In Vitro Multiplication and NMR Fingerprinting of Rare Veronica caucasica M. Bieb
Abstract
:1. Introduction
2. Results
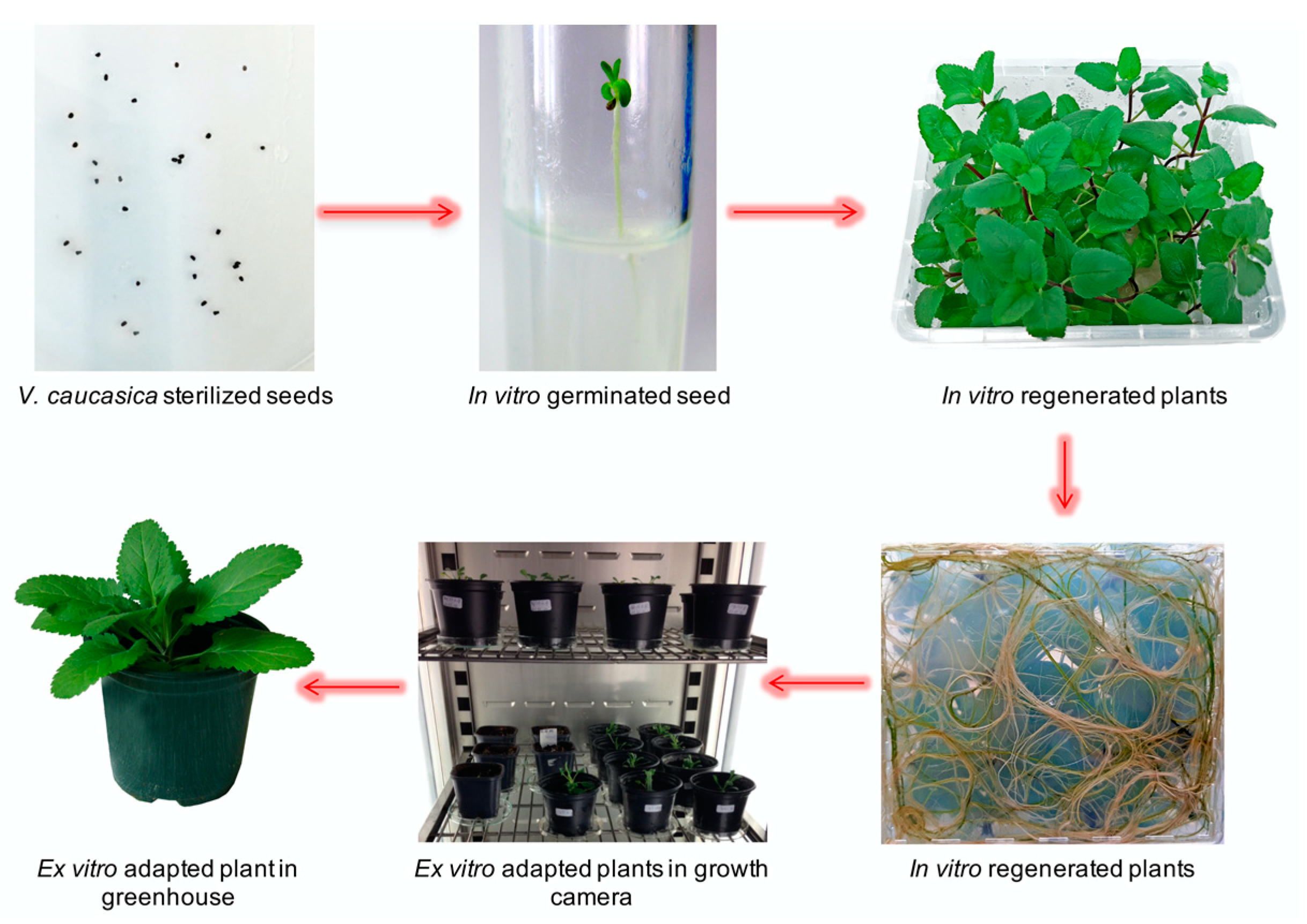
2.1. In Vitro Multiplication and Ex Vitro Acclimation of V. caucasica
2.2. Comparative Determination of Plastid Pigments, Total Phenolics, Flavonoids and ROS Accumulation in In Vitro Cultivated and Ex Vitro Adapted Plants
2.3. NMR-Based Metabolite Profiling of In Vitro Cultivated and Ex Vitro Acclimated V. caucasica
3. Discussion
3.1. In Vitro Multiplication and Ex Vitro Acclimation of V. caucasica
3.2. Physiological State of V. caucasica Plants during Ex Situ Conservation
3.3. Metabolite Profiling during V. caucasica Ex Situ Conservation
4. Materials and Methods
4.1. Plant Material and In Vitro Cultivation of V. caucasica
4.2. Ex Vitro Acclimation
4.3. Plastid Pigments Quantification
4.4. ROS Imaging
4.5. Determination of Total Phenolics Content
4.6. Determination of Flavonoids Content
4.7. Extraction Procedure and NMR Analyses
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Albach, D.C.; Martınez-Ortega, M.M.; Fischer, M.A.; Chase, M.W. A new classification of the tribe veroniceae-problems and a possible solution. Taxon 2004, 53, 429–452. [Google Scholar] [CrossRef]
- Beara, I.; Zivkovic, J.; Lesjak, M.; Ristic, J.; Savikin, K.; Maksimovic, Z.; Jankovic, T. Phenolic profile and anti-inflammatory activity of three Veronica species. Ind. Crop. Prod. 2015, 63, 276–280. [Google Scholar] [CrossRef]
- Su, B.; Zhu, Q.; Jia, Z. Aquaticol, a novel bis-sesquiterpene from Veronica anagallis-aquatica. Tetrahedron Lett. 1999, 40, 357–358. [Google Scholar] [CrossRef]
- Grundemann, C.; Garcia-Kaufer, M.; Sauer, B.; Stangenberg, E.; Konczol, M.; Merfort, I.; Zehl, M.; Huber, R. Traditionally used Veronica ocinalis inhibits proinflammatory mediators via the nf-kb signalling pathway in a human lung cell line. J. Ethnopharmacol. 2013, 145, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Kupeli, E.; Harput, U.S.; Varel, M.; Yesilada, E.; Saracoglu, I. Bioassay-guided isolation of iridoid glucosides with antinociceptive and anti-inflammatory activities from Veronica anagallis-aquatica L. J. Ethnopharmacol. 