Analysis of Volatile Compounds in Sea Bass (Lateolabrax japonicus) Resulting from Different Slaughter Methods Using Electronic-Nose (E-Nose) and Gas Chromatography-Ion Mobility Spectrometry
Abstract
:1. Introduction
2. Results
2.1. E-Nose Analysis
2.2. Sensory Evaluation
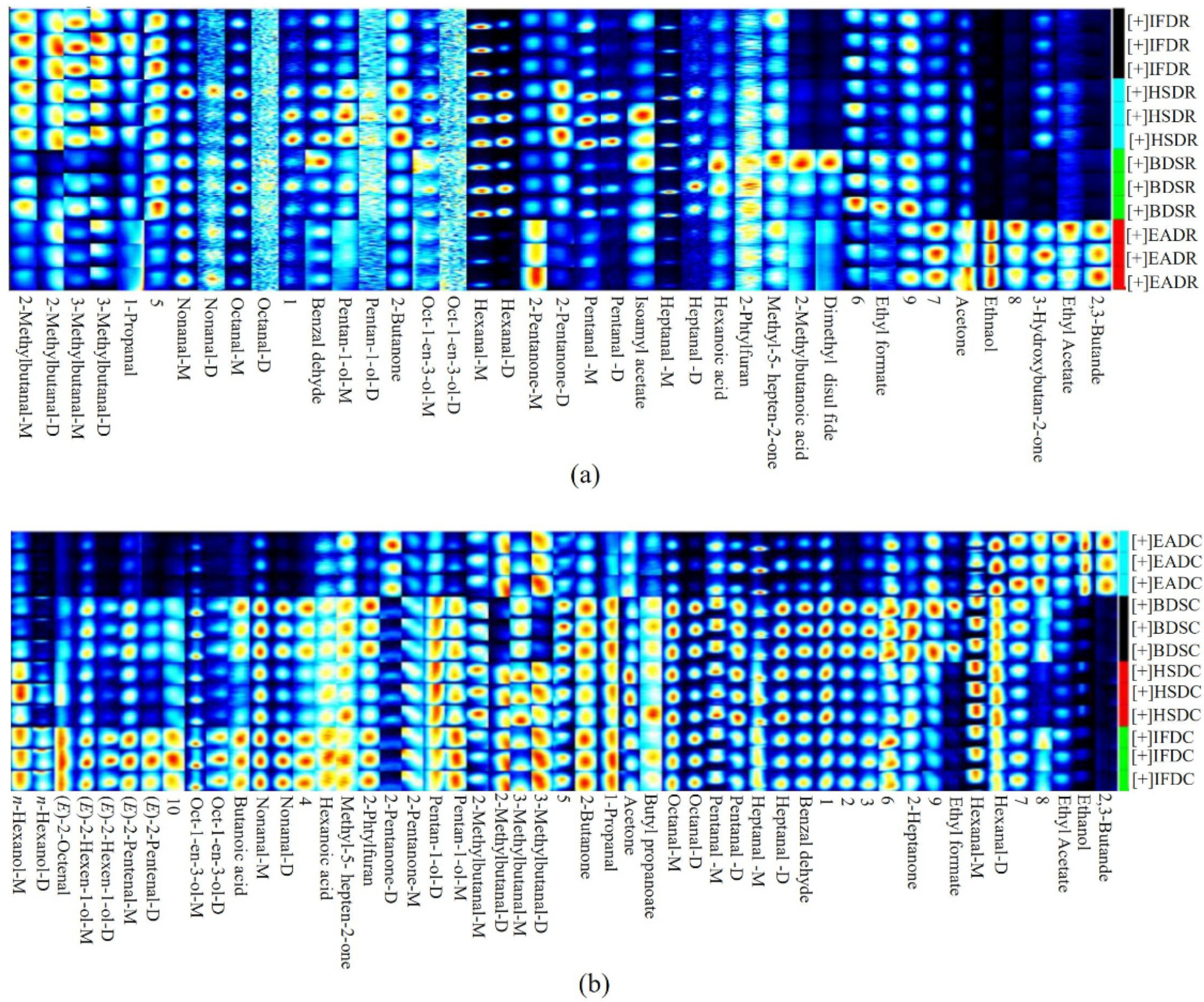
2.3. Analysis of GC-IMS Compositional Spectra and Profile Differences
2.4. Identification of VOCs
2.5. Effects of Slaughter Method on the Changes in VOCs in Raw and Cooked Sea Bass
3. Materials and Methods
3.1. Animals and Ethics Approval
3.2. Materials
3.3. E-Nose Analysis
3.4. Sensory Evaluation
3.5. GC-IMS Analysis
3.6. Data Processing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ayala, M.; Albors, O.L.; Blanco, A.; Alcázar, A.G.; Abellán, E.; Zarzosa, G.R.; Gil, F. Structural and ultrastructural changes on muscle tissue of sea bass, Dicentrarchus labrax L., after cooking and freezing. Aquaculture 2005, 250, 215–231. [Google Scholar] [CrossRef]
- Sampels, S. The effects of processing technologies and preparation on the final quality of fish products. Trends Food Sci. Technol. 2015, 44, 131–146. [Google Scholar] [CrossRef]
- Iglesias, J.; Medina, I.; Bianchi, F.; Careri, M.; Mangia, A.; Musci, M. Study of the volatile compounds useful for the characterisation of fresh and frozen-thawed cultured gilthead sea bream fish by solid-phase microextraction gas chromatography–mass spectrometry. Food Chem. 2009, 115, 1473–1478. [Google Scholar] [CrossRef]
- Poli, B.M.; Parisi, G.; Scappini, F.; Zampacavallo, G. Fish welfare and quality as affected by pre-slaughter and slaughter management. Aquac. Int. 2005, 13, 29–49. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Jia, S.; Huang, Z.; Luo, Y. Effect of different stunning methods on antioxidant status, in vivo myofibrillar protein oxidation, and the susceptibility to oxidation of silver carp (Hypophthalmichthys molitrix) fillets during 72 h postmortem. Food Chem. 2018, 246, 121–128. [Google Scholar] [CrossRef]
- Acerete, L.; Reig, L.; Alvarez, D.; Flos, R.; Tort, L. Comparison of two stunning/slaughtering methods on stress response and quality indicators of European sea bass (Dicentrarchus labrax). Aquaculture 2009, 287, 139–144. [Google Scholar] [CrossRef]
- Secci, G.; Parisi, G.; Dasilva, G.; Medina, I. Stress during slaughter increases lipid metabolites and decreases oxidative stability of farmed rainbow trout (Oncorhynchus mykiss) during frozen storage. Food Chem. 2016, 190, 5–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Li, Q.; Lyu, J.; Kong, C.; Song, S.; Luo, Y. The impact of stunning methods on stress conditions and quality of silver carp (Hypophthalmichthys molitrix) fillets stored at 4 °C during 72 h postmortem. Food Chem. 2017, 216, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Loulier, J.; Lefort, F.; Stocki, M.; Asztemborska, M.; Szmigielski, R.; Siwek, K.; Grzywacz, T.; Hsiang, T.; Ślusarski, S.; Oszako, T.; et al. Detection of Fungi and Oomycetes by Volatiles Using E-Nose and SPME-GC/MS Platforms. Molecules 2020, 25, 5749. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Zhao, Y.; Li, L.; Yang, X.; Wu, Y.; Chen, S.; Cen, J.; Yang, S.; Yang, D. Novel insight into the formation mechanism of volatile flavor in Chinese fish sauce (Yu-lu) based on molecular sensory and metagenomics analyses. Food Chem. 2020, 323, 126839. [Google Scholar] [CrossRef] [PubMed]
- Shvartsburg, A.A. Encyclopedia of Spectroscopy & Spectrometry, 3rd ed.; American Academic Press: Salt Lake City, UT, USA, 2010; pp. 1140–1148. [Google Scholar]
- Yang, Y.; Zhang, X.; Wang, Y.; Pan, D.; Sun, Y.; Cao, J. Study on the volatile compounds generated from lipid oxidation of Chinese bacon (unsmoked) during processing. Eur. J. Lipid Sci. Technol. 2017, 119, 1600512–1600522. [Google Scholar] [CrossRef]
- Josephson, D.B.; Lindsay, R.C.; Stuiber, D.A. Enzymic Hydroperoxide Initiated Effects in Fresh Fish. J. Food Sci. 1987, 52, 596–600. [Google Scholar] [CrossRef]
- Saldaña, E.; Saldarriaga, L.; Cabrera, J.; Behrens, J.H.; Selani, M.M.; Rios-Mera, J.; Contreras-Castillo, C.J. Descriptive and hedonic sensory perception of Brazilian consumers for smoked bacon. Meat Sci. 2019, 147, 60–69. [Google Scholar] [CrossRef]
- Resconi, V.C.; Bueno, M.; Escudero, A.; Magalhaes, D.; Ferreira, V.; Campo, M.M. Ageing and retail display time in raw beef odour according to the degree of lipid oxidation. Food Chem. 2018, 242, 288–300. [Google Scholar] [CrossRef] [Green Version]
- Gu, S.; Chen, W.; Wang, Z.; Wang, J.; Huo, Y. Rapid detection of Aspergillus spp. infection levels on milled rice by headspace-gas chromatography ion-mobility spectrometry (HS-GC-IMS) and E-nose. LWT 2020, 132, 109758. [Google Scholar] [CrossRef]
- Yu, D.; Xu, Y.; Regenstein, J.M.; Xia, W.; Yang, F.; Jiang, Q.; Wang, B. The effects of edible chitosan-based coatings on flavor quality of raw grass carp (Ctenopharyngodon idellus) fillets during refrigerated storage. Food Chem. 2018, 242, 412–420. [Google Scholar] [CrossRef]
- Lu, F.; Nielsen, N.; Baron, C.; Diehl, B.; Jacobsen, C. Impact of primary amine group from aminophospholipids and amino acids on marine phospholipids stability: Non-enzymatic browning and lipid oxidation. Food Chem. 2013, 141, 879–888. [Google Scholar] [CrossRef]
- Insausti, K.; Murillo-Arbizu, M.; Urrutia, O.; Mendizabal, J.; Beriain, M.; Colle, M.; Bass, P.; Arana, A. Volatile Compounds, Odour and Flavour Attributes of Lamb Meat from the Navarra Breed as Affected by Ageing. Foods 2021, 10, 493. [Google Scholar] [CrossRef]
- Shi, W.-Z.; Wang, X.-C.; Tao, N.-P.; Liu, Y.; Xu, Q. Effect of slaughter methods on the volatile composition of cultured grass carp. J. Fish. China 2011, 35, 456–465. [Google Scholar]
