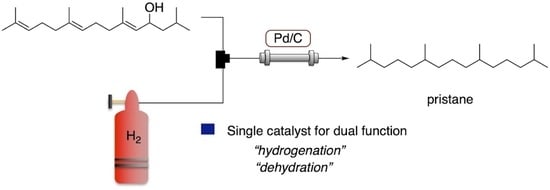
Greener Synthesis of Pristane by Flow Dehydrative Hydrogenation of Allylic Alcohol Using a Packed-Bed Reactor Charged by Pd/C as a Single Catalyst
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Typical Procedure for the Batch Synthesis of 2,6-Dimethylheptane 6
3.3. Typical Procedure for the Batch Synthesis of Pristane 3
3.4. Procedure for the Flow Synthesis of Pristane 3
- 2,6,10,14-Tetramethylpentadecane (pristane, 3) (see Supplementary Materials)
- 2,6,10,14-Tetramethylpentadecan-4-ol (4) (see Supplementary Materials)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matthews, L.H. Reproduction in the basking shark, Cetorhinus maximus (Gunner). Philos. Trans. R. Soc. London Ser. B 1950, 234, 247–316. [Google Scholar]
- Parker, H.W.; Scott, F.C. Age, size and vertebral calcification in the basking shark, Cetorhinus maximus (Gunnerus). Zool. Meded. 1965, 40, 305–319. [Google Scholar]
- Satoh, M.; Richards, H.B.; Shaheen, V.M.; Yoshida, H.; Shaw, M.; Naim, J.O.; Wooley, P.H.; Reeves, W.H. Widespreadsuscepti- bility among inbred mouse strains to the induction of lupus auto- antibodies by pristane. Clin. Exp. Immunol. 2000, 121, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Holmdahl, R.; Lorentzen, J.C.; Lu, S.; Olofsson, P.; Wester, L.; Holmberg, J.; Peterson, U. Arthritis induced in rats with non-immunogenic adjuvants as models for rheumatoid arthritis. Immunol. Rev. 2001, 184, 184–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gado, K.; Silva, S.; Paloczi, K.; Domjan, G.; Falus, A. Mouse plasmacytoma: An experimental model of human multiple myeloma. Haematologica 2001, 86, 227–236. [Google Scholar] [PubMed]
- Tanaka, K.; Motomatsu, S.; Koyama, K.; Tanaka, S.; Fukase, K. Large-scale synthesis of immunoactivating natural product, pristane, by continuous microfluidic dehydration as the key step. Org. Lett. 2007, 9, 299–302. [Google Scholar] [CrossRef]
- Gholamzadeh, P.; Ziarani, G.M.; Lashgari, N.; Badiei, A.; Asadiatouei, P. Silica functionalized propyl sulfonic acid (SiO2-Pr-SO3H): An efficient catalyst in organic reactions. J. Mol. Catal. A Chem. 2014, 391, 208–222. [Google Scholar] [CrossRef]
- Ziarani, G.M.; Lashgari, N.; Badiei, A. Sulfonic acid-functionalized mesoporous silica (SBA-Pr-SO3H) as solid acid catalyst in organic reactions. J. Mol. Catal. A Chem. 2015, 397, 166. [Google Scholar] [CrossRef]
- Furuta, A.; Fukuyama, T.; Ryu, I. Efficient flow Fischer esterification of carboxylic acids with alcohols using sulfonic acid-functionalized silica as supported catalyst. Bull. Chem. Soc. Jpn. 2017, 90, 607–612. [Google Scholar] [CrossRef]
- Furuta, A.; Hirobe, Y.; Fukuyama, T.; Ryu, I.; Manabe, Y.; Fukase, K. Flow dehydration and hydrogenation of allylic alcohols: Application to the waste-free synthesis of pristane. Eur. J. Org. Chem. 2017, 2017, 1365–1368. [Google Scholar] [CrossRef]
- Kasakado, T.; Hyodo, M.; Furuta, A.; Kamardine, A.; Ryu, I.; Fukuyama, T. Flow Friedel–Crafts alkylation of 1-adamantanol with arenes using HO-SAS as an immobilized acid catalyst. J. Chin. Chem. Soc. 2020, 67, 2253–2257. [Google Scholar] [CrossRef]
