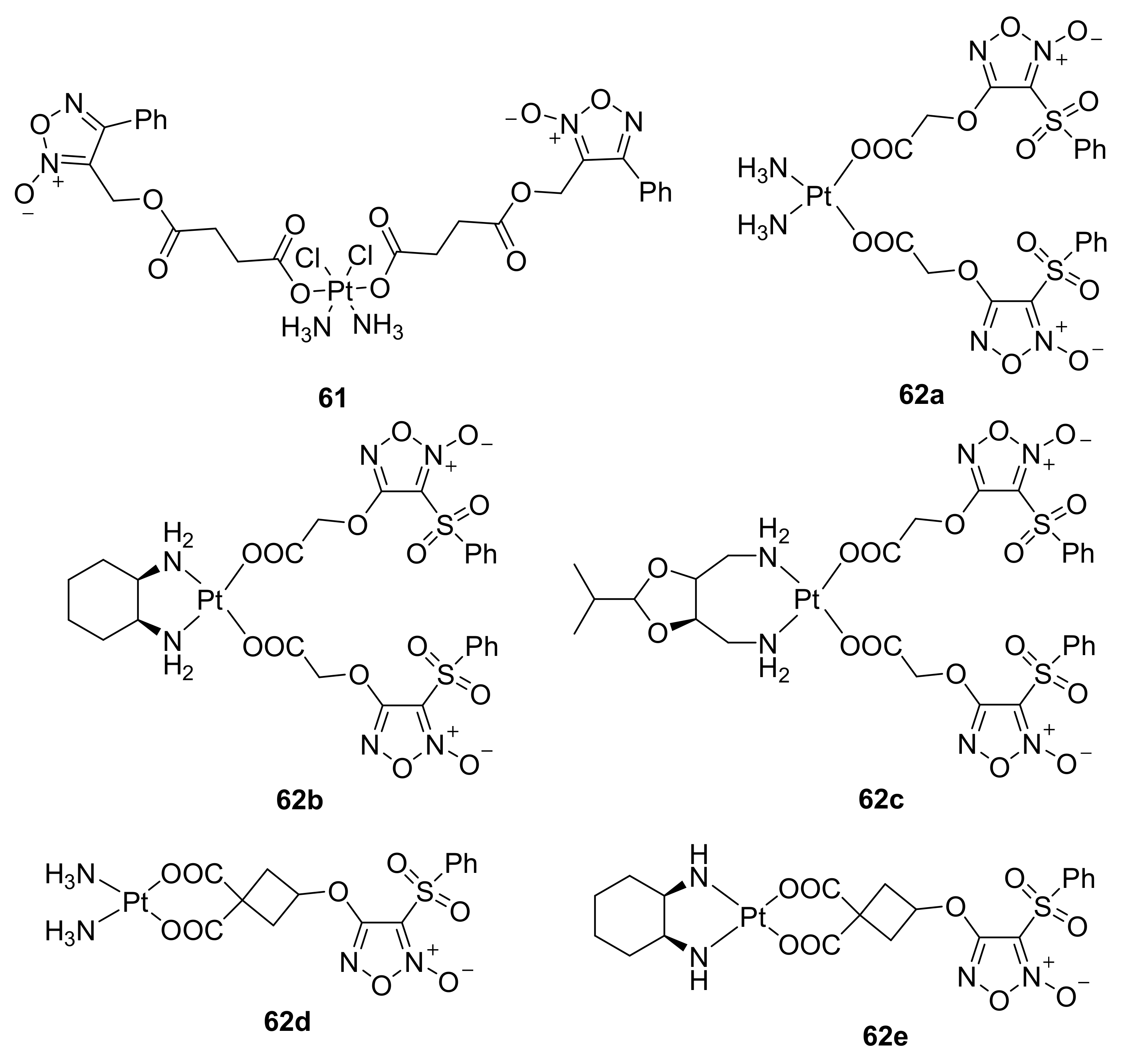
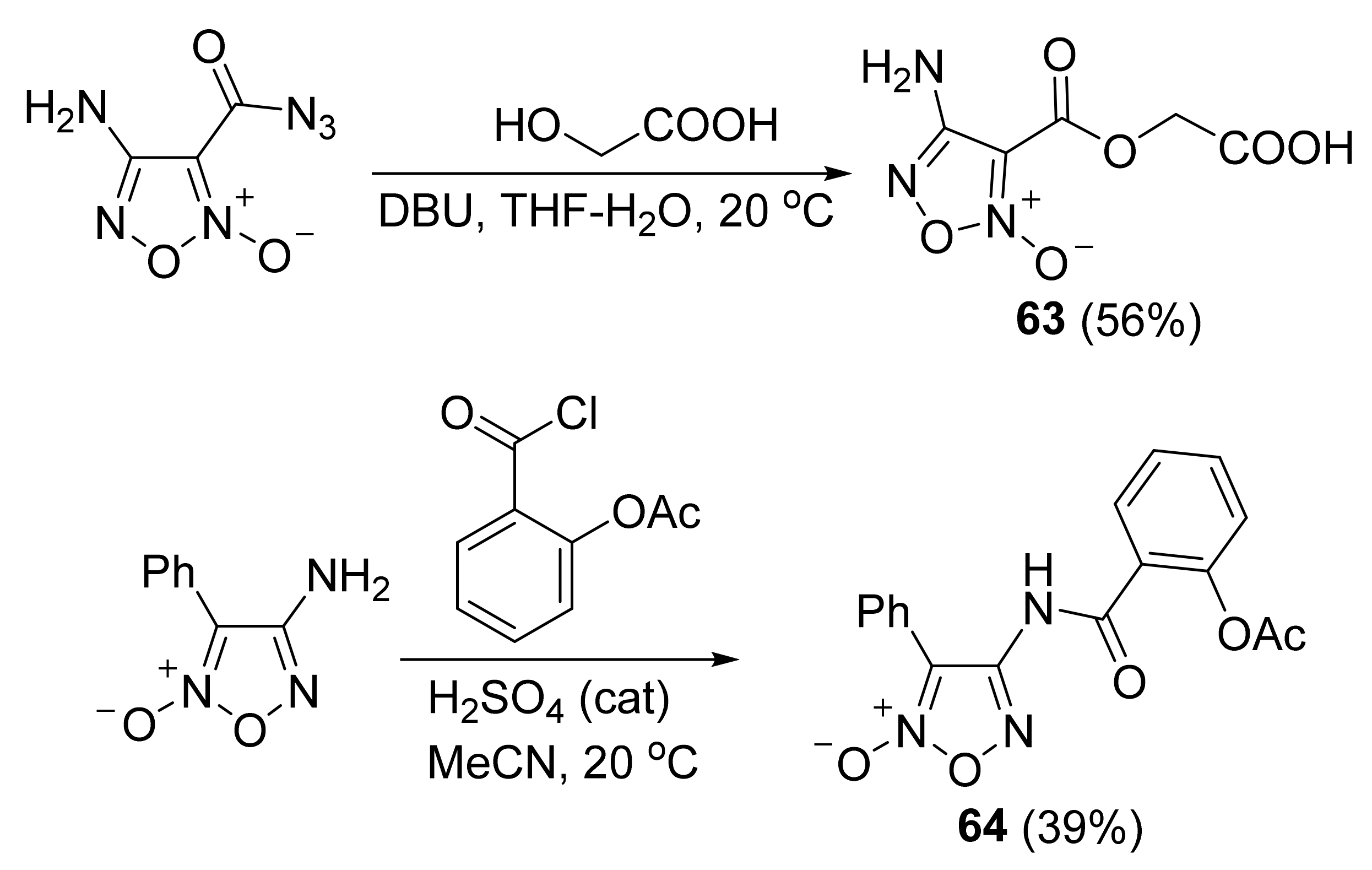
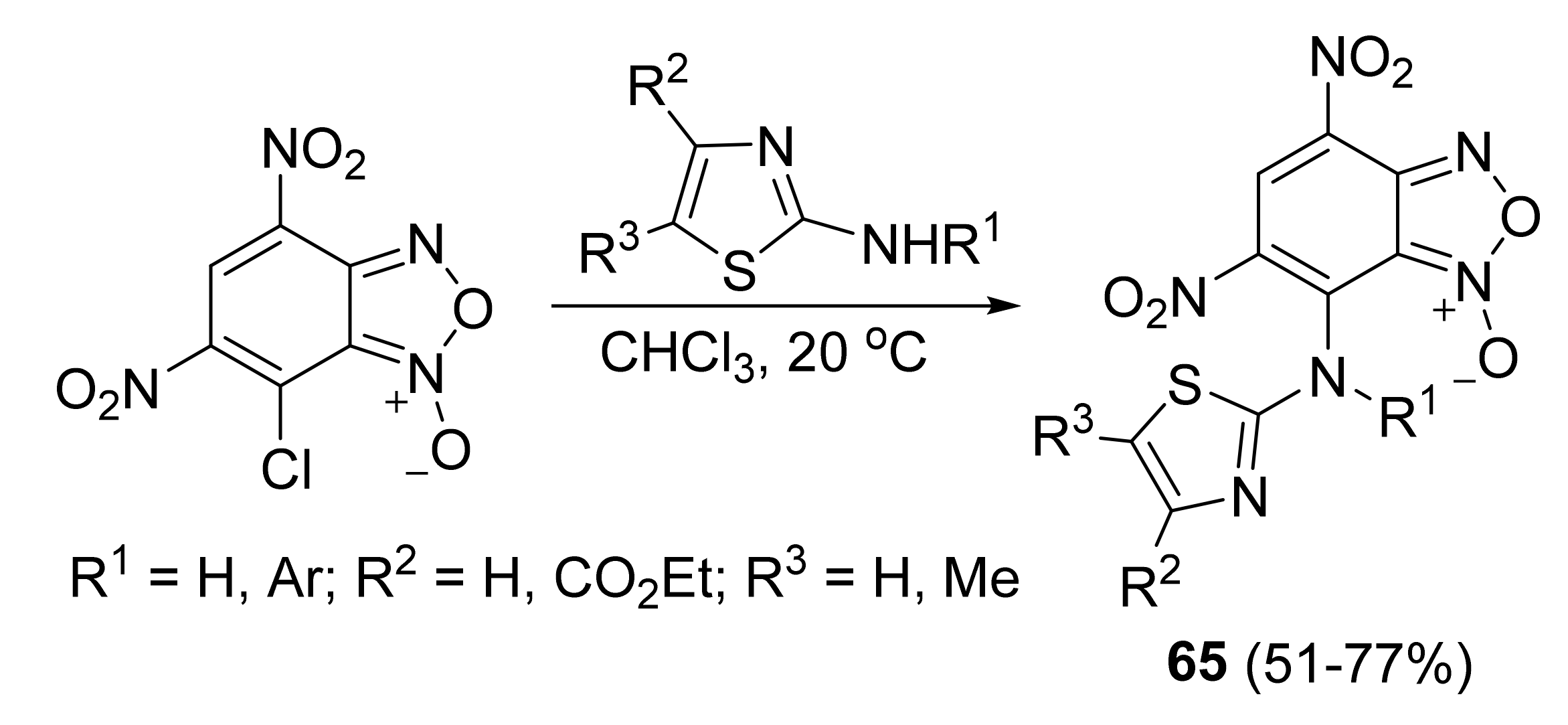
3. Furoxans
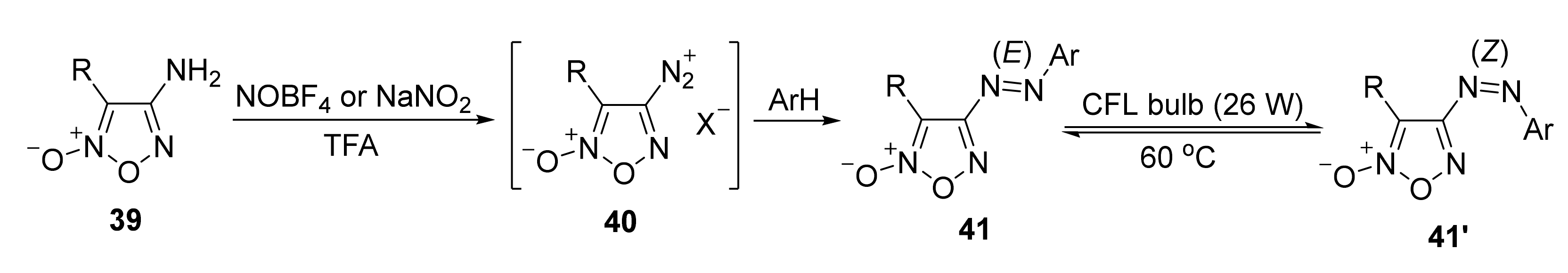
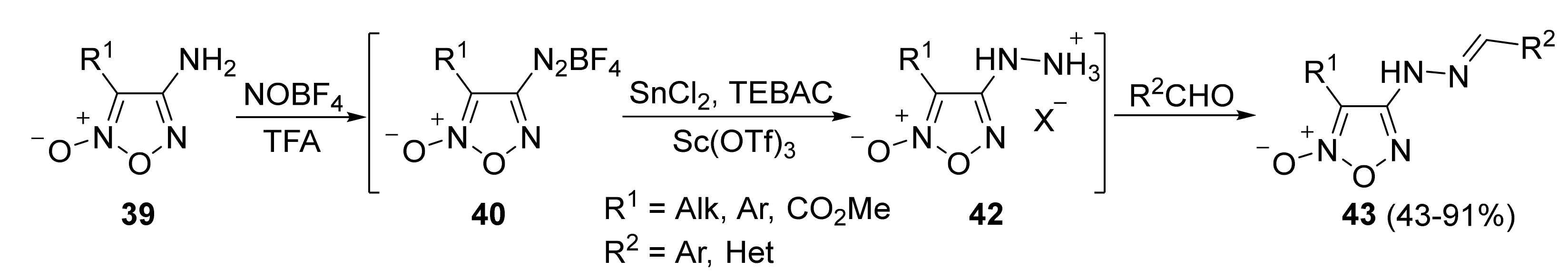
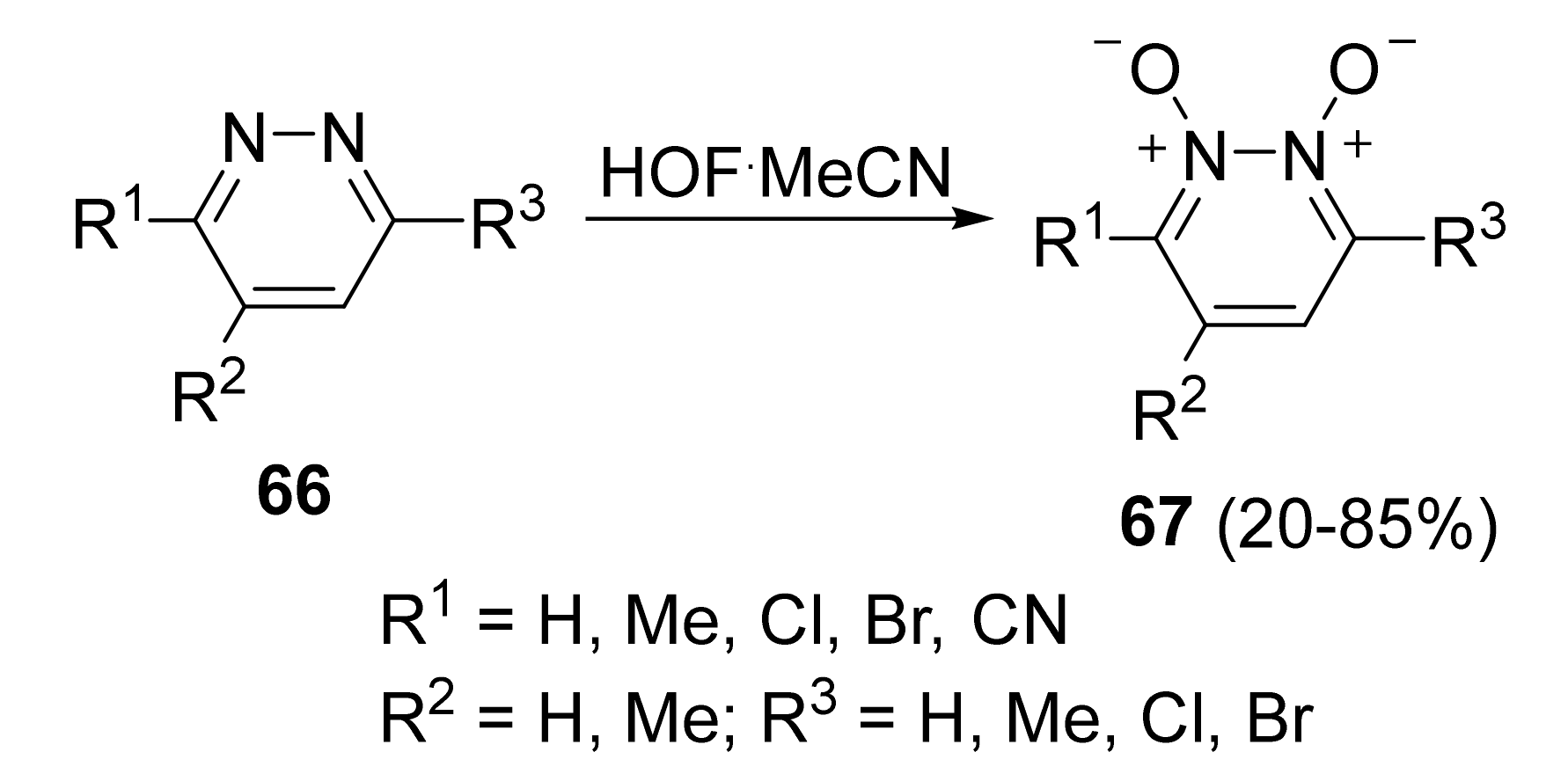
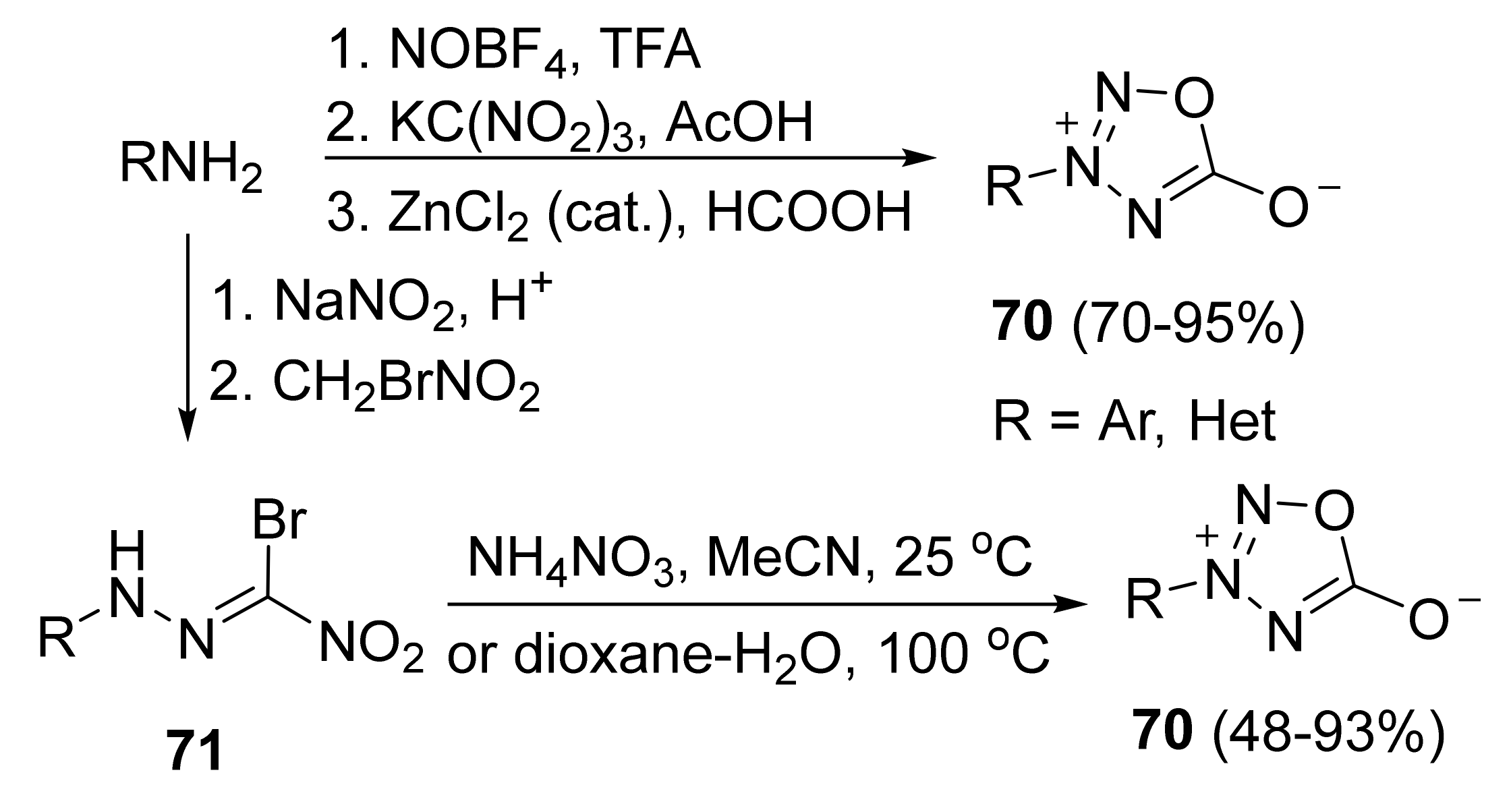
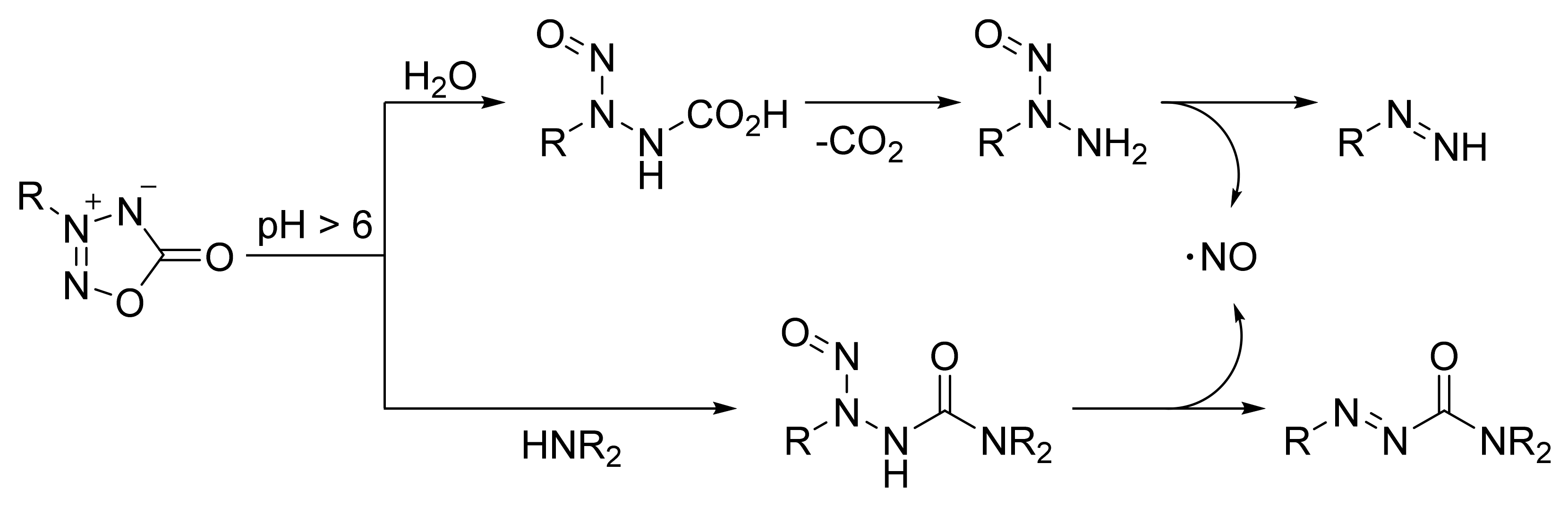
4. Pyridazine Dioxides and Azasydnones
5. Conclusions and Future Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Vanhoutte, P.M.; Zhao, Y.; Xu, A.; Leung, S.W.S. Thirty Years of Saying NO: Sources, Fate, Actions, and Misfortunes of the Endothelium-Derived Vasodilator Mediator. Circ. Res. 2016, 119, 375–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Huang, Z.; Li, L.-L. Advanced nitric oxide donors: Chemical structure of NO drugs, NO nanomedicines and biomedical applications. Nanoscale 2021, 13, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Paulo, M.; Costa, D.E.F.R.; Bonaventura, D.; Lunardi, C.N.; Bendhack, L.M. Nitric Oxide Donors as Potential Drugs for the Treatment of Vascular Diseases Due to Endothelium Dysfunction. Curr. Pharm. Des. 2020, 26, 3748–3759. [Google Scholar] [CrossRef] [PubMed]
- Gkaliagkousi, E.; Ritter, J.; Ferro, A. Platelet-Derived Nitric Oxide Signaling and Regulation. Circ. Res. 2007, 101, 654–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krol, M.; Kepinska, M. Human Nitric Oxide Synthase—Its Functions, Polymorphisms, and Inhibitors in the Context of Inflammation, Diabetes and Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 56. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Giuffrida Stella, A.M. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef]
- Furchgott, R.F. Endothelium-Derived Relaxing Factor: Discovery, Early Studies, and Identifcation as Nitric Oxide (Nobel Lecture). Angew. Chem. Int. Ed. 1999, 38, 1870–1880. [Google Scholar] [CrossRef]
- Ignarro, L.J. Nitric Oxide: A Unique Endogenous Signaling Molecule in Vascular Biology (Nobel Lecture). Angew. Chem. Int. Ed. 1999, 38, 1882–1892. [Google Scholar] [CrossRef]
- Murad, F. Discovery of Some of the Biological Effects of Nitric Oxide and Its Role in Cell Signaling (Nobel Lecture). Angew. Chem. Int. Ed. 1999, 38, 1856–1868. [Google Scholar] [CrossRef]
- Khan, F.H.; Dervan, E.; Bhattacharyya, D.D.; McAuliffe, J.D.; Miranda, K.M.; Glynn, S.A. The Role of Nitric Oxide in Cancer: Master Regulator or NOt? Int. J. Mol. Sci. 2020, 21, 9393. [Google Scholar] [CrossRef]
- Heinrich, T.A.; da Silva, R.S.; Miranda, K.M.; Switzer, C.H.; Wink, D.A.; Fukuto, J.M. Biological nitric oxide signalling: Chemistry and terminology. Br. J. Pharmacol. 2013, 169, 1417–1429. [Google Scholar] [CrossRef] [Green Version]
- Basudhar, D.; Ridnour, L.A.; Cheng, R.; Kesarwala, A.H.; Heinecke, J.; Wink, D.A. Biological signaling by small inorganic molecules. Coord. Chem. Rev. 2016, 306, 708–723. [Google Scholar] [CrossRef] [Green Version]
- Majumder, S.; Sinha, S.; Siamwala, J.H.; Muley, A.; Seerapu, H.R.; Kolluru, G.K.; Veeriah, V.; Nagarajan, S.; Sridhara, S.R.C.; Priya, M.K.; et al. A comparative study of NONOate based NO donors: Spermine NONOate is the best suited NO donor for angiogenesis. Nitric Oxide 2014, 36, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Behera, J.; Nagarajan, S.; Saran, U.; Kumar, R.; Keshri, G.K.; Suryakumar, G.; Chatterjee, S. Nitric oxide restores peripheral blood mononuclear cell adhesion against hypoxia via NO-cGMP signaling. Cell Biochem. Funct. 2020, 38, 319–329. [Google Scholar] [CrossRef]
- Swaminathan, A.; Kasiviswanathan, D.; Balaguru, U.M.; Kolluru, G.K.; Suryakumar, G.; Chatterjee, S. Hypoxia perturbs endothelium by re-organizing cellular actin architecture: Nitric oxide offers limited protection. Tissue Cell 2018, 50, 114–124. [Google Scholar] [CrossRef]
- Siamwala, J.H.; Kumar, P.; Veeriah, V.; Muley, A.; Rajendran, S.; Konikkat, S.; Majumder, S.; Mani, K.P.; Chatterjee, S. Nitric Oxide Reverses the Position of the Heart during Embryonic Development. Int. J. Mol. Sci. 2019, 20, 1157. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Sundaresan, L.; Chatterjee, S. Nitrosative Stress and Cardiogenesis: Cardiac Remodelling Perturbs Embryonic Metabolome. In Modulation of Oxidative Stress in Heart Disease; Chakraborti, S., Dhalla, N.S., Dikshit, M., Ganguly, N.K., Eds.; Springer: Singapore, 2019; pp. 377–392. [Google Scholar]
