GSH/ROS Dual-Responsive Supramolecular Nanoparticles Based on Pillar[6]arene and Betulinic Acid Prodrug for Chemo–Chemodynamic Combination Therapy
Abstract
:1. Introduction
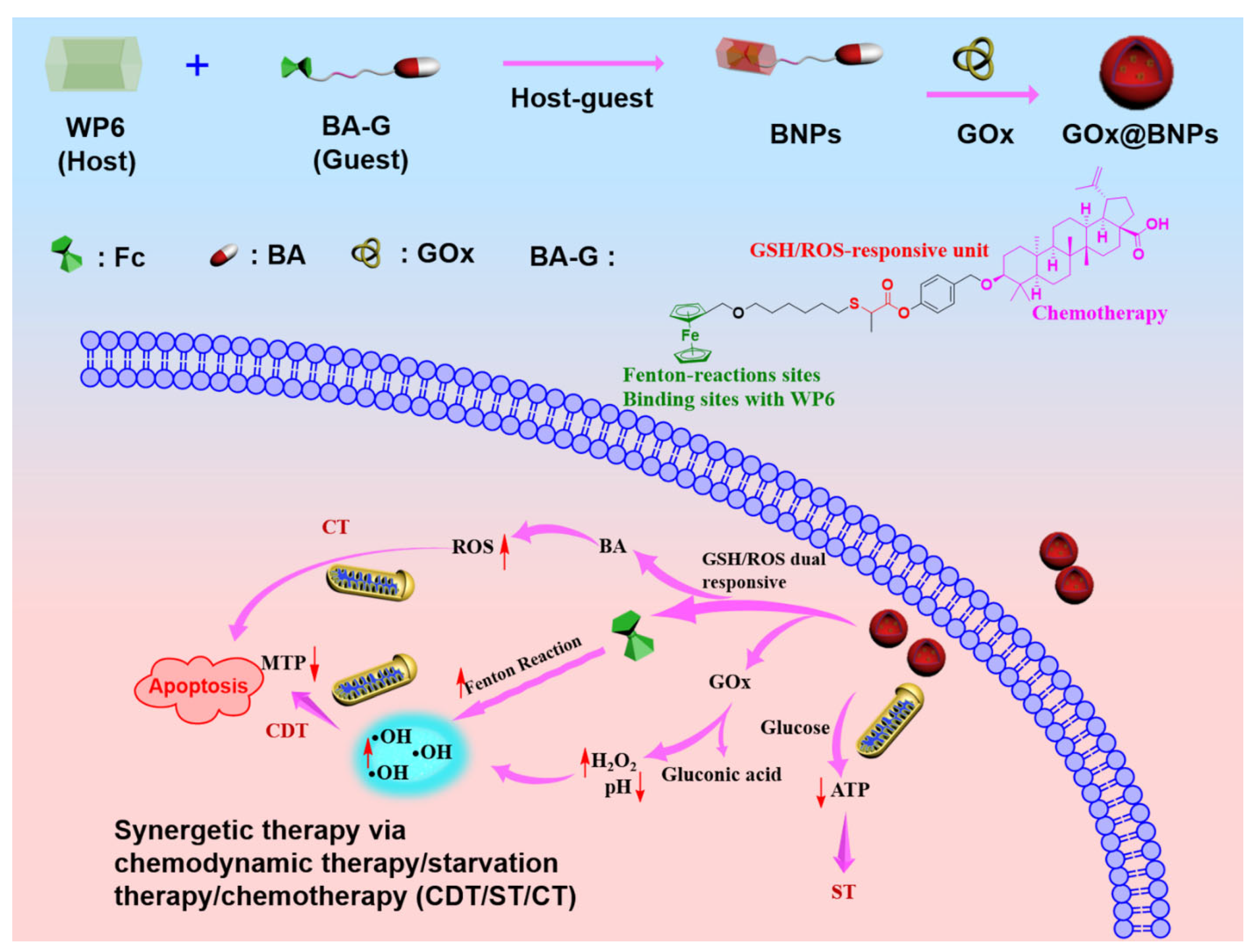
2. Results and Discussion
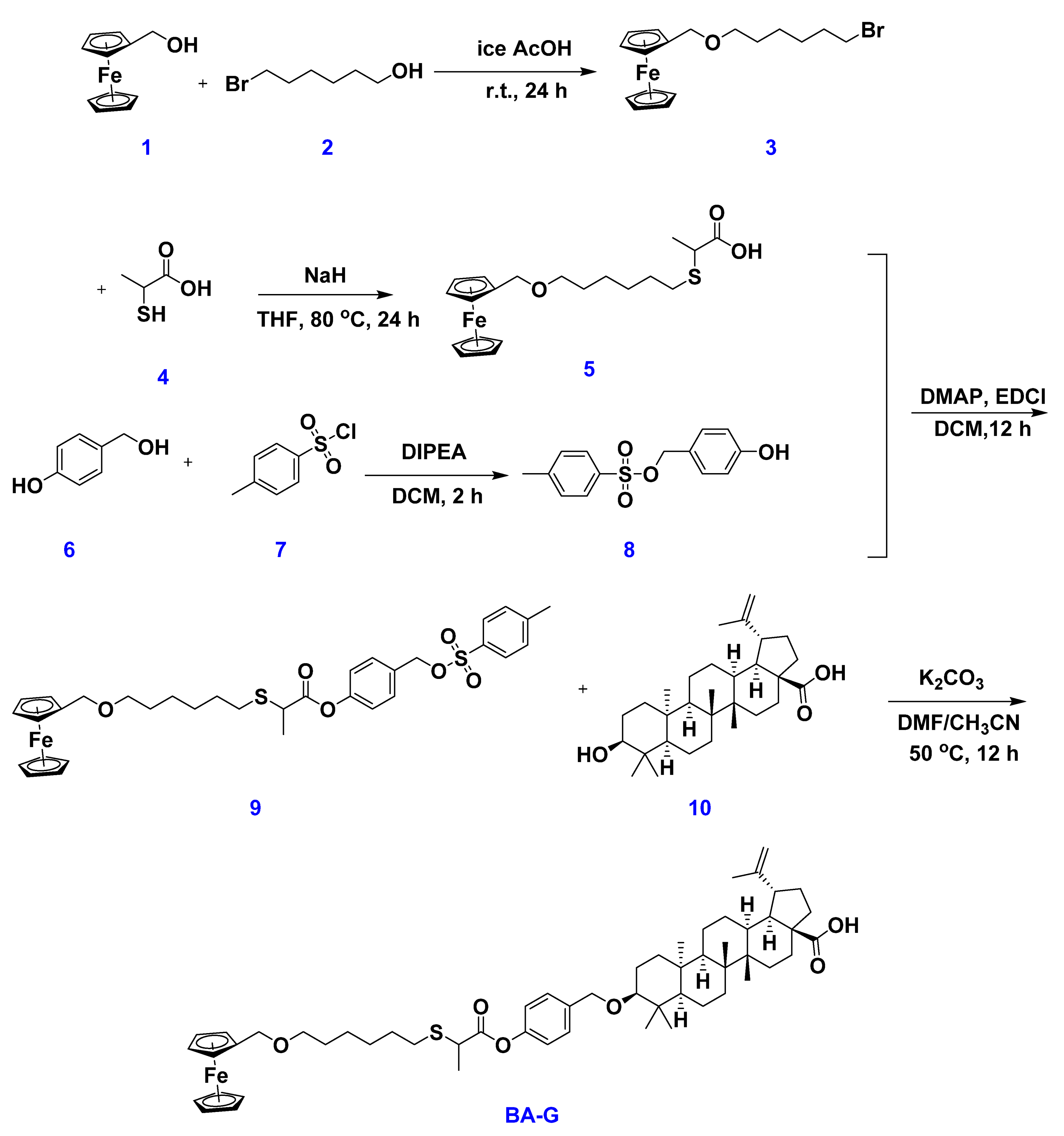
2.1. Synthesis of the Host WP6 and Guest BA-G
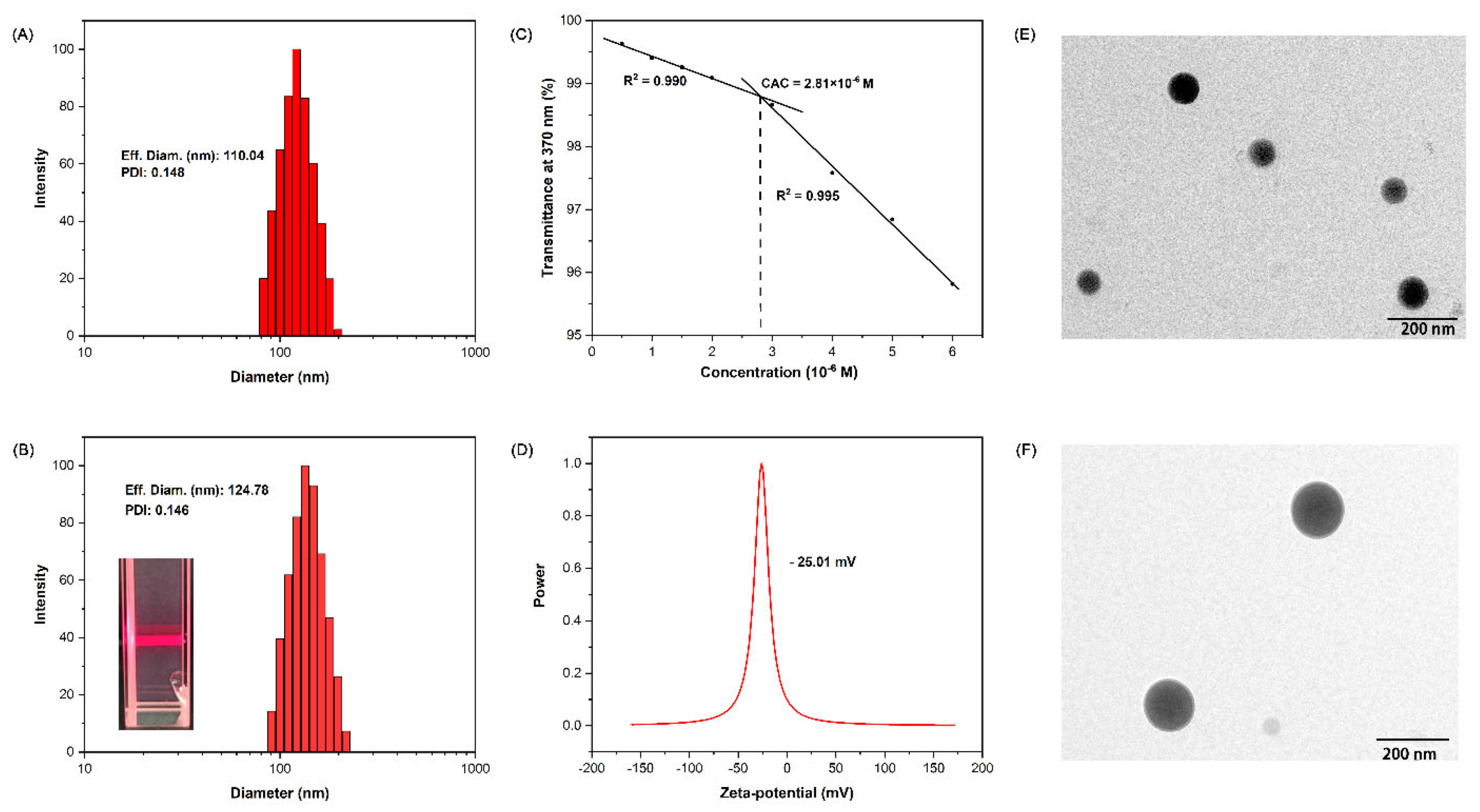
2.2. Fabrication of Supramolecular Nanoparticles: BNPs and GOx@BNPs
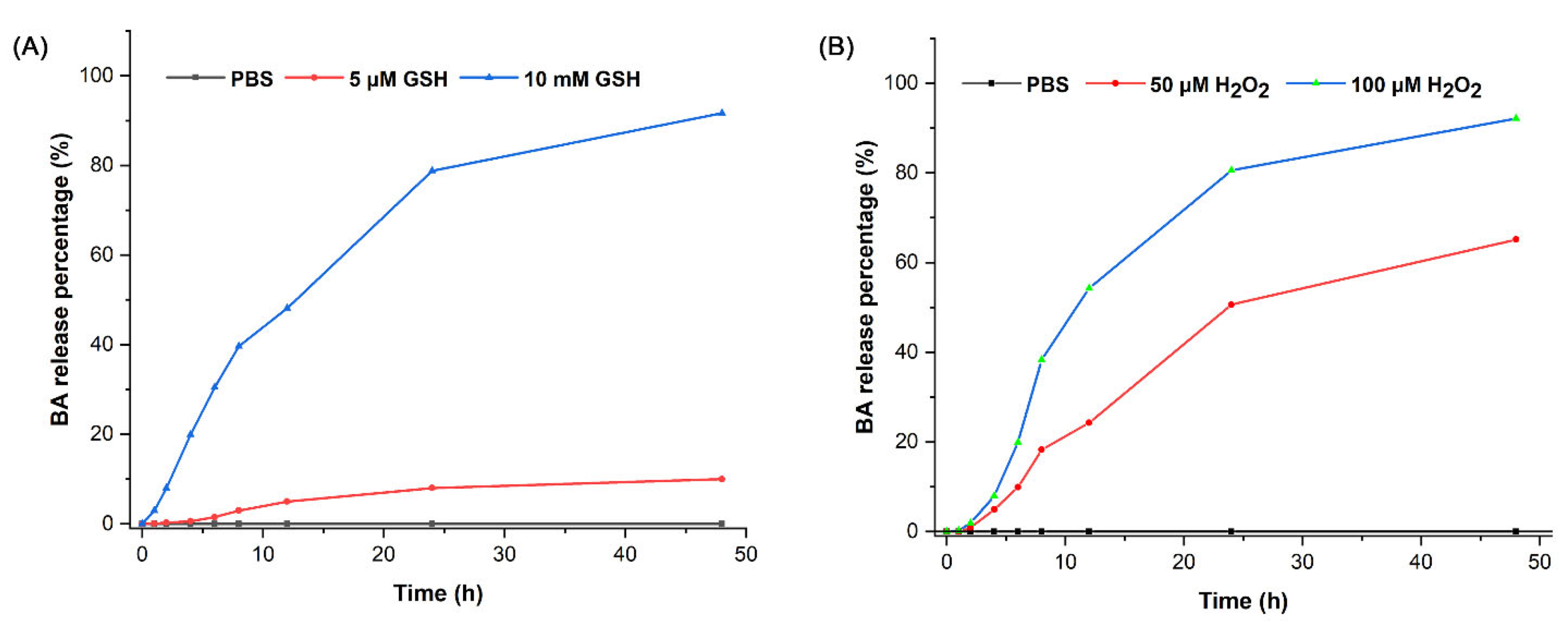
2.3. Drug Release Behavior
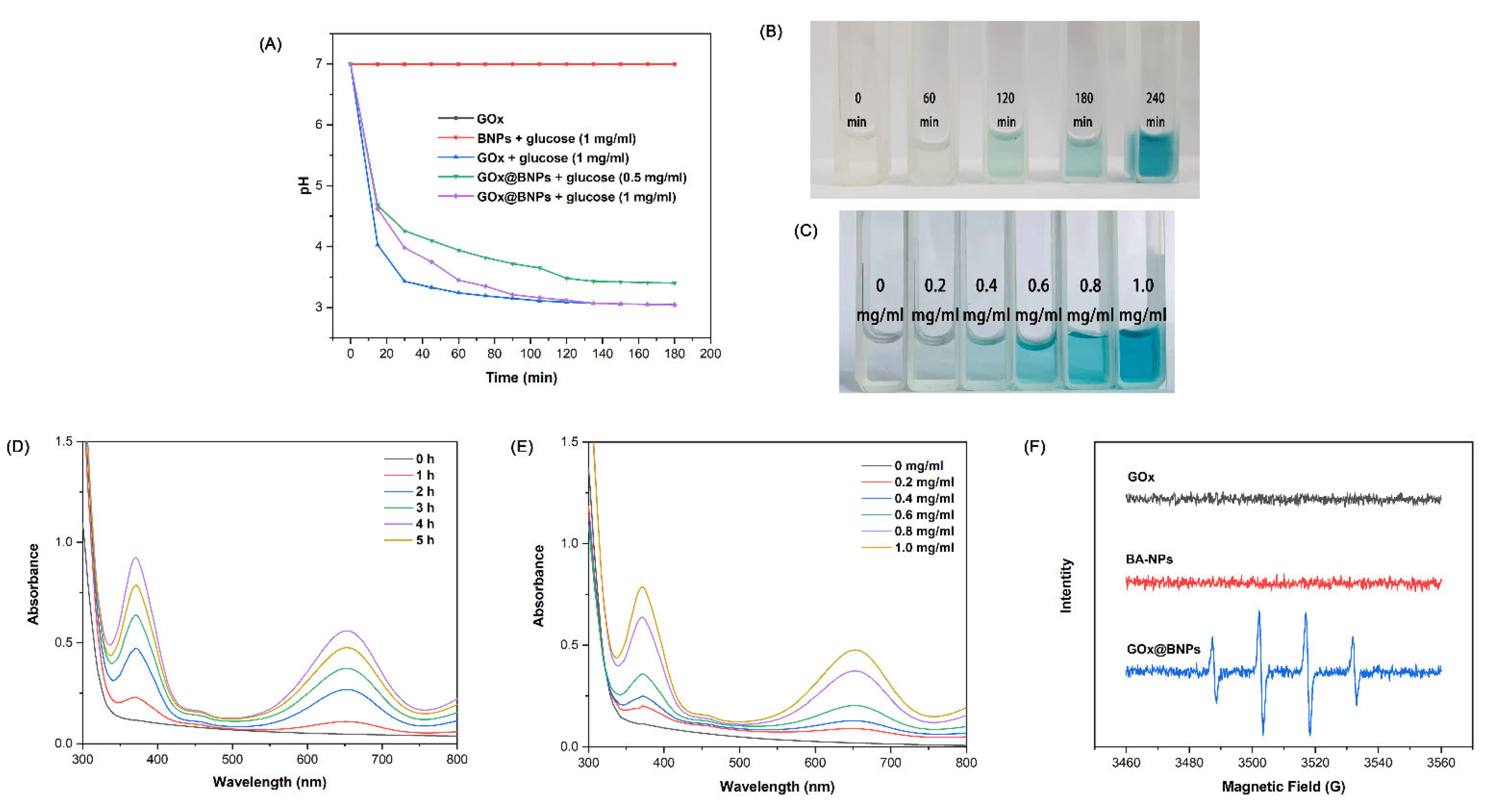
2.4. GOx@BNPs Induced Cascade Reactions
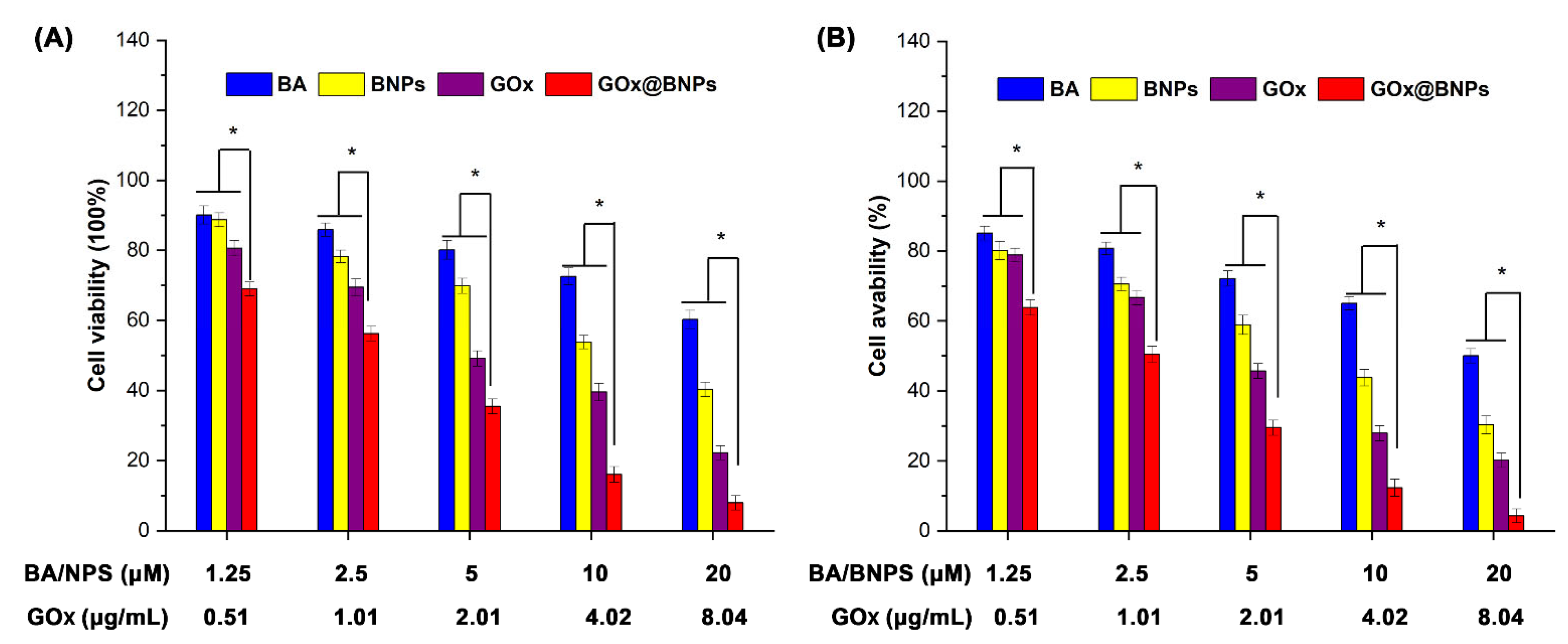
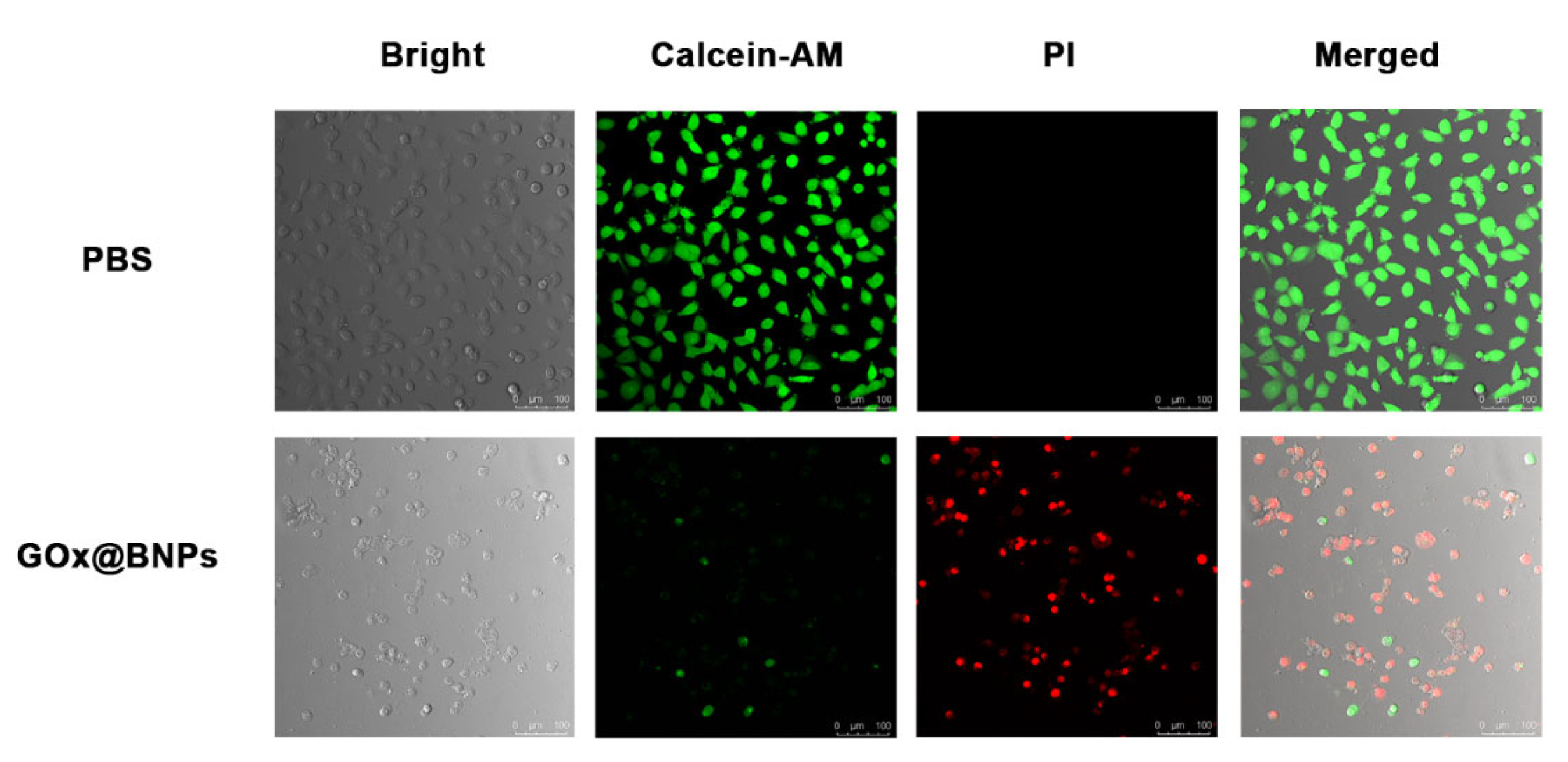
2.5. Cytotoxicity of GOx@BNPs
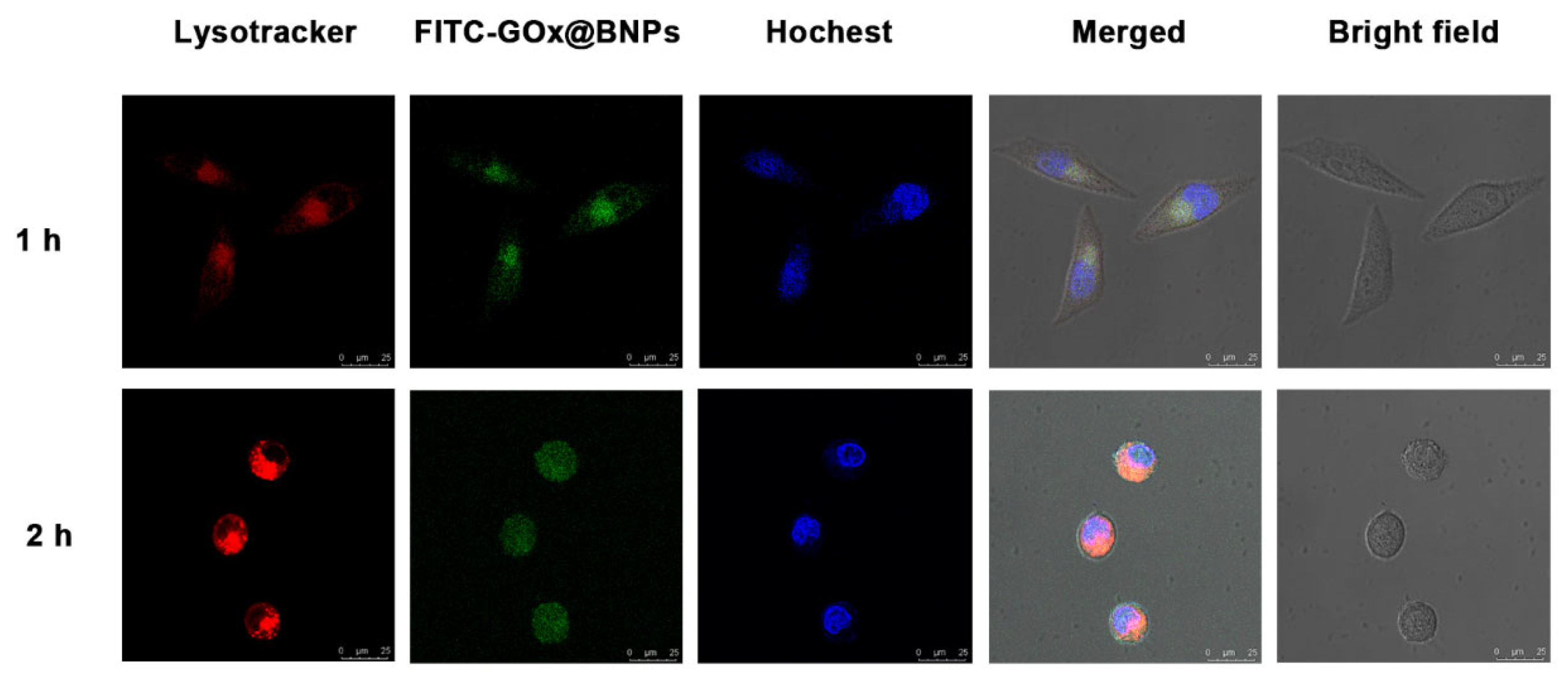
2.6. Cellular Internalization and Subcellullar Distribution
2.7. Mechanism of Cancer Inhibition
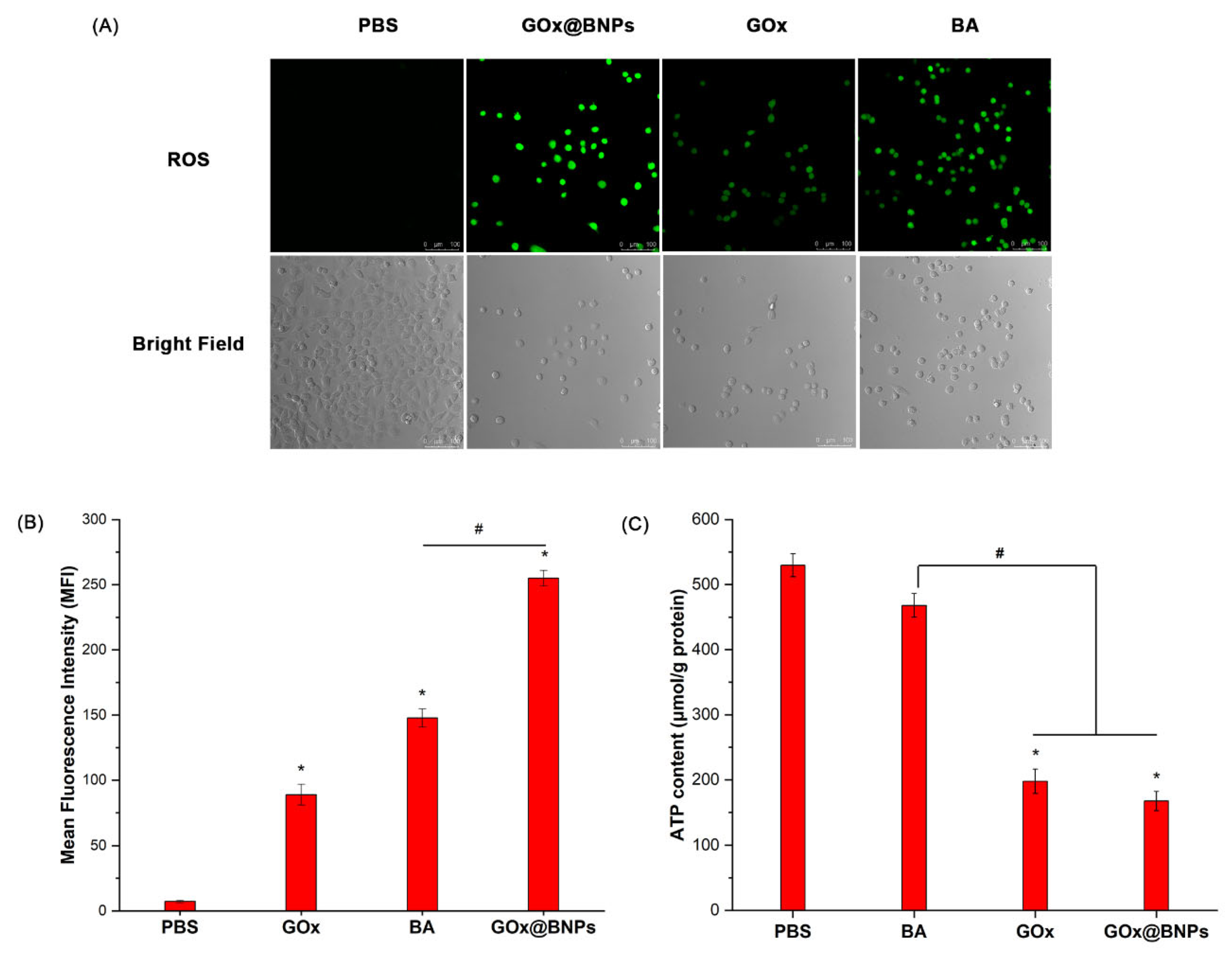
2.7.1. Elevation of ROS
2.7.2. Decrease in ATP
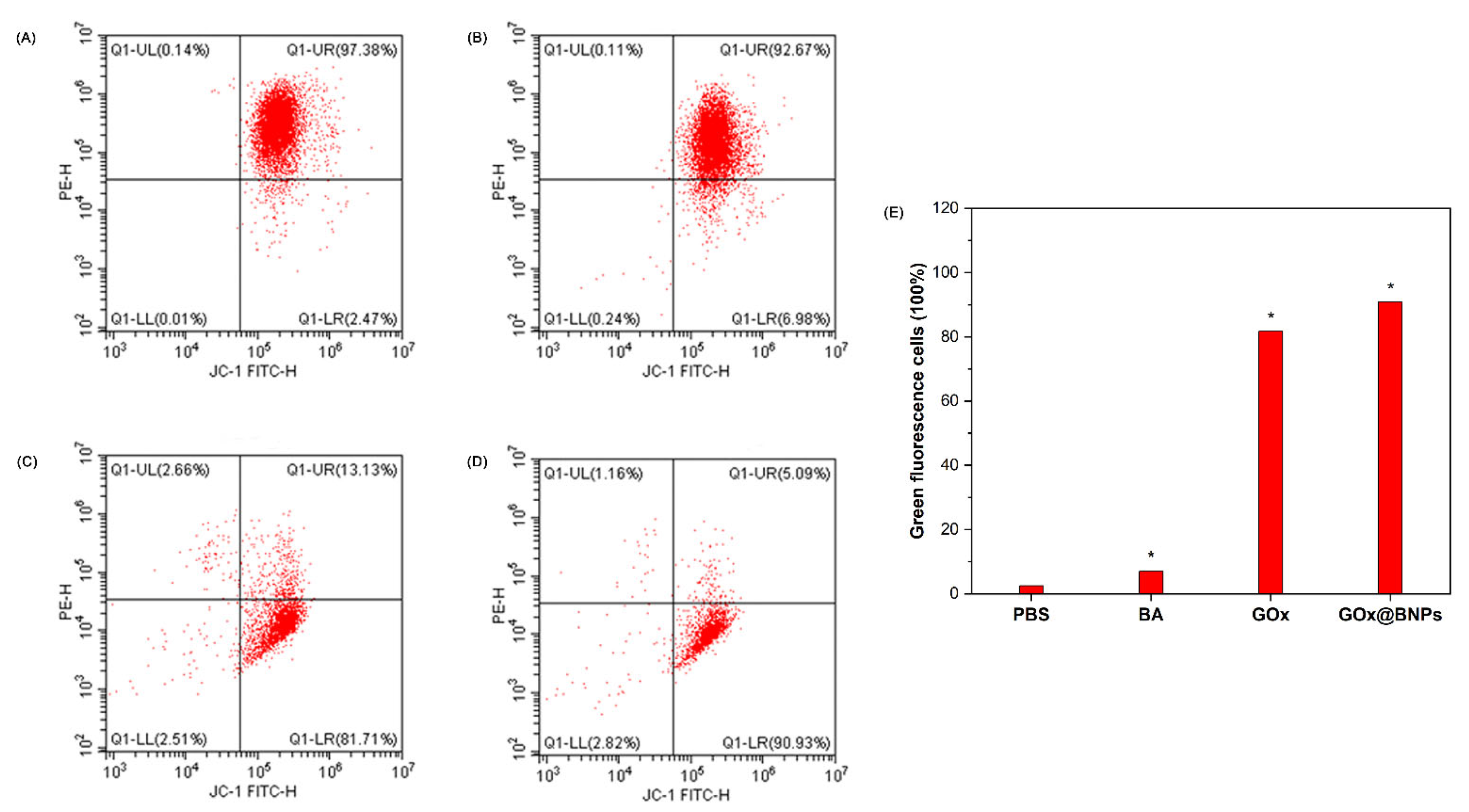
2.7.3. Evaluation of Mitochondrial Transmembrane Potential (MTP)
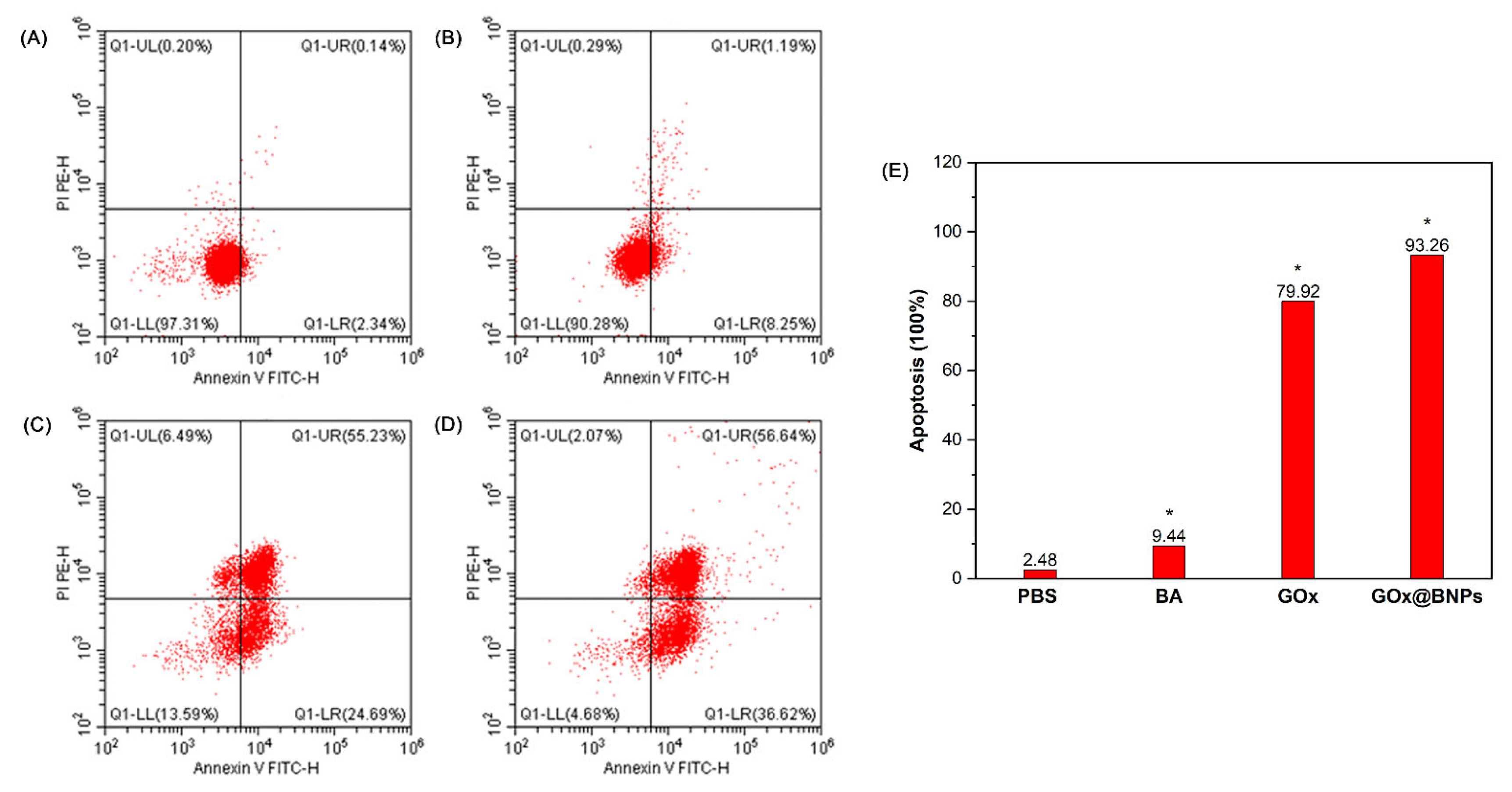
2.7.4. Induction of Apoptosis
3. Materials and Methods
3.1. General Information
3.2. Preparation of BA-G
3.2.1. Synthesis of Compound 3
3.2.2. Synthesis of Compound 5
3.2.3. Synthesis of Compound 8
3.2.4. Synthesis of Compound 9
3.2.5. Synthesis of Copound BA-G
3.3. Preparation of BNPs and GOx@BNPs
where mGOx-loaded, mGOx, mWP6, and mBA-G are the mass of GOx encapsulated into the vesicles and the mass of GOx, WP6, and BA-G added, respectively. The mass of GOx was measured via fluorescence spectroscopy at 520 nm and calculated according to a standard calibration curve of GOx in deionized water, with concentrations from 10 to 100 μg/mL.Loading content (wt %) = (mGOx-loaded/mGOx-loaded + mWP6 + mBA-G) × 100Encapsulation efficiency (%) = (mGOx-loaded/mGOx) × 100