- Guillén, M.D.; Carton, I.; Salmerón, J.; Casas, C. Headspace composition of cod liver oil and its evolution in storage after opening. First evidence of the presence of toxic aldehydes. Food Chem. 2009, 114, 1291–1300. [Google Scholar] [CrossRef]
- Chu, F.L.; Yaylayan, V.A. Model Studies on the Oxygen-Induced Formation of Benzaldehyde from Phenylacetaldehyde Using Pyrolysis GC-MS and FTIR. J. Agric. Food Chem. 2008, 56, 10697–10704. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.P.; Hultin, H.O. Contributions of Blood and Blood Components to Lipid Oxidation in Fish Muscle. J. Agric. Food Chem. 2002, 50, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, L.; Song, G.; Feng, J.; Zhang, Y.; Shen, Q. Development of a laser irradiation based headspace solid-phase microextraction method for high-throughput guiding the production of Maillard reaction products of tuna hydrolysate. LWT 2019, 107, 291–298. [Google Scholar] [CrossRef]
- Gardner, K.; Legako, J.F. Volatile flavor compounds vary by beef product type and degree of doneness1. J. Anim. Sci. 2018, 96, 4238–4250. [Google Scholar] [CrossRef] [Green Version]
- Hallier, A.; Prost, C.; Sérot, T. Influence of Rearing Conditions on the Volatile Compounds of Cooked Fillets of Silurus glanis(European Catfish). J. Agric. Food Chem. 2005, 53, 7204–7211. [Google Scholar] [CrossRef]
- Paleari, M.A.; Moretti, V.M.; Beretta, G.; Caprino, F. Chemical parameters, fatty acids and volatile compounds of salted and ripened goat thigh. Small Rumin. Res. 2008, 74, 140–148. [Google Scholar] [CrossRef]
- Grigorakis, K.; Alexis, M.N.; Taylor, K.D.A.; Hole, M. Comparison of wild and cultured gilthead sea bream (Sparus aurata); composition, appearance and seasonal variations. Int. J. Food Sci. Technol. 2002, 37, 477–484. [Google Scholar] [CrossRef]
- Grigorakis, K.; Taylor, K.; Alexis, M. Organoleptic and volatile aroma compounds comparison of wild and cultured gilthead sea bream (Sparus aurata): Sensory differences and possible chemical basis. Aquaculture 2003, 225, 109–119. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, F.P.; Yang, X.Y.; Du, X.L.; Liu, Y.M.; Wang, X.C. Determination of volatile flavor components of Leiocassis longirostris muscle by head space solid phase micro-extraction and gas chromatography-mass spectrometry. LWT 2015, 41, 160–165. [Google Scholar]
- Kunyaboon, S.; Thumanu, K.; Park, J.W.; Khongla, C.; Yongsawatdigul, J. Evaluation of lipid oxidation, volatile compounds and vibrational spectroscopy of silver carp (Hypophthalmichthys molitrix) during ice storage as related to the quality of its washed mince. Foods 2021, 3, 495. [Google Scholar] [CrossRef]
- Timón, M.; Ventanas, J.; Carrapiso, A.I.; Jurado, A.; Garcia, C. Subcutaneous and intermuscular fat characterisation of dry-cured Iberian hams. Meat Sci. 2001, 58, 85–91. [Google Scholar] [CrossRef]
