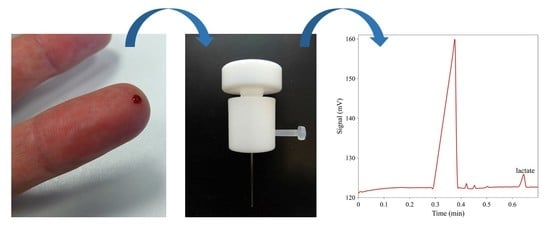
Sub-Minute Analysis of Lactate from a Single Blood Drop Using Capillary Electrophoresis with Contactless Conductivity Detection in Monitoring of Athlete Performance
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection of Separation Conditions
2.2. Comparison of the Blood Plasma Separation Device with Centrifugation
2.3. Analytical Parameters of the Method
2.3.1. Intra-Day and Inter-Day Precision of Migration Times and Peak Areas
2.3.2. Recoveries
2.3.3. Calibration, Limit of Detection, Limit of Quantitation
2.4. Correlation between the Developed CE Method and a Hand-Held Device
2.5. Content of Lactate in Blood Plasma during Exercise
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. CE Instrumentation
3.3. Blood Plasma Separation Device
3.4. Hand-Held Lactate Analyzer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Richard, A.; Kress, T. Measuring serum lactate. Nurs. Crit. Care 2009, 4, 56. [Google Scholar]
- Fischbach, F.T.; Dunning, M.B. A Manual of Laboratory and Diagnostic Tests, 8th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Kee, J.L. Laboratory and Diagnostic Tests with Nursing Implications, 6th ed; Prentice Hall: Upper Saddle River, NJ, USA, 2002. [Google Scholar]
- Kruse, O.; Grunnet, N.; Barfod, C. Blood lactate as a predictor for in-hospital mortality in patients admitted acutely to hospital: A systematic review. Scand. J. Trauma Resusc. Emerg. Med. 2011, 19, 74–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svedahl, K.; MacIntosh, B.R. Anaerobic Threshold: The Concept and Methods of Measurement. Can. J. Appl. Physiol. 2003, 28, 299–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodwin, M.L.; Harris, J.E.; Hernández, A.; Gladden, L.B. Blood Lactate Measurements and Analysis during Exercise:A Guide for Clinicians. J. Diabetes Sci. Technol. 2007, 1, 558–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumagai, S.; Tanaka, K.; Matsuura, Y.; Matsuzaka, A.; Hirakoba, K.; Asano, K. Relationships of the anaerobic threshold with the 5 km, 10 km, and 10 mile races. Eur. J. Appl. Physiol. Occup. Physiol. 1982, 49, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Alderman, J.A.; Cross, R.E. Adaptation to the centrifugal analyzer of an enzymatic method for the measurement of lactate in plasma and cerebrospinal fluid. Clin. Chem. 1977, 23, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- de Keijzer, M.H.; Brandts, R.W.; Brans, P.G.W. Evaluation of a biosensor for the measurement of lactate in whole blood. Clin. Biochem. 1999, 32, 109–112. [Google Scholar] [CrossRef]
- Hasegawa, H.; Fukushima, T.; Lee, J.A.; Tsukamoto, K.; Moriya, K.; Ono, Y.; Imai, K. Determination of serum d-lactic and l-lactic acids in normal subjects and diabetic patients by column-switching HPLC with pre-column fluorescence derivatization. Anal. Bioanal. Chem. 2003, 377, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Paik, M.J.; Cho, E.Y.; Kim, H.; Choi, S.; Ahn, Y.H.; Lee, G. Simultaneous clinical monitoring of lactic acid, pyruvic acid and ketone bodies in plasma as methoxime/tert-butyldimethylsilyl derivatives by gas chromatography-mass spectrometry in selected ion monitoring mode. Biomed. Chromatogr. 2008, 22, 450–453. [Google Scholar]
- Bonaventura, J.M.; Sharpe, K.; Knight, E.; Fuller, K.L.; Tanner, R.K.; Gore, C.J. Reliability and accuracy of six hand-held blood lactate analysers. J. Sci. Med. Sports 2015, 14, 203–214. [Google Scholar]