3.4. Synthesis of FITC-GOx
3.5. In Vitro Release of BA
3.6. pH Variation under Different Concentrations of Glucose
3.7. Measurement of ·OH Generation
3.8. In Vitro Cytotoxicity by Intracellular Drug Delivery
3.9. Live/Dead Cell Staining
3.10. Cellular Uptake and Subcellular Distribution
3.11. Intracellular Reactive Oxygen Species (ROS) Detection
3.12. Intracellular ATP Level Measurements
3.13. Evaluation of Mitochondrial Transmembrane Potential (MTP)
3.14. Flow Cytometric Analysis of Apoptosis
3.15. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- D’Arcy, M.S. A review of the chemopreventative and chemotherapeutic properties of the phytochemicals berberine, resveratrol and curcumin, and their influence on cell death via the pathways of apoptosis and autophagy. Cell Biol. Int. 2020, 44, 1781–1791. [Google Scholar] [CrossRef]
- Butler, M.S.; Robertson, A.A.; Cooper, M.A. Natural product and natural product derived drugs in clinical trials. Nat. Prod. Rep. 2014, 31, 1612–1661. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Liu, X.; Deng, R.; Gao, S.; Jiang, Q.; Liu, R.; Li, H.; Miao, Y.; Zhai, Y.; Zhang, S.; et al. Betulinic acid attenuated bleomycin-induced pulmonary fibrosis by effectively intervening Wnt/beta-catenin signaling. Phytomedicine 2021, 81, 153428. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, J.; Chen, Y. Betulinic acid and the pharmacological effects of tumor suppression. Mol. Med. Rep. 2016, 14, 4489–4495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.M.; Xu, H.G.; Wang, L.; Li, Y.J.; Sun, P.H.; Wu, X.M.; Wang, G.J.; Chen, W.M.; Ye, W.C. Betulinic Acid and its Derivatives as Potential Antitumor Agents. Med. Res. Rev. 2015, 35, 1127–1155. [Google Scholar] [CrossRef] [PubMed]