- Song, G.; Dai, Z.; Shen, Q.; Peng, X.; Zhang, M. Analysis of the Changes in Volatile Compound and Fatty Acid Profiles of Fish Oil in Chemical Refining Process. Eur. J. Lipid Sci. Technol. 2018, 120, 1700219–1700227. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Wang, L.; Kang, C.-J.; Shi, W.-Z.; Wang, Y.T. Effects of different slaughter methods on the volatile components of rainbow trout meat. J. Gansu Agric. Univ. 2019, 54, 158–168. [Google Scholar]
- Martin, D.; Antequera, T.; Muriel, E.; Andres, A.I.; Ruiz, J. Oxidative changes of fresh loin from pig, caused by dietary conjugated linoleic acid and monounsaturated fatty acids, during refrigerated storage. Food Chem. 2008, 111, 730–737. [Google Scholar] [CrossRef]
- Lorenzo, J.M. Changes on physico-chemical, textural, lipolysis and volatile compounds during the manufacture of dry-cured foal “cecina”. Meat Sci. 2014, 96, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.; Bouckaert, C.; Lancker, F.V.; Meulenaer, B.D.; Kimpe, N. Det. Amino acid catalysis of 2-Alkylfuran formation from lipid oxidation-derived α,β-unsaturated aldehydes. J. Agr. Food Chem. 2011, 59, 11058–11062. [Google Scholar] [CrossRef] [PubMed]
- Caprino, F.; Moretti, V.M.; Bellagamba, F.; Turchini, G.M.; Busetto, M.L.; Giani, I.; Paleari, M.A.; Pazzaglia, M. Fatty acid composition and volatile compounds of caviar from farmed white sturgeon (Acipenser transmontanus). Anal. Chim. Acta 2008, 617, 139–147. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Zhu, Y.; Wang, X.; Shi, W. Volatile components present in different parts of grass carp. J. Food Biochem. 2018, 42, 12668. [Google Scholar] [CrossRef]
- Chen, J.; Tao, L.; Zhang, T.; Zhang, J.; Wu, T.; Luan, D.; Ni, L.; Wang, X.; Zhong, J. Effect of four types of thermal processing methods on the aroma profiles of acidity regulator-treated tilapia muscles using E-nose, HS-SPME-GC-MS, and HS-GC-IMS. LWT 2021, 147, 111585–111597. [Google Scholar] [CrossRef]
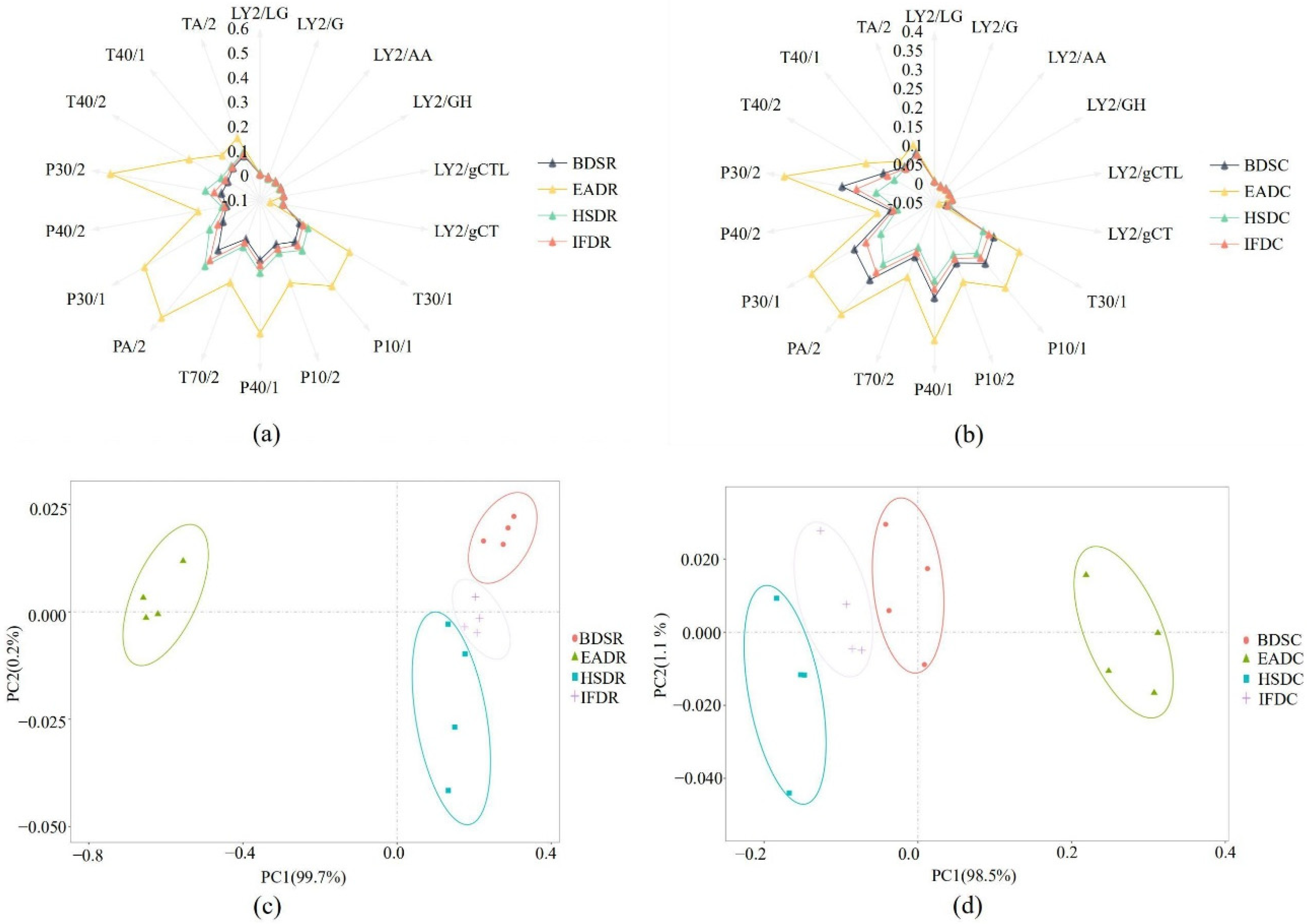
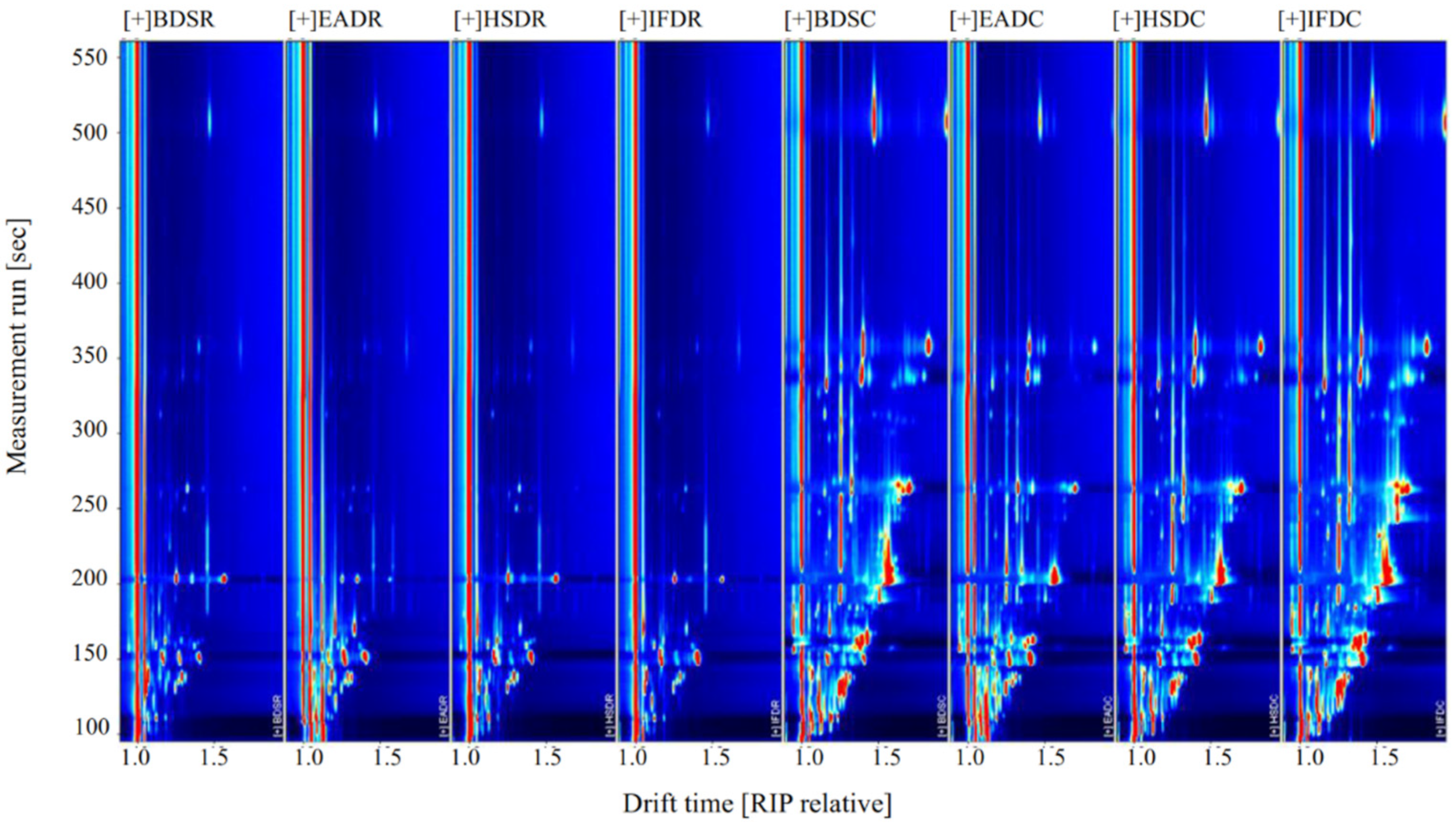

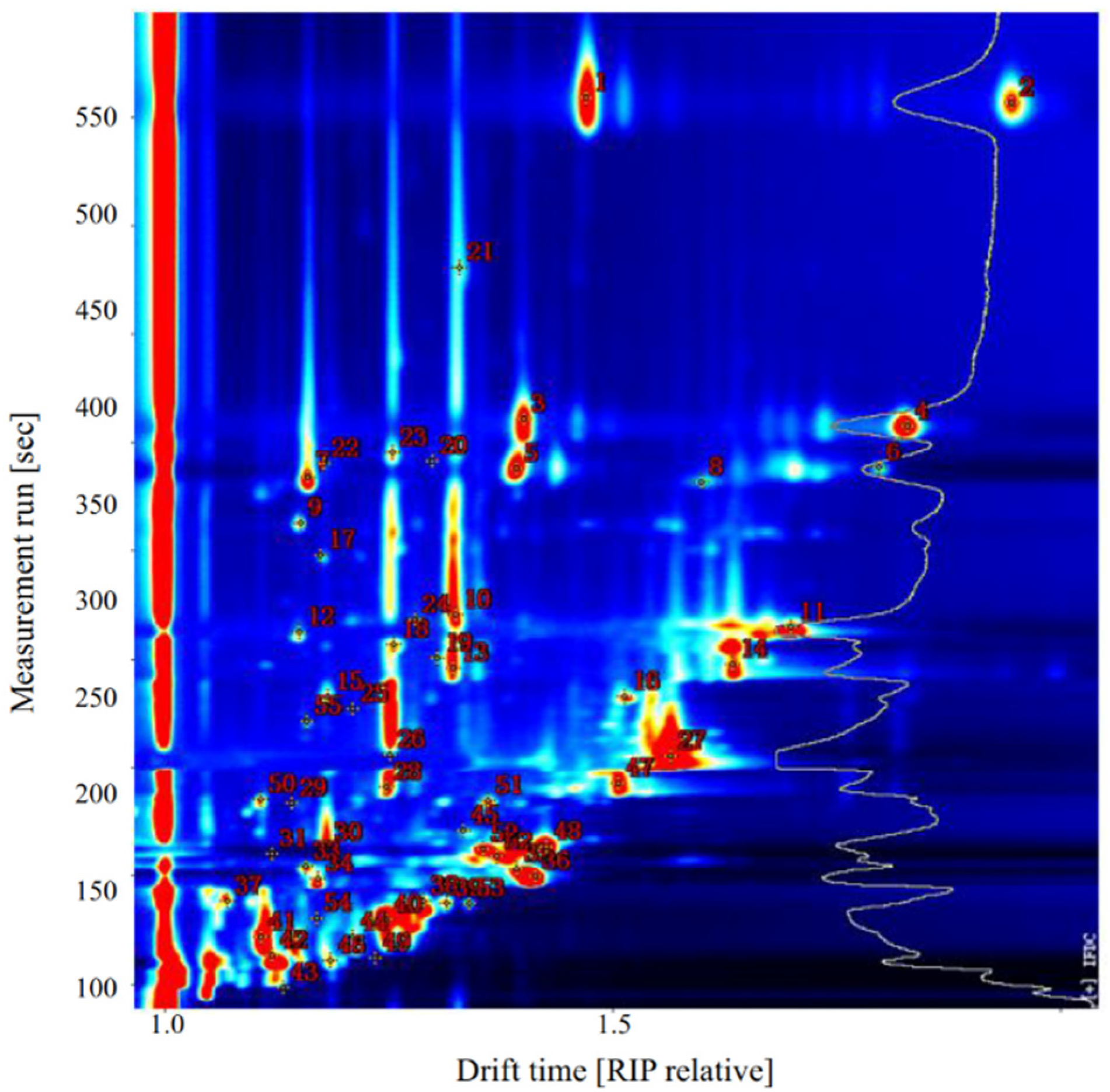
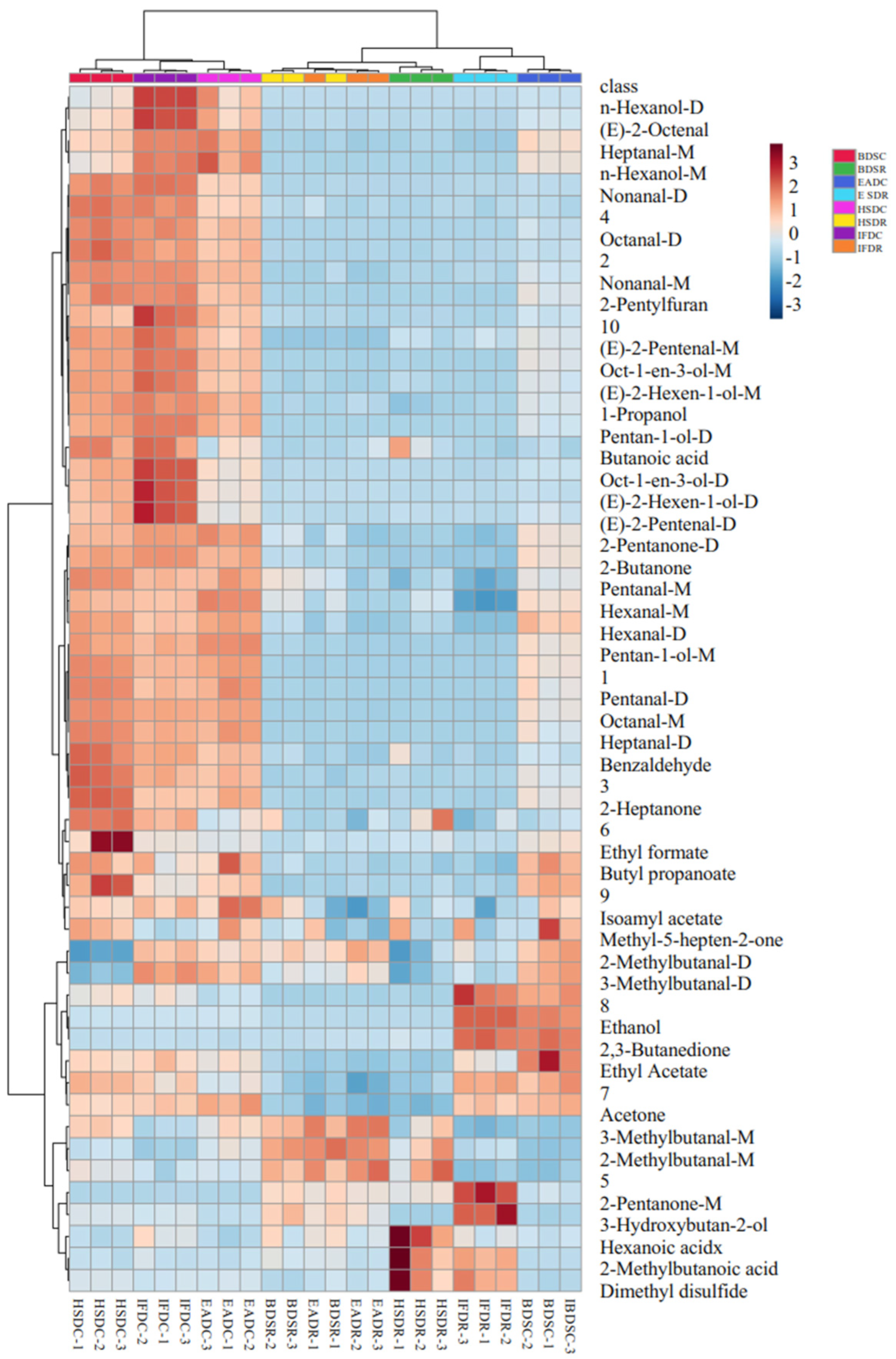
| Evaluation Dimension | Steaming Samples | |||
|---|---|---|---|---|
| EADC | HSDC | IFDC | BDSC | |
| Flavor | 3.75 ± 0.90 d | 6.06 ± 0.58 c | 7.44 ± 0.58 b | 8.40 ± 0.48 a |
| Texture | 7.25 ± 0.89 b | 7.69 ± 0.84 ab | 5.88 ± 0.69 a | 8.38 ± 0.58 a |
| Comprehensive score | 5.56 ± 0.78 d | 6.81 ± 0.46 c | 7.50 ± 0.46 b | 8.44 ± 0.56 a |
| Count. | Compound | CAS# | RI | Rt (s) | Dt (ms) | Peak Volume | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HSDR | IFDR | BDSR | EADR | EADC | HSDC | BDSC | IFDC | ||||||
| Aldehydes | |||||||||||||