2005, 102, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Tayeboon, G.S.; Niknam, F.; Sharifi-Rad, M.; Mohajeri, M.; Salehi, B.; Iriti, M.; Sharifi-Rad, M. Veronica persica poir. extract-antibacterial, antifungal and scolicidal activities, and inhibitory potential on acetylcholinesterase, tyrosinase, lipoxygenase and xanthine oxidase. Cell. Mol. Biol. 2018, 64, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Taskova, R.; Gotfredsen, C.; Jensen, S. Chemotaxonomy of Veroniceae and its allies in the Plantaginaceae. Phytochemistry 2006, 67, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Johansen, M.; Larsen, T.; Mattebjerg, M.; Gotfredsen, C.; Jensen, S. Chemical markers in Veronica sect. Hebe. Biochem. Syst. Ecol. 2007, 35, 614–620. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Georgiev, M.I.; Fierascu, I.; Ungureanu, C.; Avramescu, S.M.; Ortan, A.; Velescu, B.S. Mitodepressive, antioxidant, antifungal and anti-inflammatory effects of wild-growing Romanian native Arctium lappa L. (Asteraceae) and Veronica persica Poiret (Plantaginaceae). Food Chem. Toxicol. 2018, 111, 44–52. [Google Scholar] [CrossRef]
- Saracoglu, I.; Oztunca, F.; Nagatsu, A.; Harput, S. Iridoid content and biological activities of Veronica cuneifolia subsp. cuneifolia and V. cymbalaria. Pharm. Biol. 2011, 49, 1150–1157. [Google Scholar] [CrossRef] [Green Version]
- Jensen, S.; Opitz, S.; Gotfredsen, C. A new phenylethanoid triglycoside in Veronica beccabunga L. Biochem. Syst. Ecol. 2011, 39, 193–197. [Google Scholar] [CrossRef] [Green Version]
- Barreira, J.C.; Dias, M.I.; Živković, J.; Stojković, D.; Soković, M.; Santos-Buelga, C.; Ferreira, I.C. Phenolic profiling of Veronica spp. grown in mountain, urban and sandy soil environments. Food Chem. 2014, 163, 275–283. [Google Scholar] [CrossRef]
- Harput, U.; Saracoglu, I.; Inoue, M.; Ogihara, Y. Phenylethanoid and iridoid glycosides from Veronica persica. Chem. Pharm. Bull. 2002, 50, 869–871. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Tan, S.; Gu, W.; Li, F.; Hua, W.; Zhang, S.; Tang, L. Phytochemical composition, isolation and hepatoprotective activity of active fraction from Veronica ciliata against acetaminophen-induced acute liver injury via p62-Keap1-Nrf2 signaling pathway. J. Ethnopharmacol. 2019, 243, 112089. [Google Scholar] [CrossRef]
- Engelmann, F. Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cell. Dev. Biol. Plant 2011, 47, 5–16. [Google Scholar] [CrossRef]
- Yordanova, Z.P.; Rogova, M.A.; Zhiponova, M.K.; Georgiev, M.I.; Kapchina-Toteva, V.M. Comparative determination of the essential oil composition in Bulgarian endemic plant Achillea thracica Velen. during the process of ex situ conservation. Phytochem. Lett. 2017, 20, 456–461. [Google Scholar] [CrossRef]