- Giri, S.; Thakar, S.; Majumder, S.; Chatterjee, S. Regulation of Oxidative Stress by Nitric Oxide Defines Lung Development and Diseases. In Oxidative Stress in Lung Diseases; Chakraborti, S., Parinandi, N.L., Ghosh, R., Ganguly, N.K., Chakraborti, T., Eds.; Springer: Singapore, 2019; pp. 445–464. [Google Scholar]
- Serafim, R.A.M.; Pernichelle, F.G.; Ferreira, E.I. The latest advances in the discovery of nitric oxide hybrid drug compounds. Expert Opin. Drug. Discov. 2017, 12, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.S. Natural Product Chemistry for Nitric Oxide Based Therapeutics. Isr. J. Chem. 2019, 59, 414–419. [Google Scholar] [CrossRef]
- Huang, Z.; Fu, J.; Zhang, Y. Nitric Oxide Donor-Based Cancer Therapy: Advances and Prospects. J. Med. Chem. 2017, 60, 7617–7635. [Google Scholar] [CrossRef]
- Wang, P.G.; Xian, M.; Tang, X.; Wu, X.; Wen, Z.; Cai, T.; Janczuk, A.J. Nitric Oxide Donors: Chemical Activities and Biological Applications. Chem. Rev. 2002, 102, 1091–1134. [Google Scholar] [CrossRef] [PubMed]
- Gasco, A.; Schoenafinger, K. Nitric Oxide Donors: For Pharmaceutical and Biological Applications; Wang, P.G., Cai, T.B., Taniguchi, N., Eds.; Wiley-VCH: Weinheim, Germany, 2005; pp. 131–175. [Google Scholar]
- Bath, P.M.W.; Krishnan, K.; Appleton, J.P. Nitric oxide donors (nitrates), L-arginine, or nitric oxide synthase inhibitors for acute stroke (Review). Cochrane Database Syst. Rev. 2017, 4, CD000398. [Google Scholar]
- Steven, S.; Oelze, M.; Hausding, M.; Roohani, S.; Kashani, F.; Kröller-Schön, S.; Helmstädter, J.; Jansen, T.; Baum, C.; Iglarz, M.; et al. Oxidative Stress and Cardiovascular Dysfunction: From Basic Science to Applied Investigations. Oxid. Med. Cell. Longevity 2018, 2018, 7845629. [Google Scholar]
- Kuchurov, I.V.; Arabadzhi, S.S.; Zharkov, M.N.; Fershtat, L.L.; Zlotin, S.G. Sustainable Synthesis of Polynitroesters in the Freon Medium and their in Vitro Evaluation as Potential Nitric Oxide Donors. ACS Sustain. Chem. Eng. 2018, 6, 2535–2540. [Google Scholar] [CrossRef]
- Schönafinger, K. Heterocyclic NO prodrugs. Il Pharmaco 1999, 54, 316–320. [Google Scholar] [CrossRef]
- Feelisch, M.; Ostrowski, J.; Noack, E. On the mechanism of NO release from sydnonimines. J. Cardiovasc. Pharmacol. 1989, 14, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Ullrich, T.; Oberle, S.; Abate, A.; Schröder, H. Photoactivation of the nitric oxide donor SIN-1. FEBS Lett. 1997, 406, 66–68. [Google Scholar] [CrossRef] [Green Version]
- Cherepanov, I.A.; Moiseev, S.K. Recent developments in the chemistry of sydnones and sydnone imines. Adv. Heterocycl. Chem. 2020, 131, 49–164. [Google Scholar]
- Beal, E.N.; Tumbull, K. An efficient, one-pot synthesis of 3-alkyl or aryl sydnoneimines. Synth. Commun. 1992, 22, 673–676. [Google Scholar] [CrossRef]
- Gotz, M.; Grozinger, K. 3-Hydroxysydnone imines. Tetrahedron 1971, 27, 4449–4456. [Google Scholar] [CrossRef]
- Cherepanov, I.A.; Samarskaya, A.S.; Godovikov, I.A.; Lyssenko, K.A.; Pankratova, A.A.; Kalinin, V.N. N6-tert-Butoxycarbonyl derivatives of sydnone imines: Preparation and synthetic use. Tetrahedron Lett. 2018, 59, 727–729. [Google Scholar] [CrossRef]
- Acharya, S.; Rogers, P.; Krishnamoorthy, R.R.; Stankowska, D.L.; Dias, H.V.R.; Yorio, T. Design and synthesis of novel hybrid sydnonimine and prodrug useful for glaucomatous optic neuropathy. Bioorg. Med. Chem. Lett. 2016, 26, 1490–1494. [Google Scholar] [CrossRef]
- Cai, T.B.; Lu, D.; Tang, X.; Zhang, Y.; Landerholm, M.; Wang, P.G. New glycosidase activated nitric oxide donors: Glycose and 3-morphorlinosydnonimine conjugates. J. Org. Chem. 2005, 70, 3518–3524. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.; Audisio, D.; Riomet, M.; Bregant, S.; Sallustrau, A.; Plougastel, A.; Decuypere, E.; Gabillet, S.; Kumar, R.A.; Elyian, J.; et al. Bioorthogonal Click and Release Reaction of Iminosydnones with Cycloalkynes. Angew. Chem., Int. Ed. 2017, 56, 15612–15616. [Google Scholar] [CrossRef]
- Samarskaya, A.S.; Cherepanov, I.A.; Godovikov, I.A.; Kalinin, V.A. N6-α-haloacyl sydnone imine derivatives. Dokl. Chem. 2015, 463, 199–203. [Google Scholar] [CrossRef]
- Shao, Z.; Liu, W.; Tao, H.; Liu, F.; Zeng, R.; Champagne, P.A.; Cao, Y.; Houk, K.N.; Liang, Y.; Cao, Y.; et al. Bioorthogonal release of sulfonamides and mutually orthogonal liberation of two drugs. Chem. Commun. 2018, 54, 14089–14092. [Google Scholar] [CrossRef] [PubMed]
- Riomet, M.; Decuypere, E.; Porte, K.; Bernard, S.; Plougastel, L.; Kolodych, S.; Audisio, D.; Taran, F. Design and Synthesis of Iminosydnones for Fast Click and Release Reactions with Cycloalkynes. Chem. Eur. J. 2018, 24, 8535–8541. [Google Scholar] [CrossRef]