| 1 | Nonanal-M | 124,196 | 1109.7 | 508.8 | 1.47 | 784.3 ± 203.0 a | 528.7 ± 86.8 b | 815.9 ± 41.9 a | 874.8 ± 59.0 a | 1400.4 ± 136.2 C | 3204.3 ± 12.3 B | 3931.2 ± 92.1 A | 3924.7 ± 37.7 A |
| 2 | Nonanal-D | 124,196 | 1108 | 506.3 | 1.95 | 84.9 ± 22.7 a | 72.6 ± 10.5 a | 86.6 ± 8.6 a | 97.0 ± 9.1 a | 172.9 ± 27.6 D | 944.5 ± 26.1 C | 1548.1 ± 110.1 B | 1731.3 ± 39.5 A |
| 9 | Benzaldehyde | 100,527 | 957.6 | 312.7 | 1.15 | 116.3 ± 8.6 a | 88.5 ± 5.1 a | 131.3 ± 51.9 a | 99.4 ± 10.9 a | 135.7 ± 10.9 D | 244.6 ± 12.7 C | 325.0 ± 20.5 A | 276.1 ± 5.0 B |
| 10 | Heptanal-M | 111,717 | 907.7 | 270.2 | 1.33 | 419 ± 23.4 a | 297.6 ± 31 b | 433.8 ± 56.9 a | 232.6 ± 9.5 b | 1567.3 ± 148.8 B | 2664.4 ± 145.8 A | 1872.3 ± 118.2 B | 2870.9 ± 28.3 A |
| 11 | Heptanal-D | 111,717 | 901.2 | 264.6 | 1.70 | 56.2 ± 9.8 a | 32.3 ± 1.6 b | 50.6 ± 9.6 a | 33.2 ± 4.3 b | 796.0 ± 107.4 C | 2108.0 ± 261.3 AB | 2454.3 ± 76.2 A | 1974.6 ± 74.6 B |
| 21 | (E)-2-octenal | 2,548,870 | 1055.1 | 430.2 | 1.33 | 40.1 ± 7.8 ab | 44.4 ± 7.7 a | 44.0 ± 4.7 a | 31.3 ± 3.4 b | 98.3 ± 5.6 C | 288.9 ± 73.6 B | 211.7 ± 39.9 B | 529.0 ± 20.4 A |
| 26 | Hexanal-M | 66,251 | 796.1 | 205.4 | 1.25 | 1267.4 ± 34.3 a | 925.3 ± 20.7 c | 1095.6 ± 159.1 b | 432.3 ± 52.2 d | 1498.4 ± 54.9 C | 2144.9 ± 35.0 A | 1842.1 ± 55.0 B | 1761.1 ± 17.4 B |
| 27 | Hexanal-D | 66,251 | 795.6 | 205.1 | 1.57 | 1741.0 ± 122.3 a | 541.2 ± 57.8 c | 1255.3 ± 173.5 b | 256.6 ± 26.2 c | 4895.1 ± 14.4 B | 5950.6 ± 164.4 A | 6035.4 ± 130.7 A | 4906.4 ± 169.7 B |
| 30 | Pentanal-M | 110,623 | 694.2 | 162.5 | 1.18 | 594.1 ± 68.9 a | 451.9 ± 103.5 ab | 389.0 ± 89.5 bc | 273.2 ± 35.9 c | 584.5 ± 19.4 C | 877.4 ± 65.5 B | 955.1 ± 19.2 A | 822.4 ± 22.1 B |
| 33 | 2-methylbutanal-M | 96,173 | 665.6 | 154.1 | 1.16 | 535.7 ± 47.1 a | 538.5 ± 10.7 a | 421.0 ± 116.1 a | 268.4 ± 18.8 b | 195.7 ± 6.0 C | 323.9 ± 28.7 A | 280.1 ± 10 B | 216.5 ± 11.5 C |
| 34 | 3-methylbutanal-M | 590,863 | 644.6 | 148.4 | 1.17 | 602.6 ± 13.9 b | 734.6 ± 7.2 a | 441.1 ± 149.2 c | 239.7 ± 19.2 c | 275.2 ± 15.3 C | 496.7 ± 53.3 A | 550.9 ± 21.7 A | 340.4 ± 19.1 B |
| 35 | 2-methylbutanal-D | 96,173 | 662.6 | 153.3 | 1.39 | 1268.0 ± 101.6 ab | 1383.0 ± 232.4 a | 558.3 ± 283.5 c | 938.2 ± 162.6 bc | 1497.3 ± 150.8 A | 1269.8 ± 92.3 B | 361.8 ± 46.3 C | 1430.6 ± 59.1 AB |
| 36 | 3-methylbutanal-D | 590,863 | 649.6 | 149.8 | 1.41 | 1276.6 ± 160.3 ab | 1494.3 ± 274.9 a | 545.0 ± 106.0 c | 995.1 ± 174.9 b | 2230.6 ± 153.2 AB | 2126.1 ± 188.2 B | 536.1 ± 102.3 C | 2442.6 ± 12.0 A |
| 48 | Pentanal-D | 110,623 | 695.9 | 163.2 | 1.43 | 102.9 ± 9.7 a | 41.9 ± 0.7 bc | 56.2 ± 12.1 b | 39.2 ± 4.0 c | 873.1 ± 286.6 C | 1860.4 ± 232.2 AB | 2064.4 ± 37.4 A | 1502.0 ± 100.1 B |
| 50 | (E)-2-pentenal-M | 1,576,870 | 749.6 | 185.0 | 1.11 | 26.8 ± 3.0 b | 22.7 ± 1.3 b | 64.2 ± 15.4 a | 67.8 ± 12.5 a | 95.8 ± 7.5 D | 164.0 ± 15.6 C | 212.9 ± 4.3 B | 240.6 ± 21.5 A |