- Dolnik, V.; Dolnikova, J. Capillary zone electrophoresis of organic acids in serum of critically ill children. J. Chromatogr. A 1995, 716, 269–277. [Google Scholar] [CrossRef]
- Oefner, P.J. Surface-charge reversed capillary zone electrophoresis of inorganic and organic anions. Electrophoresis 1995, 16, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Markuszewski, M.J.; Szczykowska, M.; Siluk, D.; Kaliszan, R. Human red blood cells targeted metabolome analysis of glycolysis cycle metabolites by capillary electrophoresis using an indirect photometric detection method. J. Pharm. Biomed. Anal. 2005, 39, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Pormsila, W.; Morand, R.; Krahenbuhl, S.; Hauser, P.C. Quantification of plasma lactate concentrations using capillary electrophoresis with contactless conductivity detection. Electrophoresis 2011, 32, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Oyet, C.; Okongo, B.; Onyuthi, R.A.; Muwanguzi, E. Biochemical changes in stored donor units: Implications on the efficacy of blood transfusion. J. Blood. Med. 2018, 9, 111–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ďurč, P.; Foret, F.; Kubáň, P. Fast blood plasma separation device for point-of-care applications. Talanta 2018, 183, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Kubáň, P.; Foret, F.; Bocek, R. Capillary electrophoresis with contactless conductometric detection for rapid screening of formate in blood serum after methanol intoxication. J. Chromatogr. A 2013, 1281, 142–147. [Google Scholar] [CrossRef] [PubMed]
- De Pauw, K.; Roelands, B.; Cheung, S.S.; De Geus, B.; Rietjens, G.; Meeusen, R. Guidelines to classify subject groups in sport-science research. Int J. Sports Physiol Perform. 2013, 8, 111–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Parameter | Lactate |
|---|---|
| Linearity (μmol/L) | 0–800 |
| Slope | 0.013 |
| Intercept | 0.1361 |
| R-squared | 0.9993 |
| LOD (μmol/L) | 6 |
| LOQ (μmol/L) | 19 |
| Intra-day precision 1, CV (%): | |
| 100 μmol/L standard | 0.7 (2.7) 2 |
| Blood plasma | 0.9 (2.9) 2 |
| Inter-day precision 1, CV (%): | |
| 100 μmol/L standard | 1.3 (3.5) 2 |
| Blood plasma | 1.3 (3.5) 2 |
| Recovery (%): | |
| 100 μmol/L standard added | 105% |
| 600 μmol/L standard added | 103% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubáň, P.; Dosedělová, V.; Martma, K.; Rannama, I.; Reinpold, K.; Shimmo, R. Sub-Minute Analysis of Lactate from a Single Blood Drop Using Capillary Electrophoresis with Contactless Conductivity Detection in Monitoring of Athlete Performance. Molecules 2021, 26, 5817. https://doi.org/10.3390/molecules26195817
Kubáň P, Dosedělová V, Martma K, Rannama I, Reinpold K, Shimmo R. Sub-Minute Analysis of Lactate from a Single Blood Drop Using Capillary Electrophoresis with Contactless Conductivity Detection in Monitoring of Athlete Performance. Molecules. 2021; 26(19):5817. https://doi.org/10.3390/molecules26195817
Chicago/Turabian StyleKubáň, Petr, Věra Dosedělová, Kert Martma, Indrek Rannama, Karmen Reinpold, and Ruth Shimmo. 2021. "Sub-Minute Analysis of Lactate from a Single Blood Drop Using Capillary Electrophoresis with Contactless Conductivity Detection in Monitoring of Athlete Performance" Molecules 26, no. 19: 5817. https://doi.org/10.3390/molecules26195817
APA StyleKubáň, P., Dosedělová, V., Martma, K., Rannama, I., Reinpold, K., & Shimmo, R. (2021). Sub-Minute Analysis of Lactate from a Single Blood Drop Using Capillary Electrophoresis with Contactless Conductivity Detection in Monitoring of Athlete Performance. Molecules, 26(19), 5817. https://doi.org/10.3390/molecules26195817