- Riomet, M.; Porte, K.; Madegard, L.; Thuéry, P.; Audisio, D.; Taran, F. Access to N-Carbonyl Derivatives of Iminosydnones by Carbonylimidazolium Activation. Org. Lett. 2020, 22, 2403–2408. [Google Scholar] [CrossRef] [PubMed]
- Samarskaya, A.S.; Cherepanov, I.A.; Godovikov, I.A.; Dmitrienko, A.O.; Moiseev, S.K.; Kalinin, V.N.; Hey-Hawkins, E. Synthesis of N6-phosphorylated sydnone imines and their functionalization via 4-Li derivatives. Novel bicyclic sydnone imines. Tetrahedron 2018, 74, 2693–2702. [Google Scholar] [CrossRef]
- Beal, E.N.; Turnbull, K. Bromination/Debromination of 6-Benzoyl-3-alkyl or 3-Aryl Sydnoneimines. Synth. Commun. 1992, 22, 1515–1522. [Google Scholar] [CrossRef]
- Cherepanov, I.A.; Kusaeva, L.H.; Godovikov, I.A.; Kalinin, V.N. 4-Formylsydnonimine derivatives. Russ. Chem. Bull. Int. Ed. 2009, 58, 2474–2477. [Google Scholar] [CrossRef]
- Freese, T.; Lücke, A.-L.; Schmidt, C.A.S.; Polamo, M.; Nieger, M.; Namyslo, J.C.; Schmidt, A. Anionic N-heterocyclic carbenes derived from sydnone imines such as molsidomine. Trapping reactions with selenium, palladium, and gold. Tetrahedron 2017, 73, 5350–5357. [Google Scholar] [CrossRef] [Green Version]
- Cherepanov, I.A.; Kalinin, V.N. Synthesis and reactivity of 4-lithium and 4-copper derivatives of sydnone imines. Mendeleev Commun. 2000, 5, 181–182. [Google Scholar] [CrossRef]
- Kalinin, V.N.; Lebedev, S.N.; Cherepanov, I.A.; Godovikov, I.A.; Lyssenko, K.A.; Hey-Hawkins, E. 4-Diphenylphosphinosydnone imines as bidentate ligands. Polyhedron 2009, 28, 2411–2417. [Google Scholar] [CrossRef]
- Cherepanov, I.A.; Lebedev, S.N.; Samarskaya, A.S.; Godovikov, I.A.; Nelyubina, Y.V.; Kalinin, V.N. 4-Thio derivatives of sydnone imines. Mendeleev Commun. 2009, 19, 322–323. [Google Scholar] [CrossRef]
- Cherepanov, I.A.; Shevaldina, E.V.; Lapshin, D.A.; Spiridonov, Y.Y.; Abubikerov, V.C.; Moiseev, S.K. 4-Lithiosydnone imines: Generation and stability. Plant growth regulating activity of 4-hydroxymethyl derivatives of sydnone imines. J. Organomet. Chem. 2021, 943, 121841. [Google Scholar] [CrossRef]
- Freese, T.; Lücke, A.-L.; Namyslo, J.C.; Nieger, M.; Schmidt, A. Heterocycle Syntheses with Anionic N-Heterocyclic Carbenes: Ring Transformations of Sydnone Imine Anions. Eur. J. Org. Chem. 2018, 2018, 1646–1654. [Google Scholar] [CrossRef]
- Freese, T.; Nieger, M.; Namyslo, J.C.; Schmidt, A. Cycloadditions of anionic N-heterocyclic carbenes of sydnone imines. Tetrahedron Lett. 2019, 60, 1272–1276. [Google Scholar] [CrossRef]
- Freese, T.; Namyslo, J.C.; Nieger, M.; Schmidt, A. Sulfur, mercury, and boron adducts of sydnone imine derived anionic N-heterocyclic carbenes. RSC Adv. 2019, 9, 4781–4788. [Google Scholar] [CrossRef] [Green Version]
- Riomet, M.; Porte, K.; Wijkhuisen, A.; Audisio, D.; Taran, F. Fluorogenic iminosydnones: Bioorthogonal tools for double turn-on click-and-release reactions. Chem. Commun. 2020, 56, 7183–7186. [Google Scholar] [CrossRef]
- Porte, K.; Renoux, B.; Péraudeau, E.; Clarhaut, J.; Eddhif, B.; Poinot, P.; Gravel, E.; Doris, E.; Wijkhuisen, A.; Audisio, D.; et al. Controlled Release of Micelle Payload via Sequential Enzymatic and Bioorthogonal Reactions in Living Systems. Angew. Chem., Int. Ed. 2019, 58, 6366–6370. [Google Scholar] [CrossRef]
- Porte, K.; Riomet, M.; Figliola, C.; Audisio, D.; Taran, F. Click and Bio-Orthogonal Reactions with Mesoionic Compounds. Chem. Rev. 2021, 121, 6718–6743. [Google Scholar] [CrossRef] [PubMed]
- Decuypere, E.; Bernard, S.; Feng, M.; Porte, K.; Riomet, M.; Thuery, P.; Audisio, D.; Taran, F. Copper-Catalyzed Aza-Iminosydnone-Alkyne Cycloaddition Reaction Discovered by Screening. ACS Catal. 2018, 8, 11882–11888. [Google Scholar] [CrossRef] [Green Version]
- Khmel’;nitskaya, E.Y.; Levina, V.I.; Trukhacheva, L.A.; Grigoriev, N.B.; Kalinin, V.N.; Cherepanov, I.A.; Lebedev, S.N.; Granik, V.G. Sydnonimines as exogenous NO donors. Russ. Chem. Bull. Int. Ed. 2004, 53, 2840–2844. [Google Scholar] [CrossRef]
- Kikuchi, K.; Hirata, M.; Nagaoka, A. Hypotensive action of N-ethoxycarbonyl-3-morpholinosydnonimine, SIN-10. Jap. J. Pharmacol. 1970, 20, 102–115. [Google Scholar] [CrossRef] [Green Version]
- Drummer, C.; Valta-Seufzer, U.; Karrenbrock, B.; Heim, J.M.; Gerzer, R. Comparison of anti-platelet properties of molsidomine, isosorbide-5-mononitrate and placebo in healthy volunteers. Eur. Heart J. 1991, 12, 541–549. [Google Scholar] [CrossRef]