- Mantovska, D.I.; Kapchina, V.M.; Yordanova, Z.P. In vitro propagation of the Balkan endemic species Stachys leucoglossa Griseb. Bulg. J. Agric. Sci. 2019, 25, 1211–1215. [Google Scholar]
- Shahzad, A.; Parveen, S.; Fatema, M. Development of a regeneration system via nodal segment culture in Veronica anagallis-aquatica L.—An amphibious medicinal plant. J. Plant Interact. 2011, 6, 61–68. [Google Scholar] [CrossRef]
- Babber, S.; Mittal, K.; Ahlawat, R.; Varghese, T.M. Micropropagation of Cardiospermum halicacabum. Biol. Plant 2001, 44, 603–606. [Google Scholar] [CrossRef]
- Dias, M.C.; Almeida, R.; Romano, A. Rapid clonal multiplication of Lavandula viridis L’Her through in vitro axillary shoot proliferation. Plant Cell Tissue Organ Cult. 2002, 68, 99–102. [Google Scholar] [CrossRef]
- Dhaka, N.; Kothari, S.L. Micropropagation of Eclipta alba, an important medicinal plant. In Vitr. Cell. Dev. Biol. 2005, 39, 658–661. [Google Scholar] [CrossRef]
- Yordanova, Z.P.; Rogova, M.A.; Kapchina-Toteva, V.M. In vitro propagation of the Balkan endemic species Verbascum eriophorum Godr. Bulg. J. Agric. Sci. 2016, 22, 767–771. [Google Scholar]
- Zayova, E.Z.; Nedev, T.A.; Petrova, D.H.; Zhiponova, M.K.; Chaneva, G.T. Efficient protocol for mass micropropagation of Artemisia annua L. GSC Biol. Pharm. Sci. 2018, 5, 059–068. [Google Scholar] [CrossRef]
- Kapchina-Toteva, V.; Dimitrova, M.; Stefanova, M.; Koleva, D.; Stefanov, D.; Kostov, K.; Yordanova, Z.P.; Zhiponova, M. Adaptive changes in photosynthetic performance and secondary metabolites during white dead nettle micropropagation. J. Plant Physiol. 2014, 171, 1344–1353. [Google Scholar] [CrossRef]
- Nishio, J.N. Why are higher plants green? Evolution of the higher plant photosynthetic pigment complement. Plant Cell Environ. 2000, 23, 539–548. [Google Scholar] [CrossRef]
- Georgieva, E.; Petrova, D.; Yordanova, Z.P.; Kapchina-Toteva, V.; Cellarova, E.; Chaneva, G. Influence of cryopreservation on the antioxidative activity of in vitro cultivated Hypericum species. Biotechnol. Biotechnol. Equip. 2014, 28, 863–870. [Google Scholar] [CrossRef] [Green Version]
- Dragolova, D.; Stefanova, M.; Dimitrova, M.; Koleva, D.; Zhiponova, M.; Kapchina-Toteva, V. In vitro cultivation and ex vitro adaptation of Nepeta nuda ssp. nuda–correlation between regeneration potential, leaf anatomy, and plastid pigments. Bulg. J. Agric. Sci. 2015, 21, 1027–1032. [Google Scholar]