- Hoenke, S.; Heise, N.V.; Kahnt, M.; Deigner, H.P.; Csuk, R. Betulinic acid derived amides are highly cytotoxic, apoptotic and selective. Eur. J. Med. Chem. 2020, 207, 112815. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Kalva, N.; Kim, H.A.; Zhang, Y.; Kim, I. pH-Responsive Polypeptide-Based Smart Nano-Carriers for Theranostic Applications. Molecules 2019, 24, 2961. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.; Hu, Y.; Yang, Y.; Wang, L.; Yang, X.; Wang, B.; Huang, M. Betulinic Acid Induces ROS-Dependent Apoptosis and S-Phase Arrest by Inhibiting the NF-kappaB Pathway in Human Multiple Myeloma. Oxid. Med. Cell Longev. 2019, 2019, 5083158. [Google Scholar] [CrossRef] [Green Version]
- Phillips, J.; Phillips, I.; Enya, B.; Zhao, H.; Nitta, T. Effect of betulinic acid and its ionic derivatives on M-MuLV replication. Biochem. Biophys. Res. Commun. 2018, 500, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Gautam, A.K.; Kumar, U.; Bhadauria, A.S.; Singh, A.K.; Kumar, D.; Mahata, T.; Maity, B.; Bera, H.; Saha, S. Mechanistic exploration of the activities of poly (lactic-co-glycolic acid)-loaded nanoparticles of betulinic acid against hepatocellular carcinoma at cellular and molecular levels. Arch. Physiol. Biochem. 2020, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Halder, A.; Jethwa, M.; Mukherjee, P.; Ghosh, S.; Das, S.; Helal Uddin, A.B.M.; Mukherjee, A.; Chatterji, U.; Roy, P. Lactoferrin-tethered betulinic acid nanoparticles promote rapid delivery and cell death in triple negative breast and laryngeal cancer cells. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1362–1371. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, J.; Fang, Y.; Yi, J.; Lu, Z.; Chen, Y.; Guo, B. Enhancing Betulinic Acid Dissolution Rate and Improving Antitumor Activity via Nanosuspension Constructed by Anti-Solvent Technique. Drug Des. Dev. Ther. 2020, 14, 243–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Bu, W.; Ni, D.; Zhang, S.; Li, Q.; Yao, Z.; Zhang, J.; Yao, H.; Wang, Z.; Shi, J. Synthesis of Iron Nanometallic Glasses and Their Application in Cancer Therapy by a Localized Fenton Reaction. Angew. Chem. Int. Ed. Engl. 2016, 55, 2101–2106. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, Y.; He, M.; Bu, W. Chemodynamic Therapy: Tumour Microenvironment-Mediated Fenton and Fenton-like Reactions. Angew. Chem. Int. Ed. Engl. 2019, 58, 946–956. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, P.; Wang, H.; Liu, Y.; Bu, W. Biomedicine Meets Fenton Chemistry. Chem. Rev. 2021, 121, 1981–2019. [Google Scholar] [CrossRef]
- de la Cruz-Lopez, K.G.; Castro-Munoz, L.J.; Reyes-Hernandez, D.O.; Garcia-Carranca, A.; Manzo-Merino, J. Lactate in the Regulation of Tumor Microenvironment and Therapeutic Approaches. Front Oncol. 2019, 9, 1143. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Feng, L.; Liu, J.; Zhu, W.; Dong, Z.; Wu, Y.; Liu, Z. Intelligent Albumin-MnO2 Nanoparticles as pH-/H2O2 -Responsive Dissociable Nanocarriers to Modulate Tumor Hypoxia for Effective Combination Therapy. Adv. Mater. 2016, 28, 7129–7136. [Google Scholar] [CrossRef]
- Ranji-Burachaloo, H.; Karimi, F.; Xie, K.; Fu, Q.; Gurr, P.A.; Dunstan, D.E.; Qiao, G.G. MOF-Mediated Destruction of Cancer Using the Cell’s Own Hydrogen Peroxide. ACS Appl. Mater. Inter. 2017, 9, 33599–33608. [Google Scholar] [CrossRef]
- Li, J.; Ke, W.; Wang, L.; Huang, M.; Yin, W.; Zhang, P.; Chen, Q.; Ge, Z. Self-sufficing H2O2-responsive nanocarriers through tumor-specific H2O2 production for synergistic oxidation-chemotherapy. J. Control. Release 2016, 225, 64–74. [Google Scholar] [CrossRef]