- Nortcliffe, A.; Botting, N.P.; O’Hagan, D. Novel amino acids: Synthesis of furoxan and sydnonimine containing amino acids and peptides as potential nitric oxide releasing motifs. Org. Biomol. Chem. 2013, 11, 4657–5671. [Google Scholar] [CrossRef]
- Nortcliffe, A.; Fleming, I.N.; Botting, N.P.; O’Hagan, D. Synthesis and anticancer properties of RGD peptides conjugated to nitric oxide releasing functional groups and abiraterone. Tetrahedron 2014, 70, 8343–8347. [Google Scholar] [CrossRef]
- Nortcliffe, A.; Ekstrom, A.G.; Black, J.R.; Ross, J.A.; Habib, F.K.; Botting, N.P.; O’Hagan, D. Synthesis and biological evaluation of nitric oxide-donating analogues of sulindac for prostate cancer treatment. Bioorg. Med. Chem. 2014, 22, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Witkin, J.M.; Savtchenko, N.; Mashkovsky, M.; Beekman, M.; Munzar, P.; Gasior, M.; Goldberg, S.R.; Ungard, J.T.; Kim, J.; Shippenberg, T.; et al. Behavioral, Toxic, and Neurochemical Effects of Sydnocarb, a Novel Psychomotor Stimulant: Comparisons with Methamphetamine. J. Pharmacol. Exp. Ther. 1999, 288, 1298–1310. [Google Scholar]
- Marvasi, M.; Chen, C.; Carrazana, M.; Durie, I.A.; Teplitski, M. Systematic analysis of the ability of Nitric Oxide donors to dislodge biofilms formed by Salmonella enterica and Escherichia coli O157:H7. AMB Express 2014, 4, 42. [Google Scholar] [CrossRef] [Green Version]
- Soulère, L.; Hoffmanna, P.; Bringaud, F. Synthesis of sydnonimine derivatives as potential trypanocidal agents. J. Heterocycl. Chem. 2003, 40, 943–947. [Google Scholar] [CrossRef]
- Du, S.; Hu, X.; Li, M.; Jiang, X.; Xu, X.; Cheng, J.; Qian, X. Discovery of novel iminosydnone compounds with insecticidal activities based on the binding mode of triflumezopyrim. Bioorg. Med. Chem. Lett. 2021, 46, 128120. [Google Scholar] [CrossRef]
- Pruschinski, L.; Lücke, A.-L.; Freese, T.; Kahnert, S.-R.; Mummel, S.; Schmidt, A. Suzuki–Miyaura Cross-Couplings under Acidic Conditions. Synthesis 2020, 52, 882–892. [Google Scholar] [CrossRef]
- Lücke, A.-L.; Pruschinski, L.; Freese, T.; Schmidt, A. Sonogashira-Hagihara and Buchwald-Hartwig cross-coupling reactions with sydnone and sydnone imine derived catalysts. Arkivoc 2020, vii, 94–104. [Google Scholar] [CrossRef]
- Gettings, M.L.; Byrd, E.F.C.; Zeller, M.; Piercey, D. Methyl sydnone imine and its energetic salts. New J. Chem. 2021, 45, 2228–2236. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Makhova, N.N. Advances in the synthesis of non-annelated polynuclear heterocyclic systems comprising the 1,2,5-oxadiazole ring. Russ. Chem. Rev. 2016, 85, 1097–1145. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Makhova, N.N. Molecular Hybridization Tools in the Development of Furoxan-Based NO-Donor Prodrugs. ChemMedChem 2017, 12, 622–638. [Google Scholar] [CrossRef] [PubMed]
- Makhova, N.N.; Fershtat, L.L. Recent advances in the synthesis and functionalization of 1,2,5-oxadiazole 2-oxides. Tetrahedron Lett. 2018, 59, 2317–2326. [Google Scholar] [CrossRef]
- Makhova, N.N.; Belen’kii, L.I.; Gazieva, G.A.; Dalinger, I.L.; Konstantinova, L.S.; Kuznetsov, V.V.; Kravchenko, A.N.; Krayushkin, M.M.; Rakitin, O.A.; Starosotnikov, A.M.; et al. Progress in the chemistry of nitrogen-, oxygen- and sulfur-containing heterocyclic systems. Russ. Chem. Rev. 2020, 89, 55–124. [Google Scholar] [CrossRef]
- Ferioli, R.; Folco, G.C.; Ferretti, C.; Gasco, A.M.; Medana, C.; Fruttero, R.; Civelli, M.; Gasco, A. A new class of furoxan derivatives as NO donors: Mechanism of action and biological activity. Br. J. Pharmacol. 1995, 114, 816–820. [Google Scholar] [CrossRef] [Green Version]
- Gasco, A.; Fruttero, R.; Sorba, G.; Di Stilo, A.; Calvino, R. NO donors: Focus on furoxan derivatives. Pure Appl. Chem. 2004, 76, 973–981. [Google Scholar] [CrossRef] [Green Version]
- Bohn, H.; Brendel, J.; Martorana, P.A.; Schönafinger, K. Cardiovascular actions of the furoxan CAS 1609, a novel nitric oxide donor. Br. J. Pharmacol. 1995, 114, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ustyuzhanina, N.E.; Fershtat, L.L.; Gening, M.L.; Nifantiev, N.E.; Makhova, N.N. Antiaggregant activity of water-soluble furoxans. Mendeleev Commun. 2018, 28, 49–51. [Google Scholar] [CrossRef]
- Civelli, M.; Giossi, M.; Caruso, P.; Razzetti, R.; Bergamaschi, M.; Bongrani, S.; Gasco, A. The involvement of the release of nitric oxide in the pharmacological activity of the new furoxan derivative CHF 2363. Br. J. Pharmacol. 1996, 118, 923–928. [Google Scholar] [CrossRef]