- Cotelle, N. Role of flavonoids in oxidative stress. Curr. Top. Med. Chem. 2001, 1, 569–590. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Al Khateeb, W.; Hussein, E.; Qouta, L.; Alu’datt, M.; Al-Shara, B.; Abu-Zaiton, A. In vitro propagation and characterization of phenolic content along with antioxidant and antimicrobial activities of Cichorium pumilum Jacq. Plant Cell Tissue Org. Cult. 2012, 110, 103–110. [Google Scholar] [CrossRef]
- Danova, K.; Nikolova-Damianova, B.; Denev, R.; Dimitrov, D. Influence of vitamins on polyphenolic content, morphological development, and stress response in shoot cultures of Hypericum spp. Plant Cell Tissue Org. Cult. 2012, 110, 383–393. [Google Scholar] [CrossRef]
- Nikolova, M.; Petrova, M.; Zayova, E. Comparative study of in vitro, ex vitro and in vivo grown plants of Arnica montana-polyphenols and free radical scavenging activity. Acta Bot. Croat. 2013, 72, 13–22. [Google Scholar] [CrossRef]
- Yakimova, E.T.; Yordanova, Z.P.; Slavov, S.; Kapchina-Toteva, V.M.; Woltering, E.J. Alternaria alternata AT toxin induces programmed cell death in tobacco. J. Phytopathol. 2009, 157, 592–601. [Google Scholar] [CrossRef]
- Velinov, V.; Vaseva, I.; Zehirov, G.; Zhiponova, M.; Georgieva, M.; Vangheluwe, N.; Beeckman, T.; Vassileva, V. Overexpression of the NMig1 gene encoding a NudC domain protein enhances root growth and abiotic stress tolerance in Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 815. [Google Scholar] [CrossRef]
- Iantcheva, A.; Zhiponova, M.; Revalska, M.; Heyman, J.; Dincheva, I.; Badjakov, I.; De Geyter, N.; Boycheva, I.; Goormachtig, S.; De Veylder, L. A common F-box gene regulates the leucine homeostasis of Medicago truncatula and Arabidopsis thaliana. Protoplasma 2021. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Hwang, D.K.; Jeong, H.U.; Ji, H.Y.; Oh, S.R.; Lee, Y. In vitro and in vivo metabolism of verproside in rats. Molecules 2012, 17, 11990–12002. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.R.; Lee, M.Y.; Ahn, K.; Park, B.Y.; Kwon, O.K.; Joung, H. Suppressive effect of verproside isolated from Pseudolysimachion longifolium on airway inflammation in a mouse model of allergic asthma. Int. Immunopharmacol. 2006, 6, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.U.; Sung, M.H.; Ryu, H.W.; Lee, J.; Kim, H.S.; In, H.J.; Lee, Y. Verproside inhibits TNF-α-induced MUC5AC expression through suppression of the TNF-α/NF-κB pathway in human airway epithelial cells. Cytokine 2016, 77, 168–175. [Google Scholar] [CrossRef]