- Baltzly, R.; Buck, J.S. Catalytic debenzylation. The effect of substitution on the strength of the O-benzyl and N-benzyl Llinkages. J. Am. Chem. Soc. 1943, 65, 1984–1992. [Google Scholar] [CrossRef]
- Simion, A.-M.; Arimura, T.; Simion, C. Reaction of cinnamaldehyde and derivatives with Raney Ni–Al alloy and Al powder in water. Reduction or oxido-reduction? Comptes Rendus Chim. 2013, 16, 476–481. [Google Scholar] [CrossRef]
- Carless, H.A.; Malik, S.S. Synthesis of pseudo-α-L-fucopyranose from toluene. J. Chem. Soc. Chem. Commun 1995, 2447–2448. [Google Scholar] [CrossRef]
- Donaldson, W.A.; Sem, D.S.; Neumann, T.S. Preparation of (4’-hydroxyphenyl)cycloalkane compounds useful as selective agonists of the estrogen receptor beta isoform. PCT Patent 2015077611, 28 May 2015. [Google Scholar]
- Uchibayashi, M. Studies on Steroids. XIV. Transformation of steroids by pseudomonas. Chem. Pharm. Bull. 1960, 8, 122–125. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, K.; Wakamatsu, T.; Matsumura, S.; Toshima, K. Synthesis of hexopyranosyl acetates and 2,3-disubstituted tetrahydropyrans via chemoselective hydrogenation of hex-2-enopyranosyl acetates. Tetrahedron Lett. 2006, 47, 8271–8274. [Google Scholar] [CrossRef]
- Yu, T.; Jiao, J.; Song, P.; Nie, W.; Yi, C.; Zhang, Q.; Li, P. Recent progress in continuous-flow hydrogenation. ChemSusChem 2020, 13, 2876–2893. [Google Scholar] [CrossRef] [PubMed]
- Cossar, P.J.; Hizartzidis, L.; Simone, M.I.; McCluskey, A.; Gordon, C.P. The expanding utility of continuous flow hydrogenation. Org. Biomol. Chem. 2015, 13, 7119–7130. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Glasnov, T.N.; Kappe, C.O. Heterogeneous catalytic hydrogenation reactions in continuous-flow reactors. ChemSusChem 2011, 4, 300–316. [Google Scholar] [CrossRef]
- Yoswathananont, N.; Nitta, K.; Nishiuchi, Y.; Sato, M. Continuous hydrogenation reactions in a tube reactor packed with Pd/C. Chem. Commun. 2005, 40–42. [Google Scholar] [CrossRef]
- Linares, N.; Hartmann, S.; Galarneau, A.; Barbaro, P. Continuous partial hydrogenation reactions by Pd/unconventional bimodal porous titania monolith catalysts. ACS Catal. 2012, 2, 2194–2198. [Google Scholar] [CrossRef]
- Ouchi, T.; Battilocchio, C.; Hawkins, J.M.; Ley, S.V. Process intensification for the continuous flow hydrogenation of ethyl nicotinate. Org. Process Res. Dev. 2014, 18, 1560–1566. [Google Scholar] [CrossRef]
- Spare, L.K.; Harman, D.G.; Aldrich-Wright, J.R.; Nguyen, T.V.; Gordon, C.P. Chemoselective flow hydrogenation approaches to diversify the cytotoxic tetrahydroepoxyisoindole carboxamide scaffold. Adv. Synth. Catal. 2018, 360, 1209–1217. [Google Scholar] [CrossRef]
- Gardiner, J.; Nguyen, X.; Genet, C.; Horne, M.D.; Hornung, C.H.; Tsanaktsidis, J. Catalytic static mixers for the continuous flow hydrogenation of a key intermediate of linezolid (Zyvox). Org. Process. Res. Dev. 2018, 22, 1448–1452. [Google Scholar] [CrossRef]
- Jerome, P.; Haribabu, J.; Bhuvanesh, N.S.P.; Karvembu, R. Pd(II)-NNN pincer complexes for catalyzing transfer hydrogenation of Ketones. ChemistrySelect 2020, 5, 13591–13597. [Google Scholar] [CrossRef]
- Nisanci, B.; Dagalan, Z. A facile and highly efficient transfer hydrogenation of ketones and aldehydes catalyzed by palladium nanoparticles supported on mesoporous graphitic carbon nitride. J. Chem. Res. 2020, 44, 14–19. [Google Scholar] [CrossRef]