- Balbo, S.; Lazzarato, L.; di Stilo, A.; Fruttero, R.; Lombaert, N.; Kirsch-Volders, M. Studies of the potential genotoxic effects of furoxans: The case of CAS 1609 and of the water-soluble analogue of CHF 2363. Toxic. Lett. 2008, 178, 44–51. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Bystrov, D.M.; Zhilin, E.S.; Makhova, N.N. N-Oxide-Controlled Chemoselective Reduction of Nitrofuroxans. Synthesis 2019, 51, 747–756. [Google Scholar] [CrossRef] [Green Version]
- Zhilin, E.S.; Fershtat, L.L.; Bystrov, D.M.; Kulikov, A.S.; Dmitrienko, A.O.; Ananyev, I.V.; Makhova, N.N. Renaissance of 1,2,5-Oxadiazolyl Diazonium Salts: Synthesis and Reactivity. Eur. J. Org. Chem. 2019, 2019, 4248–4259. [Google Scholar] [CrossRef]
- Zhilin, E.S.; Polkovnichenko, M.S.; Ananyev, I.V.; Fershtat, L.L.; Makhova, N.N. Novel Arylazo-1,2,5-oxadiazole Photoswitches: Synthesis, Photoisomerization and Nitric Oxide Releasing Properties. ChemPhotoChem 2020, 4, 5346–5354. [Google Scholar] [CrossRef]
- Bystrov, D.M.; Ananyev, I.V.; Fershtat, L.L.; Makhova, N.N. Direct Synthesis of N-(1,2,5-Oxadiazolyl)hydrazones through a Diazotization/Reduction/Condensation Cascade. J. Org. Chem. 2020, 85, 15466–15475. [Google Scholar] [CrossRef]
- Dos Santos Fernandes, G.F.; Pavan, A.R.; dos Santos, J.L. Heterocyclic N-oxides—A Promising Class of Agents against Tuberculosis, Malaria and Neglected Tropical Diseases. Curr. Pharm. Des. 2018, 24, 1325–1340. [Google Scholar] [CrossRef]
- Serafim, R.A.M.; Gonçalves, J.E.; de Souza, F.P.; de Melo Loureiro, A.P.; Storpirtis, S.; Krogh, R.; Andricopulo, A.D.; Dias, L.C.; Ferreira, E.I. Design, synthesis and biological evaluation of hybrid bioisoster derivatives of N-acylhydrazone and furoxan groups with potential and selective anti-Trypanosoma cruzi activity. Eur. J. Med. Chem. 2014, 82, 418–425. [Google Scholar] [CrossRef]
- Hernández, P.; Rojas, R.; Gilman, R.H.; Sauvain, M.; Lima, L.M.; Barreiro, E.J.; González, M.; Cerecetto, H. Hybrid furoxanyl N-acylhydrazone derivatives as hits for the development of neglected diseases drug candidates. Eur. J. Med. Chem. 2013, 59, 64–74. [Google Scholar] [CrossRef]
- Guglielmo, S.; Cortese, D.; Vottero, F.; Rolando, B.; Kommer, V.P.; Williams, D.L.; Fruttero, R.; Gasco, A. New praziquantel derivatives containing NO-donor furoxans and related furazans as active agents against Schistosoma mansoni. Eur. J. Med. Chem. 2014, 84, 135–145. [Google Scholar] [CrossRef] [Green Version]
- Epishina, M.A.; Kulikov, A.S.; Fershtat, L.L.; Ananyev, I.V.; Makhova, N.N. Synthesis of new pharmacologically oriented heterocyclic ensembles, [2-(1H-pyrazol-1-yl)thiazol-4-yl]furoxans. Mendeleev Commun. 2019, 29, 288–291. [Google Scholar] [CrossRef]
- Kulikov, A.S.; Epishina, M.A.; Fershtat, L.L.; Makhova, N.N. Effective synthesis of 7H-1,2,4-triazolo[3,4-b][1,3,4]thiadiazines. Chem. Heterocycl. Compd. 2018, 54, 669–672. [Google Scholar] [CrossRef]
- Kulikov, A.S.; Epishina, M.A.; Fershtat, L.L.; Romanova, A.A.; Makhova, N.N. Effective synthesis of 6-substituted 7H-tetrazolo[5,1-b][1,3,4]thiadiazines via a one-pot condensation/nitrosation/azide-tetrazole tautomerism reaction sequence. Tetrahedron Lett. 2017, 58, 3998–4002. [Google Scholar] [CrossRef]
- Kulikov, A.S.; Epishina, M.A.; Churakov, A.I.; Anikina, L.V.; Fershtat, L.L.; Makhova, N.N. Regioselective synthesis, structural diversification and cytotoxic activity of (thiazol-4-yl)furoxans. Mendeleev Commun. 2018, 28, 623–625. [Google Scholar] [CrossRef]
- Matsubara, R.; Kim, H.; Sakaguchi, T.; Xie, W.; Zhao, X.; Nagoshi, Y.; Wang, C.; Tateiwa, M.; Ando, A.; Hayashi, M.; et al. Modular Synthesis of Carbon-Substituted Furoxans via Radical Addition Pathway. Useful Tool for Transformation of Aliphatic Carboxylic Acids Based on “Build-and-Scrap” Strategy. Org. Lett. 2020, 22, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, R.; Ando, A.; Hasebe, H.; Kim, H.; Tsuneda, T.; Hayashi, M. Synthesis and Synthetic Application of Chloro- and Bromofuroxans. J. Org. Chem. 2020, 85, 5959–5972. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Hayashi, M.; Matsubara, R. Borylfuroxans: Synthesis and Applications. Org. Lett. 2021, 23, 4317–4321. [Google Scholar] [CrossRef]
- Popov, S.A.; Semenova, M.D.; Baev, D.S.; Sorokina, I.V.; Zhukova, N.A.; Frolova, T.S.; Tolstikova, T.G.; Shults, E.E.; Turks, M. Lupane-type conjugates with aminoacids, 1,3,4- oxadiazole and 1,2,5-oxadiazole-2-oxide derivatives: Synthesis, anti-inflammatory activity and in silico evaluation of target affinity. Steroids 2019, 150, 108443. [Google Scholar] [CrossRef]