- Stojković, D.S.; Živković, J.; Soković, M.; Glamočlija, J.; Ferreira, I.C.; Janković, T.; Maksimović, Z. Antibacterial activity of Veronica montana L. extract and of protocatechuic acid incorporated in a food system. Food Chem. Toxicol. 2013, 55, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Zhiponova, M.; Yordanova, Z.; Pavlova, D.; Rogova, M.; Dimitrova, M.; Dragolova, D.; Tasheva-Terzieva, E.; Kapchina-Toteva, V. Importance of phenolics in populations of Teucrium chamaedrys (Lamiaceae) from serpentine soils. Aust. J. Bot. 2020, 68, 352–362. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F.A. Revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- McKinney, G. Absorption of light by chlorophyll solutions. J. Biol. Chem. 1941, 140, 315–322. [Google Scholar] [CrossRef]
- Thordal-Christensen, H.; Zhang, Z.; Wei, Y.; Collinge, D.B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Sakamoto, M.; Tada, Y.; Nakayashiki, H.; Tosa, Y.; Mayama, S. Two phases of intracellular reactive oxygen species production during victorin-induced cell death in oats. J. Gen. Plant Pathol. 2005, 71, 387–394. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Zahmanov, G.; Alipieva, K.; Simova, S.; Georgiev, M. Metabolic differentiations of dwarf elder by NMR-based metabolomics. Phytochem. Lett. 2015, 11, 404–409. [Google Scholar] [CrossRef]
- Marchev, A.; Yordanova, Z.; Alipieva, K.; Zahmanov, G.; Rusinova-Videva, S.; Kapchina-Toteva, V.; Georgiev, M.I. Genetic transformation of rare Verbascum eriophorum Godr. plants and metabolic alterations revealed by NMR-based metabolomics. Biotechnol. Lett. 2016, 38, 1621–1629. [Google Scholar] [CrossRef]




| Variants | Length of Shoots (cm) | Number of Shoots | Root Formation | Length of Roots (cm) | Degree of Callus Formation |
|---|---|---|---|---|---|
| Control | 2.85 a ± 0.35 | 1.5 a ± 0.52 | + | 4.97 a ± 0.85 | – |
| BA 0.1 mg L–1 | 4.80 b ± 0.85 | 2.0 ab ± 0.07 | + | 2.36 b ± 1.24 | + |
| BA 0.5 mg L–1 | 4.32 b ± 1.22 | 2.5 b ± 0.71 | – | – | ++ |
| BA 1.0 mg L–1 | 0.90 c ± 0.40 | 9.14 c ± 0.64 | – | – | ++ |
| Metabolite | V.c. In Vitro a | V.c. Ex Vitro a | Selected Signals, Multiplicity and Coupling Constant b |
|---|---|---|---|
| Amino acids | |||
| Alanine | ++ | + | δ 1.47 (d, J = 7.2) |
| Valine | + | - | δ 0.99 (d, J = 7.0)/δ 1.04 (d, J = 7.0) |
| Leucine | + | - | δ 0.95 (d, J = 6.3)/δ 0.97 (d, J = 6.3) |
| Isoleucine | + | - | δ 0.94 (t, J = 7.3)/δ 1.01 (d, J = 7.3) |
| Sugars | |||
| α-Glucose | + | ++ | δ 5.17 (d, J = 3.8) |
| β-Glucose | + | ++ | δ 4.56 (d, J = 7.9)/3.18 (dd, J = 7.9, 9.2) |
| Sucrose | + | + | δ 5.39 (d, J = 3.9) |
| Organic acids | |||
| Acetic acid | - | + | δ 1.93 (s) |