| 51 | (E)-2-pentenal-D | 1,576,870 | 748.3 | 184.5 | 1.37 | 8.1 ± 2.4 ab | 5.6 ± 0.2 b | 12.1 ± 3.6 a | 6.8 ± 2.0 b | 23.7 ± 6.6 C | 51.9 ± 8.6 C | 108.6 ± 13.7 B | 202.4 ± 26.7 A |
| Alcohols | |||||||||||||
| 7 | oct-1-en-3-ol-M | 3,391,864 | 982.4 | 333.9 | 1.16 | 123.5 ± 11.7 a | 100.3 ± 19.1 ab | 124.3 ± 17.5 a | 91.6 ± 9.6 b | 588.5 ± 69.0 D | 1181.0 ± 54.3 C | 1431.6 ± 41.0 B | 1749.6 ± 51.0 A |
| 8 | oct-1-en-3-ol-D | 3,391,864 | 979.3 | 331.2 | 1.60 | 40.3 ± 3.2 a | 41.9 ± 1.5 a | 41.1 ± 3.1 a | 36.6 ± 3.4 a | 60.0 ± 1.6 D | 119.2 ± 16.2 C | 189 ± 12.6 B | 283.3 ± 14.6 A |
| 13 | n-Hexanol-M | 111,273 | 870.9 | 245.5 | 1.32 | 60.6 ± 8.6 a | 54.5 ± 2.0 a | 62.7 ± 8.0 a | 53.8 ± 9.3 a | 412.5 ± 27.3 B | 957.6 ± 206.7 A | 467.2 ± 113.2 B | 1006.3 ± 21.8 A |
| 14 | n-Hexanol-D | 111,273 | 874.4 | 247.4 | 1.64 | 41 ± 5.1 a | 35.7 ± 5.8 a | 37.7 ± 2.2 a | 41.2 ± 1.5 a | 134.3 ± 23.2 C | 928.0 ± 389.2 B | 436.3 ± 124.6 C | 1820.7 ± 26.5 A |
| 15 | (E)-2-hexen-1-ol-M | 928,950 | 846.7 | 232.6 | 1.18 | 60.6 ± 8.2 a | 62.4 ± 7.6 a | 76.5 ± 11.8 a | 61.4 ± 0.4 a | 119.3 ± 11.9 D | 214.7 ± 9.2 C | 266.2 ± 2.9 B | 313.7 ± 19.6 A |
| 16 | (E)-2-hexen-1-ol-D | 928,950 | 846.7 | 232.6 | 1.52 | 25.16 ± 4.9 a | 25.56 ± 1.3 a | 17.98 ± 0.9 b | 21.96 ± 3.8 ab | 34.2 ± 4.9 D | 134.8 ± 17.6 C | 249.4 ± 23.5 B | 435.7 ± 52.8 A |
| 28 | pentan-1-ol-M | 71,410 | 764 | 190.9 | 1.25 | 99.6 ± 10.9 a | 55.6 ± 8.4 b | 58.1 ± 10.5 b | 47.8 ± 5.7 b | 375.4 ± 48.0 C | 758.8 ± 9.0 A | 685.1 ± 35.7 B | 619.4 ± 47.6 B |
| 41 | 1-propanol | 71,238 | 544.8 | 121.5 | 1.11 | 423.6 ± 23.5 a | 451.5 ± 54.2 a | 281.9 ± 89.5 b | 390.0 ± 23.4 a | 737.4 ± 50.1 C | 1526.4 ± 119.3 B | 1660.4 ± 71.8 AB | 1779.3 ± 77.5 A |
| 43 | ethanol | 64,175 | 458.9 | 98.4 | 1.13 | 223.0 ± 7.0 b | 178.7 ± 10.5 c | 174.3 ± 23 c | 6116.3 ± 9.1 a | 5175.2 ± 243.1 A | 856.6 ± 45.6 B | 626.8 ± 30.8 B | 719.8 ± 19.4 B |
| 47 | pentan-1-ol-D | 71,410 | 767.6 | 192.3 | 1.51 | 23.9 ± 2.2 a | 23.7 ± 2.6 a | 24.9 ± 3.7 a | 23.5 ± 4.8 a | 275.6 ± 51.3 C | 959.1 ± 94.5 C | 1040.5 ± 61.5 B | 1286.2 ± 12.3 A |
| Ketones | |||||||||||||
| 18 | 2-heptanone | 110,430 | 891.6 | 256.6 | 1.23 | 80.1 ± 7.0 a | 59.5 ± 2.9 b | 87.2 ± 12.1 a | 58.4 ± 8.2 b | 275.7 ± 35.1 C | 508.4 ± 71.9 B | 736.9 ± 20.6 A | 443.5 ± 8.6 B |
| 31 | 2-Pentanone-M | 107,879 | 687.7 | 160.1 | 1.12 | 285.4 ± 11.6 b | 222.5 ± 8.2 b | 215.0 ± 25.8 b | 653.4 ± 74.7 a | 121.7 ± 20.7 A | 63.7 ± 1.4 B | 63.8 ± 1.5 B | 66.6 ± 2.4 B |
| 32 | 2-Pentanone-D | 107,879 | 682.7 | 158.7 | 1.37 | 246.7 ± 16.2 a | 104.9 ± 14.0 c | 137.6 ± 10.1 b | 87.9 ± 17.4 c | 352.9 ± 15.5 C | 607.6 ± 30.5 A | 514.0 ± 7.7 B | 588.7 ± 7.3 A |
| 40 | 2-Butanone | 78,933 | 575.7 | 129.9 | 1.25 | 481.6 ± 21.0 a | 358.4 ± 35.0 b | 319.7 ± 17.8 b | 270.5 ± 21.9 c | 841.9 ± 55.7 C | 1195.0 ± 55.3 B | 1258.2 ± 34.7 B | 1350.8 ± 7.1 A |