- Prabhuling, S.; Tamboli, Y.; Choudhari, P.B.; Bhatia, M.S.; Mohanta, T.K.; Al-Harrasi, A.; Pudukulathan, Z.K. Synthesis and Modeling Studies of Furoxan Coupled Spiro-Isoquinolino Piperidine Derivatives as NO Releasing PDE 5 Inhibitors. Biomedicines 2020, 8, 121. [Google Scholar] [CrossRef]
- Ma, J.; Chen, L.; Fan, J.; Cao, W.; Zeng, G.; Wang, Y.; Li, Y.; Zhou, Y.; Deng, X. Dual-targeting Rutaecarpine-NO donor hybrids as novel antihypertensive agents by promoting release of CGRP. Eur. J. Med. Chem. 2019, 168, 146–153. [Google Scholar] [CrossRef]
- Dos Santos Fernandes, G.F.; de Souza, P.C.; Marino, L.B.; Chegaev, K.; Guglielmo, S.; Lazzarato, L.; Fruttero, R.; Chung, M.C.; Pavan, F.R.; dos Santos, J.L. Synthesis and biological activity of furoxan derivatives against Mycobacterium tuberculosis. Eur. J. Med. Chem. 2016, 123, 523–531. [Google Scholar] [CrossRef] [Green Version]
- De Souza, P.C.; Fernandes, G.F.S.; Marino, L.B.; Ribeiro, C.M.; da Silva, P.B.; Chorilli, M.; Silva, C.S.P.; Resende, F.A.; Solcia, M.C.; de Grandis, R.A.; et al. Furoxan derivatives demonstrated in vivo efficacy by reducing Mycobacterium tuberculosis to undetectable levels in a mouse model of infection. Biomed. Pharmacother. 2020, 130, 110592. [Google Scholar] [CrossRef]
- Niu, X.; Cao, J.; Zhang, Y.; Gao, X.; Cheng, M.; Liu, Y.; Wang, W.; Yuan, Z. A glutathione responsive nitric oxide release system based on charge-reversal chitosan nanoparticles for enhancing synergistic effect against multidrug resistance tumor. Nanomed. Nanotechnol. 2019, 20, 102015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, J.; Meng, T.; Qin, Y.; Li, T.; Fu, J.; Yin, J. Nitric oxide-donating and reactive oxygen species-responsive prochelators based on 8-hydroxyquinoline as anticancer agents. Eur. J. Med. Chem. 2021, 212, 113153. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Deng, Y.; Huang, Y.; Yu, Z.; Wang, C.; Wang, K.; Dong, J.; Chen, Y. Synthesis and Antitumor Evaluation of Novel Hybrids of Phenylsulfonylfuroxan and Estradiol Derivatives. ChemistryOpen 2020, 9, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Sodano, F.; Gazzano, E.; Rolando, B.; Marini, E.; Lazzarato, L.; Fruttero, R.; Riganti, C.; Gasco, A. Tuning NO release of organelle-targeted furoxan derivatives and their cytotoxicity against lung cancer cells. Bioorg. Chem. 2021, 111, 104911. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Lai, F.; Fu, J.; Li, C.; Ma, J.; Chen, C.; Liu, K.; Zhang, T.; Chen, X.; Zhang, D. Novel nitric oxide-releasing derivatives of triptolide as antitumor and anti-inflammatory agents: Design, synthesis, biological evaluation, and nitric oxide release studies. Eur. J. Med. Chem. 2020, 190, 112079. [Google Scholar] [CrossRef]
- Dai, Y.; Zhu, Y.; Cheng, J.; Shen, J.; Huang, H.; Liu, M.; Chen, Z.; Liu, Y. Nitric oxide-releasing platinum(IV) prodrug efficiently inhibits proliferation and metastasis of cancer cells. Chem. Commun. 2020, 56, 14051. [Google Scholar] [CrossRef]
- Zhao, J.; Gou, S.; Sun, Y.; Fang, L.; Wang, Z. Antitumor Platinum(II) Complexes Containing Platinum-Based Moieties of Present Platinum Drugs and Furoxan Groups as Nitric Oxide Donors: Synthesis, DNA Interaction, and Cytotoxicity. Inorg. Chem. 2012, 51, 10317–10324. [Google Scholar] [CrossRef] [PubMed]
- Larin, A.A.; Fershtat, L.L.; Ustyuzhanina, N.E.; Gening, M.L.; Nifantiev, N.E.; Makhova, N.N. New hybrid furoxan structures with antiaggregant activity. Mendeleev Commun. 2018, 28, 595–597. [Google Scholar] [CrossRef]
- Fernandes, G.F.S.; Campos, D.L.; da Silva, I.C.; Prates, J.L.B.; Pavan, A.R.; Pavan, F.R.; dos Santos, J.L. Benzofuroxan Derivatives as Potent Agents against Multidrug-Resistant Mycobacterium tuberculosis. ChemMedChem 2021, 16, 1268–1282. [Google Scholar] [CrossRef] [PubMed]
- Fedik, N.S.; Kletskii, M.E.; Burov, O.N.; Lisovin, A.V.; Kurbatov, S.V.; Chistyakov, V.A.; Morozov, P.G. Comprehensive study of nitrofuroxanoquinolines. New perspective donors of NO molecules. Nitric Oxide 2019, 93, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Chugunova, E.; Frenna, V.; Consiglio, G.; Micheletti, G.; Boga, C.; Akylbekov, N.; Burilov, A.; Spinelli, D. On the Nucleophilic Reactivity of 4,6-Dichloro-5-nitrobenzofuroxan with Some Aliphatic and Aromatic Amines: Selective Nucleophilic Substitution. J. Org. Chem. 2020, 85, 13472–13480. [Google Scholar] [CrossRef]