| Lactic acid | ++ | + | δ 1.31 (d, J = 6.9)/δ 4.06 m |
| Succinic acid | - | + | δ 2.48 (s) |
| Formic acid | + | + | δ 8.45 (s) |
| Malic acid | + | - | δ 2.80 (dd, J = 16.9, 8.2)/δ 2.93 (dd, J = 16.9, 3.9) |
| Phenolic acids | |||
| Caffeic acid | ++ | ++ | δ 7.63 (d, J = 16.0)/δ 7.14 (d, J = 2.2)/δ 7.05 (dd, J = 8.5, 2.2)/δ 6.88 (d, J = 8.4)/δ 6.28 (d, J = 16.0) |
| Protocatechuic acid | ++ | ++++ | δ 7.41 (d, J = 2.1)/δ 7.38 (dd, J = 8.4,2.1)/δ 6.85 (d, J = 8.3) |
| Iridoid glucosides | |||
| Verproside | +++ | + | δ 7.51 (dd, J = 8.3, 2.2)/δ 7.48 (d, J = 2.2)/δ 6.92 (d, J = 8.3)/δ 6.41 (dd, J = 6.1, 2.0)/δ 5.16 (d, J = 9.7)/δ 5.13 (dd, J = 6.0, 1.7)/δ 5.02 (d, J = 10.0)/δ 4.85 (d, J = 7.9)/δ 4.23 (d, J = 13.3)/δ 2.70 (m)/δ 2.66 (m) |
| Aucubin | ++ | + | δ 6.32 (dd, J = 6.2, 1.9)/δ 5.81 (brs)/δ 5.11 (dd, J = 6.2, 3.7)/δ 5.09 (d, J = 6.1)/δ 4.34 (d, J = 15.6)/δ 4.22 (d, J = 15.2) |
| Catalpol | ++ | ++ | δ 6.38 (dd, J = 6.0, 1.7)/δ 5.11 (dd, J = 6.0, 4.6)/δ 5.03 (d, J = 9.9)/δ 4.18 (d, J = 13.3)/δ 3.98 (dd, 8.0, 1.1)/δ 3.78 (d, 13.3) /δ 2.58 (dd, J = 9.6, 7.7)/δ 2.27 m |
| Others | |||
| Choline | + | + | δ 3.19 (s) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantovska, D.I.; Zhiponova, M.K.; Georgiev, M.I.; Grozdanova, T.; Gerginova, D.; Alipieva, K.; Simova, S.; Popova, M.; Kapchina-Toteva, V.M.; Yordanova, Z.P. In Vitro Multiplication and NMR Fingerprinting of Rare Veronica caucasica M. Bieb. Molecules 2021, 26, 5888. https://doi.org/10.3390/molecules26195888
Mantovska DI, Zhiponova MK, Georgiev MI, Grozdanova T, Gerginova D, Alipieva K, Simova S, Popova M, Kapchina-Toteva VM, Yordanova ZP. In Vitro Multiplication and NMR Fingerprinting of Rare Veronica caucasica M. Bieb. Molecules. 2021; 26(19):5888. https://doi.org/10.3390/molecules26195888
Chicago/Turabian StyleMantovska, Desislava I., Miroslava K. Zhiponova, Milen I. Georgiev, Tsvetinka Grozdanova, Dessislava Gerginova, Kalina Alipieva, Svetlana Simova, Milena Popova, Veneta M. Kapchina-Toteva, and Zhenya P. Yordanova. 2021. "In Vitro Multiplication and NMR Fingerprinting of Rare Veronica caucasica M. Bieb" Molecules 26, no. 19: 5888. https://doi.org/10.3390/molecules26195888
APA StyleMantovska, D. I., Zhiponova, M. K., Georgiev, M. I., Grozdanova, T., Gerginova, D., Alipieva, K., Simova, S., Popova, M., Kapchina-Toteva, V. M., & Yordanova, Z. P. (2021). In Vitro Multiplication and NMR Fingerprinting of Rare Veronica caucasica M. Bieb. Molecules, 26(19), 5888. https://doi.org/10.3390/molecules26195888