- He, T.; Xu, H.; Zhang, Y.; Yi, S.; Cui, R.; Xing, S.; Wei, C.; Lin, J.; Huang, P. Glucose Oxidase-Instructed Traceable Self-Oxygenation/Hyperthermia Dually Enhanced Cancer Starvation Therapy. Theranostics 2020, 10, 1544–1554. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.H.; Qi, C.; Lin, J.; Huang, P. Catalytic chemistry of glucose oxidase in cancer diagnosis and treatment. Chem. Soc. Rev. 2018, 47, 6454–6472. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Outschoorn, U.E.; Peiris-Pages, M.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Cancer metabolism: A therapeutic perspective. Nat. Rev. Clin. Oncol. 2017, 14, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Counihan, J.L.; Grossman, E.A.; Nomura, D.K. Cancer Metabolism: Current Understanding and Therapies. Chem. Rev. 2018, 118, 6893–6923. [Google Scholar] [CrossRef]
- Tang, Y.; Ji, Y.; Yi, C.; Cheng, D.; Wang, B.; Fu, Y.; Xu, Y.; Qian, X.; Choonara, Y.E.; Pillay, V.; et al. Self-accelerating H2O2-responsive Plasmonic Nanovesicles for Synergistic Chemo/starving therapy of Tumors. Theranostics 2020, 10, 8691–8704. [Google Scholar] [CrossRef]
- Song, N.; Lou, X.Y.; Ma, L.; Gao, H.; Yang, Y.W. Supramolecular nanotheranostics based on pillarenes. Theranostics 2019, 9, 3075–3093. [Google Scholar] [CrossRef]
- Webber, M.J.; Langer, R. Drug delivery by supramolecular design. Chem. Soc. Rev. 2017, 46, 6600–6620. [Google Scholar] [CrossRef]
- Feng, H.T.; Li, Y.; Duan, X.; Wang, X.; Qi, C.; Lam, J.W.Y.; Ding, D.; Tang, B.Z. Substitution Activated Precise Phototheranostics through Supramolecular Assembly of AIEgen and Calixarene. J. Am. Chem. Soc. 2020, 142, 15966–15974. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, C.; Long, R.; Chen, T.; Yan, C.; Yao, Y. Pillar[5]arene Based [1]rotaxane Systems With Redox-Responsive Host-Guest Property: Design, Synthesis and the Key Role of Chain Length. Front. Chem. 2019, 7, 508. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Jie, K.; Huang, F. Supramolecular Amphiphiles Based on Host-Guest Molecular Recognition Motifs. Chem. Rev. 2015, 115, 7240–7303. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gao, L.; Hu, X.Y.; Wang, L. Supramolecular Drug Delivery Systems Based on Water-Soluble Pillar[n]arenes. Chem. Rec. 2016, 16, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Li, Z.; Lin, Q.; Yang, Y.W. Functional supramolecular gels based on pillar[n]arene macrocycles. Nanoscale 2020, 12, 2180–2200. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Meng, Z.; Li, Q.; Huang, X.; Kang, Z.; Dong, H.; Chen, J.; Sun, J.; Dong, Y.; Li, J.; et al. A pH responsive complexation-based drug delivery system for oxaliplatin. Chem. Sci. 2017, 8, 4458–4464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, Z.; Achazi, K.; Haag, R.; Dong, S.; Schalley, C.A. Supramolecular hydrophobic guest transport system based on pillar [5] arene. Chem. Commun. 2015, 51, 10326–10329. [Google Scholar] [CrossRef] [Green Version]
- Kakuta, T.; Yamagishi, T.A.; Ogoshi, T. Stimuli-Responsive Supramolecular Assemblies Constructed from Pillar[n]arenes. Acc. Chem. Res. 2018, 51, 1656–1666. [Google Scholar] [CrossRef]
- Shao, L.; Zhou, J.; Hua, B.; Yu, G. A dual-responsive supra-amphiphile based on a water-soluble pillar[7]arene and a naphthalene diimide-containing guest. Chem. Commun (Camb) 2015, 51, 7215–7218. [Google Scholar] [CrossRef]
- Zuo, M.; Qian, W.; Xu, Z.; Shao, W.; Hu, X.Y.; Zhang, D.; Jiang, J.; Sun, X.; Wang, L. Multiresponsive Supramolecular Theranostic Nanoplatform Based on Pillar [5] arene and Diphenylboronic Acid Derivatives for Integrated Glucose Sensing and Insulin Delivery. Small 2018, 14, e1801942. [Google Scholar] [CrossRef]
- Lv, X.; Xia, D.; Zuo, Y.; Wu, X.; Wei, X.; Wang, P. Gemini-Type Supramolecular Amphiphile Based on a Water-Soluble Pillar [5] arene and an Azastilbene Guest and Its Application in Stimuli-Responsive Self-Assemblies. Langmuir 2019, 35, 8383–8388. [Google Scholar] [CrossRef]