- Chugunova, E.; Micheletti, G.; Telese, D.; Boga, C.; Islamov, D.; Usachev, K.; Burilov, A.; Tulesinova, A.; Voloshina, A.; Lyubina, A.; et al. Novel Hybrid Compounds Containing Benzofuroxan and Aminothiazole Scaffolds: Synthesis and Evaluation of Their Anticancer Activity. Int. J. Mol. Sci. 2021, 22, 7497. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Fershtat, L.L.; Gening, M.L.; Nifantiev, N.E.; Makhova, N.N. New insight into the antiaggregant activity of furoxans. Mendeleev Commun. 2016, 26, 513–515. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Bolognese, F.; Rolando, B.; Guglielmo, S.; Lazzarato, L.; Fruttero, R. Anti-Pseudomonas activity of 3-nitro-4-phenylfuroxan. Microbiology 2018, 164, 1557–1566. [Google Scholar] [CrossRef]
- Fei, Y.; Wu, J.; An, H.-W.; Zhu, K.; Peng, B.; Cai, J.; Zhang, Y.; Li, L.-L.; Wang, H.; Huang, Z. Identification of New Nitric Oxide-Donating Peptides with Dual Biofilm Eradication and Antibacterial Activities for Intervention of Device-Related Infections. J. Med. Chem. 2020, 63, 9127–9135. [Google Scholar] [CrossRef]
- Huang, L.Y.; Tsui, D.Y.; Williams, C.M.; Wyse, B.D.; Smith, M.T. The furoxan nitric oxide donor, PRG150, evokes dose-dependent analgesia in a rat model of painful diabetic neuropathy. Clin. Exp. Pharmacol. Physiol. 2015, 42, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Pippin, A.B.; Arshad, Z.H.M.; Voll, R.J.; Nye, J.A.; Ghassabian, S.; Williams, C.M.; Mancini, A.; Liotta, D.C.; Smith, M.T.; Goodman, M.M. In Vitro Metabolic Stability and in Vivo Biodistribution of 3-Methyl-4-furoxancarbaldehyde Using PET Imaging in Rats. ACS Med. Chem. Lett. 2016, 7, 563–567. [Google Scholar]
- Huang, L.; Wyse, B.D.; Williams, C.M.; Smith, M.T. Nitric oxide modulates μ-opioid receptor function in vitro. Clin. Exp. Pharmacol. Physiol. 2019, 46, 676–685. [Google Scholar] [CrossRef]
- Eaton, J.K.; Ruberto, R.A.; Kramm, A.; Viswanathan, V.S.; Schreiber, S.L. Diacylfuroxans Are Masked Nitrile Oxides That Inhibit GPX4 Covalently. J. Am. Chem. Soc. 2019, 141, 20407–20415. [Google Scholar] [CrossRef] [PubMed]
- Larin, A.A.; Bystrov, D.M.; Fershtat, L.L.; Konnov, A.A.; Makhova, N.N.; Monogarov, K.A.; Meerov, D.B.; Melnikov, I.N.; Pivkina, A.N.; Kiselev, V.G.; et al. Nitro-, Cyano-, and Methylfuroxans, and Their Bis-Derivatives: From Green Primary to Melt-Cast Explosives. Molecules 2020, 25, 5836. [Google Scholar] [CrossRef]
- Larin, A.A.; Shaferov, A.V.; Epishina, M.A.; Melnikov, I.N.; Muravyev, N.V.; Ananyev, I.V.; Fershtat, L.L.; Makhova, N.N. Pushing the Energy-Sensitivity Balance with High-Performance Bifuroxans. ACS Appl. Energy Mater. 2020, 3, 7764–7771. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Makhova, N.N. 1,2,5-Oxadiazole-Based High-Energy-Density Materials: Synthesis and Performance. ChemPlusChem 2020, 85, 13–42. [Google Scholar] [CrossRef] [Green Version]
- Larin, A.A.; Muravyev, N.V.; Pivkina, A.N.; Suponitsky, K.Y.; Ananyev, I.V.; Khakimov, D.V.; Fershtat, L.L.; Makhova, N.N. Assembly of Tetrazolylfuroxan Organic Salts: Multipurpose Green Energetic Materials with High Enthalpies of Formation and Excellent Detonation Performance. Chem. Eur. J. 2019, 25, 4225–4233. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Ovchinnikov, I.V.; Epishina, M.A.; Romanova, A.A.; Lempert, D.B.; Muravyev, N.V.; Makhova, N.N. Assembly of Nitrofurazan and Nitrofuroxan Frameworks for High-Performance Energetic Materials. ChemPlusChem 2017, 82, 1315–1319. [Google Scholar] [CrossRef]
- Nakadate, M.; Sueyoshi, S.; Suzuki, I. Studies on Pyridazine 1,2-Dioxides. I. Syntheses of Pyridazine 1,2-Dioxides. Chem. Pharm. Bull. 1970, 18, 1211–1218. [Google Scholar] [CrossRef] [Green Version]
- Rozen, S.; Shaffer, A. Synthesis of N,N-Dioxopyridazines. Org. Lett. 2017, 19, 4707–4709. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, A.S.; Epishina, M.A.; Zhilin, E.S.; Shuvaev, A.D.; Fershtat, L.L.; Makhova, N.N. Design and synthesis of pyrazolo[3,4-d]pyridazine 5,6-dioxides as novel NO-donors. Mendeleev Commun. 2021, 31, 42–45. [Google Scholar] [CrossRef]
- Ivanova, O.A.; Averina, E.B.; Kuznetsova, T.S.; Zefirov, N.S. Synthesis of new 3,4-disubstituted furazans. Chem. Heterocycl. Compd. 2000, 36, 1091–1096. [Google Scholar] [CrossRef]
- Ogurtsov, V.A.; Dorovatovskii, P.V.; Zubavichus, Y.V.; Khrustalev, V.N.; Fakhrutdinov, A.N.; Zlotin, S.G.; Rakitin, O.A. [1,2,5]Oxadiazolo[3,4-d]pyridazine 1,5,6-trioxides: Efficient synthesis via the reaction of 3,4-bis(hydroxyimino)methyl)-1,2,5-oxadiazole 2-oxides with a mixture of concentrated nitric and trifluoroacetic acids and structural characterization. Tetrahedron Lett. 2018, 59, 3143–3146. [Google Scholar] [CrossRef]