- Mahato, S.K.; Ul Islam, R.; Acharya, C.; Witcomb, M.J.; Mallick, K. Polymer-stabilized palladium nanoparticles for the chemoselective transfer hydrogenation of α,β-unsaturated carbonyls. single-step bottom-up approach. ChemCatChem 2014, 6, 1419–1426. [Google Scholar]
- Hanna, S.; Wills, T.; Butcher, T.W.; Hartwig, J.F. Palladium-catalyzed oxidative dehydrosilylation for contra-thermodynamic olefin isomerization. ACS Catal. 2020, 10, 8736–8741. [Google Scholar] [CrossRef]
- Ren, W.; Sun, F.; Chu, J.; Shi, Y. A Pd-catalyzed site-controlled isomerization of terminal olefins. Org. Lett. 2020, 22, 1868–1873. [Google Scholar] [CrossRef]
- Maung, M.S.; Shon, Y.-S. Effects of noncovalent interactions on the catalytic activity of unsupported colloidal palladium nanoparticles stabilized with thiolate ligands. J. Phys. Chem. C 2017, 121, 20882–20891. [Google Scholar] [CrossRef]
- Larionov, E.; Lin, L.; Guenee, L.; Mazet, C. Scope and mechanism in palladium-catalyzed isomerizations of highly substituted allylic, homoallylic, and alkenyl alcohols. J. Am. Chem. Soc. 2014, 136, 16882–16894. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-K.; Zhang, W.-Z.; Zhang, K.; Wang, W.-L.; Lu, X.-B. Carbon dioxide-promoted palladium-catalyzed dehydration of primary allylic alcohols: Access to substituted 1,3-dienes. Org. Chem. Front. 2021, 8, 941–946. [Google Scholar] [CrossRef]
- Masuyama, Y.; Takahara, J.P.; Hashimoto, K.; Kurusu, Y. Palladium-catalyzed dehydration of propynyl alcohols with tin(II) chloride. J. Chem. Soc. Chem. Commun. 1993, 1219–1220. [Google Scholar] [CrossRef]







| Entry | Catalyst (mg) | T (°C) | H2 Pressure (atm) | Pristane 3 | 4 Yield (%) | |
|---|---|---|---|---|---|---|
| Yield (%) | Purity (%) | |||||
| 1 | 10% Pd/C (20) | rt | 1 | 30 | 99 | 48 |
| 2 | 5% Pd/Al2O3 (40) | rt | 1 | Trace | - | 92 |
| 3 | 10% Pd/C (20) | 90 | 10 | 30 | 98 | 63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasakado, T.; Hirobe, Y.; Furuta, A.; Hyodo, M.; Fukuyama, T.; Ryu, I. Greener Synthesis of Pristane by Flow Dehydrative Hydrogenation of Allylic Alcohol Using a Packed-Bed Reactor Charged by Pd/C as a Single Catalyst. Molecules 2021, 26, 5845. https://doi.org/10.3390/molecules26195845
Kasakado T, Hirobe Y, Furuta A, Hyodo M, Fukuyama T, Ryu I. Greener Synthesis of Pristane by Flow Dehydrative Hydrogenation of Allylic Alcohol Using a Packed-Bed Reactor Charged by Pd/C as a Single Catalyst. Molecules. 2021; 26(19):5845. https://doi.org/10.3390/molecules26195845
Chicago/Turabian StyleKasakado, Takayoshi, Yuki Hirobe, Akihiro Furuta, Mamoru Hyodo, Takahide Fukuyama, and Ilhyong Ryu. 2021. "Greener Synthesis of Pristane by Flow Dehydrative Hydrogenation of Allylic Alcohol Using a Packed-Bed Reactor Charged by Pd/C as a Single Catalyst" Molecules 26, no. 19: 5845. https://doi.org/10.3390/molecules26195845
APA StyleKasakado, T., Hirobe, Y., Furuta, A., Hyodo, M., Fukuyama, T., & Ryu, I. (2021). Greener Synthesis of Pristane by Flow Dehydrative Hydrogenation of Allylic Alcohol Using a Packed-Bed Reactor Charged by Pd/C as a Single Catalyst. Molecules, 26(19), 5845. https://doi.org/10.3390/molecules26195845