| 42 | acetone | 67,641 | 512.8 | 112.9 | 1.12 | 574.7 ± 56.6 b | 356.8 ± 43.1 c | 430.4 ± 57.2 c | 1408.8 ± 89.2 a | 1540.5 ± 79.8 AB | 1646.2 ± 119.2 A | 1291.5 ± 30.0 D | 1394.9 ± 58 BC |
| 45 | 3-hydroxybutan-2-one | 513,860 | 715.1 | 171.0 | 1.33 | 382.9 ± 44.0 b | 266.6 ± 39.9 b | 63.4 ± 6.3 c | 707.6 ± 119.7 a | 79.0 ± 12.4 C | 137.1 ± 9.0 B | 198.8 ± 4.1 A | 184.9 ± 12.4 A |
| 54 | 2,3-butanedione | 431,038 | 576.7 | 130.1 | 1.17 | 43.8 ± 9.3 b | 50.7 ± 4.2 b | 68.2 ± 19.6 b | 449.1 ± 29.1 a | 422.7 ± 26.7 A | 45.0 ± 10.2 B | 44.3 ± 2.2 B | 48.4 ± 3.4 B |
| 19 | isoamyl acetate | 123,922 | 880.3 | 250.6 | 1.31 | 62.5 ± 25.8 a | 39.0 ± 12.1 a | 60.0 ± 14.0 a | 44.4 ± 12.3 a | 68.5 ± 15.1 A | 91.8 ± 17.0 A | 74.5 ± 3.8 A | 82.1 ± 5.9 A |
| 24 | butyl propanoate | 590,012 | 905 | 267.8 | 1.28 | 12.9 ± 1.7 a | 11.1 ± 1.2 ab | 10.6 ± 1.7 ab | 8.7 ± 2.3 b | 30.7 ± 3.6 A | 31.0 ± 8.6 A | 31.0 ± 4.5 A | 23.8 ± 6.5 A |
| 49 | Ethyl formate | 109,944 | 509.8 | 112.2 | 1.24 | 23.7 ± 2.2 c | 37.6 ± 4.0 b | 49.3 ± 9.3 a | 25.3 ± 4.8 c | 92.6 ± 9.7 B | 71.0 ± 6.7 B | 280.2 ± 142.8 A | 89.6 ± 1.8 B |
| 53 | Ethyl Acetate | 141,786 | 602.7 | 137.1 | 1.34 | 35.0 ± 5.9 b | 19.4 ± 2.6 b | 27.9 ± 6.6 b | 88.0 ± 17.2 a | 208.0 ± 46.0 A | 97.2 ± 11.8 B | 111.6 ± 5.8 B | 122.8 ± 17.9 B |
| Acids | |||||||||||||
| 20 | Hexanoic acid | 142,621 | 991.2 | 341.4 | 1.30 | 74.1 ± 12.2 b | 66.7 ± 6.2 b | 126.0 ± 26.8 a | 67.6 ± 6.6 b | 61.0 ± 2.1 B | 54.2 ± 4.0 B | 55.7 ± 2.7 B | 72.1 ± 8.2 A |
| 25 | 2-Methylbutanoic acid | 116,530 | 835.6 | 226.6 | 1.21 | 27.1 ± 4.1 bc | 19.8 ± 4.7 c | 86.2 ± 40.9 a | 62.7 ± 4.8 ab | 15.0 ± 0.6 A | 17.9 ± 2.3 A | 17.2 ± 2.7 A | 20.1 ± 5.3 A |
| 55 | Butanoic acid | 107,926 | 825.4 | 221.1 | 1.16 | 12.1 ± 0.3 a | 15.0 ± 3.0 a | 23.7 ± 11.9 a | 11.4 ± 1.3 a | 13.1 ± 2.3 B | 21.8 ± 6.1 B | 39.4 ± 4.1 A | 41.6 ± 5.2 A |
| Furans | |||||||||||||
| 23 | 2-Pentylfuran | 3,777,693 | 995.7 | 345.2 | 1.26 | 22.2 ± 3.6 a | 16.4 ± 2.9 b | 21.0 ± 0.8 ab | 16.5 ± 0.9 b | 97.4 ± 17.4 C | 205.1 ± 9.6 B | 279.0 ± 28.2 A | 284.0 ± 8.7 A |
| Sulfur compounds | |||||||||||||
| 29 | Dimethyl disulfide | 624,920 | 745.7 | 183.5 | 1.14 | 36.5 ± 14.0 b | 41.9 ± 4.3 b | 182.6 ± 91.1 a | 148.5 ± 21.4 a | 30.7 ± 4.4 C | 53.7 ± 2.3 AB | 58.9 ± 1.0 A | 50.0 ± 4.1 B |
| Hydrocarbons | |||||||||||||
| 3 | Octanal-M | 124,130 | 1006.8 | 360.8 | 1.40 | 262.7 ± 26.0 a | 200.1 ± 32.0 b | 260.2 ± 27.0 a | 184.2 ± 14.0 b | 1215.8 ± 198.3 C | 2396.4 ± 93.9 B | 2648.9 ± 69.7 A | 2368.9 ± 60.5 B |
| 4 | Octanal-D | 124,130 | 1004.3 | 357.1 | 1.83 | 70.6 ± 5.3 a | 75.8 ± 11.3 a | 70.5 ± 7.2 a | 71.9 ± 5.9 a | 354.2 ± 134.2 C | 1629.9 ± 151.2 B | 2355.7 ± 129.7 A | 2220.2 ± 104.4 A |
| Sensors | Sensitive Compounds |
|---|---|
| LY2/LG | Oxynitride, sulfide, chloride, fluorine |