- Obruchnikova, N.V.; Novikov, R.A.; Zlotin, S.G.; Dorovatovskii, P.V.; Khrustalev, V.N.; Rakitin, O.A. Synthesis and structural investigation of 4,4’;-dimethyl-[3,3’;-bi(1,2,5-oxadiazole)] 5,5’;-dioxide. Russ. Chem. Bull. Int. Ed. 2018, 67, 2044–2048. [Google Scholar] [CrossRef]
- Spyroudis, S.; Varvoglis, A. A New Synthesis of Pyridazine 1,2-Dioxides. Synthesis 1976, 1976, 837–838. [Google Scholar] [CrossRef]
- Ohsawa, A.; Arai, H.; Igeta, H. Oxidative Cyclization of 2-Unsaturated 1,4-Dioximes. Heterocycles 1978, 9, 1367–1373. [Google Scholar]
- Ohsawa, A.; Arai, H.; Igeta, H.; Akimoto, T.; Tsuji, A.; Iitaka, Y. Oxidative cyclization of dioximes and bis(hydrazones) of 2-unsaturated 1,4-diketones. J. Org. Chem. 1979, 44, 3524–3529. [Google Scholar] [CrossRef]
- Kots, A.Y.; Grafov, M.A.; Khropov, Y.V.; Betin, V.L.; Belushkina, N.N.; Busygina, O.G.; Yazykova, M.Y.; Ovchinnikov, I.V.; Kulikov, A.S.; Makhova, N.N.; et al. Vasorelaxant and antiplatelet activity of 4,7-dimethyl-1,2,5-oxadiazolo[3,4-d]pyridazine 1,5,6-trioxide: Role of soluble guanylate cyclase, nitric oxide and thiols. Br. J. Pharmacol. 2000, 129, 1163–1177. [Google Scholar] [CrossRef] [Green Version]
- Shevelev, S.A.; Dalinger, I.L.; Gulevskaya, V.I.; Cherkasova, T.I.; Vinogradov, V.M.; Ugrak, B.I.; Starosotnikov, A.M. Synthesis of mesoionic 3-aryl(hetaryl)-1,2,3,4-oxatriazol-5-ones based on N-aryl- and N-hetarylhydrazones of bromonitroformaldehyde. Chem. Heterocycl. Compd. 1999, 35, 363–373. [Google Scholar] [CrossRef]
- Zhilin, E.S.; Bystrov, D.M.; Ananyev, I.V.; Fershtat, L.L.; Makhova, N.N. Straightforward Access to the Nitric Oxide Donor Azasydnone Scaffold by Cascade Reactions of Amines. Chem. Eur. J. 2019, 25, 14284–14289. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.Q.; Kier, L.B.; Glennon, R.A.; Egle, J.L., Jr. Preliminary studies of mesoionic 3-(substituted-aryl)-.psi.-oxatriazoles as potential antihypertensive agents. J. Med. Chem. 1982, 25, 1503–1505. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.L.; Fedorchuk, M.; Shetty, B.V.; Anderson, F.E. Synthesis and activity of some 3-substituted 1,2,3,4-pseudooxatriazol-5-ones and their precursors and related compounds. J. Med. Chem. 1970, 13, 196–203. [Google Scholar] [CrossRef]
- Zhilin, E.S.; Ustyuzhanina, N.E.; Fershtat, L.L.; Nifantiev, N.E.; Makhova, N.N. Antiaggregant effects of (1,2,5-oxadiazolyl)azasydnone ring assemblies as novel antiplatelet agents. Chem. Biol. Drug Des. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Gettings, M.; Piercey, D. Azasydnones and their use in Energetic Materials. Energ. Mater. Front. 2020, 1, 136–140. [Google Scholar] [CrossRef]
- Gettings, M.L.; Thoenen, M.T.; Byrd, E.F.C.; Sabatini, J.J.; Zeller, M.; Piercey, D.G. Tetrazole Azasydnone (C2N7O2H) And Its Salts: High-Performing Zwitterionic Energetic Materials Containing A Unique Explosophore. Chem. Eur. J. 2020, 26, 14530–14535. [Google Scholar] [CrossRef]
- Dalinger, I.L.; Serushkina, O.V.; Muravyev, N.V.; Meerov, D.B.; Miroshnichenko, E.A.; Kon’kova, T.S.; Suponitsky, K.Y.; Vener, M.V.; Sheremetev, A.B. Azasydnone—Novel “green” building block for designing high energetic compounds. J. Mater. Chem. A 2018, 6, 18669–18676. [Google Scholar] [CrossRef]
- Serushkin, V.V.; Sinditskii, V.P.; Filatov, S.A.; Kulagina, P.D.; Nguyen, V.T.; Vatsadze, I.A.; Dalinger, I.L.; Sheremetev, A.B. Thermal stability and combustion behaviors of energetic materials based on a new heterocycle azasydnone. Int. J. Energ. Mater. Chem. Propul. 2018, 17, 147–170. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fershtat, L.L.; Zhilin, E.S. Recent Advances in the Synthesis and Biomedical Applications of Heterocyclic NO-Donors. Molecules 2021, 26, 5705. https://doi.org/10.3390/molecules26185705
Fershtat LL, Zhilin ES. Recent Advances in the Synthesis and Biomedical Applications of Heterocyclic NO-Donors. Molecules. 2021; 26(18):5705. https://doi.org/10.3390/molecules26185705
Chicago/Turabian StyleFershtat, Leonid L., and Egor S. Zhilin. 2021. "Recent Advances in the Synthesis and Biomedical Applications of Heterocyclic NO-Donors" Molecules 26, no. 18: 5705. https://doi.org/10.3390/molecules26185705
APA StyleFershtat, L. L., & Zhilin, E. S. (2021). Recent Advances in the Synthesis and Biomedical Applications of Heterocyclic NO-Donors. Molecules, 26(18), 5705. https://doi.org/10.3390/molecules26185705