- Luo, C.; Sun, J.; Liu, D.; Sun, B.; Miao, L.; Musetti, S.; Li, J.; Han, X.; Du, Y.; Li, L.; et al. Self-Assembled Redox Dual-Responsive Prodrug-Nanosystem Formed by Single Thioether-Bridged Paclitaxel-Fatty Acid Conjugate for Cancer Chemotherapy. Nano. Lett. 2016, 16, 5401–5408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, G.; Xue, M.; Zhang, Z.; Li, J.; Han, C.; Huang, F. A water-soluble pillar [6] arene: Synthesis, host-guest chemistry, and its application in dispersion of multiwalled carbon nanotubes in water. J. Am. Chem. Soc. 2012, 134, 13248–13251. [Google Scholar] [CrossRef] [PubMed]
- Durand, N.; Storz, P. Targeting reactive oxygen species in development and progression of pancreatic cancer. Expert Rev. Anticanc. Ther. 2017, 17, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Cao, Y.; Li, Y.; Hu, X.; Xiao, T.; Lin, C.; Pan, Y.; Wang, L. pH-Responsive Supramolecular Vesicles Based on Water-Soluble Pillar[6]arene and Ferrocene Derivative for Drug Delivery. J. Am. Chem. Soc. 2013, 135, 10542–10549. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, R.; Liu, S.; Zhao, Y.; Gao, J.; Zhu, L. ZT-25, a new vacuolar H(+)-ATPase inhibitor, induces apoptosis and protective autophagy through ROS generation in HepG2 cells. Eur. J. Pharmacol. 2016, 771, 130–138. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Liu, G.; Wang, X.; Hu, R.; Liang, X. PIG11 over-expression predicts good prognosis and induces HepG2 cell apoptosis via reactive oxygen species-dependent mitochondrial pathway. Biomed. Pharmacother. 2018, 108, 435–442. [Google Scholar] [CrossRef]
- Xin, J.; Deng, C.; Aras, O.; Zhou, M.; Wu, C.; An, F. Chemodynamic nanomaterials for cancer theranostics. J. Nanobiotechnol. 2021, 19, 192. [Google Scholar] [CrossRef]