| LY2/G | Carbon oxide, amines, ammonia |
| LY2/AA | Ammonia, ethanol, acetone |
| LY2/GH | Amines, ammonia |
| LY2/gCTL | Hydrogen sulfide |
| LY/gCT | Propane, butane |
| T30/1 | Chloride |
| P10/1 | Hydrocarbon, ammonia, chlorine |
| P10/2 | Methane, ethane |
| P40/1 | Chlorine, fluorine |
| T70/1 | Toluene, xylene, carbon oxide |
| PA/2 | Amines, ammonium hydroxide, ethanol |
| P30/1 | Hydrocarbon, ammonia, ethanol |
| P40/2 | Hydrogen sulfide, chlorine, fluorine |
| P30/2 | Ketone, hydrogen sulfide |
| T40/2 | Chlorine, fluorine |
| T40/1 | Fluorine |
| TA/2 | Ethanol |
| Evaluation Project | Evaluation Content | Evaluation Standard | |
|---|---|---|---|
| Flavor | Whether there is an inherent flavor of sea bass, and whether there is any peculiar flavor | Samples have a strong chicken flavor and taste, a unique umami taste of soft-boiled chicken and no bloody taste | 9 |
| Samples have a light umami taste, a little bit of peculiar, delicious flavor and slightly bloody taste | 5 | ||
| The samples have a bloody taste without the unique umami taste and flavor | 1 | ||
| Texture profile | Based on the intuitive steaming sea bass quality and state of the skin during the oral processing | The sample has elastic, tender skin | 9 |
| The sample is tender, but the elasticity is weak | 5 | ||
| The texture of the sea bass is like chewing wax, and without elasticity | 1 | ||
| Comprehensive scores | Preference | Highly like | 9 |
| Average | 5 | ||
| Highly dislike | 1 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, J.; Wu, Y.; Yang, S.; Wang, D.; Liu, Q. Analysis of Volatile Compounds in Sea Bass (Lateolabrax japonicus) Resulting from Different Slaughter Methods Using Electronic-Nose (E-Nose) and Gas Chromatography-Ion Mobility Spectrometry. Molecules 2021, 26, 5889. https://doi.org/10.3390/molecules26195889
Wang Y, Li J, Wu Y, Yang S, Wang D, Liu Q. Analysis of Volatile Compounds in Sea Bass (Lateolabrax japonicus) Resulting from Different Slaughter Methods Using Electronic-Nose (E-Nose) and Gas Chromatography-Ion Mobility Spectrometry. Molecules. 2021; 26(19):5889. https://doi.org/10.3390/molecules26195889
Chicago/Turabian StyleWang, Yueqi, Jinxing Li, Yanyan Wu, Shengyuan Yang, Di Wang, and Qiang Liu. 2021. "Analysis of Volatile Compounds in Sea Bass (Lateolabrax japonicus) Resulting from Different Slaughter Methods Using Electronic-Nose (E-Nose) and Gas Chromatography-Ion Mobility Spectrometry" Molecules 26, no. 19: 5889. https://doi.org/10.3390/molecules26195889
APA StyleWang, Y., Li, J., Wu, Y., Yang, S., Wang, D., & Liu, Q. (2021). Analysis of Volatile Compounds in Sea Bass (Lateolabrax japonicus) Resulting from Different Slaughter Methods Using Electronic-Nose (E-Nose) and Gas Chromatography-Ion Mobility Spectrometry. Molecules, 26(19), 5889. https://doi.org/10.3390/molecules26195889