- Xia, M.; Yang, M.; Wang, Y.; Tian, F.; Hu, J.; Yang, W.; Tao, S.; Lu, L.; Ding, X.; Jiang, S.; et al. dl-Mandelic acid exhibits high sperm-immobilizing activity and low vaginal irritation: A potential non-surfactant spermicide for contraception. Biomed. Pharmacother. 2020, 126, 110104. [Google Scholar] [CrossRef]
- Kumar, P.; Bhadauria, A.S.; Singh, A.K.; Saha, S. Betulinic acid as apoptosis activator: Molecular mechanisms, mathematical modeling and chemical modifications. Life Sci. 2018, 209, 24–33. [Google Scholar] [CrossRef]
- Li, J.; Dirisala, A.; Ge, Z.; Wang, Y.; Yin, W.; Ke, W.; Toh, K.; Xie, J.; Matsumoto, Y.; Anraku, Y.; et al. Therapeutic Vesicular Nanoreactors with Tumor-Specific Activation and Self-Destruction for Synergistic Tumor Ablation. Angew. Chem. Int. Ed. Engl. 2017, 56, 14025–14030. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, P.; Luo, W.; Qian, J.; Meng, C.; Shan, W.; Xu, Z.; Zhang, W.; Liu, X.; Ling, Y. GSH/ROS Dual-Responsive Supramolecular Nanoparticles Based on Pillar[6]arene and Betulinic Acid Prodrug for Chemo–Chemodynamic Combination Therapy. Molecules 2021, 26, 5900. https://doi.org/10.3390/molecules26195900
Zhu P, Luo W, Qian J, Meng C, Shan W, Xu Z, Zhang W, Liu X, Ling Y. GSH/ROS Dual-Responsive Supramolecular Nanoparticles Based on Pillar[6]arene and Betulinic Acid Prodrug for Chemo–Chemodynamic Combination Therapy. Molecules. 2021; 26(19):5900. https://doi.org/10.3390/molecules26195900
Chicago/Turabian StyleZhu, Peng, Weidan Luo, Jianqiang Qian, Chi Meng, Wenpei Shan, Zhongyuan Xu, Wei Zhang, Xin Liu, and Yong Ling. 2021. "GSH/ROS Dual-Responsive Supramolecular Nanoparticles Based on Pillar[6]arene and Betulinic Acid Prodrug for Chemo–Chemodynamic Combination Therapy" Molecules 26, no. 19: 5900. https://doi.org/10.3390/molecules26195900





